Facile and Rapid Synthesis of Porous Hydrated V2O5 Nanoflakes for High-Performance Zinc Ion Battery Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Hydrated V2O5
2.2. Materials Characterization
2.3. Electrochemical Measurements
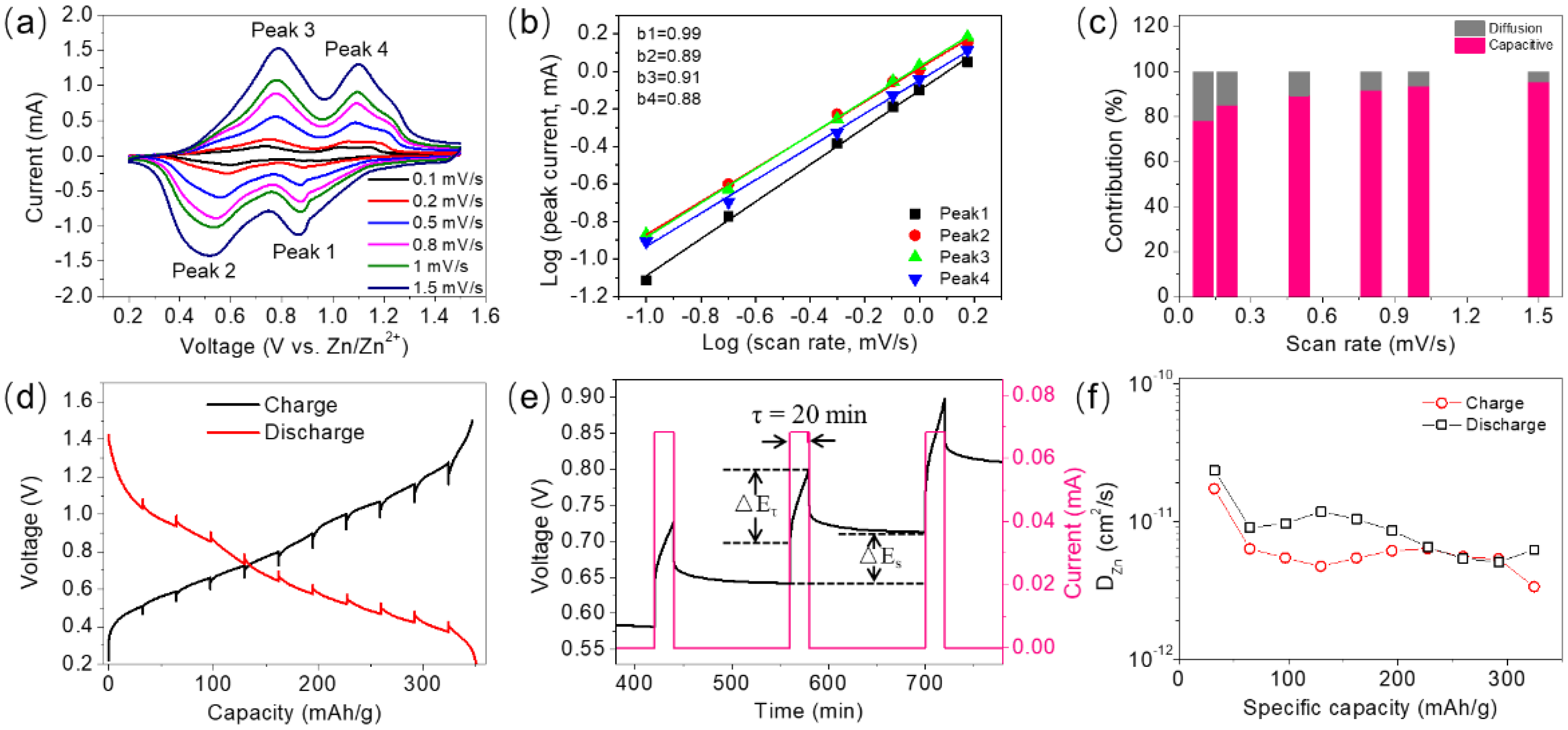
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, K.; Han, J.; Hirata, A.; Fujita, T.; Shen, Y.; Ning, S.; Liu, P.; Kashani, H.; Tian, Y.; Ito, Y.; et al. Lithium intercalation into bilayer graphene. Nat. Commun. 2019, 10, 275. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Yu, N.; Xiong, R.; Wang, Y.; Zhou, C.; Li, Y.; Pang, C.; Li, Z.; Zou, L.; Guo, K. Facile fabrication of low-cost and scalable graphite tape as novel current collectors for flexible supercapacitors. J. Alloys Compd. 2021, 861, 158476. [Google Scholar] [CrossRef]
- Guo, K.; Wan, Y.; Yu, N.; Hu, L.; Zhai, T.; Li, H. Hand-drawing patterned ultra-thin integrated electrodes for flexible micro supercapacitors. Energy Storage Mater. 2018, 11, 144–151. [Google Scholar] [CrossRef]
- Guo, K.; Wang, X.; Hu, L.; Zhai, T.; Li, H.; Yu, N. Highly Stretchable Waterproof Fiber Asymmetric Supercapacitors in an Integrated Structure. ACS Appl. Mater. Interfaces 2018, 10, 19820–19827. [Google Scholar] [CrossRef]
- Yu, N.; Zou, L.; Li, C.; Guo, K. In-situ growth of binder-free hierarchical carbon coated CoSe2 as a high performance lithium ion battery anode. Appl. Surf. Sci. 2019, 483, 85–90. [Google Scholar] [CrossRef]
- Shi, C.; Xiang, K.; Zhu, Y.; Chen, X.; Zhou, W.; Chen, H. Preparation and electrochemical properties of nanocable-like Nb2O5/surface-modified carbon nanotubes composites for anode materials in lithium ion batteries. Electrochim. Acta 2017, 246, 1088–1096. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kim, J.A.; Kim, S.-H.; Uhm, I.S.; Kang, S.J.; Kim, G.; Lee, S.-Y.; Yeon, S.-H.; Lee, S.-Y. All-Nanomat Lithium-Ion Batteries: A New Cell Architecture Platform for Ultrahigh Energy Density and Mechanical Flexibility. Adv. Energy Mater. 2017, 7, 1701099. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8, 14424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasiri, G.; Glenneberg, J.; Bani Hashemi, A.; Kun, R.; la Mantia, F. Mixed copper-zinc hexacyanoferrates as cathode materials for aqueous zinc-ion batteries. Energy Storage Mater. 2019, 19, 360–369. [Google Scholar] [CrossRef]
- Yang, Q.; Mo, F.; Liu, Z.; Ma, L.; Li, X.; Fang, D.; Chen, S.; Zhang, S.; Zhi, C. Activating C-Coordinated Iron of Iron Hexacyanoferrate for Zn Hybrid-Ion Batteries with 10 000-Cycle Lifespan and Superior Rate Capability. Adv. Mater. 2019, 31, 1901521. [Google Scholar] [CrossRef] [PubMed]
- Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [CrossRef]
