High-Dispersed V2O5-CuOX Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterization
2.3. Catalytic Performance Test
3. Results and Discussion
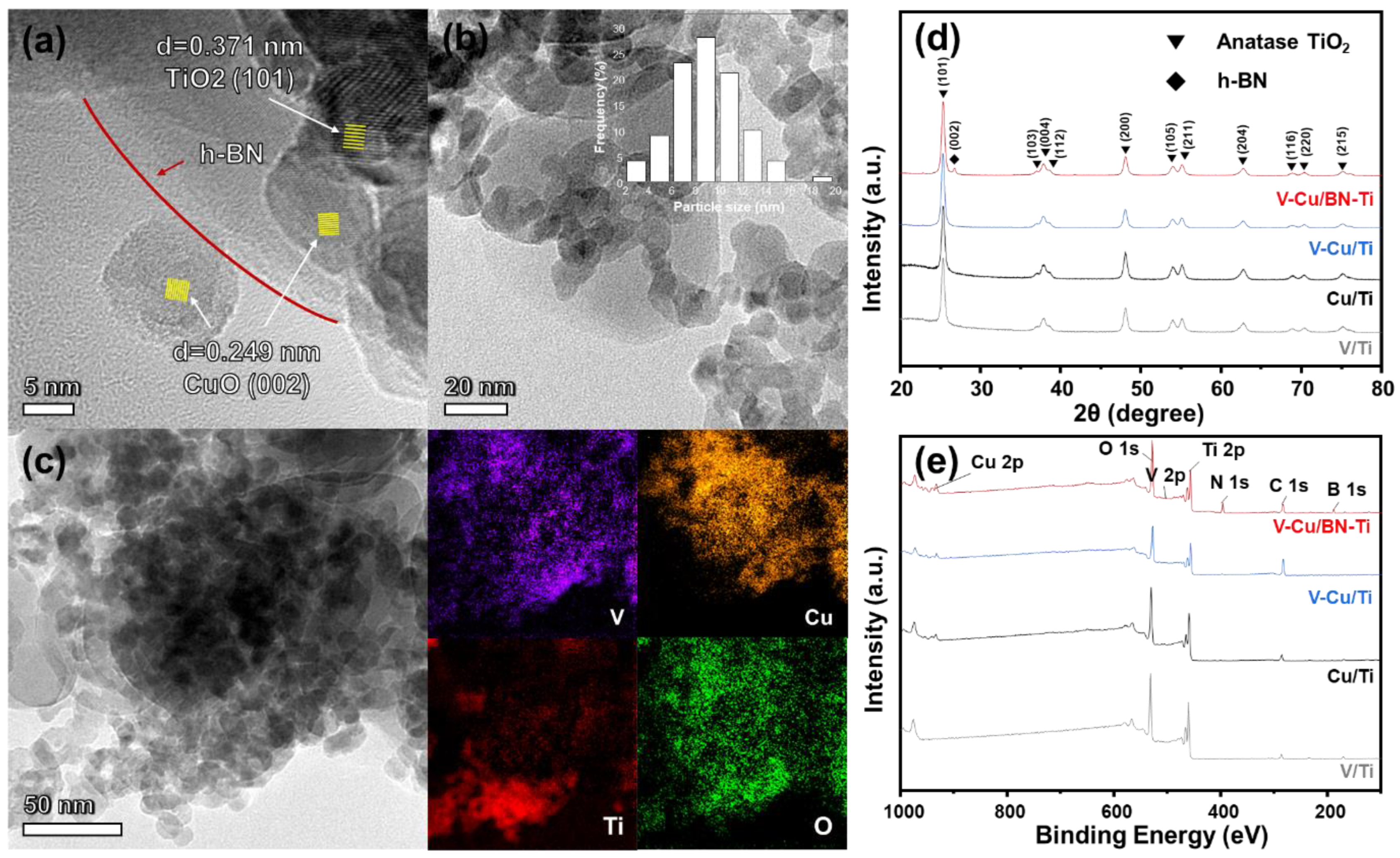
3.1. Morphology and Textile Properties Analysis
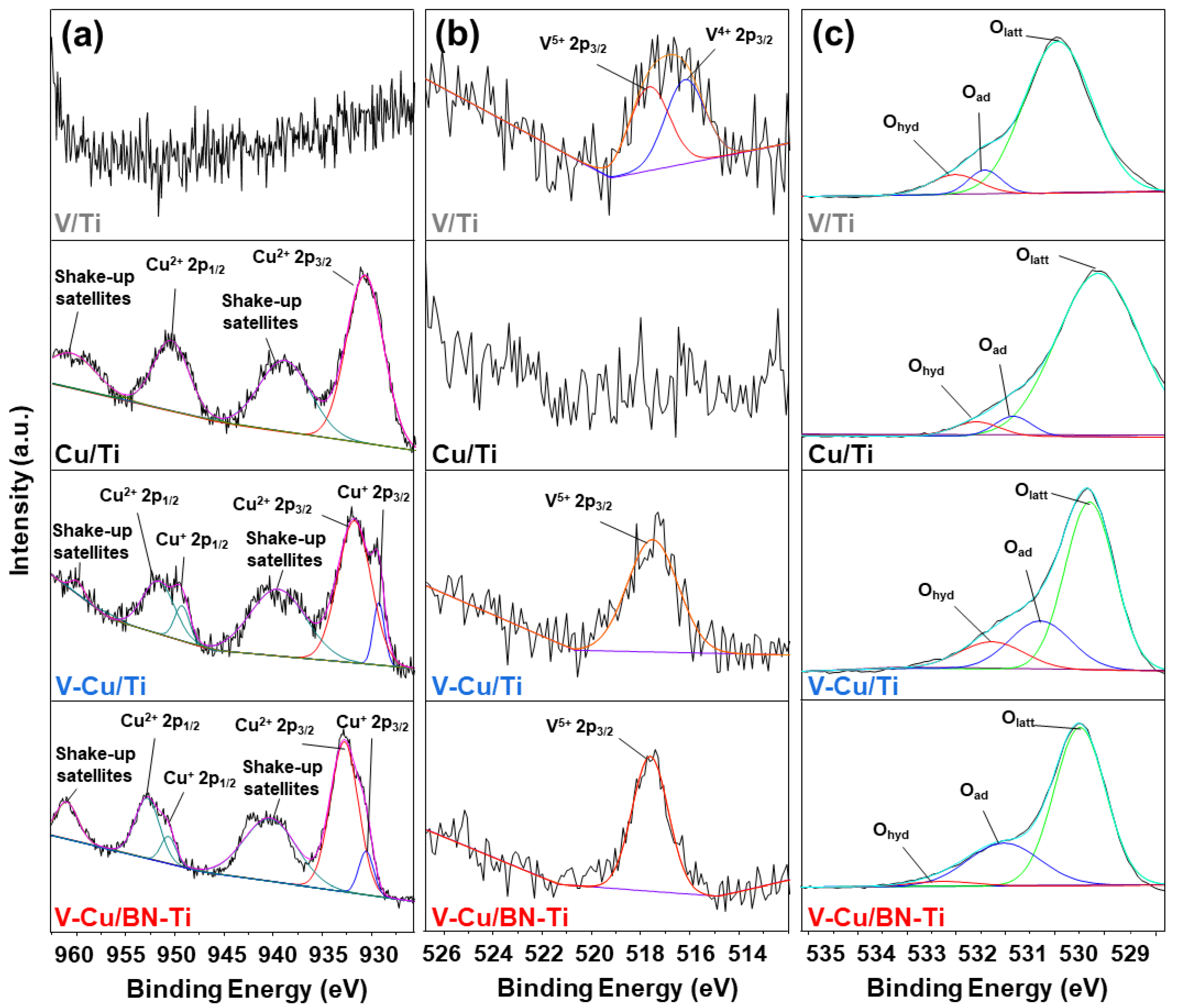
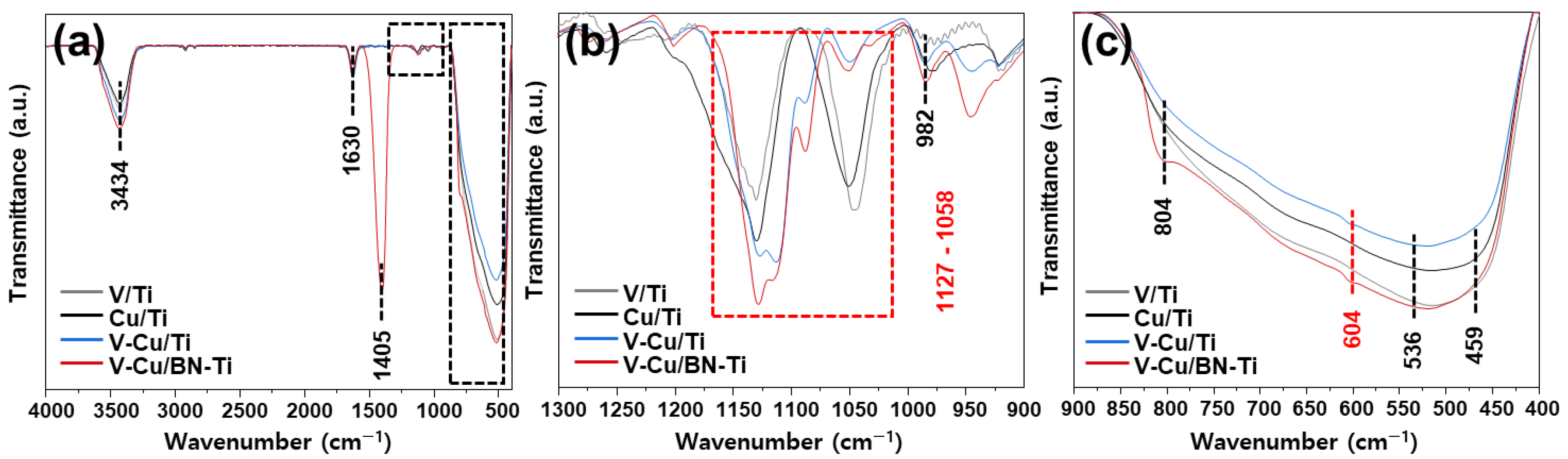
3.2. Characterization of the NH3-SCR and NH3-SCO Catalysts
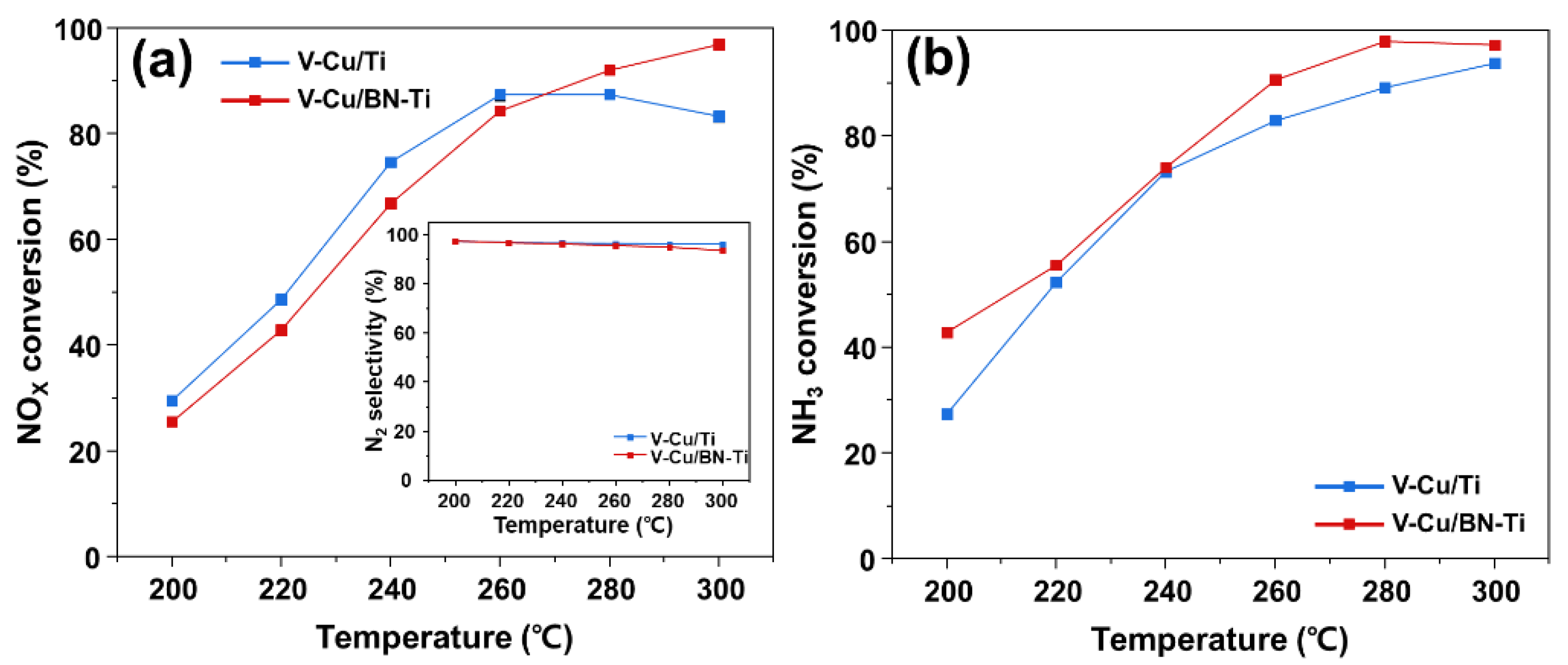
3.3. Catalytic Performance of NH3-SCR and NH3-SCO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, X.; Chen, P.; Zhu, B. Research status and prospect on vanadium-based catalysts for NH3-SCR denitration. Materials 2018, 11, 1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Kim, S.I.; Lee, M.J.; Ye, B.; Kim, T.; Kim, H.D.; Lee, J.W.; Lee, D.H. Effect of catalyst crystallinity on V-based selective catalytic reduction with ammonia. Nanomaterials 2021, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Ye, B.; Lee, M.-j.; Chun, S.-Y.; Jeong, B.; Kim, H.-D.; Jae, J.; Kim, T. Selective catalytic reduction of NO by NH3 over V2O5-WO3 supported by titanium isopropoxide (TTIP)-treated TiO2. J. Ind. Eng. Chem. 2022, 109, 422–430. [Google Scholar] [CrossRef]
- Frost, G.J.; McKeen, S.A.; Trainer, M.; Ryerson, T.B.; Neuman, J.A.; Roberts, J.M.; Swanson, A.; Holloway, J.S.; Sueper, D.T.; Fortin, T.; et al. Effects of changing power plant NOX emissions on ozone in the eastern United States: Proof of concept. J. Geophys. Res. 2006, 111, D12306. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jiao, M.; Chen, Z.; He, H.; Liu, L. Effects of montmorillonite and anatase TiO2 support on CeO2 catalysts during NH3-SCR reaction. Microporous Mesoporous Mater. 2021, 320, 111072. [Google Scholar] [CrossRef]
- Ye, B.; Kim, J.; Lee, M.-J.; Chun, S.-Y.; Jeong, B.; Kim, T.; Lee, D.H.; Kim, H.-D. Mn-Ce oxide nanoparticles supported on nitrogen-doped reduced graphene oxide as low-temperature catalysts for selective catalytic reduction of nitrogen oxides. Microporous Mesoporous Mater. 2021, 310, 110588. [Google Scholar] [CrossRef]
