Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
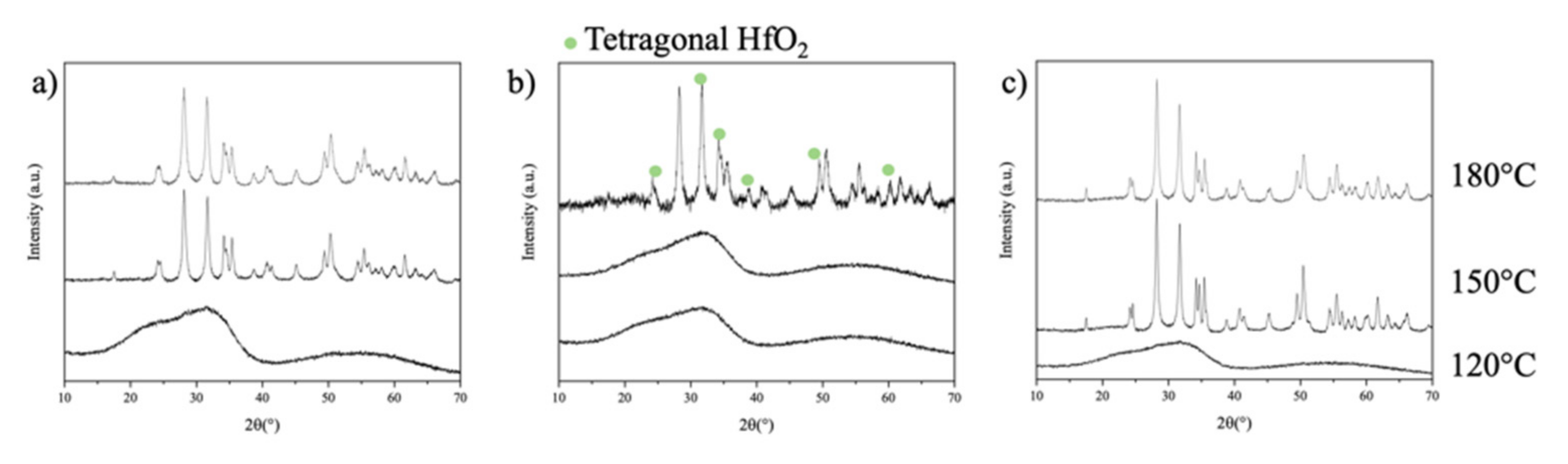
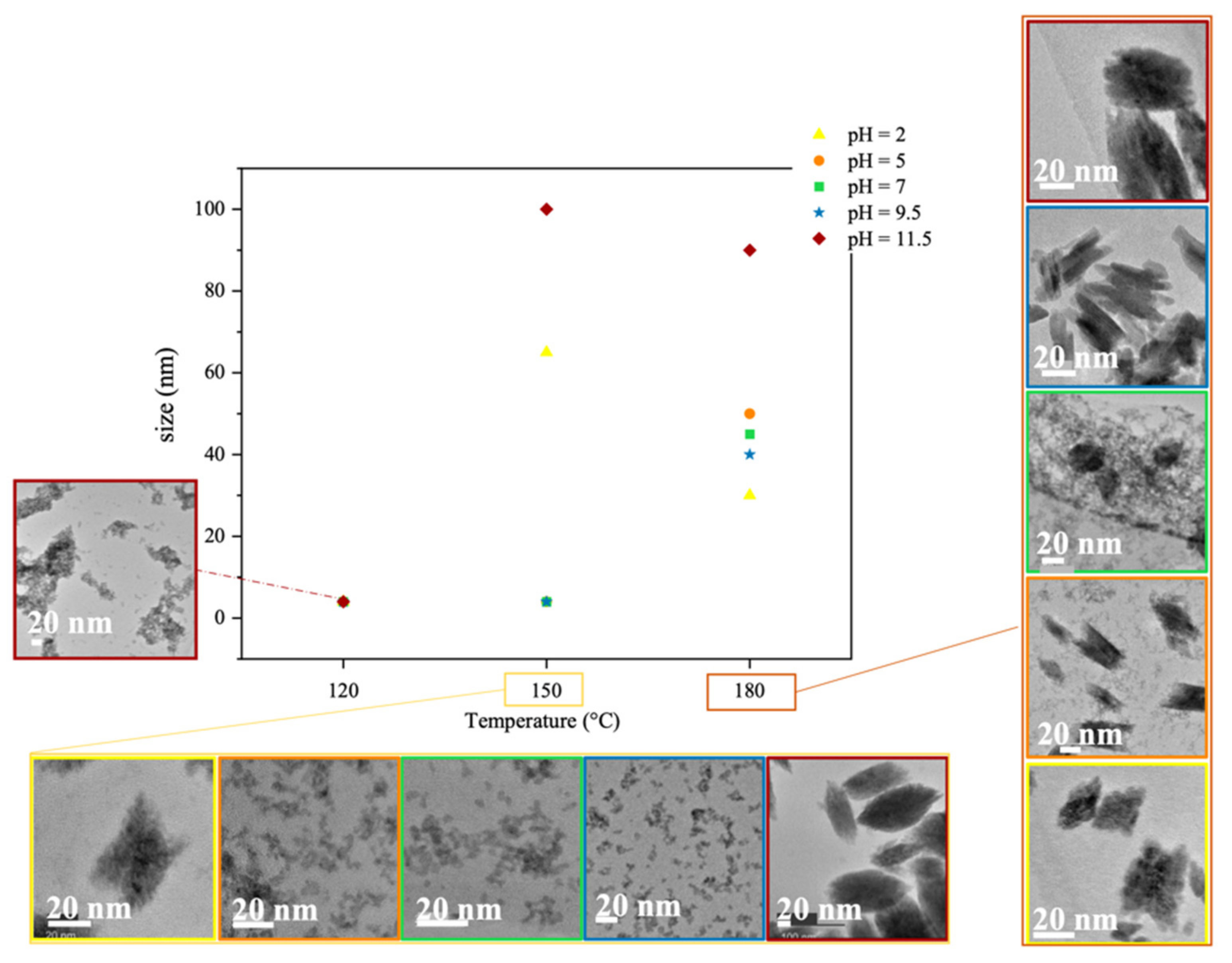
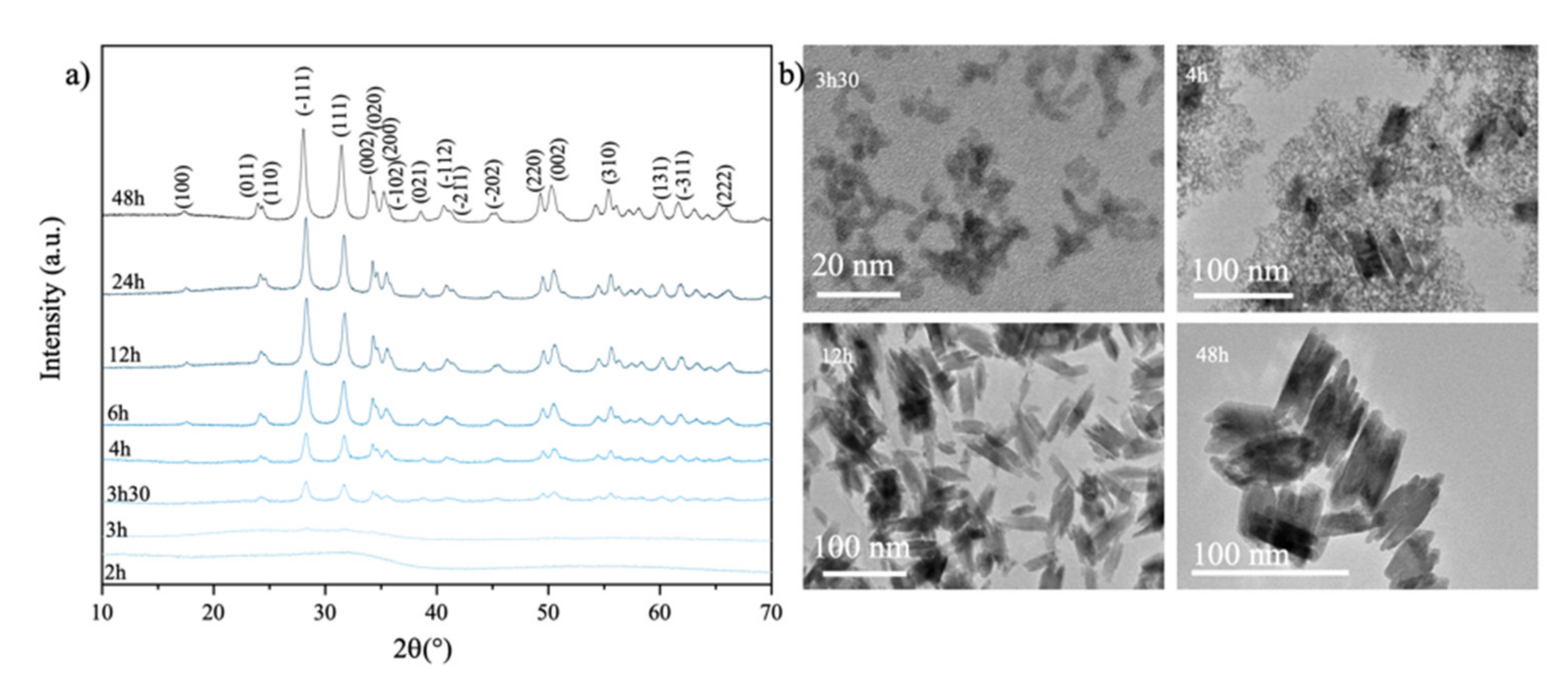
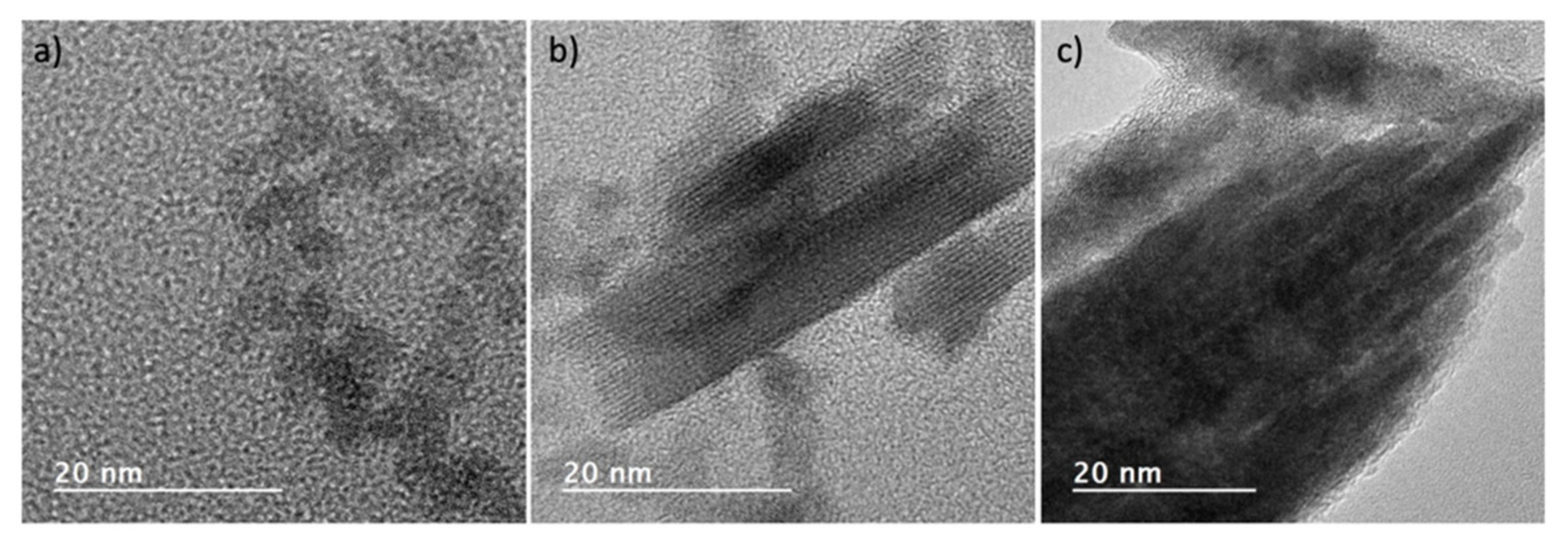
2.2. Synthesis of the Sol-Gel Solutions and the Monoclinic HfO2 Nanocrystals
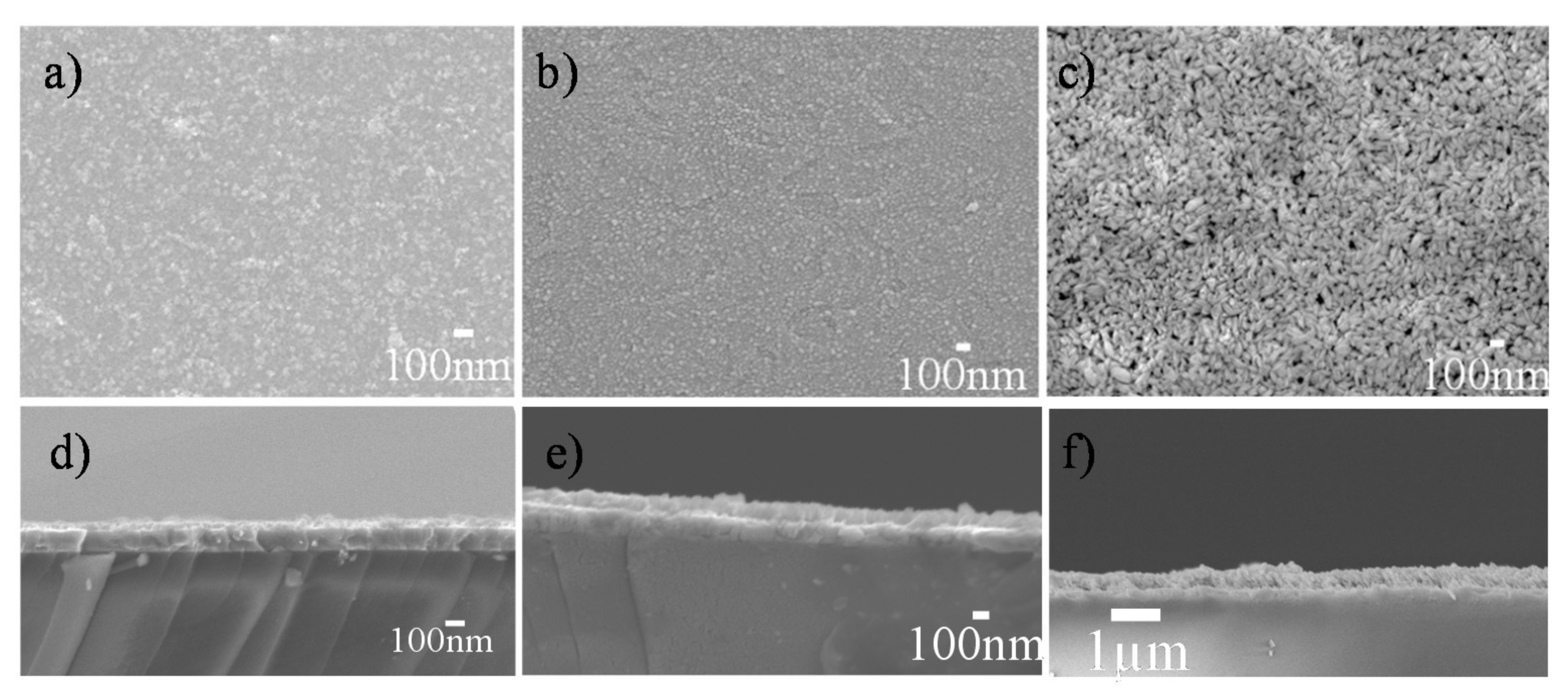
2.3. Preparation of the Polymorphic Nanostructured HfO2 Thin Films
2.4. Characterization and Analytical Techniques
3. Results and Discussion
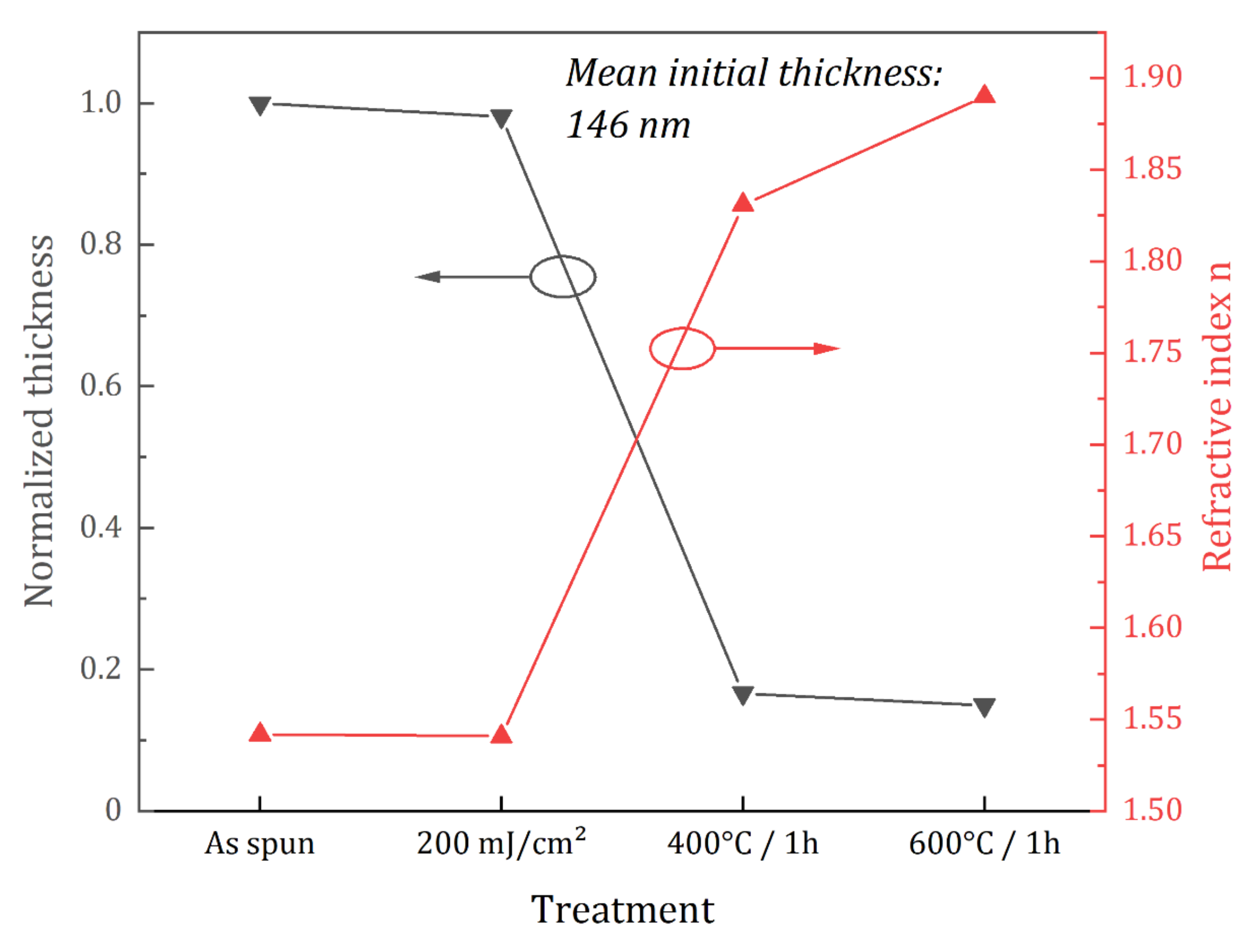
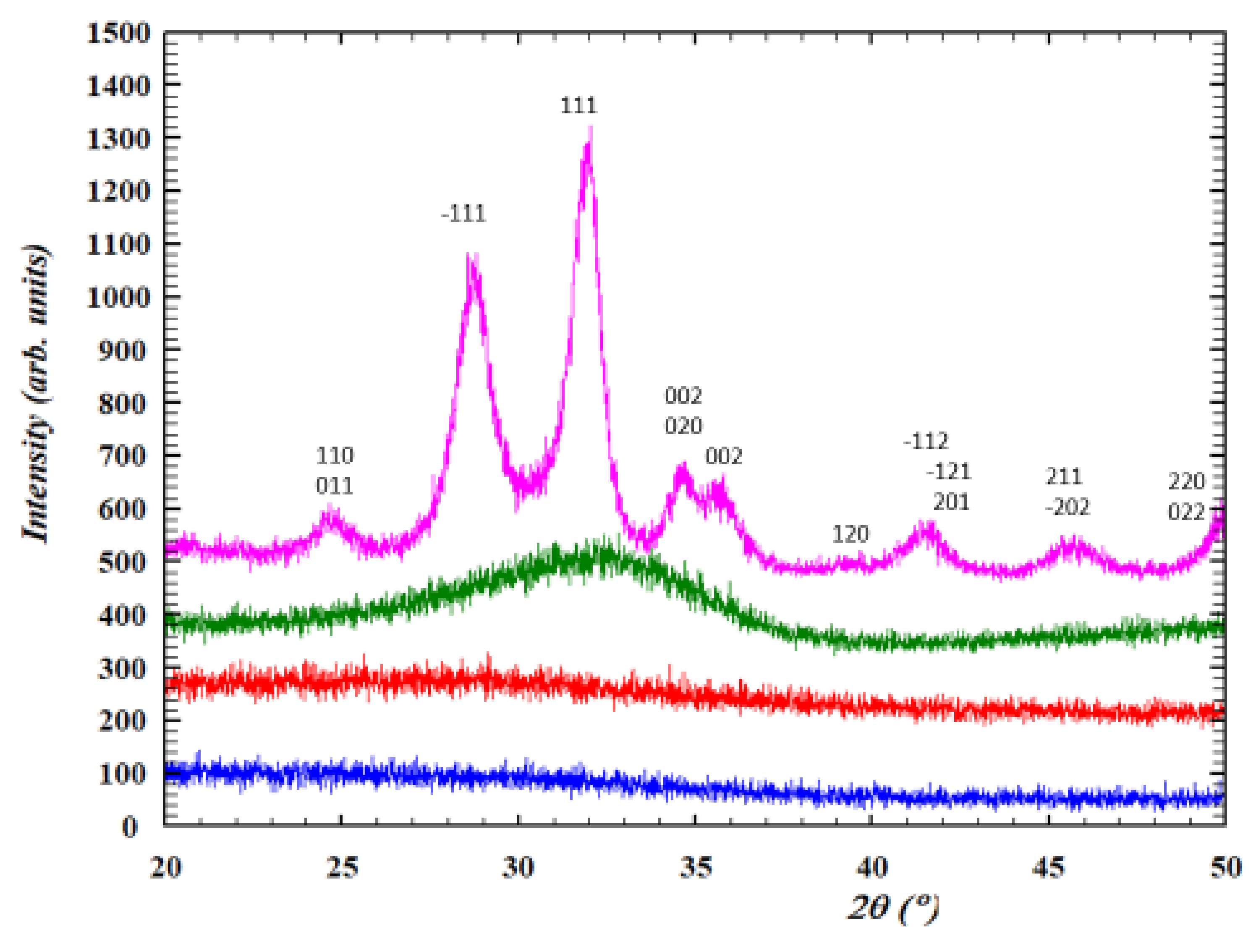
3.1. Amorphous and Monoclinic HfO2-DUV Micropatterned Thin Films
3.2. Continuous, Dense, Monoclinic HfO2-EPD Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikolajick, T.; Schroeder, U.; Park, M.H. Special topic on ferroelectricity in hafnium oxide: Materials and devices. Appl. Phys. Lett. 2021, 118, 180402. [Google Scholar] [CrossRef]
- Ali, F.; Zhou, D.; Ali, M.; Ali, H.W.; Daaim, M.; Khan, S.; Hussain, M.M.; Sun, N. Recent progress on energy-related applications of HfO2-based ferroelectric and antiferroelectric materials. ACS Appl. Electron. Mater. 2020, 2, 2301. [Google Scholar] [CrossRef]
- Francois, T.; Coignus, J.; Grenouillet, L.; Barnes, J.P.; Vaxelaire, N.; Ferrand, J.; Bottala-Gambetta, I.; Gros-Jean, M.; Jeannot, S.; Boivin, P.; et al. Ferroelectric HfO2 for Memory Applications: Impact of Si Doping Technique and Bias Pulse Engineering on Switching Performance. In Proceedings