Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives
Abstract
:1. Introduction
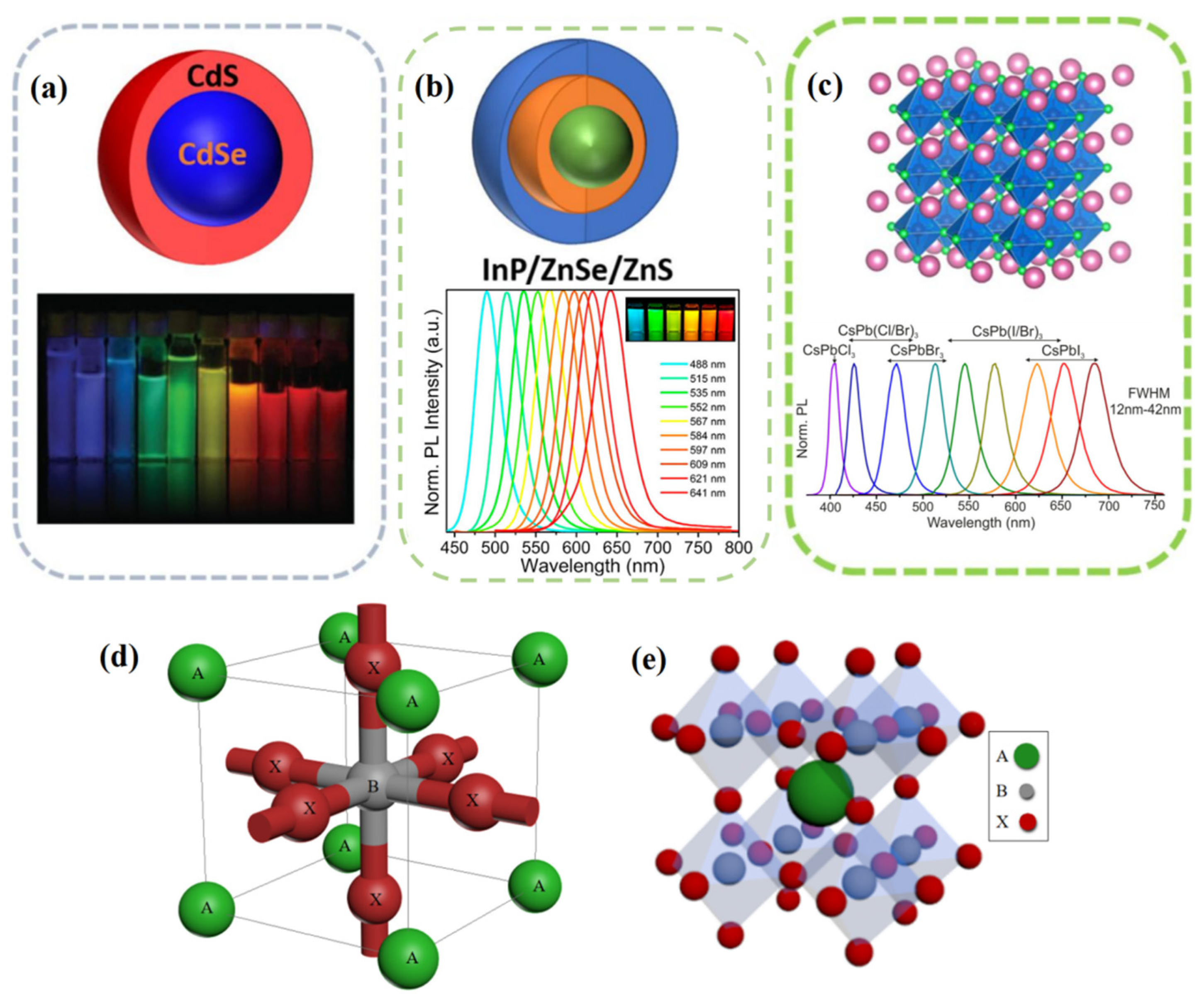
