Copper Zinc Sulfide (CuZnS) Quantum Dot-Decorated (NiCo)–S/Conductive Carbon Matrix as the Cathode for Li–S Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Oxidized CNT (oxdCNT)
2.2. Synthesis of CuZnS Quantum Dots (CZSQDs)
2.3. Synthesis of (NiCo)–S@rGO/oxdCNT Matrix
2.4. Preparation of CuZnS QDs-Decorated (NiCo)–S@rGO/CNT Composite and Sulfur Composite
2.5. Characterization of the Materials
3. Results and Discussion
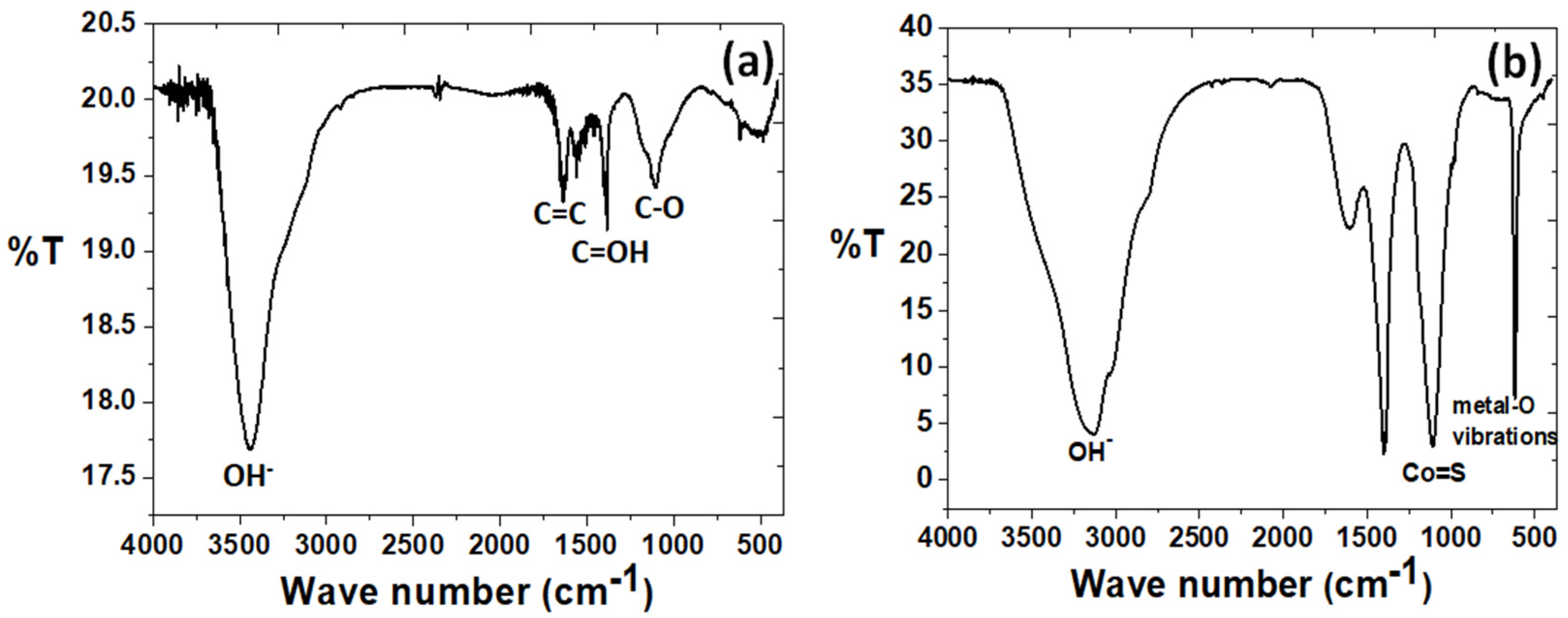
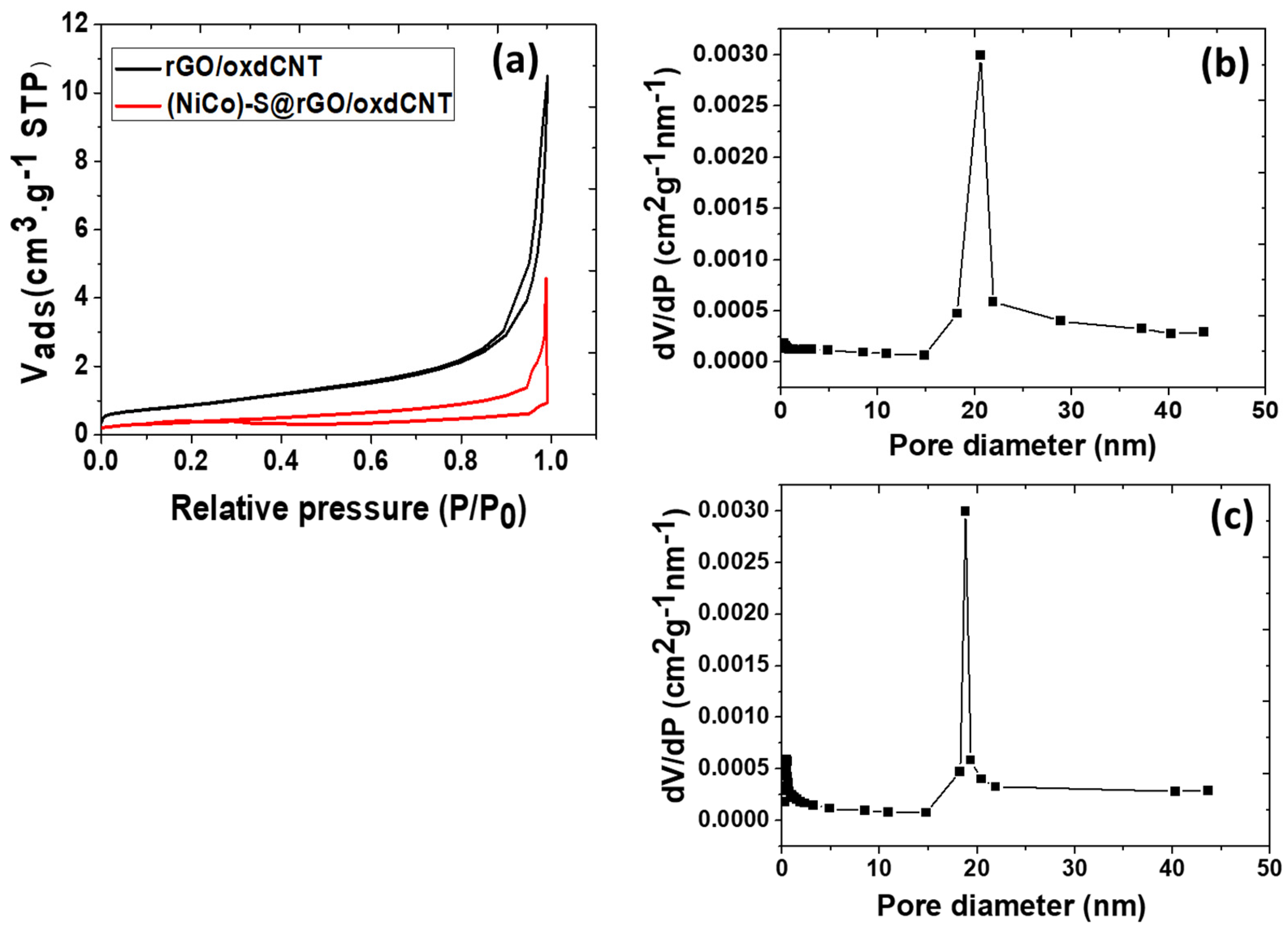
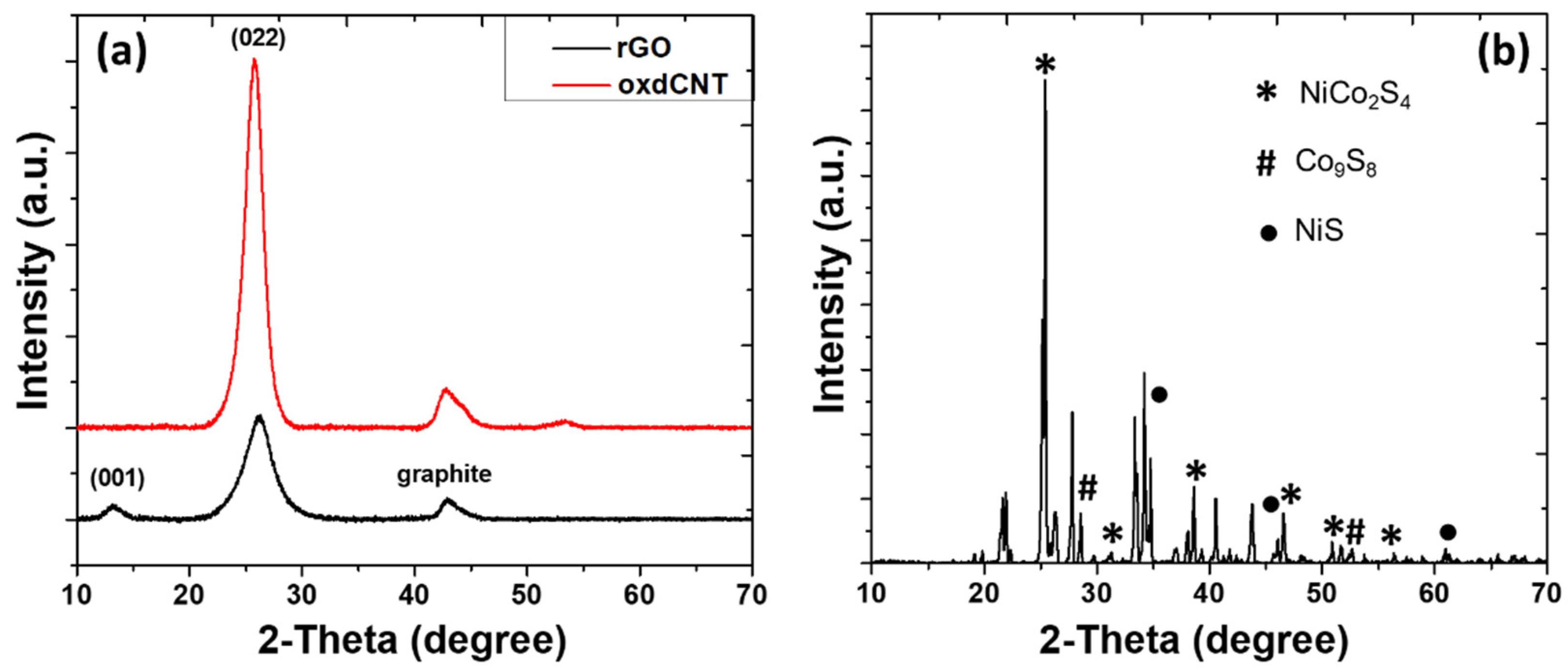
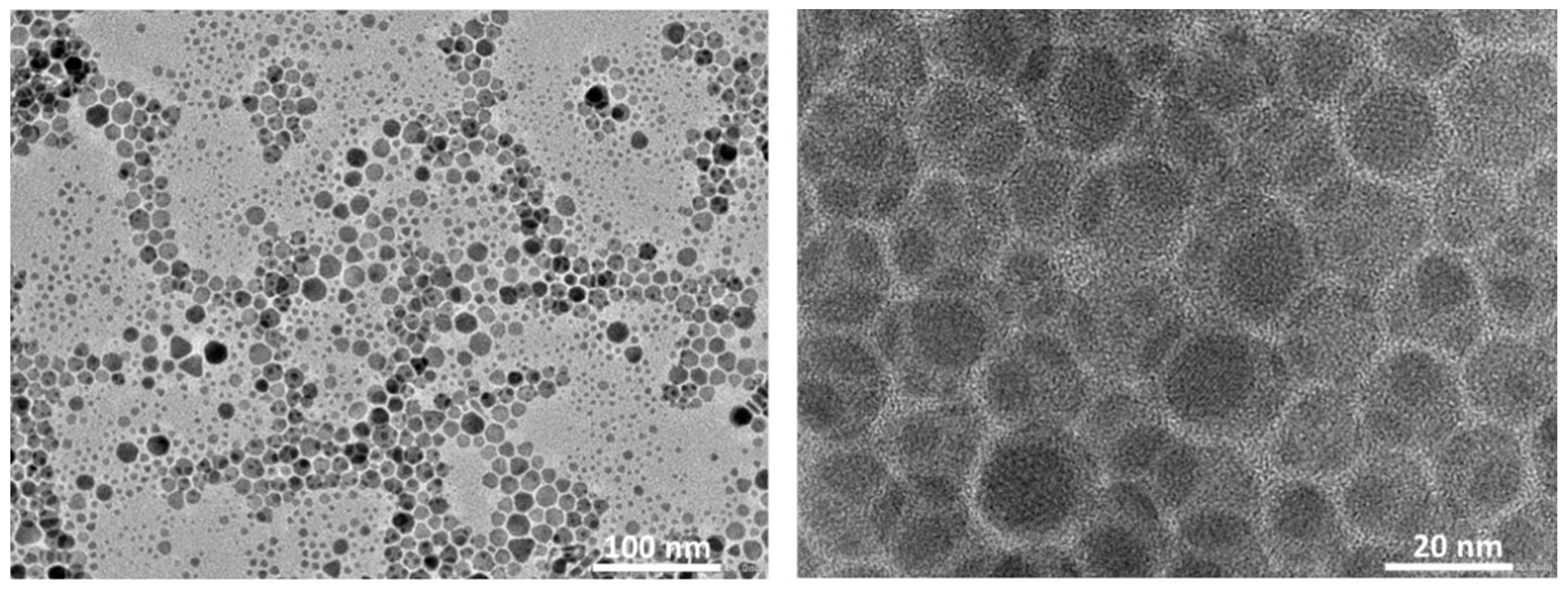
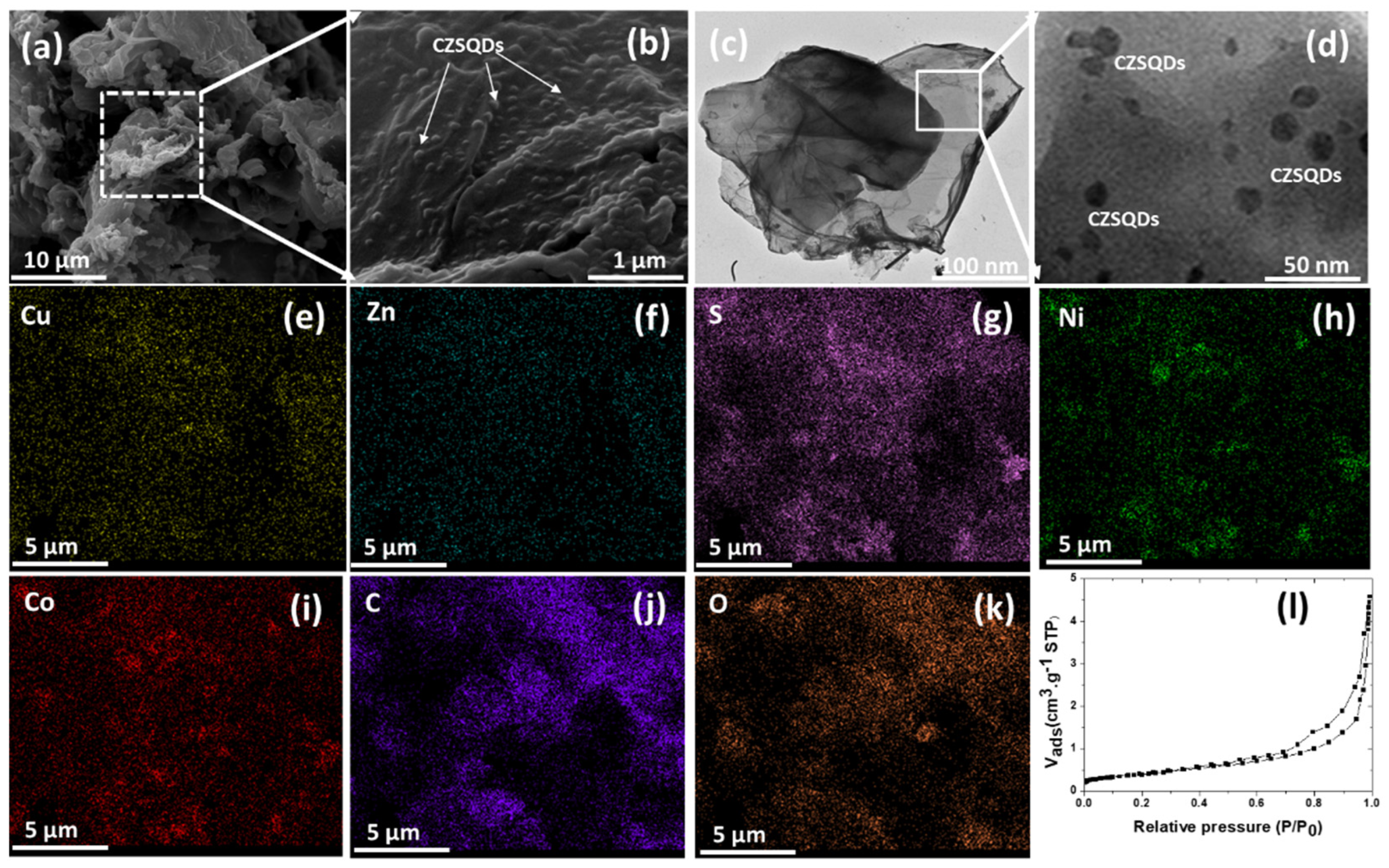
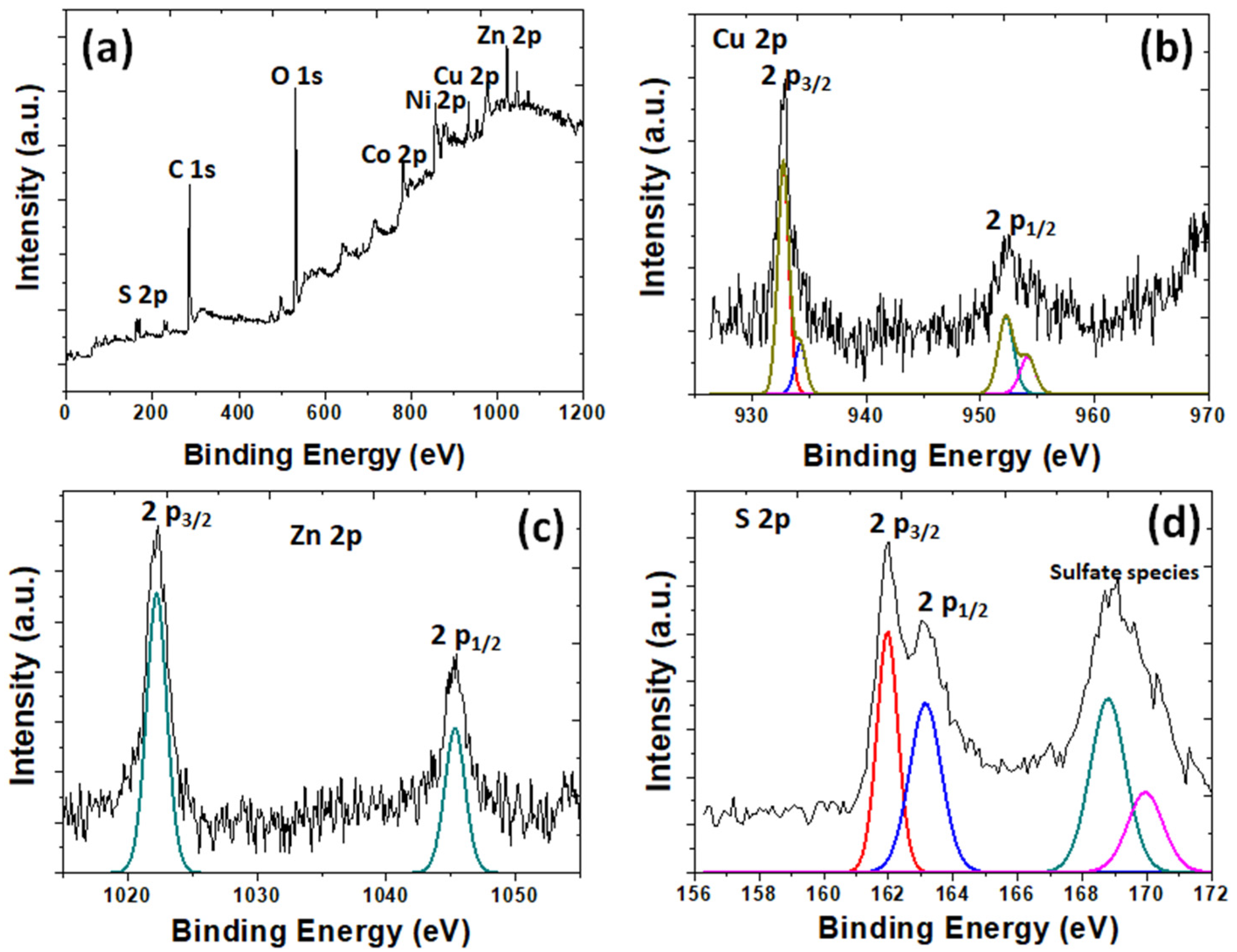
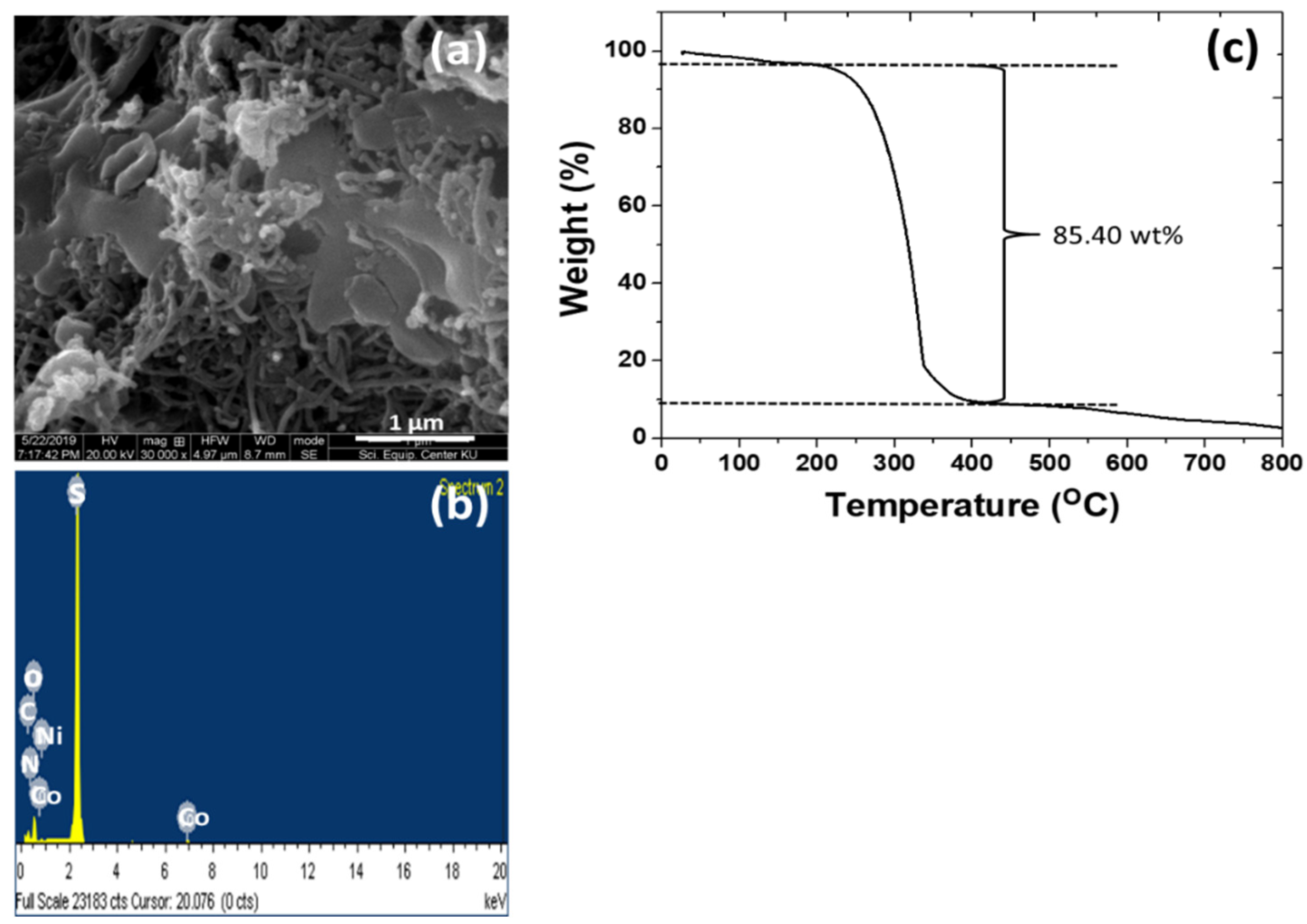
3.1. Physico-Chemical Properties of the As-Prepared rGO/oxdCNT, the (NiCo)–S@rGO/oxdCNT and the QDs Loading Materials
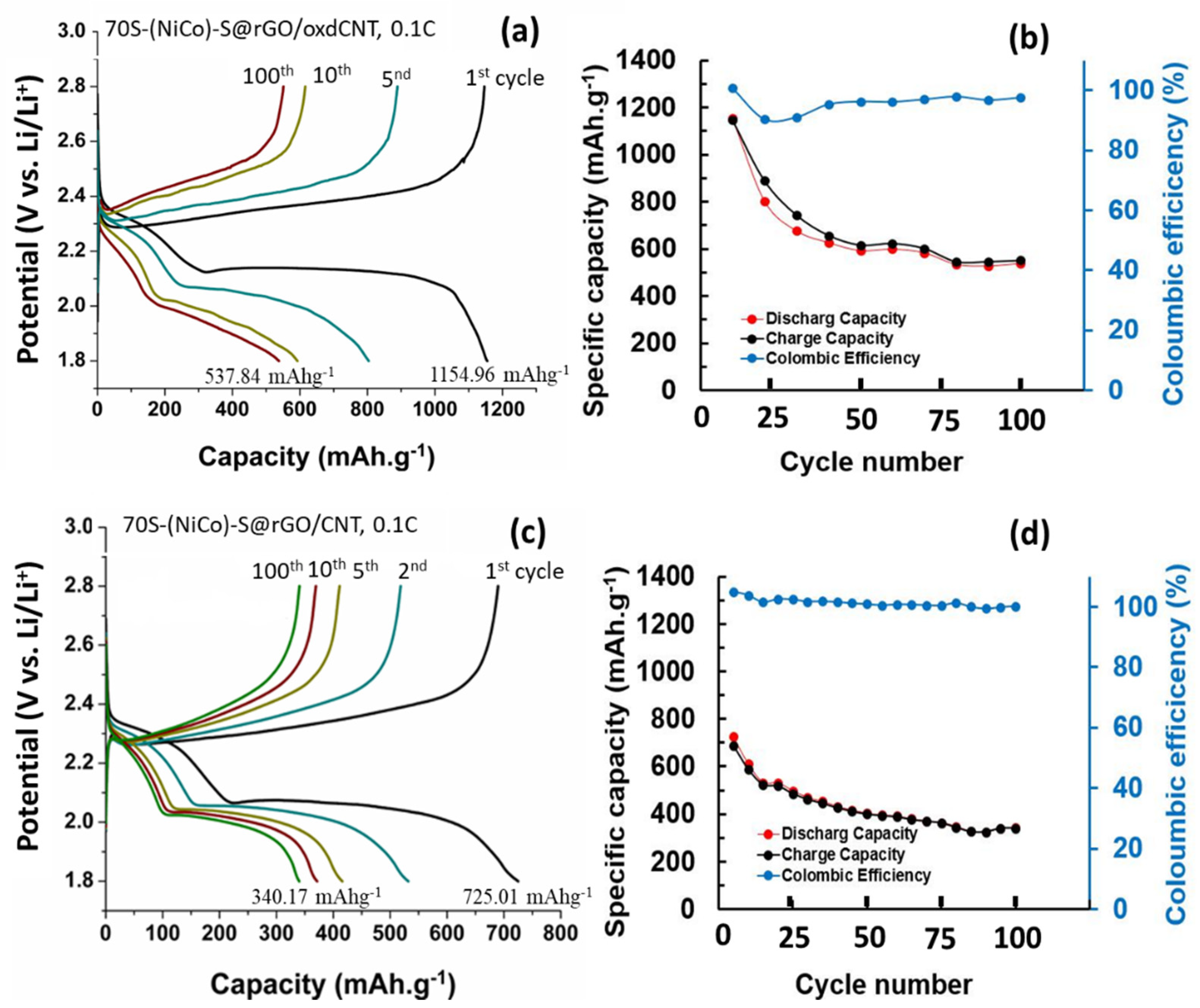
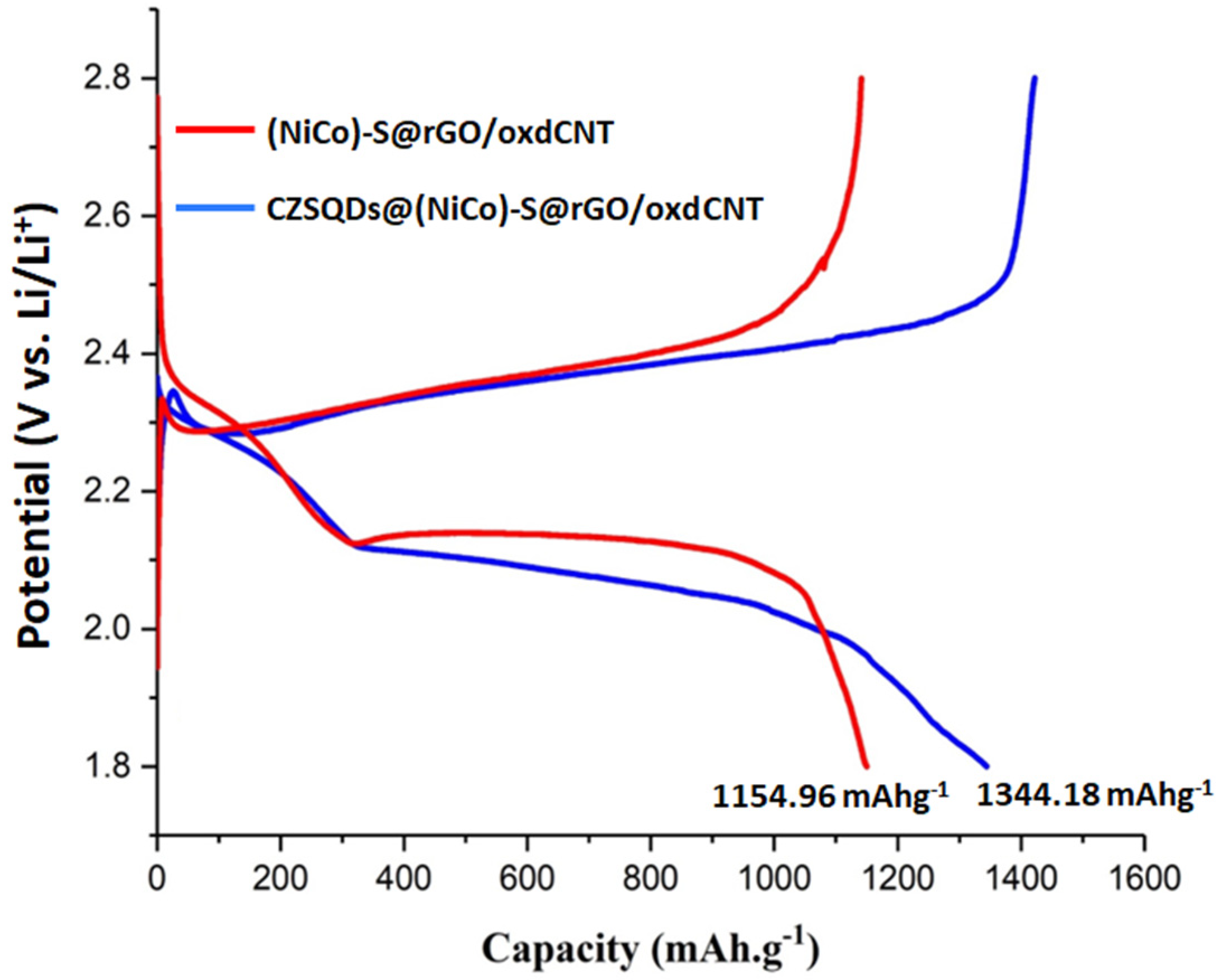
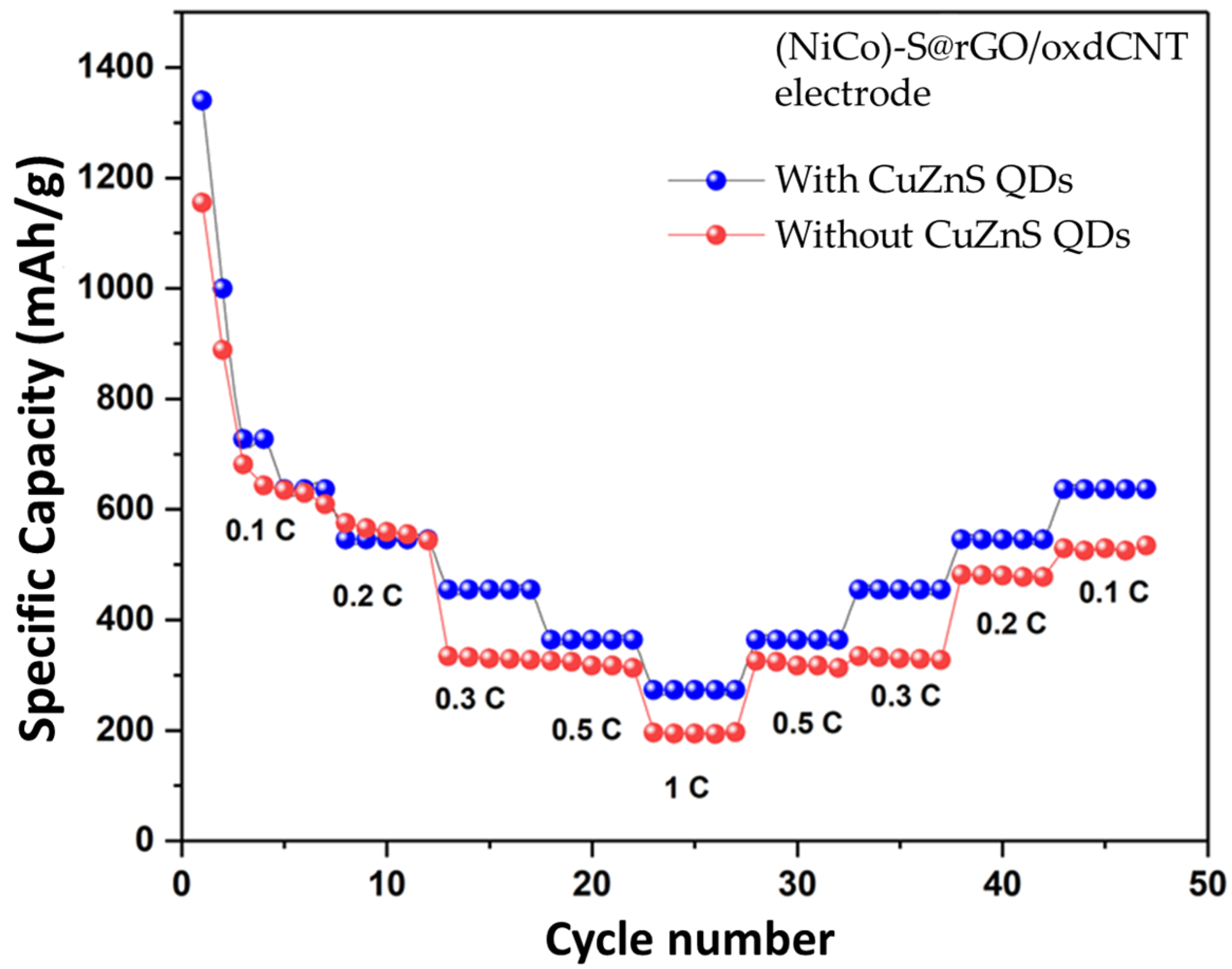
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, S.H.; Sung, Y.E. Design of structural and functional nanomaterials for lithium-sulfur batteries. Nano Today 2018, 18, 35–64. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium−Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Ely, T.O.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium-Sulfur Batteris: State of the Art and Future Directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar]