of the 2019 IEEE 11th International Memory Workshop (IMW), Monterey, CA, USA, 12–15 May 2019; Monterey, CA, USA, 2019; pp. 1–4. Available online: https://hal.archives-ouvertes.fr/hal-02399691/document (accessed on 24 May 2022).
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Kumar Yadav, B.; Dhar Malhotra, B. Highly sensitive protein functionalized nanostructured hafnium oxide based biosensing platform for non-invasive oral cancer detection. Sens. Actuator B-Chem. 2016, 235, 10. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, W.; Zhang, G.; Han, G.; Liu, Y.; Hao, Y. Synaptic behaviors in ferroelectric-like field-effect transistors with ultrathin amorphous HfO2 film. Nanoscale Res. Lett. 2022, 17, 17. [Google Scholar] [CrossRef]
- Chouprik, A.; Negrov, D.; Tsymbal, E.Y.; Zenkevichv, A. Defects in ferroelectric HfO2. Nanoscale 2021, 13, 11635. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Stipa, P.; Pierantoni, L.; Mencarelli, D. Phase properties of different HfO2 polymorphs: A DFT-based study. Crystals 2022, 12, 90. [Google Scholar] [CrossRef]
- Kaiser, N.; Vogel, T.; Zintler, A.; Petzold, S.; Arzumanov, A.; Piros, E.; Eilhardt, R.; Molina-Luna, L.; Alff, L. Defect-stabilized substoichiometric polymorphs of hafnium oxide with semiconducting properties. ACS Appl. Mater. Interfaces 2022, 14, 1290. [Google Scholar] [CrossRef]
