Ti3C2Tx as a Sensor for SF6/N2 Nitrogen-Containing Fault Decomposition Characteristic Products: A Theoretical Study
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
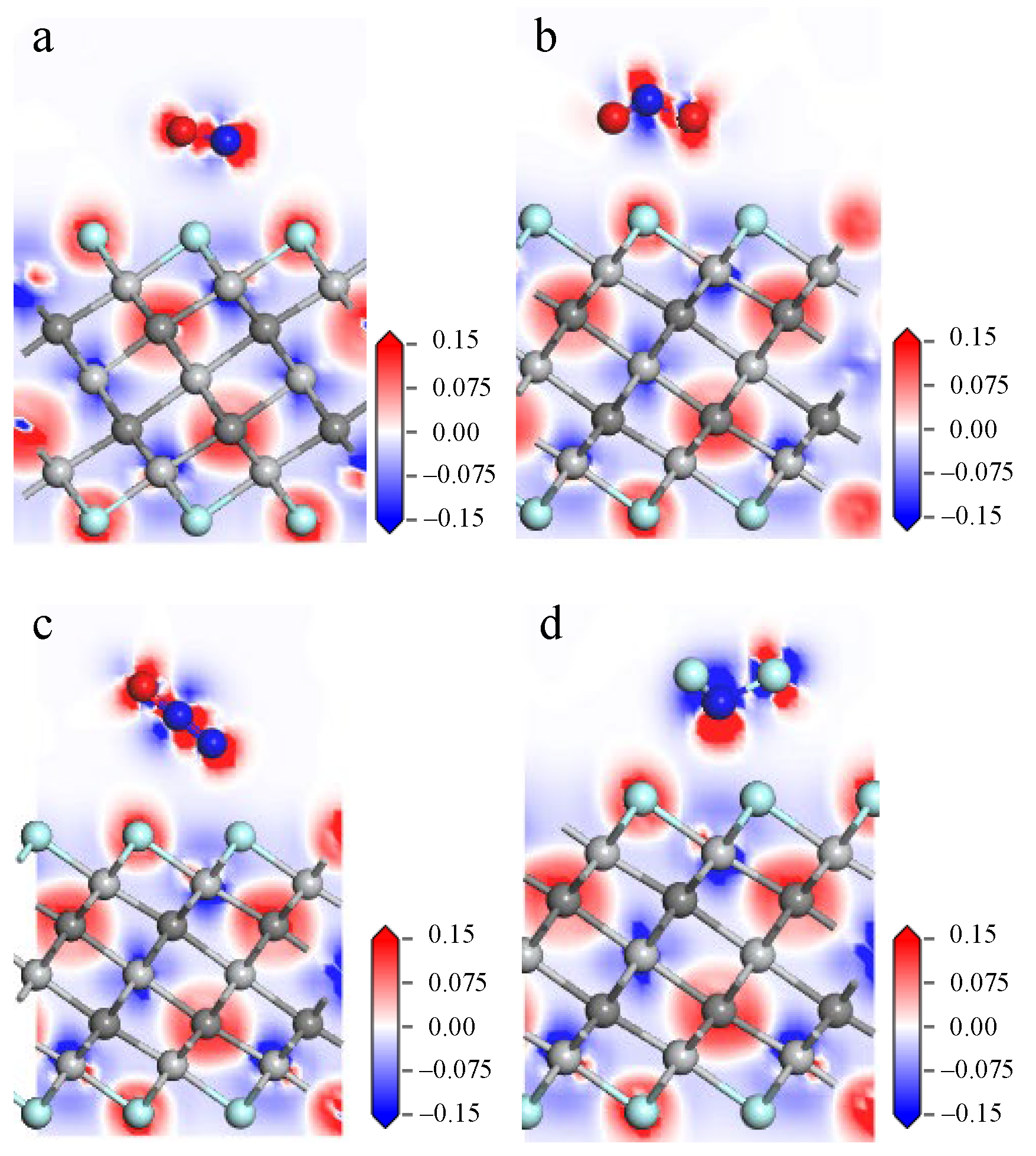
3.1. Gas Adsorption on Ti3C2F2
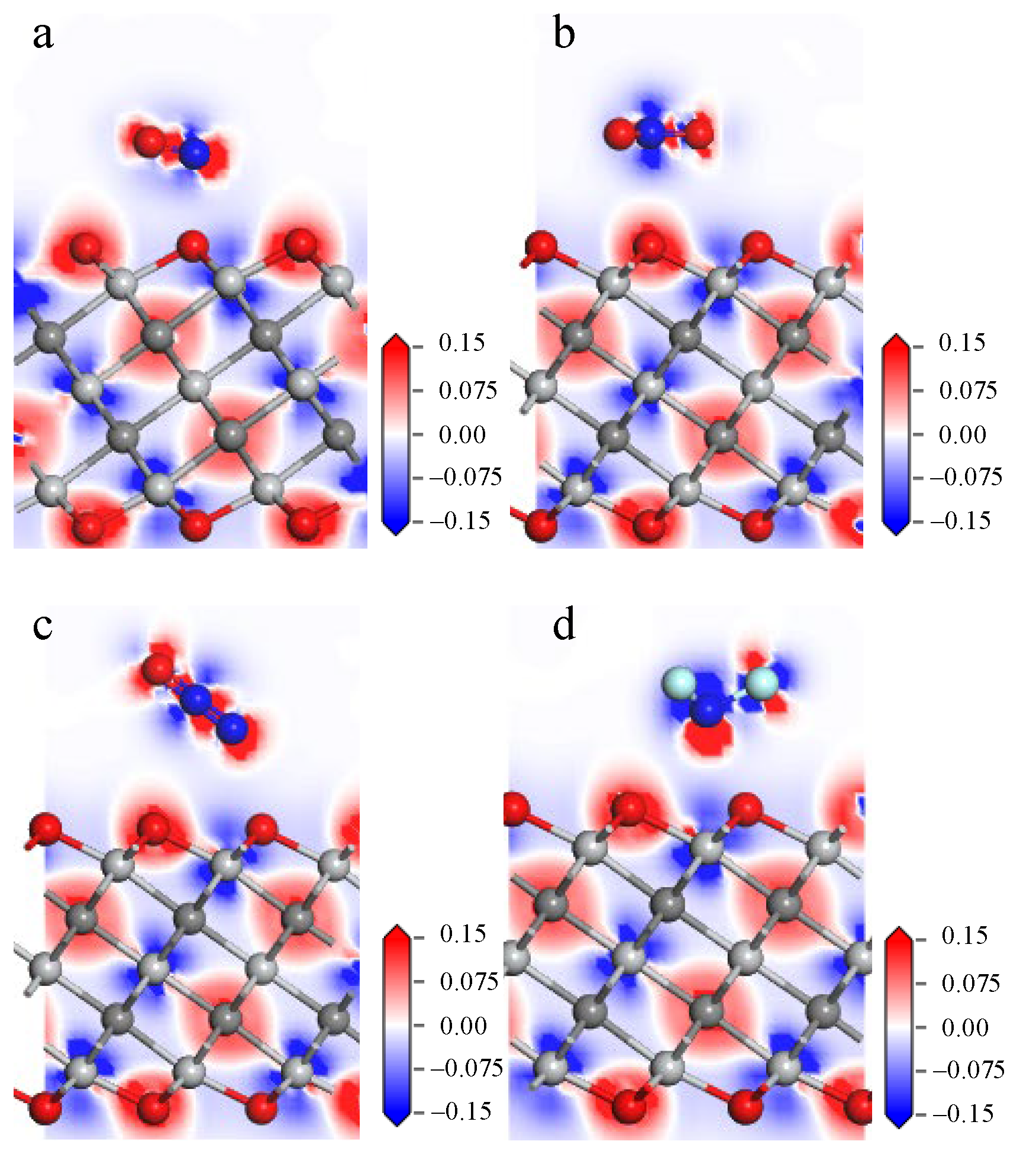
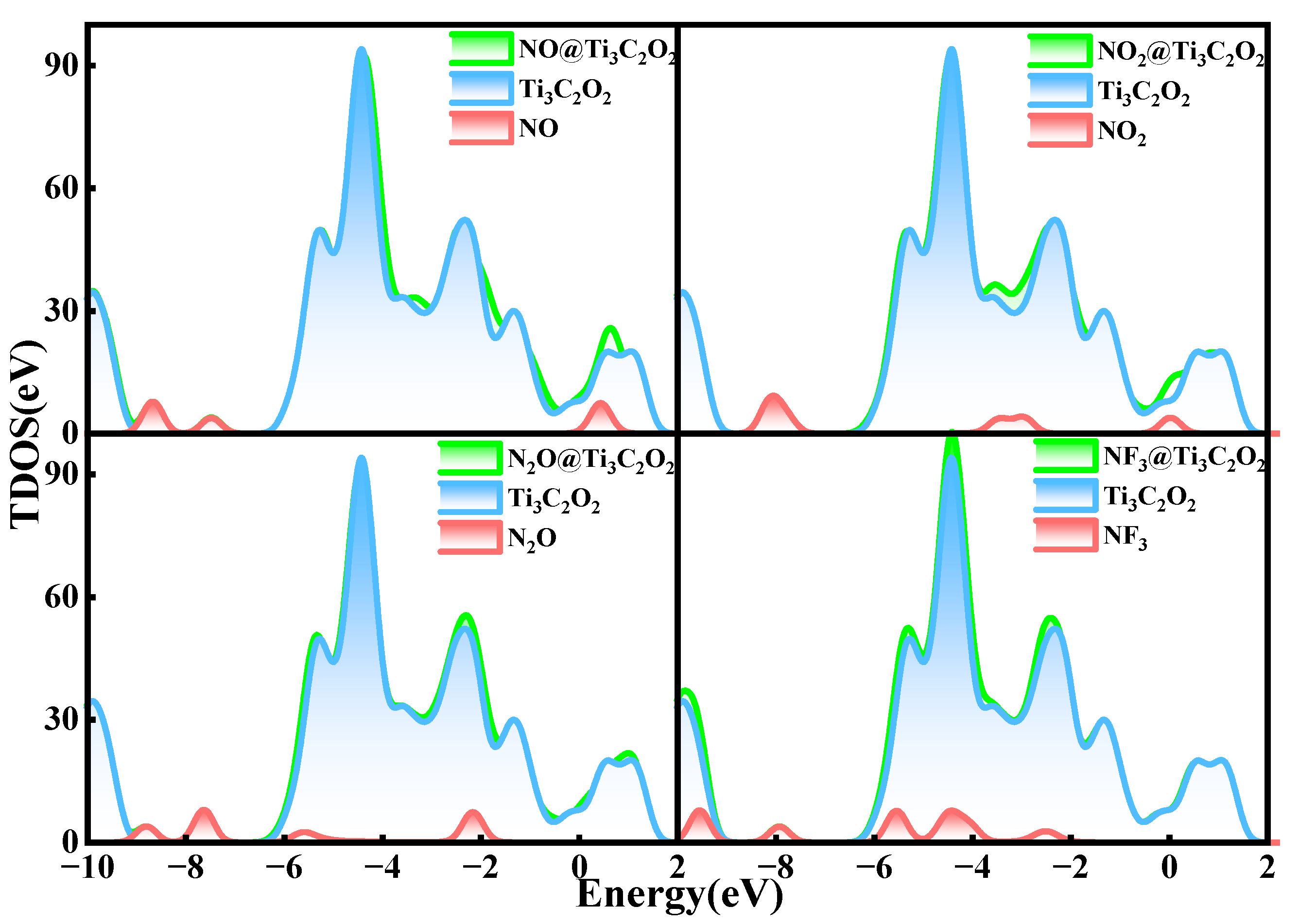
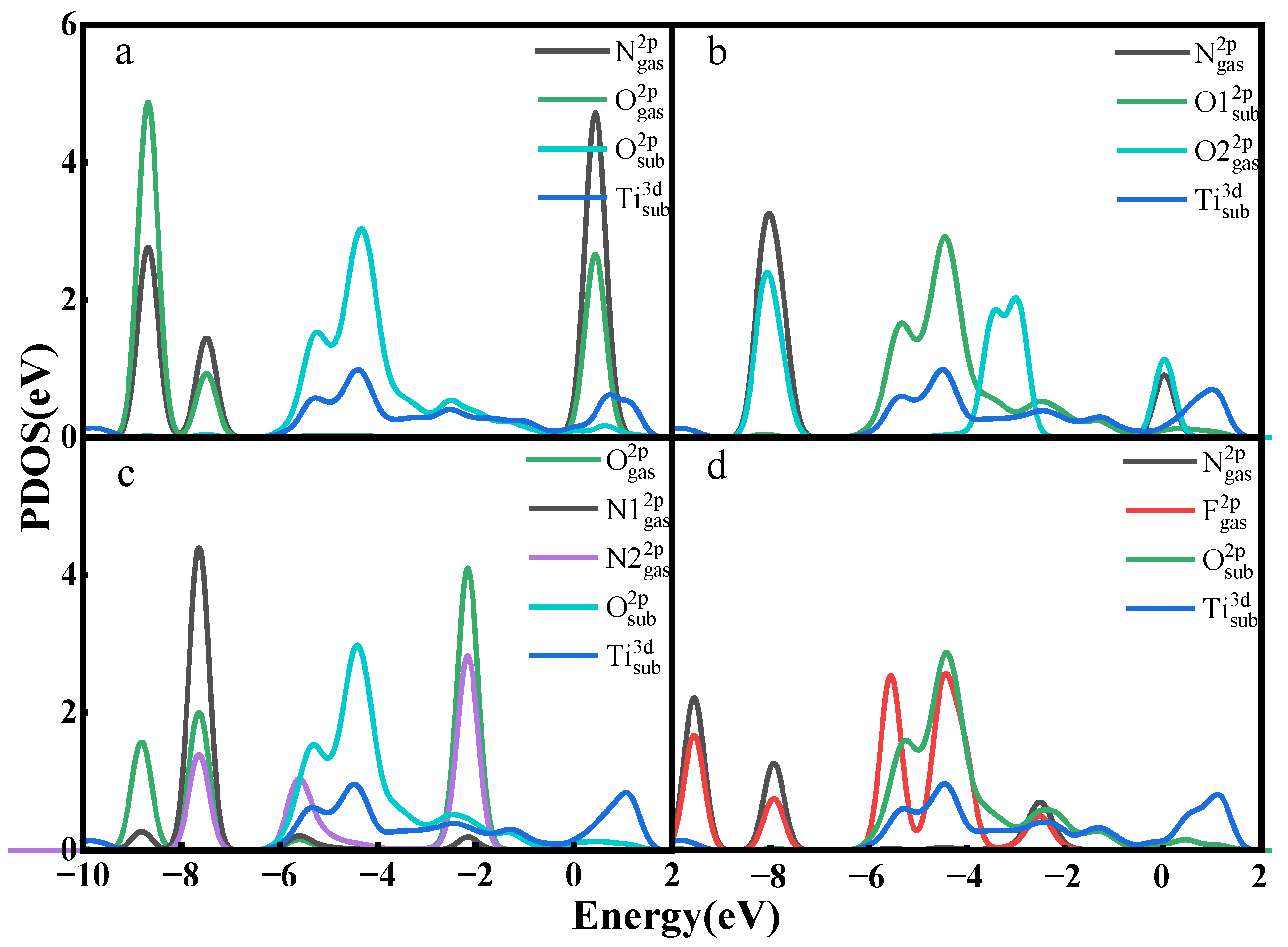
