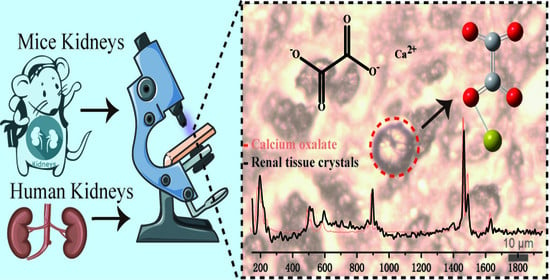
In Situ Identification of Unknown Crystals in Acute Kidney Injury Using Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Information
2.2. Mouse Model
2.3. Tissue Processing and Slide Preparation
2.4. Raman Spectrum and Imaging
3. Results
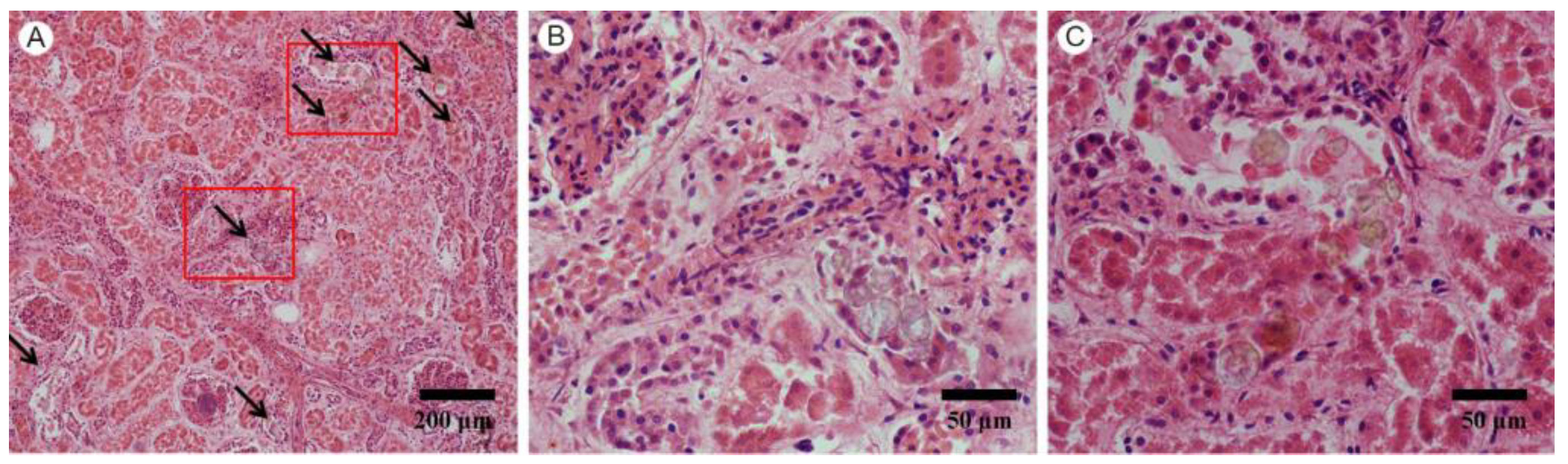
3.1. Pathological Findings
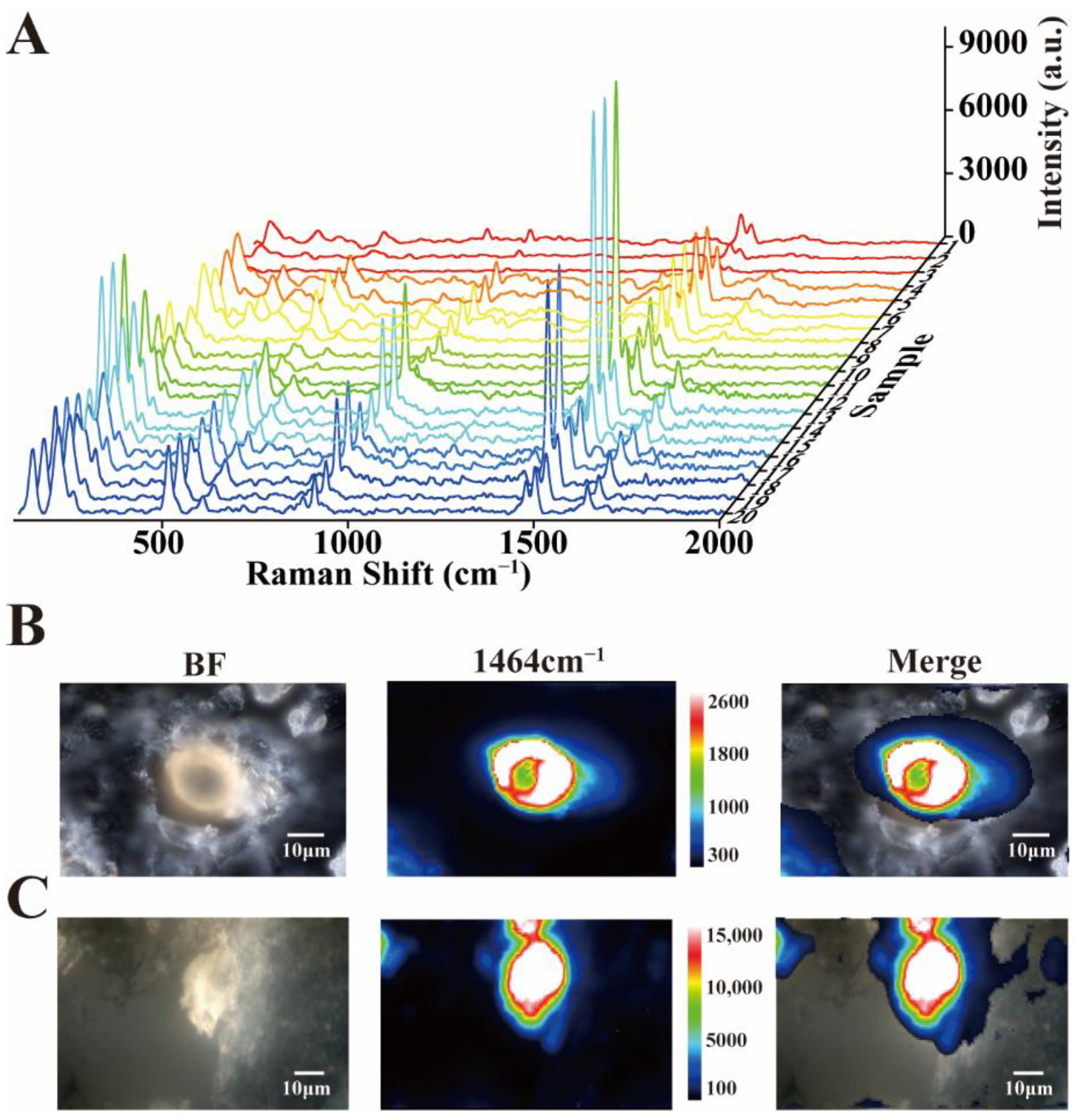
3.2. Raman Detection of Unknown Crystals
3.3. Reproducibility and Raman Imaging of Calcium Oxalate Dihydrate Crystals
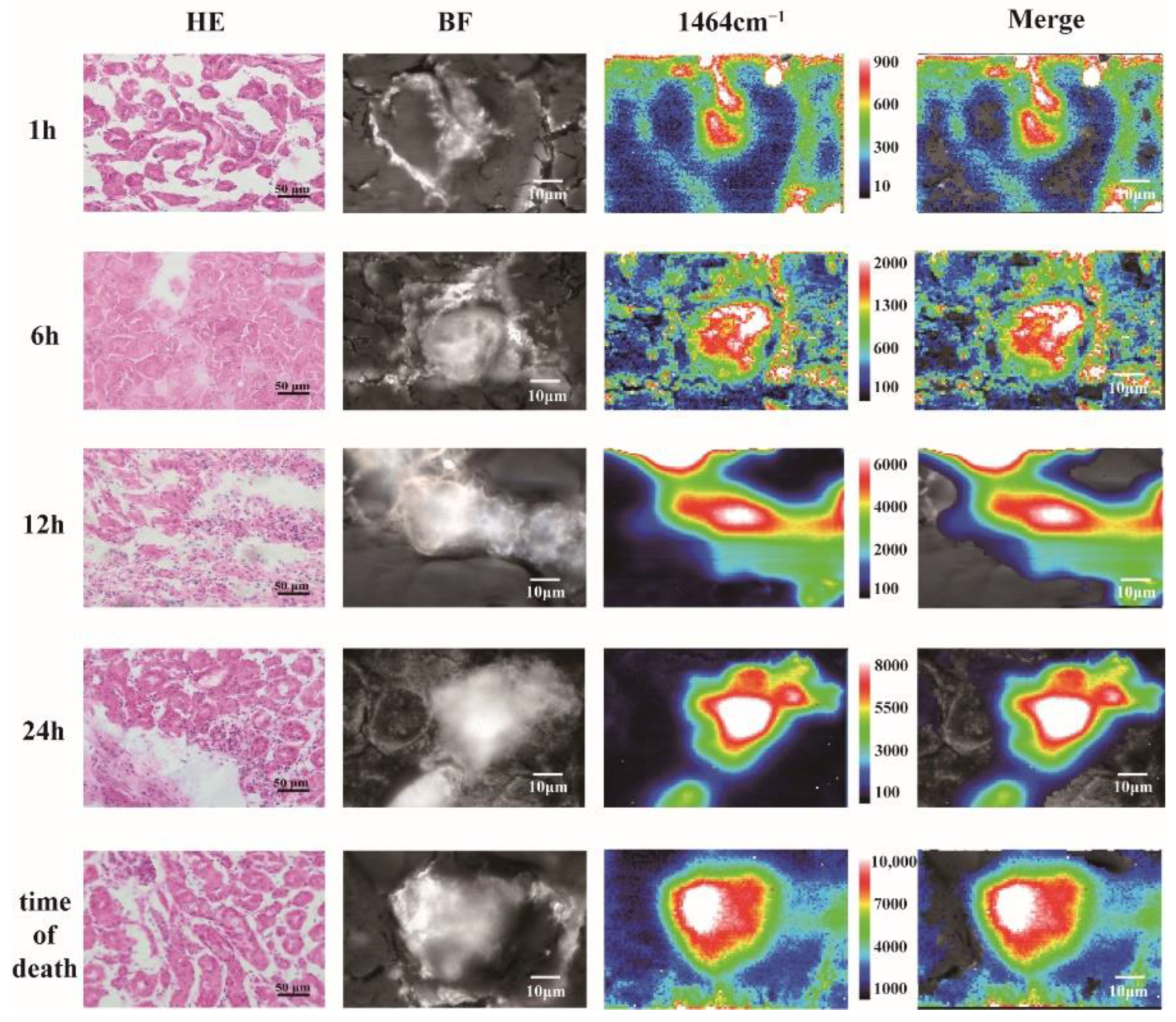
3.4. Application of Raman Spectroscopy in Biopsy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordero, E.; Rüger, J.; Marti, D.; Mondol, A.S.; Hasselager, T.; Mogensen, K.; Hermann, G.G.; Popp, J.; Schie, I.W. Bladder Tissue Characterization Using Probe-Based Raman Spectroscopy: Evaluation of Tissue Heterogeneity and Influence on the Model Prediction. J. Biophotonics 2020, 13, e201960025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Y.; Cui, X.; Sun, Y.; Mao, Z.; Wang, J.; Chen, F.; Wang, J.; Cao, Y. Crown Monitoring: Trace the Dynamic Changes of Caspase-3 and H2O2 in Real-Time Imaging Based on FRET/SERS. Biosens. Bioelectron. 2021, 192, 113539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Y.; Li, D.; Liu, Y.; Mao, Z.S.; Yu, Y.; Zhu, P.; Xu, Q.; Sun, Y.; Hu, L.; Wang, J.; et al. Recyclable and Green AuBPs@MoS2@tinfoil Box for High Throughput SERS Tracking of Diquat in Complex Compounds. Sens. Actuators B Chem. 2021, 344, 130290. [Google Scholar] [CrossRef]
- De Jong, B.W.D.; Bakker Schut, T.C.; Wolffenbuttel, K.P.; Nijman, J.M.; Kok, D.J.; Puppels, G.J. Identification of Bladder Wall Layers by Raman Spectroscopy. J. Urol. 2002, 168, 1771–1778. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Lin, K.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; So, J.B.Y.; Huang, Z. In Vivo Diagnosis of Esophageal Cancer Using Image-Guided Raman Endoscopy and Biomolecular Modeling. Technol. Cancer Res. Treat. 2011, 10, 103–112. [Google Scholar] [CrossRef]