- Yao, X.; Huang, N.; Han, F.; Zhang, Q.; Wan, H.; Mwizerwa, J.P.; Wang, C.; Xu, X. High-Performance All-Solid-State Lithium–Sulfur Batteries Enabled by Amorphous Sulfur-Coated Reduced Graphene Oxide Cathodes. Adv. Energy. Mater. 2017, 7, 1602923. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Rational Design of Statically and Dynamically Stable Lithium–Sulfur Batteries with High Sulfur Loading and Low Electrolyte/Sulfur Ratio. Adv. Mater. 2017, 30, 1705951. [Google Scholar] [CrossRef]
- Luo, L.; Manthiram, A. Rational Design of High-Loading Sulfur Cathodes with a Poached-Egg-Shaped Architecture for Long-Cycle Lithium–Sulfur Batteries. ACS. Energy. Lett. 2017, 2, 2205–2211. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Popcorn Inspired Porous Macrocellular Carbon: Rapid Puffing Fabrication from Rice and Its Applications in Lithium–Sulfur Batteries. Adv. Energy. Mater. 2018, 8, 1701110. [Google Scholar] [CrossRef]
- Cheng, Z.; Pan, H.; Chen, J.; Meng, X.; Wang, R. Separator Modified by Cobalt-Embedded Carbon Nanosheets Enabling Chemisorption and Catalytic Effects of Polysulphides for High-Energy-Density Lithium-Sulfur Batteries. Adv. Energy. Mater. 2019, 9, 1901609. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Li, X.; Xiao, W.; Chen, Z.; Lu, J. Interlayer Material Selection for Lithium-Sulfur Batteries. Joule 2019, 3, 361–386. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Huang, F.; Wen, Z.; Wang, Q.; Hong, X.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur batteries with conductive polymer modified separators. J. Mater. Chem. A 2016, 4, 16968–16974. [Google Scholar] [CrossRef]