3.2. Gas Adsorption on Ti3C2O2
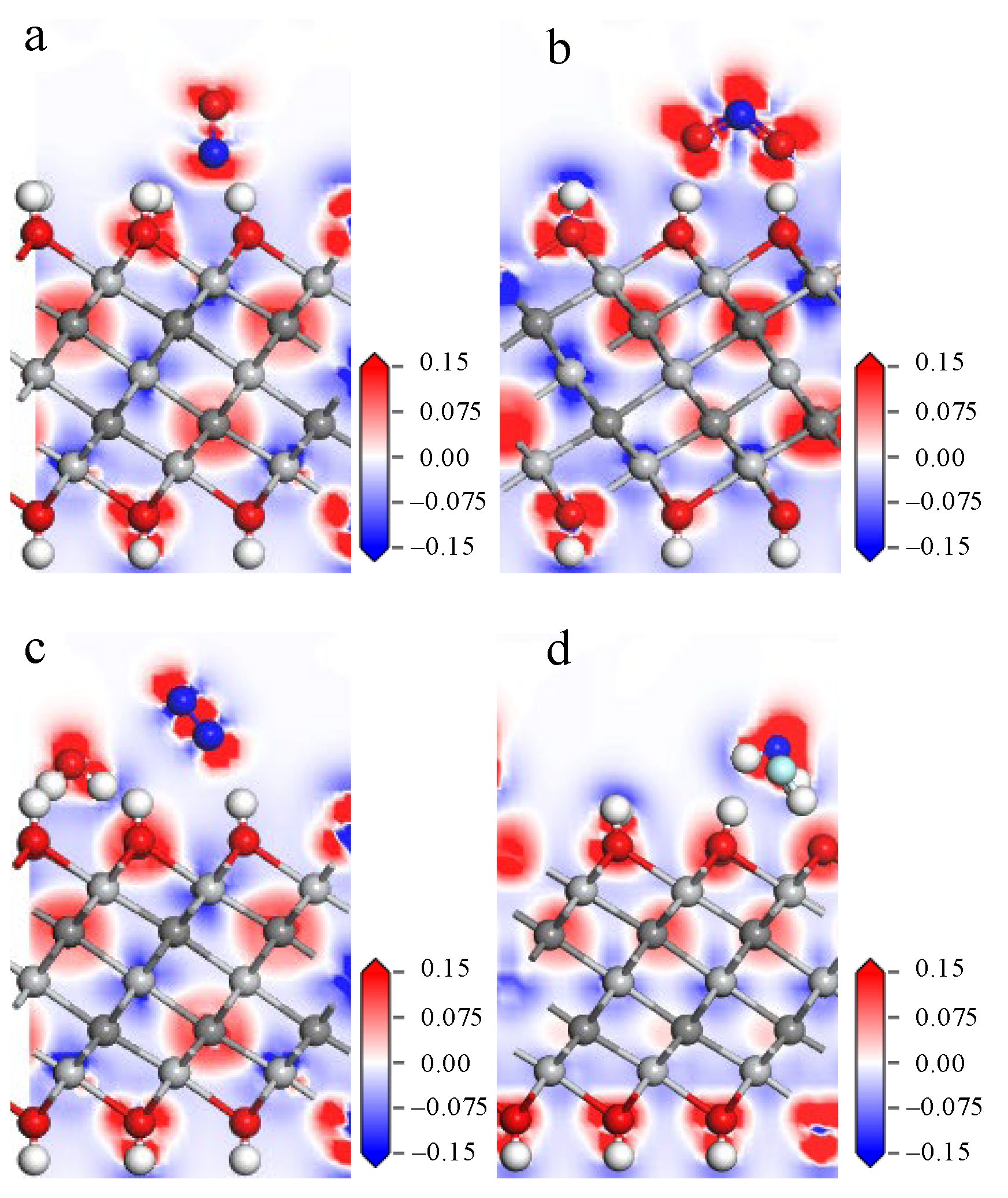
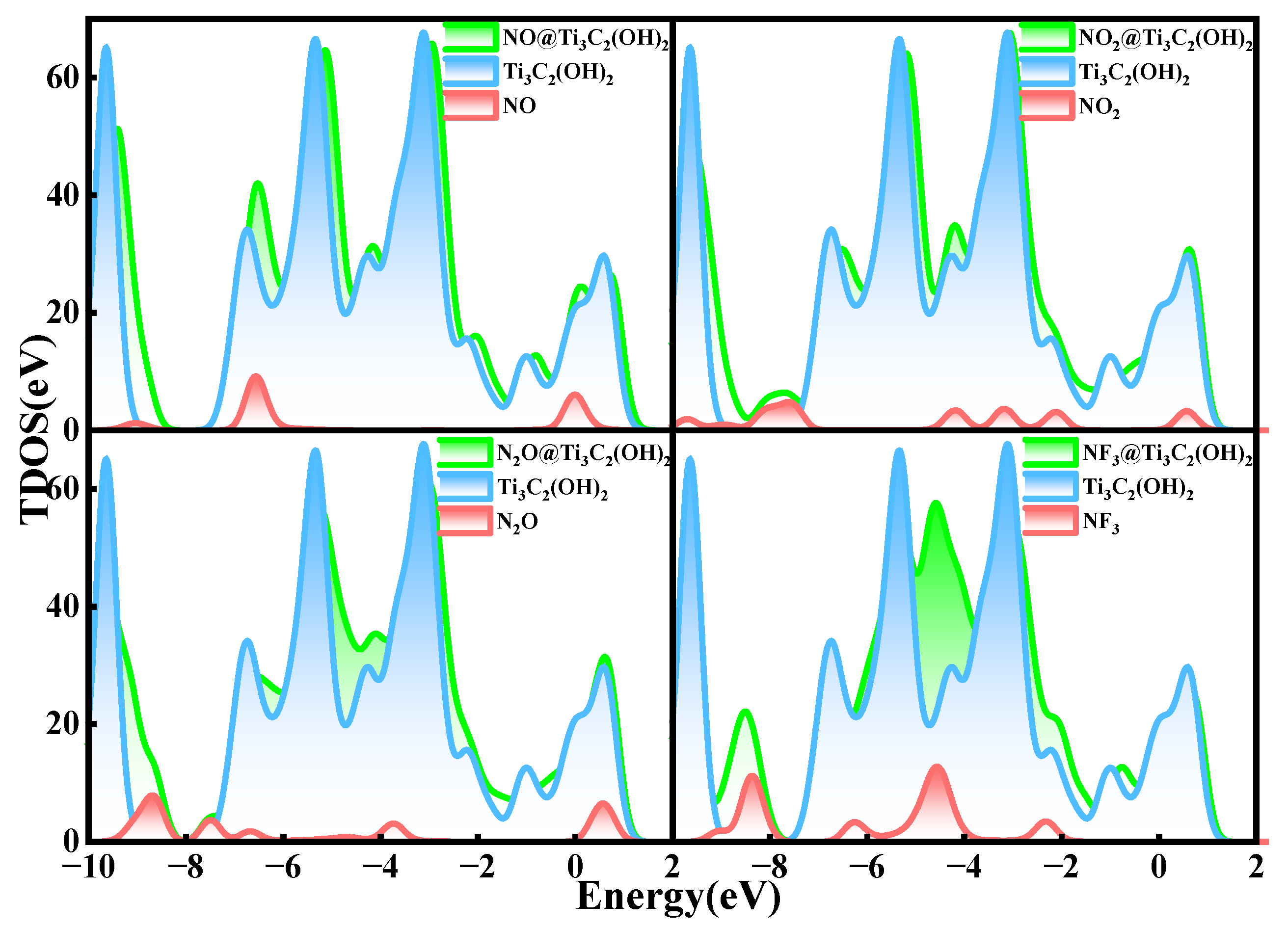
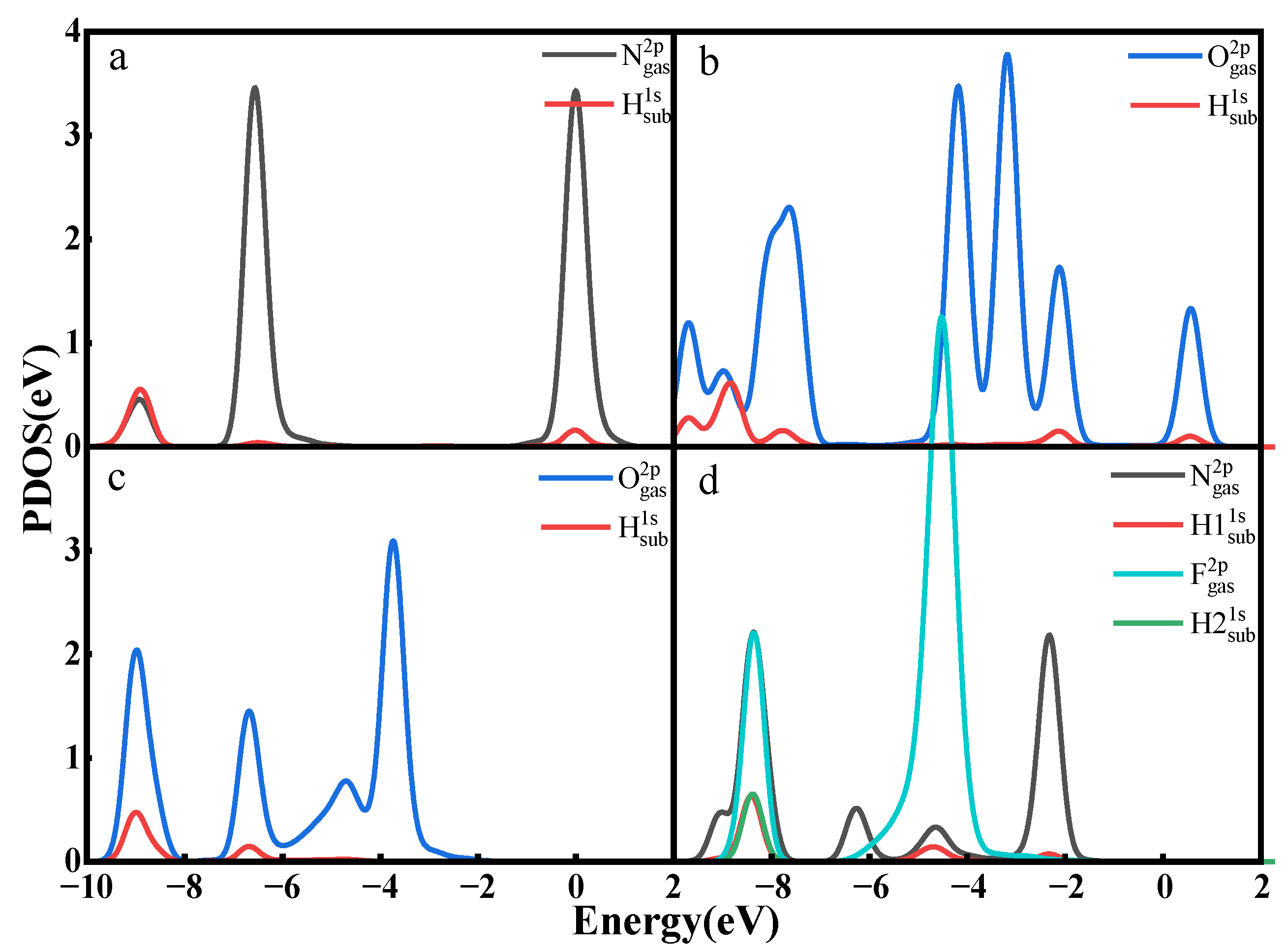