- Richter, M.; Trunschke, A.; Bentrup, U.; Brzezinka, K.W.; Schreier, E.; Schneider, M.; Pohl, M.M.; Fricke, R. Selective Catalytic Reduction of Nitric Oxide by Ammonia over Egg-Shell MnOX/NaY Composite Catalysts. J. Catal. 2002, 206, 98–113. [Google Scholar] [CrossRef]
- Phil, H.H.; Reddy, M.P.; Kumar, P.A.; Ju, L.K.; Hyo, J.S. SO2 resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOX at low temperatures. Appl. Catal. B Environ. 2008, 78, 301–308. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Y.; Wang, J.; Luo, L.; Zhu, T. Ca Doping Effect on the Competition of NH3–SCR and NH3 Oxidation Reactions over Vanadium-Based Catalysts. J. Phys. Chem. C 2021, 125, 6128–6136. [Google Scholar] [CrossRef]
- Guo, K.; Fan, G.; Gu, D.; Yu, S.; Ma, K.; Liu, A.; Tan, W.; Wang, J.; Du, X.; Zou, W.; et al. Pore Size Expansion Accelerates Ammonium Bisulfate Decomposition for Improved Sulfur Resistance in Low-Temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef]
- Jabłońska, M.; Nocuń, M.; Gołąbek, K.; Palkovits, R. Effect of preparation procedures on catalytic activity and selectivity of copper-based mixed oxides in selective catalytic oxidation of ammonia into nitrogen and water vapour. Appl. Surf. Sci. 2017, 423, 498–508. [Google Scholar] [CrossRef]
- Ko, A.; Woo, Y.; Jang, J.; Jung, Y.; Pyo, Y.; Jo, H.; Lim, O.; Lee, Y.J. Availability of NH3 adsorption in vanadium-based SCR for reducing NOX emission and NH3 slip. J. Ind. Eng. Chem. 2019, 78, 433–439. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chu, H.; Cho, C.M. Absorption and reaction kinetics of amines and ammonia solutions with carbon dioxide in flue gas. J. Air Waste Manag. Assoc. 2003, 53, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Seara, J.; Sieres, J.; Rodríguez, C.; Vázquez, M. Ammonia–water absorption in vertical tubular absorbers. Int. J. Therm. Sci. 2005, 44, 277–288. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Zhang, T.; Wang, J.; Wei, G.; Liu, M.; Ning, P. Structural tuning and NH3-SCO performance optimization of CuO-Fe2O3 catalysts by impact of thermal treatment. Appl. Surf. Sci. 2019, 485, 81–91. [Google Scholar] [CrossRef]
- Shah, S.; Abrol, S.; Balram, S.; Barve, J. Optimal ammonia injection for emissions control in power plants. IFAC-PapersOnLine 2015, 48–30, 379–384. [Google Scholar] [CrossRef]
- Jabłońska, M.; Beale, A.M.; Nocuń, M.; Palkovits, R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal. B Environ. 2018, 232, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Wang, H.; Wang, S.; Cheng, H.; Qin, Y.; Wang, Z. Role of the support on the behavior of Ag-based catalysts for NH 3 selective catalytic oxidation (NH3-SCO). Appl. Surf. Sci. 2014, 316, 373–379. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, D.H.; Hong, S.C. The Selective Catalytic Oxiation of Ammonia: Effect of Physicochemical Properties on Pt/TiO2. Appl. Chem. Eng. 2017, 28, 279–285. [Google Scholar] [CrossRef]
- Shrestha, S.; Harold, M.P.; Kamasamudram, K.; Yezerets, A. Selective oxidation of ammonia on mixed and dual-layer Fe-ZSM-5+Pt/Al2O3 monolithic catalysts. Catal. Today 2014, 231, 105–115. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Mamedov, S.; Boolchand, P.; Smirniotis, P.G. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl. Catal. B Environ. 2007, 76, 123–134. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, S.; Xin, Q.; Zou, R.; Zheng, C.; Gao, X.; Cen, K. Mechanistic investigation of NH3 oxidation over V-0.5Ce(SO4)2/Ti NH3-SCR catalyst. Catal. Commun. 2018, 112, 1–4. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Qu, Z.; Tade, M.; Liu, S. The role of copper species on Cu/γ-Al2O3 catalysts for NH3–SCO reaction. Appl. Surf. Sci. 2012, 258, 3738–3743. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Jraba, N.; Makhlouf, T.; Delahay, G.; Tounsi, H. Catalytic activity of Cu/eta-Al2O3 catalysts prepared from aluminum scraps in the NH3-SCO and in the NH3-SCR of NO. Environ. Sci. Pollut. Res. Int. 2022, 29, 9053–9064. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, D.; Tong, Z.; Wang, J.; Chen, J.; He, C. Rationally engineered ReO-CuSO4/TiO2 catalyst with superior NH3-SCO efficiency and remarkably boosted SO2 tolerance: Synergy of acid sites and surface adsorbed oxygen. Chem. Eng. J. 2022, 442, 136356. [Google Scholar] [CrossRef]
- Darvell, L.I.; Heiskanen, K.; Jones, J.M.; Ross, A.B.; Simell, P.; Williams, A. An investigation of alumina-supported catalysts for the selective catalytic oxidation of ammonia in biomass gasification. Catal. Today 2003, 81, 681–692. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Rafalska-Łasocha, A.; Dziembaj, R. Selective oxidation of ammonia to nitrogen on transition metal containing mixed metal oxides. Appl. Catal. B Environ. 2005, 58, 235–244. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Jia, W. The effect of SO2 on NH3-SCO and SCR properties over Cu/SCR catalyst. Appl. Surf. Sci. 2020, 507, 145153. [Google Scholar] [CrossRef]
- Wang, Z.-y.; Guo, R.-t.; Shi, X.; Pan, W.-g.; Liu, J.; Sun, X.; Liu, S.-w.; Liu, X.-y.; Qin, H. The enhanced performance of Sb-modified Cu/TiO2 catalyst for selective catalytic reduction of NOX with NH3. Appl. Surf. Sci. 2019, 475, 334–341. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Z.; Huang, S.; Zhang, W. Cu-SSZ-13 zeolite–metal oxide hybrid catalysts with enhanced SO2-tolerance in the NH3-SCR of NOX. Appl. Catal. B Environ. 2020, 269, 118825. [Google Scholar] [CrossRef]
- Wu, P.; Lu, L.; He, J.; Chen, L.; Chao, Y.; He, M.; Zhu, F.; Chu, X.; Li, H.; Zhu, W. Hexagonal boron nitride: A metal-free catalyst for deep oxidative desulfurization of fuel oils. Green Energy Environ. 2020, 5, 166–172. [Google Scholar] [CrossRef]