2. Fundamental Structure and Optical Properties of PQDs
2.1. Fundamental Structure of PQDs
2.2. Optical Properties of PQDs
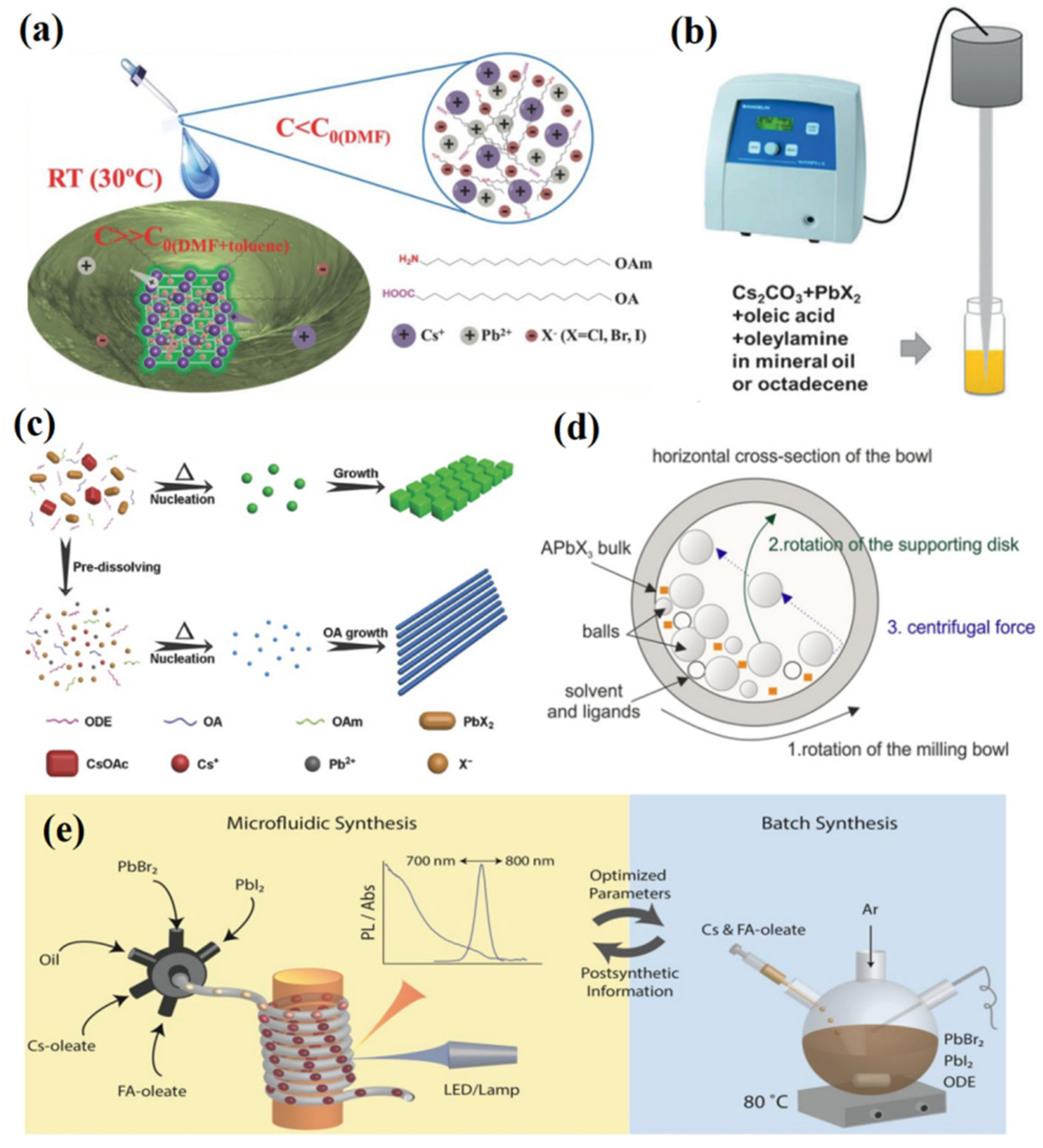
3. Synthesis Methods of PQDs
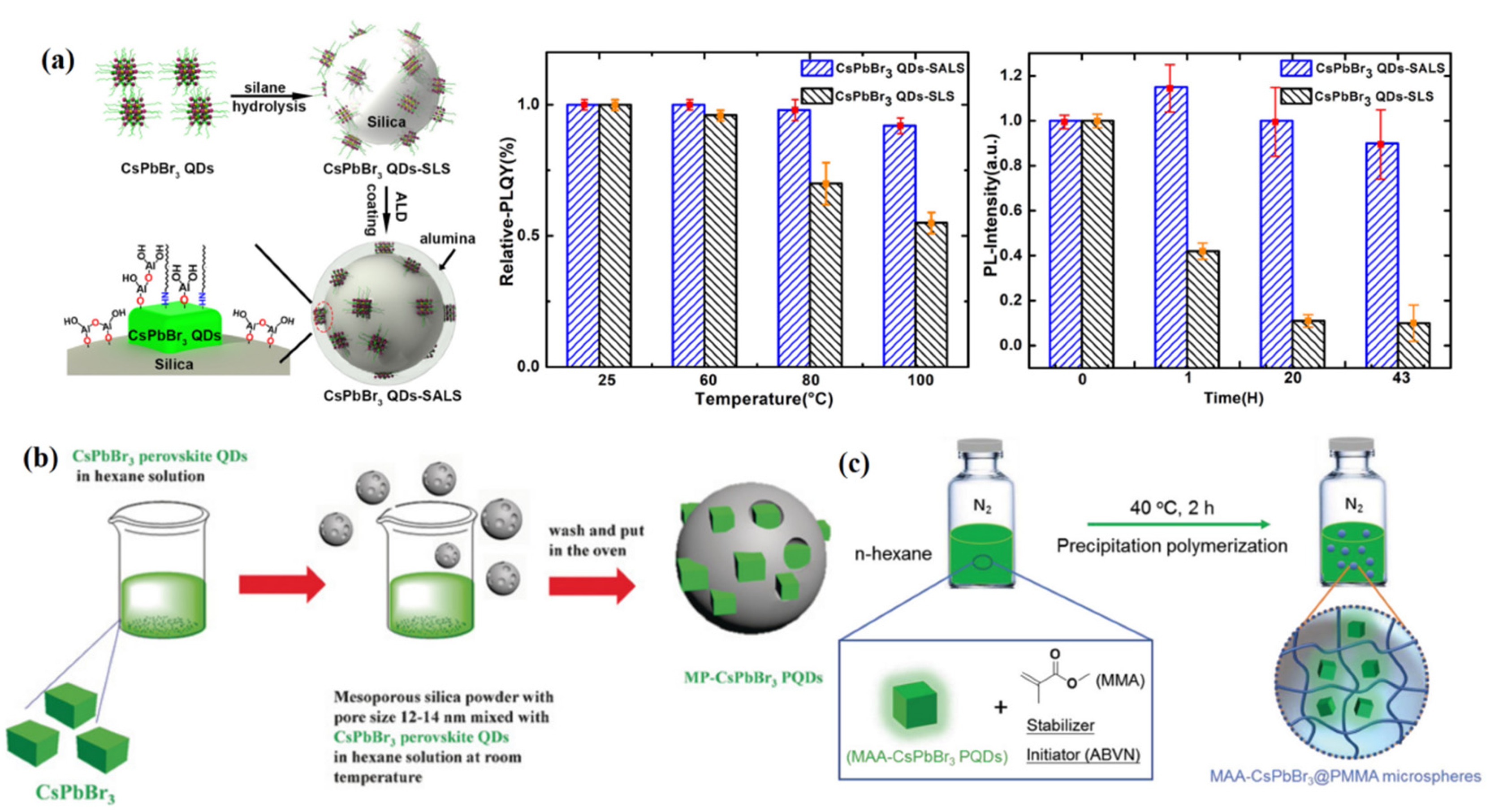