- Krauß, S.D.; Roy, R.; Yosef, H.K.; Lechtonen, T.; El-Mashtoly, S.F.; Gerwert, K.; Mosig, A. Hierarchical Deep Convolutional Neural Networks Combine Spectral and Spatial Information for Highly Accurate Raman-Microscopy-Based Cytopathology. J. Biophotonics 2018, 11, e201800022. [Google Scholar] [CrossRef]
- Yosef, H.K.; Krauß, S.D.; Lechtonen, T.; Jütte, H.; Tannapfel, A.; Käfferlein, H.U.; Brüning, T.; Roghmann, F.; Noldus, J.; Mosig, A.; et al. Noninvasive Diagnosis of High-Grade Urothelial Carcinoma in Urine by Raman Spectral Imaging. Anal. Chem. 2017, 89, 6893–6899. [Google Scholar] [CrossRef]
- Gu, H.X.; Xue, L.; Zhang, Y.F.; Li, D.W.; Long, Y.T. Facile Fabrication of a Silver Dendrite-Integrated Chip for Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2015, 7, 2931–2936. [Google Scholar] [CrossRef]
- Shi, X.; Li, H.W.; Ying, Y.L.; Liu, C.; Zhang, L.; Long, Y.T. In Situ Monitoring of Catalytic Process Variations in a Single Nanowire by Dark-Field-Assisted Surface-Enhanced Raman Spectroscopy. Chem. Commun. 2016, 52, 1044–1047. [Google Scholar] [CrossRef]
- Li, D.W.; Qu, L.L.; Hu, K.; Long, Y.T.; Tian, H. Monitoring of Endogenous Hydrogen Sulfide in Living Cells Using Surface-Enhanced Raman Scattering. Angew. Chemie—Int. Ed. 2015, 54, 12758–12761. [Google Scholar] [CrossRef]
- Austin, L.A.; Osseiran, S.; Evans, C.L. Raman Technologies in Cancer Diagnostics. Analyst 2016, 141, 476–503. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.B.; Xanthopoulos, P.; Pyrgiotakis, G.; Grobmyer, S.R.; Pardalos, P.M.; Hench, L.L. Raman Spectroscopy for Clinical Oncology. Adv. Opt. Technol. 