Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
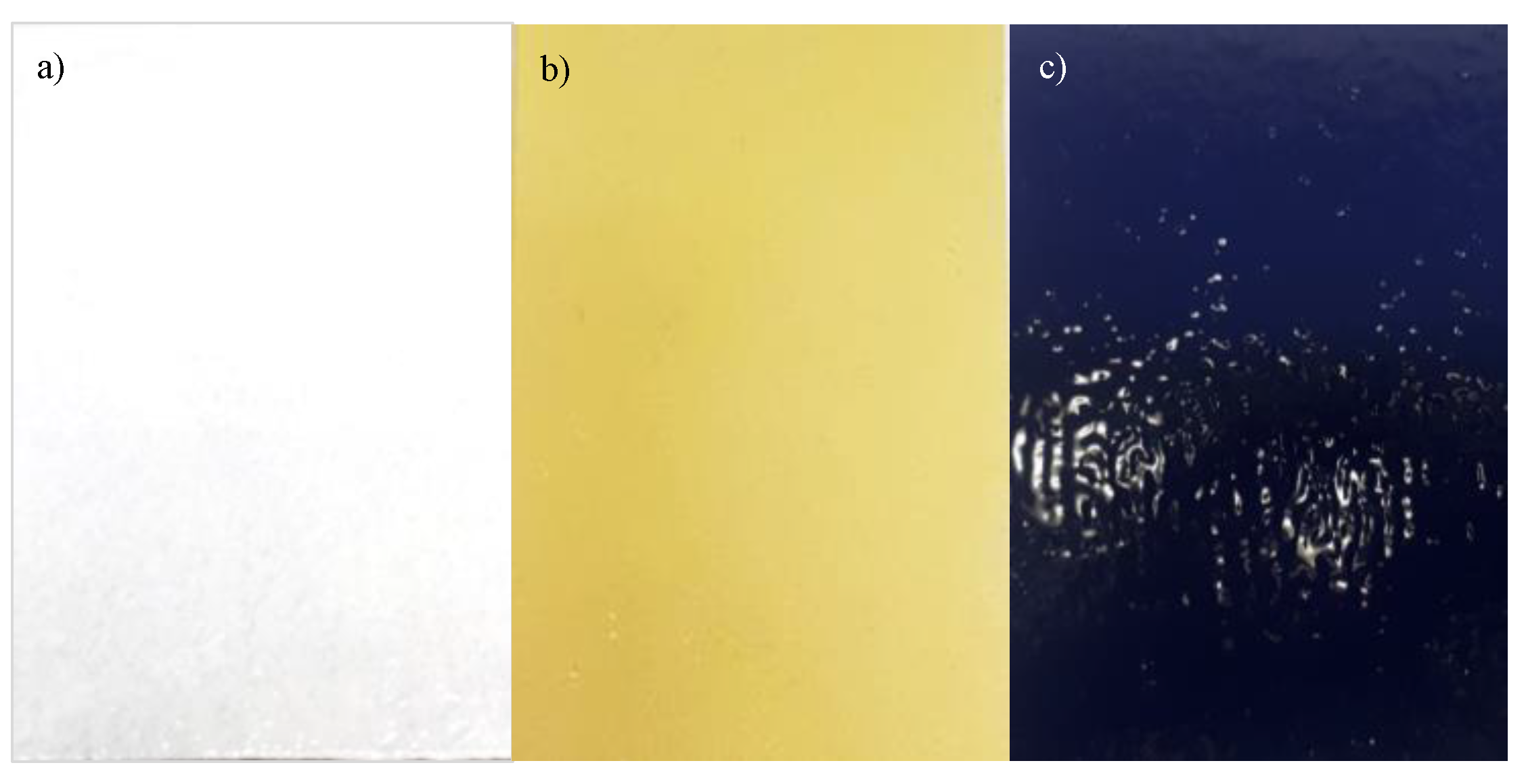
2.3. Coatings Preparation
2.4. Coatings Characterization
2.5. Microtox Assay
2.6. Bacteria, Culture Conditions, and Bacteriostatic Activity Tests of Biocidal Agents
3. Results and Discussion
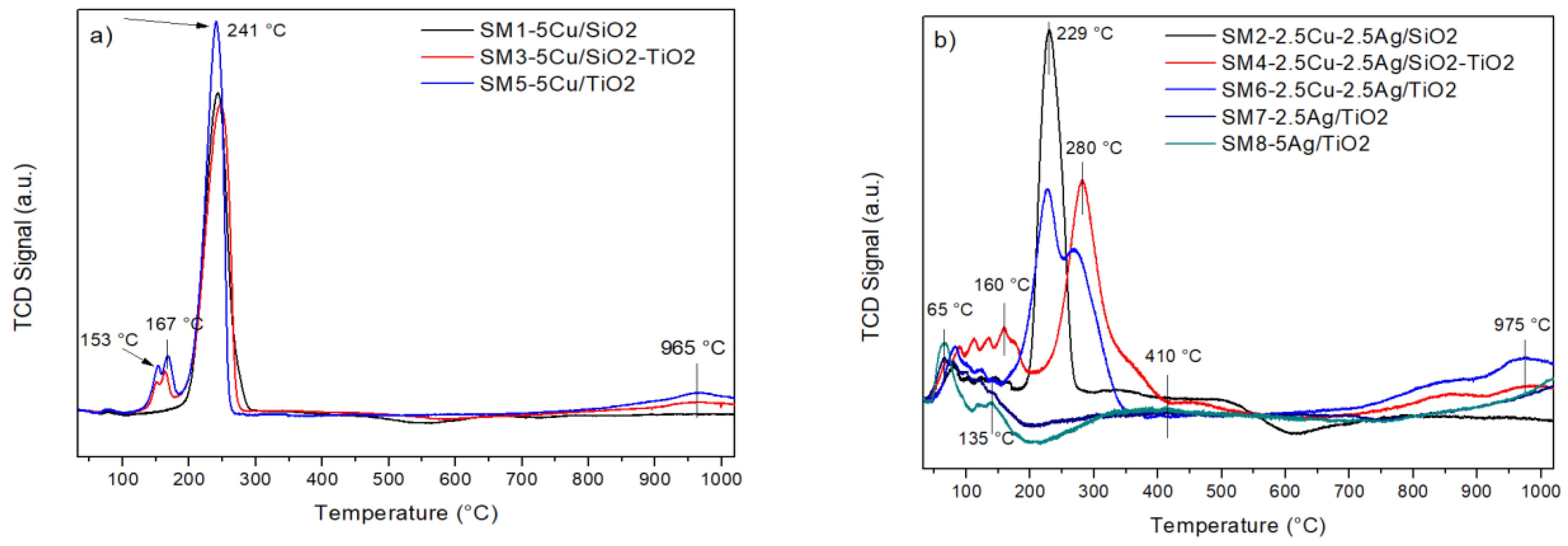
3.1. Samples Characterization
3.2. Evaluation of the Adhesion Power of the Coatings and Their Rheological Features
3.3. Ecotoxicological Assays (Microtox Toxicity Tests)
3.4. Antibacterial Activity Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolter, R.; Greenberg, E.P. The superficial life of microbes. Nature 2006, 441, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Jägerbrand, A.K.; Brutemark, A.; Barthel Svedén, J.; Gren, I.-M. A review on the environmental impacts of shipping on aquatic and nearshore ecosystems. Sci. Total Environ. 2019, 695, 133637. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef]
- Gule, N.P.; Begum, N.M.; Klumperman, B. Advances in biofouling mitigation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 535–555. [Google Scholar] [CrossRef]
- Elashnikov, R.; Ulbrich, P.; Vokatá, B.; Pavlíčková, V.S.; Švorčík, V.; Lyutakov, O.; Rimpelová, S. Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials 2021, 11, 3083. [Google Scholar] [CrossRef]
- Pang, L.; Meier-Haack, J.; Huang, S.; Qi, L.; Cui, H.; Ruan, S.; Zeng, Y.-J. Antibiofouling Thin-Film Nanocomposite Membranes for Sustainable Water Purification. Adv. Sustain. Syst. 2021, 5, 2000279. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Karimi, E. Antimicrobial Activities of Nanoparticles. In Nanotechnology for Agriculture: Crop Production & Protection; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2019; pp. 171–206. [Google Scholar] [CrossRef]
- Calabrese, C.; La Parola, V.; Testa, M.L.; Liotta, L.F. Antifouling and antimicrobial activity of Ag, Cu and Fe nanoparticles supported on silica and titania. Inorg. Chim. Acta 2022, 529, 120636. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel’s Silic One. Available online: https://www.hempel.com/products/hempels-silic-one-77450 (accessed on 11 June 2022).