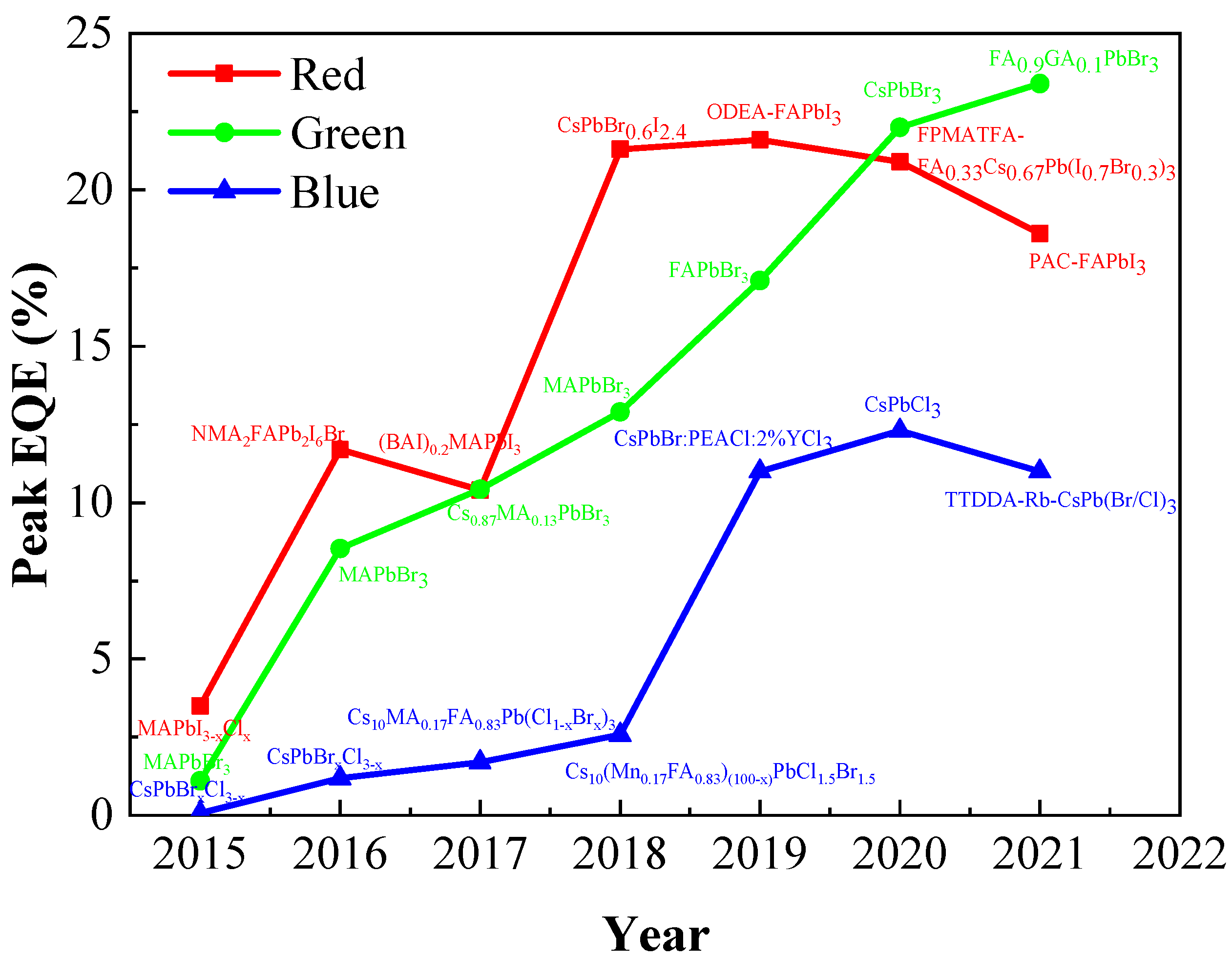
4. Performance Improvement of PQDs
4.1. Ion Doping of PQDs