2011, 2011, 1–20. [Google Scholar] [CrossRef][Green Version]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman Spectroscopy for Medical Diagnostics—From in-Vitro Biofluid Assays to in-Vivo Cancer Detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Lochocki, B.; Morrema, T.H.J.; Ariese, F.; Hoozemans, J.J.M.; De Boer, J.F. The Search for a Unique Raman Signature of Amyloid-Beta Plaques in Human Brain Tissue from Alzheimer’s Disease Patients. Analyst 2020, 145, 1724–1736. [Google Scholar] [CrossRef]
- Michael, R.; Lenferink, A.; Vrensen, G.F.J.M.; Gelpi, E.; Barraquer, R.I.; Otto, C. Hyperspectral Raman Imaging of Neuritic Plaques and Neurofibrillary Tangles in Brain Tissue from Alzheimer’s Disease Patients. Sci. Rep. 2017, 7, 15603. [Google Scholar] [CrossRef]
- Bratchenko, L.A.; Bratchenko, I.A.; Khristoforova, Y.A.; Artemyev, D.N.; Konovalova, D.Y.; Lebedev, P.A.; Zakharov, V.P. Raman Spectroscopy of Human Skin for Kidney Failure Detection. J. Biophotonics 2021, 14, e202000360. [Google Scholar] [CrossRef]
- Yay, A.; Onses, M.S.; Sahmetlioglu, E.; Ceyhan, A.; Pekdemir, S.; Onder, G.O.; Sezer, G.; Sarica, Z.S.; Aydin, F. Raman Spectroscopy: A Novel Experimental Approach to Evaluating Cisplatin Induced Tissue Damage. Talanta 2020, 207, 120343. [Google Scholar] [CrossRef]
- Clark, A.; Neyra, J.A.; Madni, T.; Imran, J.; Phelan, H.; Arnoldo, B.; Wolf, S.E. Acute Kidney Injury after Burn. Burns 2017, 43, 898–908. [Google Scholar] [CrossRef]
- Steinvall, I.; Bak, Z.; Sjoberg, F. Acute Kidney Injury Is Common, Parallels Organ Dysfunction or Failure, and Carries Appreciable Mortality in Patients with Major Burns: A Prospective Exploratory Cohort Study. Crit. Care 2008, 12, R124. [Google Scholar] [CrossRef]
