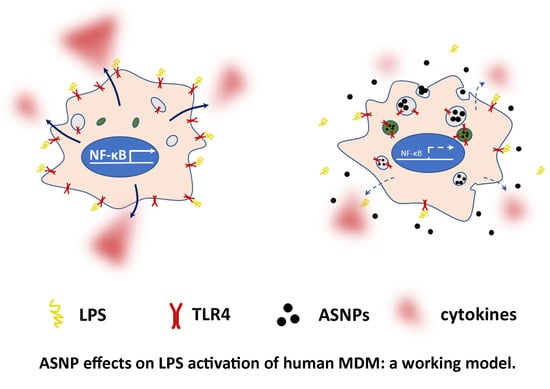
The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monocyte Isolation and Macrophage Differentiation
2.3. ASNP Suspension and Experimental Treatments
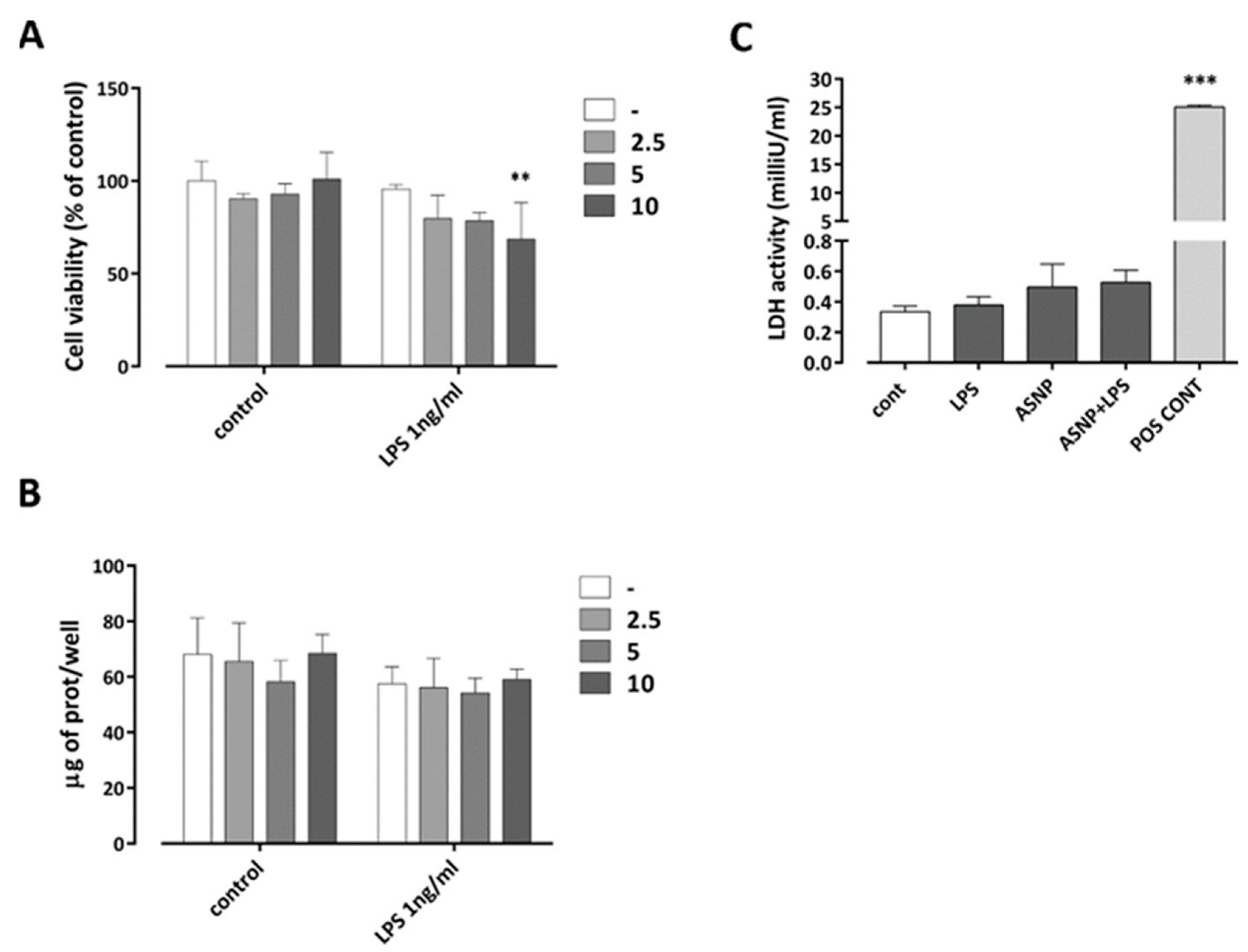
2.4. Cell Viability and Cytotoxicity
2.5. Western Blot Analysis
2.6. Immunocytochemistry and Confocal Microscopy
2.7. Gene Silencing
2.8. RNA Extraction and Real Time PCR
2.9. Statistics
3. Results
3.1. The Exposure to ASNP Did Not Cause Cytotoxicity in Human Macrophages
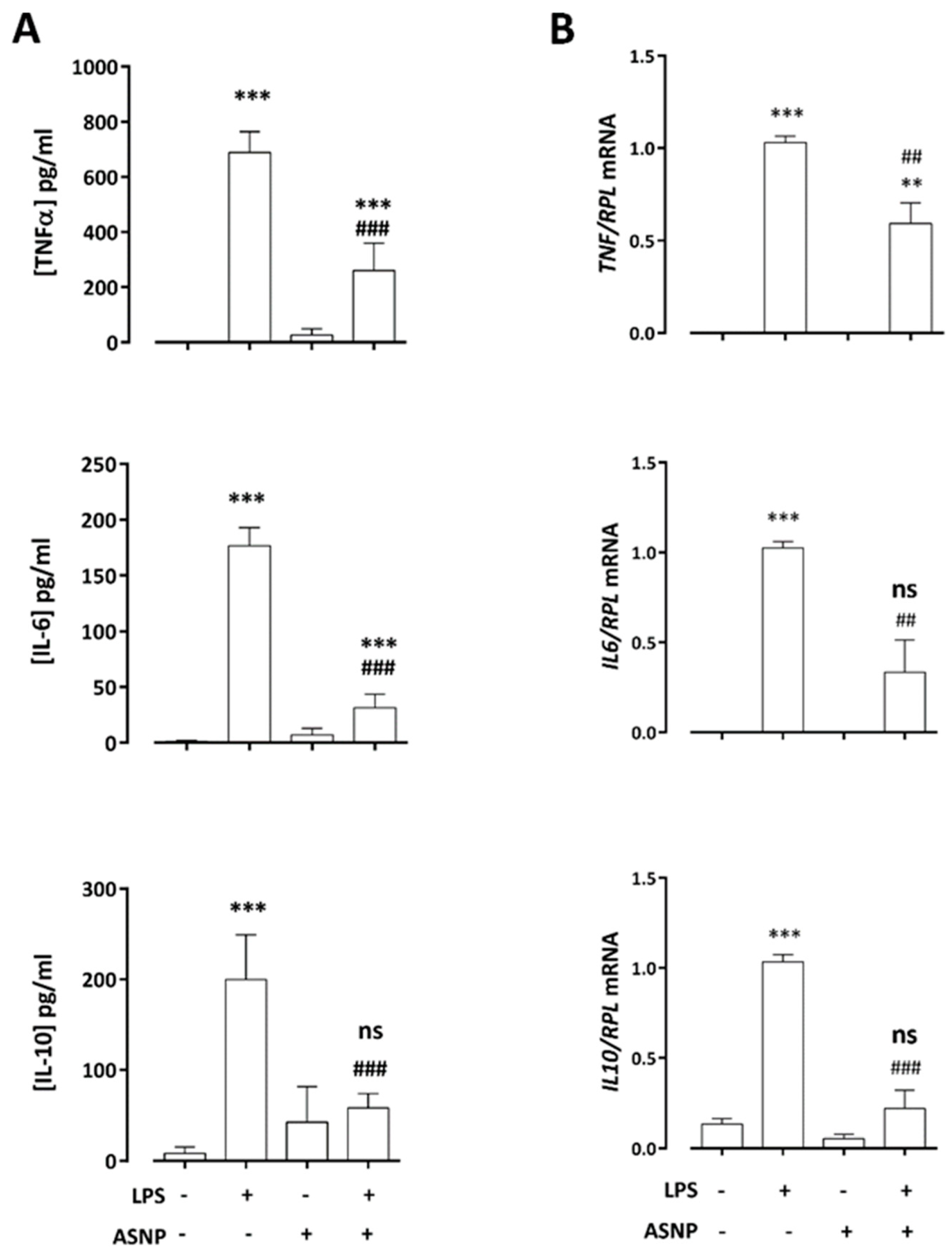
3.2. ASNPs Dampen the Secretion of Inflammatory Cytokines
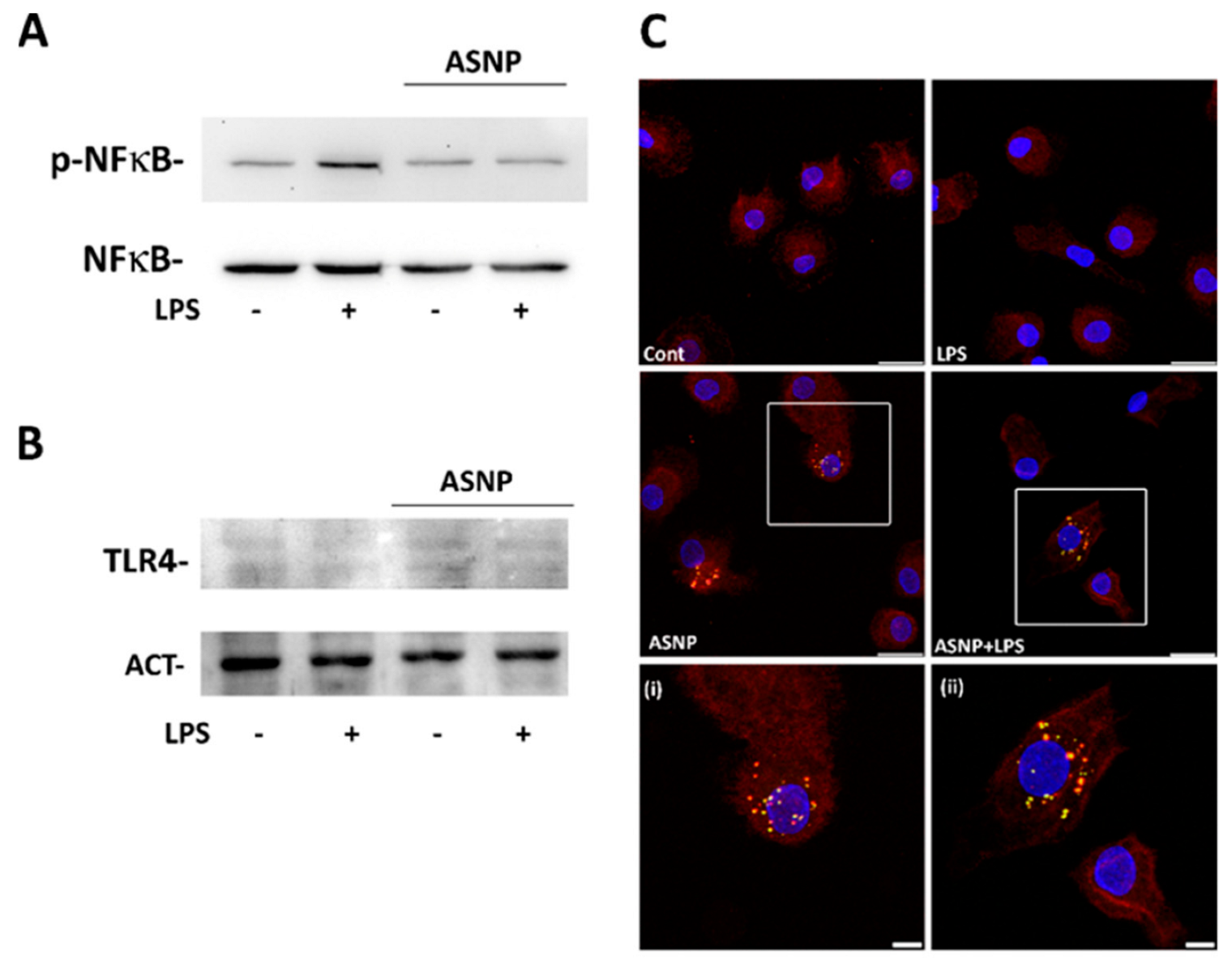
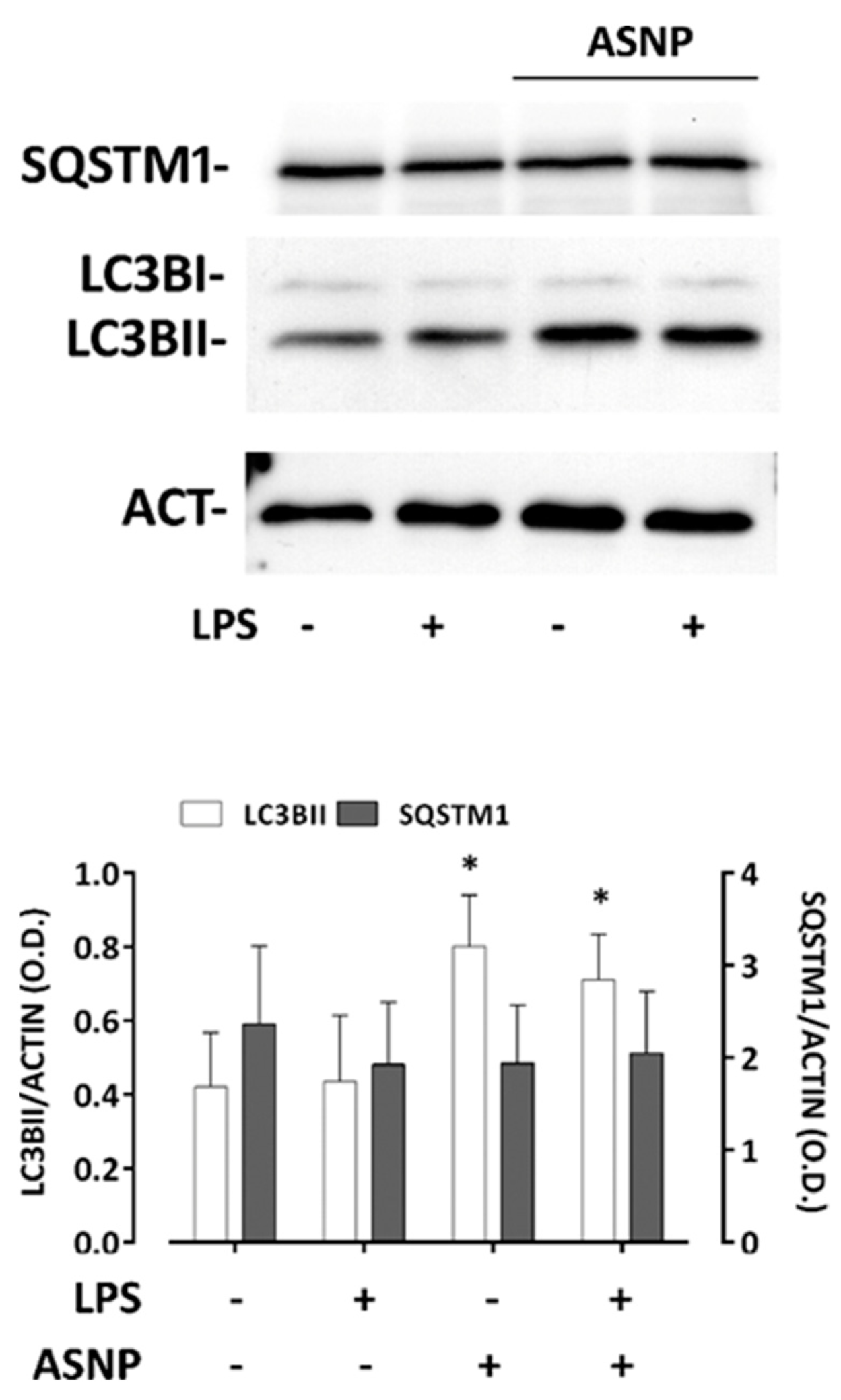
3.3. ASNP Inhibit NFκB Activation, Affect Autophagic Flux and Promote TLR4 Internalization
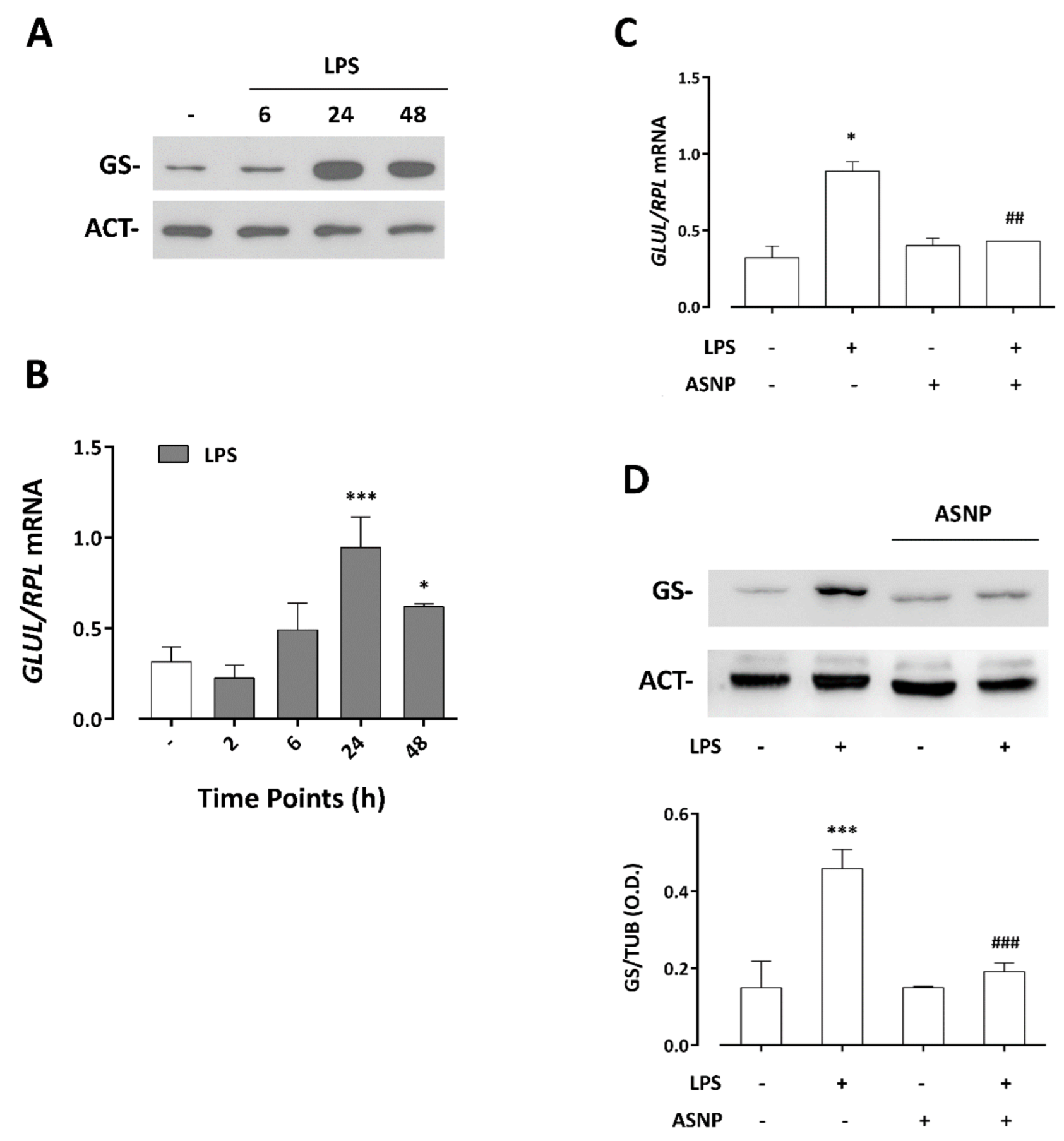
3.4. LPS-Dependent Induction of Glutamine Synthetase Gene and Protein Expression Are Prevented by ASNP Exposure
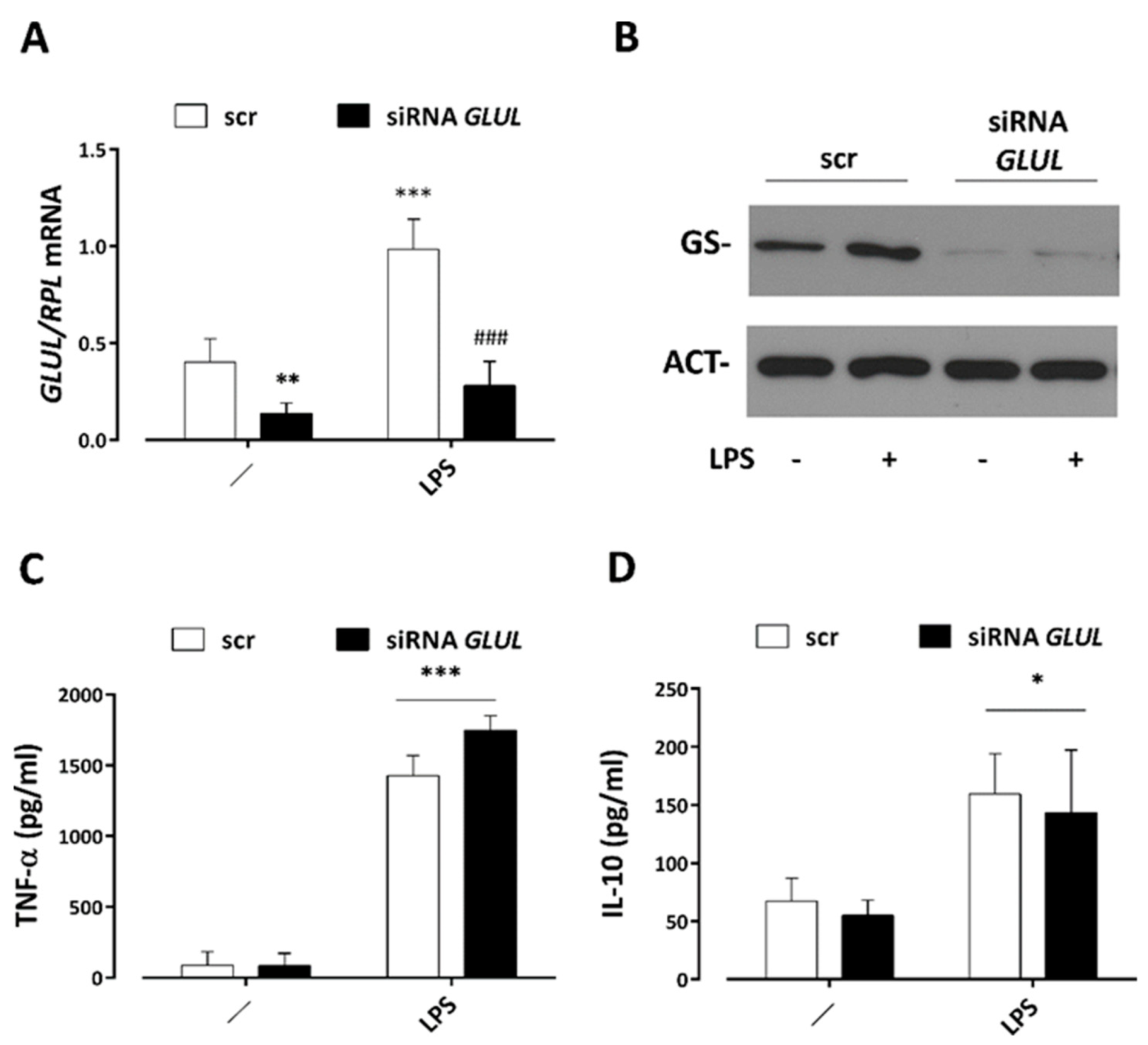
3.5. GS Silencing Does Not Affect Cytokine Secretion by LPS-Stimulated Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, H.C.; Suter, M.; Naegeli, H. Critical review of the safety assessment of nano-structured silica additives in food. J. Nanobiotechnol. 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Jha, S. Amorphous nanosilica induced toxicity, inflammation and innate immune responses: A critical review. Toxicology 2020, 441, 152519. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Y.; Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard. Mater. 2021, 406, 124626. [Google Scholar] [CrossRef]