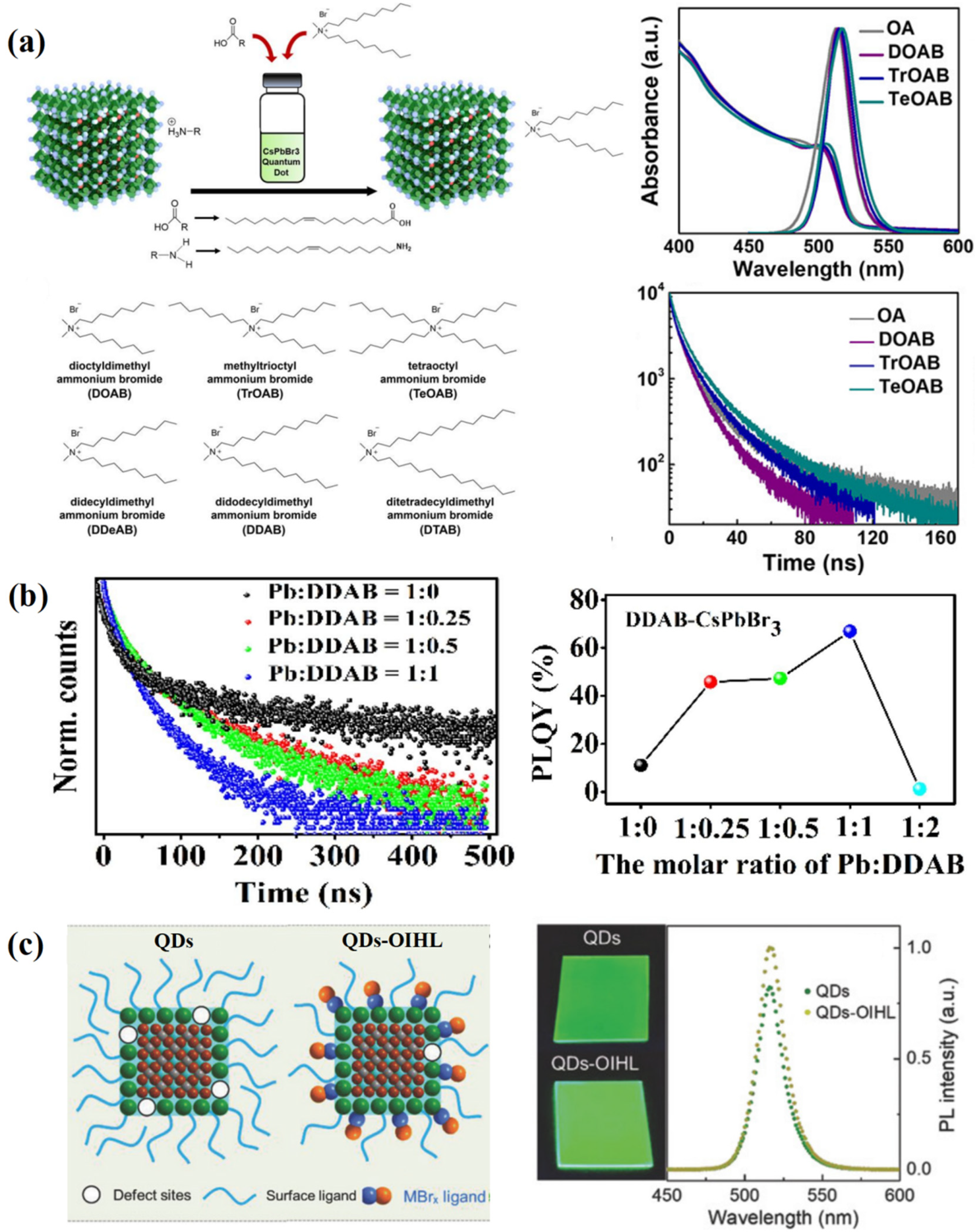
4.2. Ligand Modification of PQDs
4.3. Coating of PQDs