- Soliman, N.A. Orphan Kidney Diseases. Nephron—Clin. Pract. 2012, 120, c194–c199. [Google Scholar] [CrossRef]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.; Kellum, J.A.; Chawla, L.S.; Cruz, D.; Ince, C.; Okusa, M.D. Potential Use of Biomarkers in Acute Kidney Injury: Report and Summary of Recommendations from the 10th Acute Dialysis Quality Initiative Consensus Conference. Kidney Int. 2014, 85, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Anders, H.J. Crystal Nephropathies: Mechanisms of Crystal-Induced Kidney Injury. Nat. Rev. Nephrol. 2017, 13, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Shi, C.; Ma, X.; Anders, H.J. Novel Insights into Crystal-Induced Kidney Injury. Kidney Dis. 2018, 4, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhao, Z.; Zhang, G.; Chen, S.; Zhao, Y.; Lu, J. Analysis and Classification of Kidney Stones Based on Raman Spectroscopy. Biomed. Opt. Express 2018, 9, 4175. [Google Scholar] [CrossRef]
- Cooper, D.; Doucet, L.; Pratt, M. Understanding in Multinational Organizations. J. Organ. Behav. 2007, 28, 303–325. [Google Scholar] [CrossRef]
- Kodati, V.R.; Tomasi, G.E.; Turumin, J.L.; Tu, A.T. Raman Spectroscopic Identification of Calcium-Oxalate-Type Kidney Stone. Appl. Spectrosc. 1990, 44, 1408–1411. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Bouropoulos, N.C.; Koutsoukos, P.G. Urinary Stone Layer Analysis of Mineral Components by Raman Spectroscopy, IR Spectroscopy, and X-Ray Powder Diffraction: A Comparative Study. Appl. Spectrosc. 1997, 51, 1205–1209. [Google Scholar] [CrossRef]
- Dassanayake, U.; Gnanathasan, C.A. Acute Renal Failure Following Oxalic Acid Poisoning: A Case Report. J. Occup. Med. Toxicol. 2012, 7, 2–5. [Google Scholar] [CrossRef]
- Herlitz, L.C.; D’Agati, V.D.; Markowitz, G.S. Crystalline Nephropathies. Arch. Pathol. Lab. Med. 2012, 136, 713–720. [Google Scholar] [CrossRef]