- Fruijtier-Polloth, C. The safety of nanostructured synthetic amorphous silica (SAS) as a food additive (E 551). Arch. Toxicol. 2016, 90, 2885–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, W.; van Kesteren, P.C.E.; Peters, R.J.B.; Oomen, A.G. Issues currently complicating the risk assessment of synthetic amorphous silica (SAS) nanoparticles after oral exposure. Nanotoxicology 2021, 15, 905–933. [Google Scholar] [CrossRef]
- Nemmar, A.; Albarwani, S.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Attoub, S.; Ali, B.H. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int. J. Nanomed. 2014, 9, 2779–2789. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, F.; Blasche, R.; Kasper, M.; Barth, K. A co-culture system with an organotypic lung slice and an immortal alveolar macrophage cell line to quantify silica-induced inflammation. PLoS ONE 2015, 10, e0117056. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Wang, R.; Jin, J.; Zhu, Y.Z. Short-Term Oral Administration of Mesoporous Silica Nanoparticles Potentially Induced Colon Inflammation in Rats Through Alteration of Gut Microbiota. Int. J. Nanomed. 2021, 16, 881–893. [Google Scholar] [CrossRef]
- Winkler, H.C.; Kornprobst, J.; Wick, P.; von Moos, L.M.; Trantakis, I.; Schraner, E.M.; Bathke, B.; Hochrein, H.; Suter, M.; Naegeli, H. MyD88-dependent pro-interleukin-1beta induction in dendritic cells exposed to food-grade synthetic amorphous silica. Part. Fibre Toxicol. 