- Li, M.; Wan, Y.; Huang, J.K.; Assen, A.H.; Hsiung, C.E.; Jiang, H.; Han, Y.; Eddaoudi, M.; Lai, Z.; Ming, J.; et al. Metal–Organic Framework-Based Separators for Enhancing Li–S Battery Stability: Mechanism of Mitigating Polysulfide Diffusion. ACS. Energy. Lett. 2017, 2, 2362–2367. [Google Scholar] [CrossRef]
- Yang, C.; Suo, L.; Borodin, O.; Wang, F.; Sun, W.; Gao, T.; Fan, X.; Hou, S.; Ma, Z.; Amine, K.; et al. Unique aqueous Li-ion/sulphur chemistry with high energy density and reversibility. Proc. Natl. Acad. Sci. USA 2017, 114, 6197–6202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or Not Radical: Revisiting Lithium–Sulfur Electrochemistry in Nonaqueous Electrolytes. Adv. Energy. Mater. 2015, 5, 1401801. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries. Carbon 2019, 141, 400–416. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Chen, P. Three-dimensional porous architectures of carbon nanotubes and graphene sheets for energy applications. Front. Energy Res. 2014, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Ding, Y.; Niu, J.; Xia, Z.; Roy, A.; Chen, H.; Qu, J.; Wang, Z.L.; Dai, L. Rationally designed graphene-nanotube 3D architectures with a seamless nodal junction for efficient energy conversion and storage. Sci. Adv. 2015, 1, 1400198. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zheng, X.; Jiang, L.; Yao, D.; He, G.; Chen, H.; Che, J. CNT-intercalated rGO/sulfur laminated structure for high-rate and long-life lithium-sulfur batteries. Mater. Lett. 2018, 219, 68–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J.; Zhao, Y.; Tan, T.; Yin, F.; Wang, Y. A porous 3D-RGO@MWCNT hybrid material as Li–S battery cathode, Beilstein. J. Nanotechnol. 2019, 10, 514–521. [Google Scholar]
- Köse, H.; Kurt, B.S.; Dombaycıoğlu, Ş.; Aydın, A.O. Rational design of cathode structure based on free-standing S/rGO/CNT nanocomposite for Li-S batteries. Synth. Met. 2020, 267, 116471. [Google Scholar] [CrossRef]
- Doñoro, Á.; Muñoz-Mauricio, Á.; Etacheri, V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries 2021, 7, 26. [Google Scholar] [CrossRef]
- Dong, W.; Meng, L.; Hong, X.; Liu, S.; Shen, D.; Xia, Y.; Yang, S. MnO2/rGO/CNTs Framework as a Sulfur Host for High-Performance Li-S Batteries. Molecules 2020, 25, 1989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Meng, Z.; Ying, H.; Xu, X.; Xu, F.; Han, W. A novel CoS2/reduced graphene oxide/multiwall carbon nanotubes composite as cathode for high performance lithium ion battery. Chem. Phys. Lett. 2017, 684, 191–196. [Google Scholar] [CrossRef]