- Trikeriotis, M.; Bae, W.J.; Schwartz, E.; Krysak, M.; Lafferty, N.; Xie, P.; Smith, B.; Zimmerman, P.A.; Ober, C.K.; Giannelis, E.P. Development of an inorganic photoresist for DUV, EUV, and electron beam imaging. In Advances in Resist Materials and Processing Technology XXVII; SPIE: San Jose, CA, USA, 2010. [Google Scholar] [CrossRef]
- Stehlin, F.; Bourgin, Y.; Spangenberg, A.; Jourlin, Y.; Parriaux, O.; Reynaud, S.; Wieder, F.; Soppera, O. Direct nanopatterning of 100 nm metal oxide periodic structures by Deep-UV immersion lithography. Opt. Lett. 2012, 37, 4651. [Google Scholar] [CrossRef]
- Stehlin, F.; Wieder, F.; Spangenberg, A.; Le Meins, J.M.; Soppera, O. Room-temperature preparation of metal-oxide nanostructures by DUV lithography from metal-oxo clusters. J. Mater. Chem. C 2014, 2, 277. [Google Scholar] [CrossRef]
- Yeh, C.C.; Liu, H.C.; Chuang, M.Y.; Denzer, J.; Berling, D.; Zan, H.W.; Soppera, O. Controllable formation of zinc oxide micro- and nanostructures via DUV direct patterning. Adv. Mater. Interfaces 2016, 3, 1600373. [Google Scholar] [CrossRef]
- Yeh, C.C.; Zan, H.W.; Soppera, O. Solution-based micro-and nanoscale metal oxide structures formed by direct patterning for electro-optical applications. Adv. Mater. 2018, 30, 1800923. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, H.; Sakai, K.; Kosma, V.; Giannelis, E.P.; Ober, C.K. Radical sensitive zinc-based nanoparticle EUV photoresists. In Advances in Patterning Materials and Processes XXXVI; SPIE: San Jose, CA, USA, 2019; p. 109601R. [Google Scholar] [CrossRef]