2017, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Tsugita, M.; Morimoto, N.; Tashiro, M.; Kinoshita, K.; Nakayama, M. SR-B1 Is a Silica Receptor that Mediates Canonical Inflammasome Activation. Cell Rep. 2017, 18, 1298–1311. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Kang, M.S.; Jin, S.W.; Lee, T.G.; Lee, G.H.; Kim, D.W.; Lee, E.W.; Park, J.; Choi, I.; Pak, Y.K. Multiple pathways of alveolar macrophage death contribute to pulmonary inflammation induced by silica nanoparticles. Nanotoxicology 2021, 15, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Cho, J.W.; Seong, E.; Park, E.J.; Lee, G.H.; Kim, D.W.; Yang, Y.S.; Oh, J.H.; Yoon, S.; Lee, T.G.; et al. Amorphous silica nanoparticle-induced pulmonary inflammatory response depends on particle size and is sex-specific in rats. Toxicol. Appl. Pharmacol. 2020, 390, 114890. [Google Scholar] [CrossRef]
- Boudard, D.; Aureli, F.; Laurent, B.; Sturm, N.; Raggi, A.; Antier, E.; Lakhdar, L.; Marche, P.N.; Cottier, M.; Cubadda, F.; et al. Chronic Oral Exposure to Synthetic Amorphous Silica (NM-200) Results in Renal and Liver Lesions in Mice. Kidney Int. Rep. 2019, 4, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Tassinari, R.; Di Felice, G.; Butteroni, C.; Barletta, B.; Corinti, S.; Cubadda, F.; Aureli, F.; Raggi, A.; Narciso, L.; Tait, S.; et al. Hazard identification of pyrogenic synthetic amorphous silica (NM-203) after sub-chronic oral exposure in rat: A multitarget approach. Food Chem. Toxicol. 