- Tiruneh, S.N.; Kang, B.K.; Kwag, S.H.; Humayoun, U.B.; Yoon, D.H. Nickel cobalt sulphide anchored in crumpled and porous graphene framework for electrochemical energy storage. Curr. Appl. Phys. 2018, 18, s37–s43. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulphides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lou, X.W. Mixed Metal Sulphides for Electrochemical Energy Storage and Conversion. Adv. Energy. Mater. 2017, 8, 1701592. [Google Scholar] [CrossRef]
- Balach, J.; Linnemann, J.; Jaumann, T.; Giebeler, L. Metal-based nanostructured materials for advanced lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 23127–23168. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Tang, S.; Qu, R. Urchin-like NiCo2S4 infused sulfur as cathode for lithium–sulfur battery. J. Mater. Sci: Mater. Electro. 2018, 30, 189–199. [Google Scholar] [CrossRef]
- Lv, J.; Bai, D.; Yang, L.; Guo, Y.; Yan, H.; Xu, S. Bimetallic sulphide nanoparticles confined by dual-carbon nanostructures as anodes for lithium-/sodium-ion batteries. Chem. Commun. 2018, 54, 8909–8912. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Yang, D.; Mao, Q.; Liu, Y.; Bao, L.; Chen, Y.; Xiong, Q.; Ji, Z.; Ling, C.D.; Liu, H.; et al. In-situ synthesis of Ni-Co-S nanoparticles embedded in novel carbon bowknots and flowers with pseudocapacitance-boosted lithium ion storage. Nanotechnology 2019, 30, 155701. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.F.; Pan, J.J.; Wei, T.T.; Li, Y.; Cao, G. NiCo2S4-based nanocomposites for energy storage in supercapacitors and batteries. Nano Today 2020, 33, 100894. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Yan, Y.; Zhao, W.; Bakenov, Z. NiCo2S4 nanoparticles embedded in nitrogen-doped carbon nanotubes networks as effective sulfur carriers for advanced Lithium-Sulfur batteries. Micropor. Mesopor. Mat. 2021, 316, 110924. [Google Scholar] [CrossRef]
- Lu, H.; Guo, Q.; Fan, Q.; Xue, L.; Lu, X.; Zan, F.; Xia, H. Cobalt sulfide quantum dot embedded in nitrogen/sulfur-doped carbon nanosheets as a polysulfide barrier in Li-S batteries. J. Alloys Compd. 2021, 870, 159341. [Google Scholar] [CrossRef]
- Li, X.; Hu, K.; Tang, R.; Zhao, K.; Ding, Y. CuS quantum dot modified carbon aerogel as an immobilizer lithium polysulphides for high-performance lithium-sulfur batteries. RSC Adv. 2016, 6, 71319–71327. [Google Scholar] [CrossRef]
- Cai, D.; Wang, L.; Li, L.; Zhang, Y.; Li, J.; Chen, D.; Tu, H.; Han, W. Self-assembled CdS quantum dots in carbon nanotubes: Induced polysulfide trapping and redox kinetics enhancement for improved lithium-sulfur battery performance. J. Mater. Chem. A 2019, 7, 806–815. [Google Scholar] [CrossRef]
- Fang, D.; Chen, S.; Wang, X.; Bando, Y.; Golberg, D.; Zhang, S. ZnS quantum dots@multilayered carbon: Geological-plate-movement-inspired design for high-energy Li-ion batteries. J. Mater. Chem. A 2018, 6, 8358–8365. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kuang, C.; Zhao, M.; Zhao, C.; Liu, L.; Lu, F.; Wang, N.; Huang, C.; Duan, C.; Jian, H.; et al. Ternary CuZnS Nanocrystals: Synthesis, Characterization, and Interfacial Application in Perovskite Solar Cells. Inorg. Chem. 2018, 57, 8375–8381. [Google Scholar] [CrossRef]