- Lee, M.-j.; Ye, B.; Jeong, B.; Chun, S.-y.; Kim, T.; Kim, D.-H.; Lee, H.; Kim, H.-D. MnOX–CeOX Nanoparticles supported on porous hexagonal boron nitride nanoflakes for selective catalytic reduction of nitrogen oxides. ACS Appl. Nano Mater. 2020, 3, 11254–11265. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Pantaleo, G.; Liotta, L.F. WO3–V2O5 Active Oxides for NOX SCR by NH3: Preparation methods, catalysts’ composition, and deactivation mechanism—A review. Catalysts 2019, 9, 527. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Gopalan, A.I.; Saianand, G.; Lee, K.P.; Kim, W.J. Manganese and graphene included titanium dioxide composite nanowires: Fabrication, characterization and enhanced photocatalytic activities. Nanomaterials 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.; Ma, L.; Ma, H.; Yue, M. In situ characterization of Cu–Fe–OX catalyst for water–gas shift reaction. J. Mater. Sci. 2017, 53, 1065–1075. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, G.; Tang, Z.; Zhang, J. Morphology-controlled synthesis of TiO2 with different structural units and applied for the selective catalytic reduction of NOX with NH3. Catal. Surv. Asia 2020, 24, 300–312. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Zhao, W.; Xu, X.; Jin, Q.; Qi, J.; Yu, R.; Wang, D. Enhanced catalytic activity of Au-CeO2/Al2O3 monolith for low-temperature CO oxidation. Catal. Commun. 2019, 129, 105729. [Google Scholar] [CrossRef]
- Kimura, J.; Ohkubo, T.; Nishina, Y.; Urita, K.; Kuroda, Y. Adsorption enhancement of nitrogen gas by atomically heterogeneous nanospace of boron nitride. RSC Adv. 2020, 11, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; Taitt, B.J.; Choi, K.-S. Copper-based catalytic anodes to produce 2,5-Furandicarboxylic acid, a biomass-derived alternative to terephthalic acid. ACS Catal. 2018, 8, 1197–1206. [Google Scholar] [CrossRef]