- Luo, C.; Xu, C.; Lv, L.; Li, H.; Huang, X.; Liu, W. Review of recent advances in inorganic photoresists. RSC Adv. 2020, 10, 8385. [Google Scholar] [CrossRef] [PubMed]
- Manouras, T.; Argitis, P. High Sensitivity Resists for EUV Lithography: A review of material design strategies and performance results. Nanomaterials 2020, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.; Ramzan, M.; Ahmed, E.; Rana, A.M.; Khalid, N.R.; Niaz, N.A.; Shakoor, A.; Ali, S.; Asghar, U.; Nadeem, M.Y. Effects of vacuum annealing on surface and optical constants of hafnium oxide thin films. Mater. Sci. Semicond. Process. 2015, 23, 3222. [Google Scholar] [CrossRef]
- Trikeriotis, M.; Krysaki, M.; Chung, Y.S.; Ouyang, C.; Cardineau, B.; Brainard, R.; Ober, C.K.; Giannelis, E.P.; Cho, K. Nanoparticle photoresists from HfO2 and ZrO2 for EUV patterning. J. Photopolym. Sci. Technol. 2012, 25, 583. [Google Scholar] [CrossRef] [Green Version]
- Bae, W.J.; Trikeriotis, M.; Sha, J.; Schwartz, E.L.; Rodriguez, R.; Zimmerman, P.; Giannelisa, E.P.; Ober, C.K. High refractive index and high transparency HfO2 nanocomposites for next generation lithography. J. Mater. Chem. 2010, 20, 5186. [Google Scholar] [CrossRef]
- Tongpeng, S.; Makbun, K.; Peanporm, P.; Sangkorna, R.; Namsar, O.; Janphuang, P.; Pojprapai, S.; Jainsirisomboon, S. Fabrication characterization of hafnium oxide thin films. Mater. Today Proc. 2019, 17, 1555. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Kumar Shrestha, L.