3.3. Gas Adsorption on Ti3C2(OH)2
3.4. Ti3C2Tx Gas Sensing Performance Evaluation
4. Conclusions
- (1)
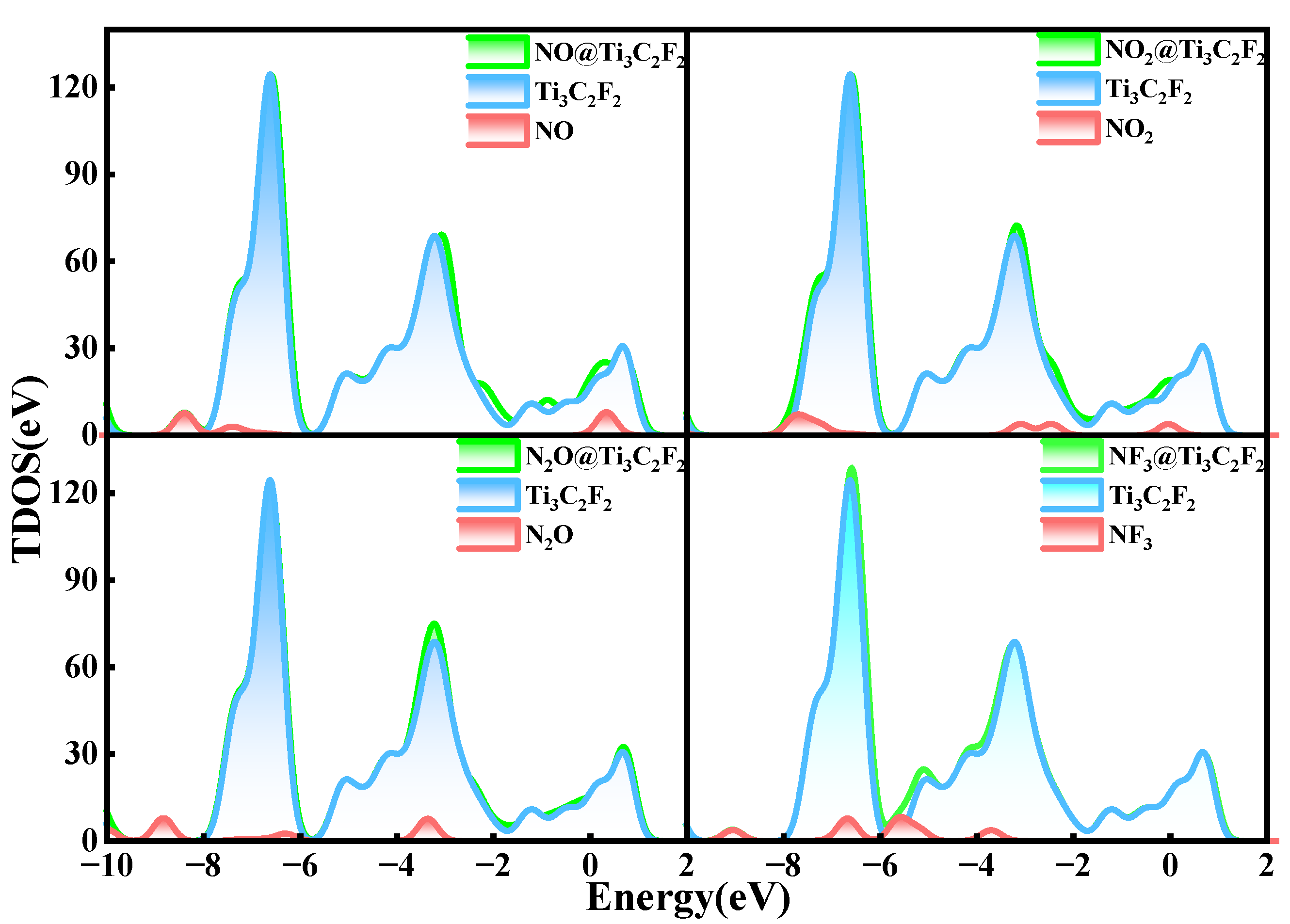
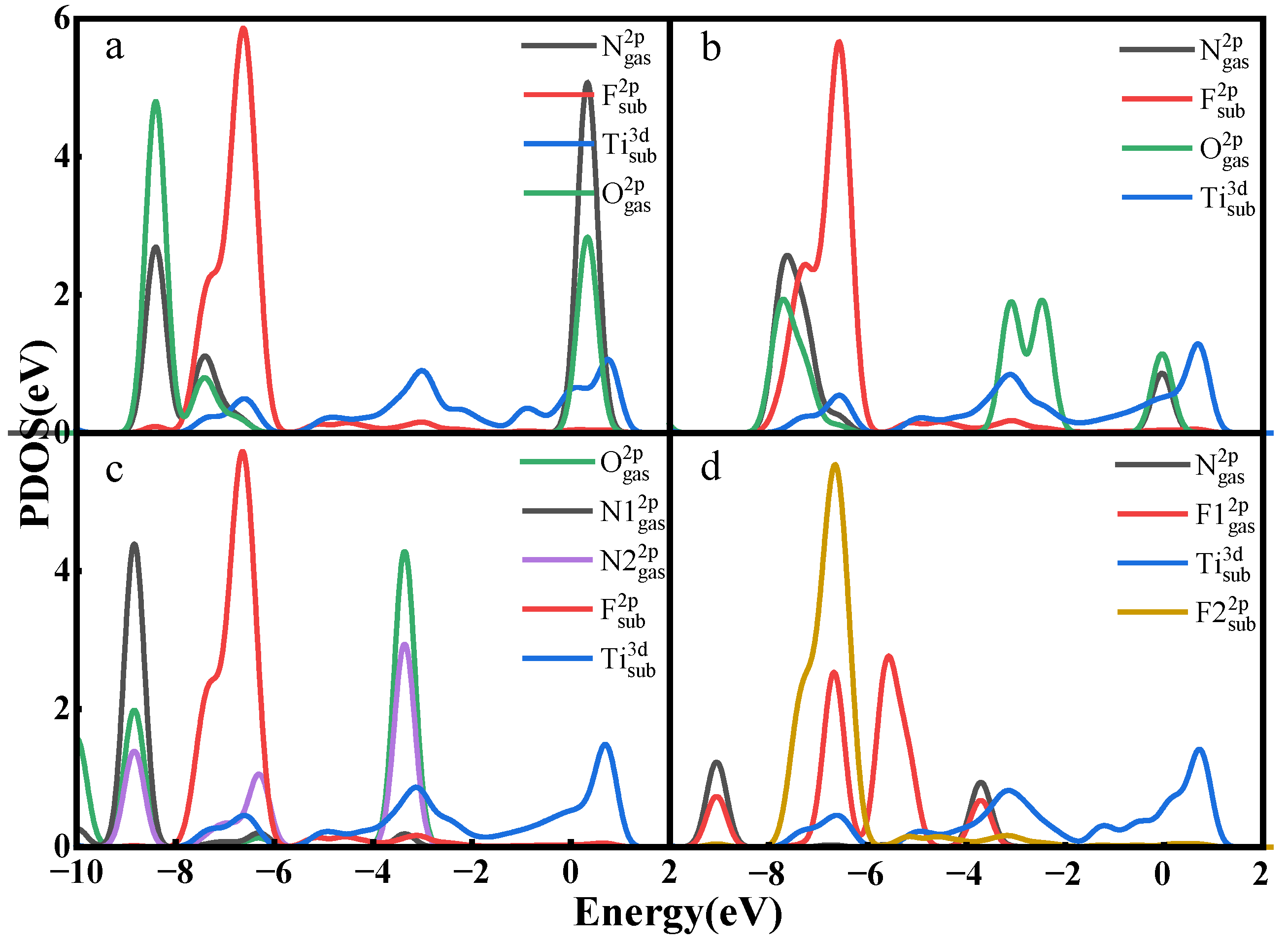
- The adsorption energies of NO on Ti3C2F2, Ti3C2O2, and Ti3C2(OH)2 are −0.216eV, −0.507 eV, and −1.709 eV, respectively, and the charge transfers are 0.111 e, 0.284 e, and −0.607 e, respectively. After NO adsorption, the density of states of the adsorbed systems all increase near the Fermi energy level, and conductivity is enhanced.