- Ren, X.; Ou, Z.; Wu, B. Low-temperature selective catalytic reduction DeNOX and regeneration of Mn-Cu catalyst supported by activated coke. Materials 2021, 14, 5958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. The catalytic properties of Cu modified attapulgite in NH3–SCO and NH3–SCR reactions. Appl. Surf. Sci. 2019, 480, 537–547. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Guo, X.; Bartholomew, C.; Hecker, W.; Baxter, L.L. Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems. Appl. Catal. B Environ. 2009, 92, 30–40. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Mao, L.; Fu, M.; Zhao, H.; Sun, L.; Xiao, Y.; Dong, G. A relationship between the V4+/V5+ ratio and the surface dispersion, surface acidity, and redox performance of V2O5-WO3/TiO2 SCR catalysts. RSC Adv. 2018, 8, 31081–31093. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Long, Y.; Tong, X.; Yin, Y.; Li, X.; Hu, J. Transition metals modified commercial SCR catalysts as efficient catalysts in NH3-SCO and NH3-SCR reactions. Mol. Catal. 2021, 515, 111888. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ouda, A.A.; Ridha, N.J.; Abud, S.H. Structure and optical properties of TiO2 nanorods prepared using polyol solvothermal method. In Proceedings of the The 7th International Conference on Applied Science and Technology (Icast 2019), Karbala City, Iraq, 27–28 March 2019. [Google Scholar]
- Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized boron nitride nanosheets/poly(l-lactide) nanocomposites and their crystallization behavior. Polymers 2019, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Bosco, M.V.; Bañares, M.A.; Martínez-Huerta, M.V.; Bonivardi, A.L.; Collins, S.E. In situ FTIR and Raman study on the distribution and reactivity of surface vanadia species in V2O5/CeO2 catalysts. J. Mol. Catal. A Chem. 2015, 408, 75–84. [Google Scholar] [CrossRef]