- Charles, D.S.; Feygenson, M.; Page, K.; Neuefeind, J.; Xu, W.; Teng, X. Structural water engaged disordered vanadium oxide nanosheets for high capacity aqueous potassium-ion storage. Nat. Commun. 2017, 8, 15520. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Cui, F.; Meng, J.; Chen, H.; Zeng, X. Enhanced rate and cycling performances of hollow V2O5 nanospheres for aqueous zinc ion battery cathode. Appl. Surf. Sci. 2020, 507, 145137. [Google Scholar] [CrossRef]
- Gökdemir, F.P.; Menda, U.D.; Kavak, P.; Saatci, A.E.; Özdemir, O.; Kutlu, K. Influence of water expulsion on structural properties of V2O5 nH2O sol-gel films. AIP Conf. Proc. 2012, 1476, 279–284. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Zhang, P.; Wang, H.; Li, S.; Ji, S.; Wen, Z.; Sun, J. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl. Surf. Sci. 2020, 502, 144207. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, X.; Bai, Y.; Wu, C.; Gu, S.; Chen, S.; Wu, F.; Amine, K.; Lu, J. Open-Structured V2O5·nH2O Nanoflakes as Highly Reversible Cathode Material for Monovalent and Multivalent Intercalation Batteries. Adv. Energy Mater. 2016, 7, 1602720. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as Cathode Material for High-Performance Aqueous Zinc Ion Batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Li, Y.; Li, C.; Yu, N.; Li, H. Compact self-standing layered film assembled by V2O5·nH2O/CNTs 2D/1D composites for high volumetric capacitance flexible supercapacitors. Sci. China Mater. 2019, 62, 936–946. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Hamdan, O.H.C.; Valverde, S.A.; Guerra, E.M.; Bianchi, R.F. Synthesis and characterization of V2O5/PANI thin films for application in amperometric ammonia gas sensors. Org. Electron. 2019, 65, 116–120. [Google Scholar] [CrossRef]
- Xiong, C.; Aliev, A.E.; Gnade, B.; Balkus, K.J. Fabrication of Silver Vanadium Oxide and V2O5 Nanowires for Electrochromics. ACS Nano 2008, 2, 293–301. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, J.; Feng, W.; Sheng, J.; Tian, X.; He, L.; An, Q.; Mai, L. Hydrated vanadium pentoxide with superior sodium storage capacity. J. Mater. Chem. A 2015, 3, 8070–8075. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Han, S.-D.; Kim, S.; Lipson, A.L.; Tepavcevic, S.; Fister, T.T.; Bloom, I.D.; Burrell, A.K.; Johnson, C.S. A High Power Rechargeable Nonaqueous Multivalent Zn/V2O5 Battery. Adv. Energy Mater. 2016, 6, 1600826. [Google Scholar] [CrossRef]
- Moretti, A.; Passerini, S. Bilayered Nanostructured V2O5·nH2O for Metal Batteries. Adv. Energy Mater. 2016, 6, 1600868. [Google Scholar] [CrossRef]
- Li, H.; He, P.; Wang, Y.; Hosono, E.; Zhou, H. High-surface vanadium oxides with large capacities for lithium-ion batteries: From hydrated aerogel to nanocrystalline VO2(B), V6O13 and V2O5. J. Mater. Chem. 2011, 21, 10999–11009. [Google Scholar] [CrossRef]