- Hung, L.X.; Bass‘ene, P.D.; Thang, P.N.; Loan, N.T.; Daney de Marcillac, W.; Dhawan, A.R.; Feng, F.; Esparza-Villa, J.U.; Thuc Hien, N.T.; Liem, N.Q.; et al. Near-infrared emitting CdTeSe alloyed quantum dots: Raman scattering, photoluminescence and single-emitter optical properties. RSC Adv. 2017, 7, 47966–47974. [Google Scholar] [CrossRef] [Green Version]
- Xinru, H.; Qun, C.; Hong, Z.; Yonghong, N.; Li, Z. Template Synthesis of NiCo2S4/Co9S8 Hollow Spheres for High-Performance. Chem. Eng. J. 2019, 368, 513–524. [Google Scholar]
- Abdelaal, M.M.; Hung, T.C.; Mohamed, S.G.; Yang, C.C.; Huang, H.P.; Hung, T.F. A Comparative Study of the Influence of Nitrogen Content and Structural Characteristics of NiS/Nitrogen-Doped Carbon Nanocomposites on Capacitive Performances in Alkaline Medium. Nanomaterials 2021, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Wang, J.; Sun, X.; Cui, L.; Yang, W.; Zheng, Y.; Liu, J. CoS2 nanoneedle array on Ti mesh: A stable and efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Electrochim. Acta. 2017, 246, 776–782. [Google Scholar] [CrossRef]
- Deng, D.R.; Xue, F.; Jia, Y.J.; Ye, J.C.; Bai, C.D.; Zheng, M.S.; Dong, Q.F. Co4N nanosheet assembled mesoporous sphere as a matrix for ultrahigh sulfur content lithium-sulfur batteries. ACS Nano. 2017, 11, 6031–6039. [Google Scholar] [CrossRef]