2020, 137, 111168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Italiani, P.; Casals, E.; Valkenborg, D.; Mertens, I.; Baggerman, G.; Nelissen, I.; Puntes, V.F.; Boraschi, D. Assessing the Immunosafety of Engineered Nanoparticles with a Novel in Vitro Model Based on Human Primary Monocytes. ACS Appl. Mater. Interfaces 2016, 8, 28437–28447. [Google Scholar] [CrossRef]
- Di Cristo, L.; Movia, D.; Bianchi, M.G.; Allegri, M.; Mohamed, B.M.; Bell, A.P.; Moore, C.; Pinelli, S.; Rasmussen, K.; Riego-Sintes, J.; et al. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol. Sci. 2016, 150, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Menga, A.; Serra, M.; Todisco, S.; Riera-Domingo, C.; Ammarah, U.; Ehling, M.; Palmieri, E.M.; Di Noia, M.A.; Gissi, R.; Favia, M.; et al. Glufosinate constrains synchronous and metachronous metastasis by promoting anti-tumor macrophages. EMBO Mol. Med. 2020, 12, e11210. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Ruotolo, R.; Marmiroli, N.; Bergamaschi, E.; Cubadda, F.; Bussolati, O. Pyrogenic and Precipitated Amorphous Silica Nanoparticles Differentially Affect Cell Responses to LPS in Human Macrophages. Nanomaterials 2020, 10, 1395. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.; Vanaudenaerde, B.; Luyts, K.; Lison, D.; Martens, J.A.; Nemery, B.; Hoet, P.H. Cytokine production by co-cultures exposed to monodisperse amorphous silica nanoparticles: The role of size and surface area. Toxicol. Lett. 2012, 211, 98–104. [Google Scholar] [CrossRef]