- (2)
- The adsorption energies of NO2 on Ti3C2F2, Ti3C2O2, and Ti3C2(OH)2 are −0.213 eV, −0.115 eV, and −3.806 eV, respectively, and the charge transfer is −0.129 e, 0.077 e, and −0.753 e, respectively, and the adsorption of NO2 leads to the enhancement of electrical conductivity of the materials.
- (3)
- The adsorption energies of N2O on Ti3C2F2, Ti3C2O2, and Ti3C2(OH)2 are −0.273 eV, −0.240 eV, and −5.461 eV, respectively, with charge transfer of 0.008 e, −0.008 e, and -0.707e. After the adsorption of N2O, only Ti3C2(OH)2 exhibits an increase in electrical conductivity, and it is the chemisorption that occurs with strong interactions.
- (4)
- The adsorption energies of NF3 on Ti3C2F2, Ti3C2O2, and Ti3C2(OH)2 are −0.323 eV, −0.386 eV, and −9.065 eV, respectively, and the charge transfers are 0.014 e, 0.036 e, and −1.428 e, respectively. NF3 exhibits strong chemisorption only on Ti3C2Tx terminated with -OH, accompanied by a large amount of charge transfer.
- (5)
- The adsorption of all four gases on the surface of Ti3C2F2 and Ti3C2O2 exhibits short recovery times, while the adsorption on the surface of Ti3C2(OH)2 makes it difficult to achieve desorption at room temperature due to the stronger adsorption effect.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maiss, M.; Brenninkmeijer, C.A.M. Atmospheric SF6: Trends, Sources, and Prospects. Environ. Sci. Technol. 1998, 32, 3077–3086. [Google Scholar] [CrossRef]
- Li, X.W.; Zhao, H.; Murphy, A.B. SF6-Alternative Gases for Application in Gas-insulated Switchgear. J. Phys. D Appl. Phys. 2018, 51, 19. [Google Scholar] [CrossRef]
- Ko, M.K.W.; Sze, N.D.; Wang, W.C.; Shia, G.; Goldman, A.; Murcray, F.J.; Murcray, D.G.; Rinsland, C.P. Atmospheric Sulfur-hexafluoride Sources, Sinks and Greenhouse Warming. J. Geophys. Res. Atmos. 1993, 98, 10499–10507. [Google Scholar] [CrossRef]
- Zhou, A.; Gao, L.; Ji, X.; Zhang, M. Research and Application of SF6/N2 Mixed Gas Used in GIS Bus. Power Syst. Technol. 2018, 42, 3429–3435. [Google Scholar]
- Zhang, B.; Xiong, J.; Chen, L.; Li, X.; Murphy, A.B. Fundamental physicochemical properties of SF6-alternative gases: A review of recent progress. J. Phys. D Appl. Phys. 2020, 53, 173001. [Google Scholar] [CrossRef]
- Qiu, Y.; Kuffel, E. Comparison of SF6/N2 and SF6/CO2 gas mixtures as alternatives to SF6 gas. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 892–895. [Google Scholar] [CrossRef]
- Zeng, F.P.; Li, H.T.; Cheng, H.T.; Tang, J.; Liu, Y.L. SF6 Decomposition and Insulation Condition Monitoring of GIE: A Review. High Volt. 2021, 6, 955–966. [Google Scholar] [CrossRef]
- Yang, J.; Ma, F.; Yu, K.; Su, Z.; Xiu, S. Experimental Study on Gas Decomposition Characteristics of SF6/N2 Mixed Gas under Breaking Current. High Volt. Eng. 2019, 45, 1616–1623. [Google Scholar]
- Li, C.; Tang, J.; Zhao, Z.; Li, H.; Miao, Y.; Zeng, F. Decomposition Characteristics of SF6/N2 under Partial Discharge of Different Degrees. IEEE Access 2020, 8, 192312–192319. [Google Scholar] [CrossRef]
- Casanovas, A.M.; Casanovas, J. Decomposition of High-pressure (400 kPa) SF6/CO2, SF6/CO, SF6/N2/CO2 and SF6/N2/CO Mixtures under Negative DC Coronas. J. Phys. D Appl. Phys. 2005, 38, 1556. [Google Scholar] [CrossRef]
- Zeng, F.P.; Lei, Z.C.; Yang, X.; Tang, J.; Yao, Q.; Miao, Y.L. Evaluating DC Partial Discharge with SF6 Decomposition Characteristics. IEEE Trans. Power Deliv. 2019, 34, 1383–1392. [Google Scholar] [CrossRef]
- Zeng, F.P.; Wu, S.Y.; Lei, Z.C.; Li, C.; Tang, J.; Yao, Q.; Miao, Y.L. SF6 Fault Decomposition Feature Component Extraction and Triangle Fault Diagnosis Method. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 581–589. [Google Scholar] [CrossRef]
- Shengxue, Y.; Chengbao, J.; Huai, W.S. Gas Sensing in 2D Materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar]
- Venkateshalu, S.; Grace, A.N. MXenes—A New Class of 2D Layered Materials: Synthesis, Properties, Applications as Supercapacitor Electrode and Beyond. Appl. Mater. Today 2020, 18, 100509. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Mater. 2017, 16, 34–50. [Google Scholar] [CrossRef]
- Pang, J.B.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.F.; Rummeli, M.H. Applications of 2D MXenes in Energy Conversion and Storage Systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The World of Two-dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef]
- Chen, W.Y.; Jiang, X.; Lai, S.N.; Peroulis, D.; Stanciu, L. Nanohybrids of A MXene and Transition Metal Dichalcogenide for Selective Detection of Volatile Organic Compounds. Nat. Commun. 2020, 11, 1302. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef]