- Pu, J.; Shen, Z.; Zheng, J.; Wu, W.; Zhu, C.; Zhou, Q.; Zhang, H.; Pan, F. Multifunctional Co3S4@sulfur nanotubes for enhanced lithium-sulfur battery performance. Nano Energy 2017, 37, 7–14. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artchuea, T.; Srikhaow, A.; Sriprachuabwong, C.; Tuantranont, A.; Tang, I.-M.; Pon-On, W. Copper Zinc Sulfide (CuZnS) Quantum Dot-Decorated (NiCo)–S/Conductive Carbon Matrix as the Cathode for Li–S Batteries. Nanomaterials 2022, 12, 2403. https://doi.org/10.3390/nano12142403
Artchuea T, Srikhaow A, Sriprachuabwong C, Tuantranont A, Tang I-M, Pon-On W. Copper Zinc Sulfide (CuZnS) Quantum Dot-Decorated (NiCo)–S/Conductive Carbon Matrix as the Cathode for Li–S Batteries. Nanomaterials. 2022; 12(14):2403. https://doi.org/10.3390/nano12142403
Chicago/Turabian StyleArtchuea, Thanphisit, Assadawoot Srikhaow, Chakrit Sriprachuabwong, Adisorn Tuantranont, I-Ming Tang, and Weeraphat Pon-On. 2022. "Copper Zinc Sulfide (CuZnS) Quantum Dot-Decorated (NiCo)–S/Conductive Carbon Matrix as the Cathode for Li–S Batteries" Nanomaterials 12, no. 14: 2403. https://doi.org/10.3390/nano12142403
APA StyleArtchuea, T., Srikhaow, A., Sriprachuabwong, C., Tuantranont, A., Tang, I.-M., & Pon-On, W. (2022). Copper Zinc Sulfide (CuZnS) Quantum Dot-Decorated (NiCo)–S/Conductive Carbon Matrix as the Cathode for Li–S Batteries. Nanomaterials, 12(14), 2403. https://doi.org/10.3390/nano12142403