- Murugadoss, S.; van den Brule, S.; Brassinne, F.; Sebaihi, N.; Mejia, J.; Lucas, S.; Petry, J.; Godderis, L.; Mast, J.; Lison, D.; et al. Is aggregated synthetic amorphous silica toxicologically relevant? Part Fibre Toxicol. 2020, 17, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugadoss, S.; Godderis, L.; Ghosh, M.; Hoet, P.H. Assessing the Toxicological Relevance of Nanomaterial Agglomerates and Aggregates Using Realistic Exposure In Vitro. Nanomaterials 2021, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Morishige, T.; Yoshioka, Y.; Inakura, H.; Tanabe, A.; Yao, X.; Tsunoda, S.; Tsutsumi, Y.; Mukai, Y.; Okada, N.; Nakagawa, S. Cytotoxicity of amorphous silica particles against macrophage-like THP-1 cells depends on particle-size and surface properties. Pharmazie 2010, 65, 596–599. [Google Scholar] [PubMed]
- Kettiger, H.; Sen Karaman, D.; Schiesser, L.; Rosenholm, J.M.; Huwyler, J. Comparative safety evaluation of silica-based particles. Toxicol. In Vitro 2015, 30, 355–363. [Google Scholar] [CrossRef]
- Breznan, D.; Das, D.D.; O’Brien, J.S.; MacKinnon-Roy, C.; Nimesh, S.; Vuong, N.Q.; Bernatchez, S.; DeSilva, N.; Hill, M.; Kumarathasan, P.; et al. Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology 2017, 11, 223–235. [Google Scholar] [CrossRef]
- Kim, H.; Zamel, R.; Bai, X.H.; Liu, M. PKC activation induces inflammatory response and cell death in human bronchial epithelial cells. PLoS ONE 2013, 8, e64182. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.W.; Park, M.H.; Lee, Y.J.; Kim, H.S.; Kwon, T.K.; Park, W.