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K. Nanoarchitectonics revolution and evolution: From small science to big technology. Small Sci. 2021, 1, 2000032. [Google Scholar] [CrossRef]
- Stefanic, G.; Music, S.; Molcanov, K. The crystallization process of HfO2 and ZrO2 under hydrothermal conditions. J. Alloys Compd. 2005, 387, 300. [Google Scholar] [CrossRef]
- Zhao, X.G.; Wang, Y.Q.; Zhang, X.Z.; Wu, Y.F.; Zhou, J.E. Hydrothermal synthesis of HfO2 nanoparticles and C/HfO2 nanopowders with core-shell structure. Chin. J. Inorg. Chem. 2008, 2, 311. [Google Scholar]
- Sahraneshin, A.; Asahina, S.; Togashi, T.; Singh, V.; Takami, S.; Hojo, D.; Arita, T.; Minami, K.; Adschiri, T. Surfactant-assisted hydrothermal synthesis of water-dispersible hafnium oxide nanoparticles in highly alkaline media. Cryst. Growth Des. 2012, 12, 5219. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, X. Formation mechanism of hafnium oxide nanoparticles by a hydrothermal route. RSC Adv. 2017, 7, 7763. [Google Scholar] [CrossRef] [Green Version]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 100. ISBN 978-0-12-386984-5. [Google Scholar]
- Doeuff, S.; Dromzee, Y.; Sanchez, C. Synthesis and structural study of the compound [Ti6(u3—0)2 (u2—O)2(u2—OAc)3(u2 —OPr)6 (OPr)6], a reference compound for the sol-gel process. C.R. Acad. Sci. Paris 1989, 308, 1409. [Google Scholar]