- Kristoffersen, H.H.; Metiu, H. Structure of V2O5·nH2O Xerogels. J. Phys. Chem. C 2016, 120, 3986–3992. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.K.; Pozarnsky, G.A.; Mecartney, M.L. The direct observation of structural development during vanadium pentoxide gelation. J. Mater. Res. 1992, 7, 2530–2537. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, H.; Chou, T.; Cao, G. Effects of Thermal Annealing on the Li+ Intercalation Properties of V2O5·nH2O Xerogel Films. J. Phys. Chem. B 2005, 109, 11361–11366. [Google Scholar] [CrossRef] [PubMed]
- Sa, N.; Kinnibrugh, T.L.; Wang, H.; Sai Gautam, G.; Chapman, K.W.; Vaughey, J.T.; Key, B.; Fister, T.T.; Freeland, J.W.; Proffit, D.L.; et al. Structural Evolution of Reversible Mg Insertion into a Bilayer Structure of V2O5·nH2O Xerogel Material. Chem. Mater. 2016, 28, 2962–2969. [Google Scholar] [CrossRef]
- Alonso, B.; Livage, J. Synthesis of Vanadium Oxide Gels from Peroxovanadic Acid Solutions: A 51V NMR Study. J. Solid State Chem. 1999, 148, 16–19. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, Y.; Chen, J.; Chen, Z.; Chen, Y.; Fu, J. Environmentally friendly room temperature synthesis of hierarchical porous α-Ni(OH)2 nanosheets for supercapacitor and catalysis applications. Green Chem. 2019, 21, 5960–5968. [Google Scholar] [CrossRef]
- Xie, Z.; Lai, J.; Zhu, X.; Wang, Y. Green Synthesis of Vanadate Nanobelts at Room Temperature for Superior Aqueous Rechargeable Zinc-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 6401–6408. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Grishanov, D.A.; Edison, E.; Srinivasan, M.; Sladkevich, S.; Gun, J.; Prikhodchenko, P.V.; Lev, O. Green Synthesis of a Nanocrystalline Tin Disulfide-Reduced Graphene Oxide Anode from Ammonium Peroxostannate: A Highly Stable Sodium-Ion Battery Anode. ACS Sustain. Chem. Eng. 2020, 8, 5485–5494. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A Deep-Cycle Aqueous Zinc-Ion Battery Containing an Oxygen-Deficient Vanadium Oxide Cathode. Angew. Chem. Int. Ed. 2020, 59, 2273–2278. [Google Scholar] [CrossRef]
- Howarth, O.W.; Hunt, J.R. Peroxo-complexes of vanadium(V); a vanadium-51 nuclear magnetic resonance study. J. Chem. Soc. Dalton Trans. 1979, 1388–1391. [Google Scholar] [CrossRef]
- Fontenot, C.J.; Wiench, J.W.; Pruski, M.; Schrader, G.L. Vanadia Gel Synthesis via Peroxovanadate Precursors. 1. In Situ Laser Raman and 51V NMR Characterization of the Gelation Process. J. Phys. Chem. B 2000, 104, 11622–11631. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, L.; Wu, Y.; Gong, Y.; Liu, Z.; Hoster, H.E.; Zhang, Y.; Zhang, S.; Yang, S.; Yan, Q.; et al. Building 3D structures of vanadium pentoxide nanosheets and application as electrodes in supercapacitors. Nano Lett. 2013, 13, 5408–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Liu, X.; Zhang, H.; Yang, Z.; Shi, X.; Liu, Q.; Lu, X. Enhancing Zn-Ion Storage Capability of Hydrated Vanadium Pentoxide by the Strategic Introduction of La3+. ChemSusChem 2020, 13, 1568–1574. [Google Scholar] [CrossRef]