- Pistone, A.; Scolaro, C.; Visco, A. Mechanical Properties of Protective Coatings against Marine Fouling: A Review. Polymers 2021, 13, 173. [Google Scholar] [CrossRef]
- Scurria, A.; Scolaro, C.; Sfameni, S.; Di Carlo, G.; Pagliaro, M.; Visco, A.; Ciriminna, R. Towards AquaSun practical utilization: Strong adhesion and lack of ecotoxicity of solar-driven antifouling sol-gel coating. Prog. Org. Coat. 2022, 165, 106771. [Google Scholar] [CrossRef]
- Pantaleo, G.; Liotta, L.F.; Venezia, A.M.; Deganello, G.; Ezzo, E.M.; El Kherbawi, M.A.; Atia, H. Support effect on the structure and CO oxidation activity of Cu-Cr mixed oxides over Al2O3 and SiO2. Mater. Chem. Phys. 2009, 114, 604–611. [Google Scholar] [CrossRef]
- Chen, C.-S.; You, J.-H.; Lin, J.-H.; Chen, Y.-Y. Effect of highly dispersed active sites of Cu/TiO2 catalyst on CO oxidation. Catal. Commun. 2008, 9, 2381–2385. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, Y.; Chen, M.; Wang, L.; Zhang, C.; He, H. Effect of Support on the Activity of Ag-based Catalysts for Formaldehyde Oxidation. Sci. Rep. 2015, 5, 12950. [Google Scholar] [CrossRef] [Green Version]
- Mamontov, G.V.; Grabchenko, M.V.; Sobolev, V.I.; Zaikovskii, V.I.; Vodyankina, O.V. Ethanol dehydrogenation over Ag-CeO2/SiO2 catalyst: Role of Ag-CeO2 interface. Appl. Catal. A Gen. 2016, 528, 161–167. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Y.; Duan, T.; Zhu, W.; Yi, Z.; Cui, X. 3D hierarchical walnut-like CuO nanostructures: Preparation, characterization and their efficient catalytic activity for CO oxidation. Phys. B Condens. Matter 2016, 493, 7–13. [Google Scholar] [CrossRef]
- Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Colón, G.; Navío, J.A.; Macías, M.; Pérez Peña, J. Effect of deposition of silver on structural characteristics and photoactivity of TiO2-based photocatalysts. Appl. Catal. B Environ. 2012, 127, 112–120. [Google Scholar] [CrossRef]
- Santos, L.M.; Machado, W.A.; França, M.D.; Borges, K.A.; Paniago, R.M.; Patrocinio, A.O.T.; Machado, A.E.H. Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Shevtsova, T.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Kprolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128525. [Google Scholar] [CrossRef]
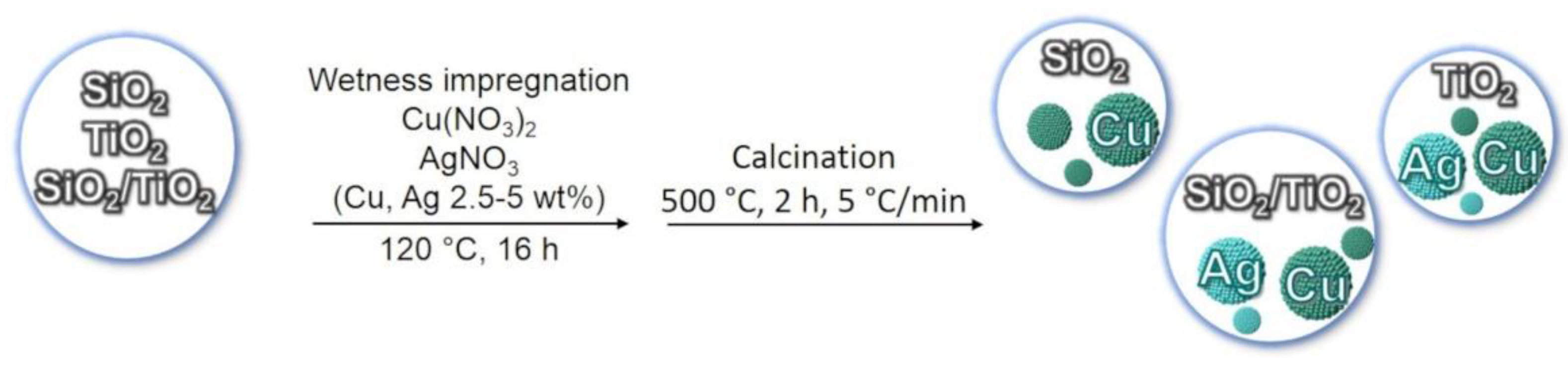





| Sample Label | Chemical Composition (Weight %) |
|---|---|
| SM1 | 5Cu/SiO2 |
| SM2 | 2.5Cu-2.5Ag/SiO2 |
| SM3 | 5Cu/SiO2–TiO2 (1:1) |
| SM4 | 2.5Cu-2.5Ag/SiO2–TiO2 (1:1) |
| SM5 | 5Cu/TiO2 |
| SM6 | 2.5Cu-2.5Ag/TiO2 |
| SM7 | 2.5Ag/TiO2 |
| SM8 | 5Ag/TiO2 |
| Sample | Tmax (°C) | Experimental H2 Consumptions (mL/g) | Theoretical H2 Consumptions (mL/g)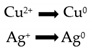 |