- Sundar, S.; Venkatachalam, G.; Kwon, S.J. Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine. Nanomaterials 2018, 8, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Qurashi, A. Shape controlled synthesis of copper vanadate platelet nanostructures, their optical band edges, and solar-driven water splitting properties. Sci. Rep. 2017, 7, 14370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leistner, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Ammonia desorption peaks can be Assigned to different copper sites in Cu/SSZ-13. Catal. Lett. 2017, 147, 1882–1890. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Liang, T.; Zhang, W.; Zheng, W.; Zhao, H.; Guo, L.; Wu, X. Catalytic performances of Cu/MCM-22 zeolites with different Cu loadings in NH3-SCR. Nanomaterials 2020, 10, 2170. [Google Scholar] [CrossRef]
- Kubota, H.; Toyao, T.; Maeno, Z.; Inomata, Y.; Murayama, T.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Shimizu, K.-i. Analogous Mechanistic Features of NH3-SCR over Vanadium Oxide and Copper Zeolite Catalysts. ACS Catal. 2021, 11, 11180–11192. [Google Scholar] [CrossRef]
- Purbia, R.; Choi, S.Y.; Kim, H.J.; Ye, B.; Jeong, B.; Lee, D.H.; Park, H.; Kim, H.-D.; Baik, J.M. Cu- and Ce-promoted nano-heterostructures on vanadate catalysts for low-temperature NH3–SCR activity with improved SO2 and water resistance. Chem. Eng. J. 2022, 437, 135427. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Ren, W.; Wang, J.; Zhu, T.; Xu, W. Effect of Ce doping on catalytic performance of Cu/TiO2 for CO oxidation. Catal. Lett. 2020, 150, 2045–2055. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 3682. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.; Wang, S.; Zhu, B.; Zhang, S.; Huang, W.; Wu, S. A comparative study of CuO/TiO2-SnO2, CuO/TiO2 and CuO/SnO2 catalysts for low-temperature CO oxidation. J. Nat. Gas Chem. 2009, 18, 449–452. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, M.; Kong, H.; Lyu, J.; Yang, H. Investigation and control technology on excessive ammonia-slipping in coal-fired plants. Energies 2020, 13, 4249. [Google Scholar] [CrossRef]