-S.; Min, D.S. Phorbol ester up-regulates phospholipase D1 but not phospholipase D2 expression through a PKC/Ras/ERK/NFkappaB-dependent pathway and enhances matrix metalloproteinase-9 secretion in colon cancer cells. J. Biol. Chem. 2008, 283, 4094–4104. [Google Scholar] [CrossRef] [Green Version]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (alpha-, gamma-, and delta-) show distinct capacities to control Nrf-2 and NfkappaB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell. Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef]
- Chiu, M.; Taurino, G.; Dander, E.; Bardelli, D.; Fallati, A.; Andreoli, R.; Bianchi, M.G.; Carubbi, C.; Pozzi, G.; Galuppo, L.; et al. ALL blasts drive primary mesenchymal stromal cells to increase asparagine availability during asparaginase treatment. Blood Adv. 2021, 5, 5164–5178. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Duan, J.; Yang, M.; Yu, Y.; Feng, L.; Yang, X.; Zhou, X.; Zhao, Z.; Sun, Z. Silica nanoparticles induce autophagosome accumulation via activation of the EIF2AK3 and ATF6 UPR pathways in hepatocytes. Autophagy 2018, 14, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.G.; Allegri, M.; Chiu, M.; Costa, A.L.; Blosi, M.; Ortelli, S.; Bussolati, O.; Bergamaschi, E. Lipopolysaccharide Adsorbed to the Bio-Corona of TiO2 Nanoparticles Powerfully Activates Selected Pro-inflammatory Transduction Pathways. Front. Immunol. 2017, 8, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detampel, P.; Tehranian, S.; Mukherjee, P.; Foret, M.; Fuerstenhaupt, T.; Darbandi, A.; Bogari, N.; Hlasny, M.; Jeje, A.; Olszewski, M.A.; et al. Caveolin-initiated macropinocytosis is required for efficient silica nanoparticles’ transcytosis across the alveolar epithelial barrier. Sci. Rep. 2022, 12, 9474. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Reker-Smit, C.; Salvati, A. Mechanisms of Uptake and Membrane Curvature Generation for the Internalization of Silica Nanoparticles by Cells. Nano Lett. 2022, 22, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Q.; Zhao, Z.; Fan, J.; Qin, L.; Alexander, D.B.; Tsuda, H.; Zhao, D.; Xu, J. Surfactant Proteins A/D-CD14 on Alveolar Macrophages Is a Common Pathway Associated With Phagocytosis of Nanomaterials and Cytokine Production. Front. Immunol. 2021, 12, 758941. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.M.; Menga, A.; Lebrun, A.; Hooper, D.C.; Butterfield, D.A.; Mazzone, M.; Castegna, A. Blockade of Glutamine Synthetase Enhances Inflammatory Response in Microglial Cells. Antioxid. Redox Signal. 2017, 26, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, E.M.; Menga, A.; Martin-Perez, R.; Quinto, A.; Riera-Domingo, C.; De Tullio, G.; Hooper, D.C.; Lamers, W.H.; Ghesquiere, B.; McVicar, D.W.; et al. Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 2017, 20, 1654–1666. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, J.; Shi, M.; Ding, H.; Ma, L.; Zhang, H.; Liu, J. Maintenance of glutamine synthetase expression alleviates endotoxin-induced sepsis via alpha-ketoglutarate-mediated demethylation. FASEB J. 2022, 36, e22281. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse | T (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| Glutamine Synthetase (GLUL) | 5′ TCA TCT TGC ATC GTG TGT GTG 3′ | 5′ CTT CAG ACC ATT CTC CTC CGG 3′ | 57 °C | 137 |