- Huang, H.S.; Chen, J.; Chen, C.F.; Ma, M.C. Vitamin E Attenuates Crystal Formation in Rat Kidneys: Roles of Renal Tubular Cell Death and Crystallization Inhibitors. Kidney Int. 2006, 70, 699–710. [Google Scholar] [CrossRef]
- Sharma, M.; Kaur, T.; Singla, S.K. Role of Mitochondria and NADPH Oxidase Derived Reactive Oxygen Species in Hyperoxaluria Induced Nephrolithiasis: Therapeutic Intervention with Combinatorial Therapy of N-Acetyl Cysteine and Apocynin. Mitochondrion 2016, 27, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Thongboonkerd, V. Proteomics of crystal–cell interactions: A model for kidney stone research. Cells 2019, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Petry, R.; Schmitt, M.; Popp, J. Raman Spectroscopy—A Prospective Tool in the Life Sciences. ChemPhysChem 2003, 4, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Jiang, Q.; Wan, H.; Li, R.; Sun, Y.; Zhang, Z.; Mao, Z.; Cao, Y.; Chen, F. In Situ Identification of Unknown Crystals in Acute Kidney Injury Using Raman Spectroscopy. Nanomaterials 2022, 12, 2395. https://doi.org/10.3390/nano12142395
Yu Y, Jiang Q, Wan H, Li R, Sun Y, Zhang Z, Mao Z, Cao Y, Chen F. In Situ Identification of Unknown Crystals in Acute Kidney Injury Using Raman Spectroscopy. Nanomaterials. 2022; 12(14):2395. https://doi.org/10.3390/nano12142395
Chicago/Turabian StyleYu, Youjia, Qiaoyan Jiang, Hua Wan, Rong Li, Yang Sun, Zhiwei Zhang, Zhengsheng Mao, Yue Cao, and Feng Chen. 2022. "In Situ Identification of Unknown Crystals in Acute Kidney Injury Using Raman Spectroscopy" Nanomaterials 12, no. 14: 2395. https://doi.org/10.3390/nano12142395
APA StyleYu, Y., Jiang, Q., Wan, H., Li, R., Sun, Y., Zhang, Z., Mao, Z., Cao, Y., & Chen, F. (2022). In Situ Identification of Unknown Crystals in Acute Kidney Injury Using Raman Spectroscopy. Nanomaterials, 12(14), 2395. https://doi.org/10.3390/nano12142395