| Catalysts | Content of Surface-Exposed Elements (at %) | Composition of Copper Species (at %) | Composition of Oxygen Species (at %) | |||
|---|---|---|---|---|---|---|
| V | Cu | Cu2+ | Cu+ | Cu+/(Cu+ + Cu2+) | Oα/(Oα + Oβ) | |
| V/Ti | 0.84 | - | - | - | - | 6.53 |
| Cu/Ti | - | 3.01 | 100 | 0 | 0 | 5.20 |
| V-Cu/Ti | 0.77 | 2.96 | 66.39 | 8.67 | 0.11 | 22.58 |
| V-Cu/BN-Ti | 3.07 | 3.70 | 57.09 | 8.22 | 0.12 | 26.73 |
| Catalysts | Brønsted Acid Sites (mmol/g) | Lewis Acid Sites (mmol/g) | H2 Consumption (μmol/g) |
|---|---|---|---|
| V/Ti | 0.72 | 0.20 | 1.03 |
| Cu/Ti | 0.80 | 0.10 | 1.28 |
| V-Cu/Ti | 0.74 | 0.47 | 1.37 |
| V-Cu/BN-Ti | 0.88 | 0.45 | 1.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.-G.; Lee, M.-J.; Kim, W.-G.; Kim, S.-J.; Jeong, B.; Ye, B.; Lee, H.; Kim, H.-D. High-Dispersed V2O5-CuOX Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance. Nanomaterials 2022, 12, 2329. https://doi.org/10.3390/nano12142329
Im H-G, Lee M-J, Kim W-G, Kim S-J, Jeong B, Ye B, Lee H, Kim H-D. High-Dispersed V2O5-CuOX Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance. Nanomaterials. 2022; 12(14):2329. https://doi.org/10.3390/nano12142329
Chicago/Turabian StyleIm, Han-Gyu, Myeung-Jin Lee, Woon-Gi Kim, Su-Jin Kim, Bora Jeong, Bora Ye, Heesoo Lee, and Hong-Dae Kim. 2022. "High-Dispersed V2O5-CuOX Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance" Nanomaterials 12, no. 14: 2329. https://doi.org/10.3390/nano12142329
APA StyleIm, H.-G., Lee, M.-J., Kim, W.-G., Kim, S.-J., Jeong, B., Ye, B., Lee, H., & Kim, H.-D. (2022). High-Dispersed V2O5-CuOX Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance. Nanomaterials, 12(14), 2329. https://doi.org/10.3390/nano12142329