|---|---|---|---|
| SM1. 5Cu/SiO2 | 241 | 17.7 | |
| 665 | 1.1 | ||
| 18.8 (Total) | 18.9 | ||
| SM2. 2.5Cu-2.5Ag/SiO2 | 81 | 2.1 | |
| 229 | 8.8 | ||
| 480 | 1.7 | ||
| 12.6 (Total) | 12.3 | ||
| SM3. 5Cu/SiO2-TiO2 | 151,164 | 1.5 | |
| 247 | 16.7 | ||
| 965 | 1.6 | ||
| 19.8 (Total) | 18.9 | ||
| SM4. 2.5Cu-2.5Ag/SiO2-TiO2 | 160 | 3.8 | |
| 280 | 7.8 | ||
| 975 | 1.9 | ||
| 13.5 (Total) | 12.3 | ||
| SM5. 5Cu/TiO2 | 153,167 | 2.4 | |
| 241 | 16.3 | ||
| 965 | 1.3 | ||
| 20.0 (Total) | 18.9 | ||
| SM6. 2.5Cu-2.5Ag/TiO2 | 83 | 2.3 | |
| 227 | 10.2 | ||
| 975 | 1.6 | ||
| 14.1 (Total) | 12.3 | ||
| SM7. 2.5 Ag/TiO2 | 67,135 | 2.7 | |
| 410 | 0.7 | ||
| 1018 | 1.2 | ||
| 4.6 (Total) | 2.8 | ||
| SM8. 5Ag/TiO2 | 65,135 | 4.9 | |
| 412 | 0.7 | ||
| 1019 | 1.5 | ||
| 7.1 (Total) | 5.6 |
| Sample | Ag3d5/2 (eV) | Cu2p3/2 (eV) | O1s (eV) | Cu/(Si + Ti) | Ag/(Si + Ti) | Ag/Cu |
|---|---|---|---|---|---|---|
| 5Cu/SiO2 (SM1) | - | 933.7 | 532.6 (100%) | 0.1 (0.05) | - | - |
| 2.5Cu-2.5Ag/SiO2 (SM2) | 368.8 | 933.9 | 532.4 (100%) | 0.04 (0.02) | 0.016 (0.014) | 0.4 (0.59) |
| 5Cu/SiO2–TiO2 (1:1) (SM3) | - | 932.9 | 529.6 (34%) 532.6 (66%) | 0.19 (0.06) | - | - |
| 2.5Cu-2.5Ag/SiO2–TiO2 (1:1) (SM4) | 368.3 | 933.5 | 529.6 (35%) 532.8 (65%) | 0.1 (0.03) | 0.13 (0.016) | 1.3 (0.59) |
| 5Cu/TiO2 (SM5) | - | 933.6 | 529.7 (48%) 531.7 (52%) | 0.34 (0.07) | - | - |
| 2.5Cu-2.5Ag/TiO2 (SM6) | 368.3 | 932.6 | 529.5 (42%) 531.7 (58%) | 0.07 (0.03) | 0.22 (0.019) | 3.0 (0.59) |
| 2.5Ag/TiO2 (SM7) | 368.1 | - | 529.5 (63%) 531.8 (37%) | - | 0.10 (0.019) | - |
| 5Ag/TiO2 (SM8) | 368.2 | - | - | 0.18 (0.042) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, C.; Parola, V.L.; Cappello, S.; Visco, A.; Scolaro, C.; Liotta, L.F. Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides. Nanomaterials 2022, 12, 2371. https://doi.org/10.3390/nano12142371
Calabrese C, Parola VL, Cappello S, Visco A, Scolaro C, Liotta LF. Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides. Nanomaterials. 2022; 12(14):2371. https://doi.org/10.3390/nano12142371
Chicago/Turabian StyleCalabrese, Carla, Valeria La Parola, Simone Cappello, Annamaria Visco, Cristina Scolaro, and Leonarda Francesca Liotta. 2022. "Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides" Nanomaterials 12, no. 14: 2371. https://doi.org/10.3390/nano12142371
APA StyleCalabrese, C., Parola, V. L., Cappello, S., Visco, A., Scolaro, C., & Liotta, L. F. (2022). Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides. Nanomaterials, 12(14), 2371. https://doi.org/10.3390/nano12142371