- Terán-Escobar, G.; Pampel, J.; Caicedo, M.J.; Lira-Cantú, M. Low-temperature, solution-processed, layered V2O5 hydrate as the hole-transport layer for stable organic solar cells. Energy Environ. Sci. 2013, 6, 3088–3098. [Google Scholar] [CrossRef] [Green Version]
- Javed, M.S.; Lei, H.; Wang, Z.; Liu, B.; Cai, X.; Mai, W. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Hydrated vanadium pentoxide/reduced graphene oxide-polyvinyl alcohol (V2O5⋅nH2O/rGO-PVA) film as a binder-free electrode for solid-state Zn-ion batteries. J. Colloid Interface Sci. 2021, 587, 845–854. [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, S.; Zhou, F.; Das, P.; Sun, C.; Zheng, S.; Wu, Z. 2D Amorphous V2O5/Graphene Heterostructures for High-Safety Aqueous Zn-Ion Batteries with Unprecedented Capacity and Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 2000081. [Google Scholar] [CrossRef]



| Vanadium Sources | Other Reagents | Temperature | Time | Ref. |
|---|---|---|---|---|
| VO(OC3H7)3 | H2O, acetone | RT * | 4 days | [31] |
| V2O5 | H2O | 800 °C | 1–2 h | [28] |
| NaVO3 | Resin, H2O | RT * | 3 days | [35] |
| V2O5 | H2O2, H2O | 205 °C | 14 h | [23] |
| V2O5 | H2O2, H2O | RT * | 26 h | [36] |
| VOSO4 | H2O | 120 °C | 20 h | [29] |
| V2O5 | H2O2, H2O | 40 °C | 0.5 h | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Cheng, W.; Chen, H.; Li, H.; Chen, J.; Liu, H.; Tu, Y.; She, W.; Huang, Z.; Wan, Y.; et al. Facile and Rapid Synthesis of Porous Hydrated V2O5 Nanoflakes for High-Performance Zinc Ion Battery Applications. Nanomaterials 2022, 12, 2400. https://doi.org/10.3390/nano12142400
Guo K, Cheng W, Chen H, Li H, Chen J, Liu H, Tu Y, She W, Huang Z, Wan Y, et al. Facile and Rapid Synthesis of Porous Hydrated V2O5 Nanoflakes for High-Performance Zinc Ion Battery Applications. Nanomaterials. 2022; 12(14):2400. https://doi.org/10.3390/nano12142400
Chicago/Turabian StyleGuo, Kai, Wenchong Cheng, Haoxiong Chen, Hanbin Li, Jinxue Chen, Haiyuan Liu, Yunliang Tu, Wenhao She, Zhengkai Huang, Yinpeng Wan, and et al. 2022. "Facile and Rapid Synthesis of Porous Hydrated V2O5 Nanoflakes for High-Performance Zinc Ion Battery Applications" Nanomaterials 12, no. 14: 2400. https://doi.org/10.3390/nano12142400
APA StyleGuo, K., Cheng, W., Chen, H., Li, H., Chen, J., Liu, H., Tu, Y., She, W., Huang, Z., Wan, Y., Zou, L., Li, Z., Zhong, X., Wu, Y., Wang, X., & Yu, N. (2022). Facile and Rapid Synthesis of Porous Hydrated V2O5 Nanoflakes for High-Performance Zinc Ion Battery Applications. Nanomaterials, 12(14), 2400. https://doi.org/10.3390/nano12142400