- Barboux-Doeuff, S.; Sanchez, C. Synthesis and characterization of titanium oxide-based Gels synthesized from acetate modified titanium butoxide Precursors. Mat. Res. Bull. 1994, 29, 1. [Google Scholar] [CrossRef]
- Chaubey, G.S.; Yao, Y.; Makongo, J.P.A.; Sahoo, P.; Misra, D.; Poudeu, P.F.P.; Wiley, J.B. Microstructural and thermal investigations of HfO2 nanoparticles. RSC Adv. 2012, 2, 9207. [Google Scholar] [CrossRef]
- Kumar, N.; Adimuriyil George, B.P.; Abrahamse, H.; Parashar, V.; Ray, S.S.; Ngil, J.C. A novel approach to low temperature synthesis of cubic HfO2 nanostructures and their cytotoxicity. Sci. Rep. 2017, 7, 9351. [Google Scholar] [CrossRef] [Green Version]
- Boccaccini, A.R.; Roether, J.A.; Thomas, B.J.C.; Shaffer, M.S.P.; Chavez, E.; Stoll, E.; Minay, E.J. The electrophoretic deposition of inorganic nanoscaled materials. J. Ceram. Soc. Jpn. 2006, 114, 1. [Google Scholar] [CrossRef] [Green Version]
- Uchikoshi, T.; Kreethawate, L.; Matsunaga, C.; Larpkiattaworn, S.; Jiemsirilers, S.; Besra, L. Fabrication of ceramic membranes on porous ceramic supports by electrophoretic deposition. Adv. Appl. Ceram. 2014, 113, 3. [Google Scholar] [CrossRef]
- Hassam, C.L.; Sciortino, F.; Nguyen, N.T.K.; Srinivasan, B.; Ariga, K.; Gascoin, F.; Grasset, F.; Mori, T.; Uchikoshi, T.; Thimont, Y.; et al. Robust, transparent hybrid thin films of phase-change material Sb2S3 prepared by electrophoretic deposition. ACS Appl. Energy Mater. 2021, 4, 9891. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Renaud, A.; Dierre, B.; Bouteille, B.; Wilmet, M.; Dubernet, M.; Ohashi, N.; Grasset, F.; Uchikoshi, T. Extended study on electrophoretic deposition process of inorganic octahedral metal clusters: Advanced multifunctional transparent nanocomposite thin films. Bull. Chem. Soc. Jpn. 2018, 91, 1763. [Google Scholar] [CrossRef] [Green Version]
- Kirakci, K.; Nguyen, T.K.N.; Grasset, F.; Uchikoshi, T.; Zelenka, J.; Kubaát, P.; Ruml, T.; Lang, K. Electrophoretically deposited layers of octahedral molybdenum cluster complexes: A promising coating for mitigation of pathogenic bacterial biofilms under blue light. ACS Appl. Mater. Interfaces 2020, 12, 52492. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.U.; Chen, Q.; Braem, A.; Shaffer, M.S.P.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes: Recent progress and remaining challenges. Int. Mater. Rev. 2021, 66, 533. [Google Scholar] [CrossRef]
- Sarkar, P.; Nicholson, P.S. Electrophoretic deposition (EPD): Mechanism, kinetics and application to ceramics. J. Am. Ceram. Soc. 1996, 79, 1987. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1. [Google Scholar] [CrossRef]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L. Electrophoretic deposition (EPD): Fundamentals and applications from nano- to microscale structures. In Handbook of Nanoelectrochemistry; Springer: Berlin/Heidelberg, Germany, 2016; p. 561. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Grasset, F.; Dierre, B.; Matsunaga, C.; Cordier, S.; Lemoine, P.; Ohashi, N.; Uchikoshi, T. Fabrication of transparent thin film of octahedral molybdenum metal clusters by electrophoretic deposition. ECS J. Solid State Sci. Technol. 2016, 5, 178. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proust, V.; Kirscher, Q.; Nguyen, T.K.N.; Obringer, L.; Ishii, K.; Rault, L.; Demange, V.; Berthebaud, D.; Ohashi, N.; Uchikoshi, T.; et al. Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning. Nanomaterials 2022, 12, 2334. https://doi.org/10.3390/nano12142334
Proust V, Kirscher Q, Nguyen TKN, Obringer L, Ishii K, Rault L, Demange V, Berthebaud D, Ohashi N, Uchikoshi T, et al. Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning. Nanomaterials. 2022; 12(14):2334. https://doi.org/10.3390/nano12142334
Chicago/Turabian StyleProust, Vanessa, Quentin Kirscher, Thi Kim Ngan Nguyen, Lisa Obringer, Kento Ishii, Ludivine Rault, Valérie Demange, David Berthebaud, Naoki Ohashi, Tetsuo Uchikoshi, and et al. 2022. "Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning" Nanomaterials 12, no. 14: 2334. https://doi.org/10.3390/nano12142334
APA StyleProust, V., Kirscher, Q., Nguyen, T. K. N., Obringer, L., Ishii, K., Rault, L., Demange, V., Berthebaud, D., Ohashi, N., Uchikoshi, T., Berling, D., Soppera, O., & Grasset, F. (2022). Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning. Nanomaterials, 12(14), 2334. https://doi.org/10.3390/nano12142334