| Tumor necrosis factor alpha (TNF) | 5’ ATG AGC ACT GAA AGC ATG ATC C 3’ | 5’ GAG GGC TGA TTA GAG AGA GGT C 3’ | 61 °C | 196 |
| Interleukin-6 (IL6) | 5’ AAC CTG AAC CTT CCA AAG ATG G 3’ | 5’ TCT GGC TTG TTC CTC ACT ACT 3’ | 54 °C | 159 |
| Interleukin-10 (IL10) | 5’ TCA AGG CGC ATG TGA ACT CC 3’ | 5’ GAT GTC AAA CTC ACT CAT GGC T 3’ | 56 °C | 176 |
| Ribosomal Protein L15 (RPL15) | 5’ GCA GCC ATC AGG TAA GCC AAG 3’ | 5’ AGC GGA CCC TCA GAA GAA AGC 3’ | 57 °C | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, M.G.; Chiu, M.; Taurino, G.; Bergamaschi, E.; Cubadda, F.; Macaluso, G.M.; Bussolati, O. The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles. Nanomaterials 2022, 12, 2307. https://doi.org/10.3390/nano12132307
Bianchi MG, Chiu M, Taurino G, Bergamaschi E, Cubadda F, Macaluso GM, Bussolati O. The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles. Nanomaterials. 2022; 12(13):2307. https://doi.org/10.3390/nano12132307
Chicago/Turabian StyleBianchi, Massimiliano G., Martina Chiu, Giuseppe Taurino, Enrico Bergamaschi, Francesco Cubadda, Guido M. Macaluso, and Ovidio Bussolati. 2022. "The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles" Nanomaterials 12, no. 13: 2307. https://doi.org/10.3390/nano12132307
APA StyleBianchi, M. G., Chiu, M., Taurino, G., Bergamaschi, E., Cubadda, F., Macaluso, G. M., & Bussolati, O. (2022). The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles. Nanomaterials, 12(13), 2307. https://doi.org/10.3390/nano12132307