- Kong, L.; Liang, X.; Deng, X.; Guo, C.; Wu, C.-M.L. Adsorption of SF6 Decomposed Species on Ti3C2O2 and Ti3C2F2 with Point Defects by DFT Study. Adv. Theory Simul. 2021, 4, 2100074. [Google Scholar] [CrossRef]
- Zeng, F.; Feng, X.; Chen, X.; Yao, Q.; Miao, Y.; Dai, L.; Li, Y.; Tang, J. First-principles Analysis of Ti3C2Tx MXene as A Promising Candidate for SF6 Decomposition Characteristic Components Sensor. Appl. Surf. Sci. 2022, 578, 152020. [Google Scholar] [CrossRef]
- Delley, B. From Molecules to Solids with the DMol3 Approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardirossian, N.; Head-Gordon, M. ωB97X-V: A 10-parameter, Range-separated Hybrid, Generalized Gradient Approximation Density Functional with Nonlocal Correlation, Designed by a Survival-of-the-fittest Strategy. Phys. Chem. Chem. Phys. 2014, 16, 9904–9924. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. Hardness Conserving Semilocal Pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 19. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type Density Functional Constructed with a Long-range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Yong, Y.; Cui, H.; Zhou, Q.; Su, X.; Kuang, Y.; Li, X. C2N monolayer as NH3 and NO sensors: A DFT study. Appl. Surf. Sci. 2019, 487, 488–495. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Hong, S.N.; Kye, Y.H.; Yu, C.J.; Jong, U.G.; Ri, G.C.; Choe, C.S.; Kim, K.H.; Han, J.M. Ab initio thermodynamic study of the SnO2(110) surface in an O2 and NO environment: A fundamental understanding of the gas sensing mechanism for NO and NO2. Phys. Chem. Chem. Phys. 2016, 18, 31566–31578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakbaz, P.; Moshayedi, M.; Hajian, S.; Soleimani, M.; Narakathu, B.B.; Bazuin, B.J.; Pourfath, M.; Atashbar, M.Z. Titanium Carbide MXene as NH3 Sensor: Realistic First-Principles Study. J. Phys. Chem. C 2019, 123, 29794–29803. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced Ammonia Response of Ti3C2Tx Nanosheets Supported by TiO2 Nanoparticles at Room Temperature. Sens. Actuators B Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Sun, P.; et al. Improvement of Gas and Humidity Sensing Properties of Organ-like MXene by Alkaline Treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, B.; Li, Q.; Fang, X.; Mao, S.; Ostrikov, K.K. H2S Sensing under Various Humidity Conditions with Ag Nanoparticle Functionalized Ti3C2Tx MXene Field-effect Transistors. J. Hazard. Mater. 2022, 424, 127492. [Google Scholar] [CrossRef]

| Ti3C2Tx | Gas | D(Å) a | L(Å) b |
|---|---|---|---|
| F | NO | 2.978 | −0.012 |
| NO2 | 2.807 | 0.011 | |
| N2O | 2.979 | −0.002 | |
| NF3 | 2.849 | 0 | |
| O | NO | 2.769 | −0.028 |
| NO2 | 2.806 | 0.005 | |
| N2O | 3.021 | −0.001 | |
| NF3 | 2.842 | −0.002 | |
| OH | NO | 1.809 | 0.08 |
| NO2 | 1.666 | 0.073 | |
| N2O | -- c | -- | |
| NF3 | -- | -- |
| Ti3C2Tx | Gas | Eads(eV) | Qt€ a |
|---|---|---|---|
| F | NO | −0.216 | 0.111 |
| NO2 | −0.213 | −0.129 | |
| N2O | −0.273 | 0.008 | |
| NF3 | −0.323 | 0.014 | |
| O | NO | −0.507 | 0.284 |
| NO2 | −0.115 | 0.077 | |
| N2O | −0.240 | −0.008 | |
| NF3 | −0.386 | 0.036 | |
| OH | NO | −1.709 | −0.607 |
| NO2 | −3.806 | −0.753 | |
| N2O | −5.461 | −0.707 | |
| NF3 | −9.065 | −1.428 |
| Ti3C2Tx | τ(NO)/s | τ(NO2)/s | τ(N2O)/s | τ(NF3)/s |
|---|---|---|---|---|
| F | 10−9 | 10−9 | 10−8 | 10−7 |
| O | 10−4 | 10−11 | 10−8 | 10−6 |
| OH | 1016 | 1052 | 1080 | 10141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Qiu, H.; Feng, X.; Chao, X.; Dai, L.; Yao, Q.; Tang, J. Ti3C2Tx as a Sensor for SF6/N2 Nitrogen-Containing Fault Decomposition Characteristic Products: A Theoretical Study. Nanomaterials 2022, 12, 2311. https://doi.org/10.3390/nano12132311
Zeng F, Qiu H, Feng X, Chao X, Dai L, Yao Q, Tang J. Ti3C2Tx as a Sensor for SF6/N2 Nitrogen-Containing Fault Decomposition Characteristic Products: A Theoretical Study. Nanomaterials. 2022; 12(13):2311. https://doi.org/10.3390/nano12132311
Chicago/Turabian StyleZeng, Fuping, Hao Qiu, Xiaoxuan Feng, Xianzong Chao, Liangjun Dai, Qiang Yao, and Ju Tang. 2022. "Ti3C2Tx as a Sensor for SF6/N2 Nitrogen-Containing Fault Decomposition Characteristic Products: A Theoretical Study" Nanomaterials 12, no. 13: 2311. https://doi.org/10.3390/nano12132311
APA StyleZeng, F., Qiu, H., Feng, X., Chao, X., Dai, L., Yao, Q., & Tang, J. (2022). Ti3C2Tx as a Sensor for SF6/N2 Nitrogen-Containing Fault Decomposition Characteristic Products: A Theoretical Study. Nanomaterials, 12(13), 2311. https://doi.org/10.3390/nano12132311