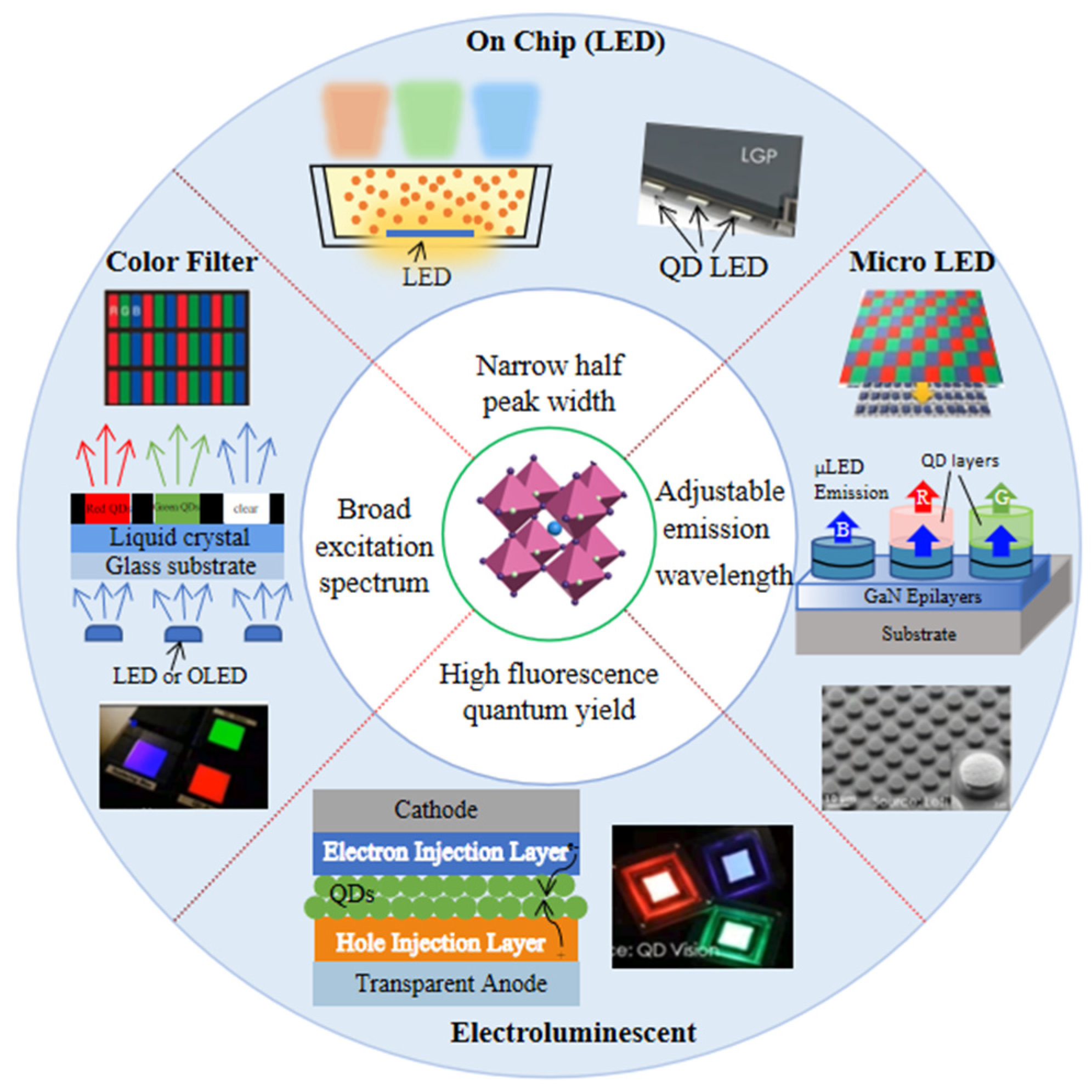
5. Progress of PQDs in Displays
5.1. Display Applications Based on PQD Photoluminescence
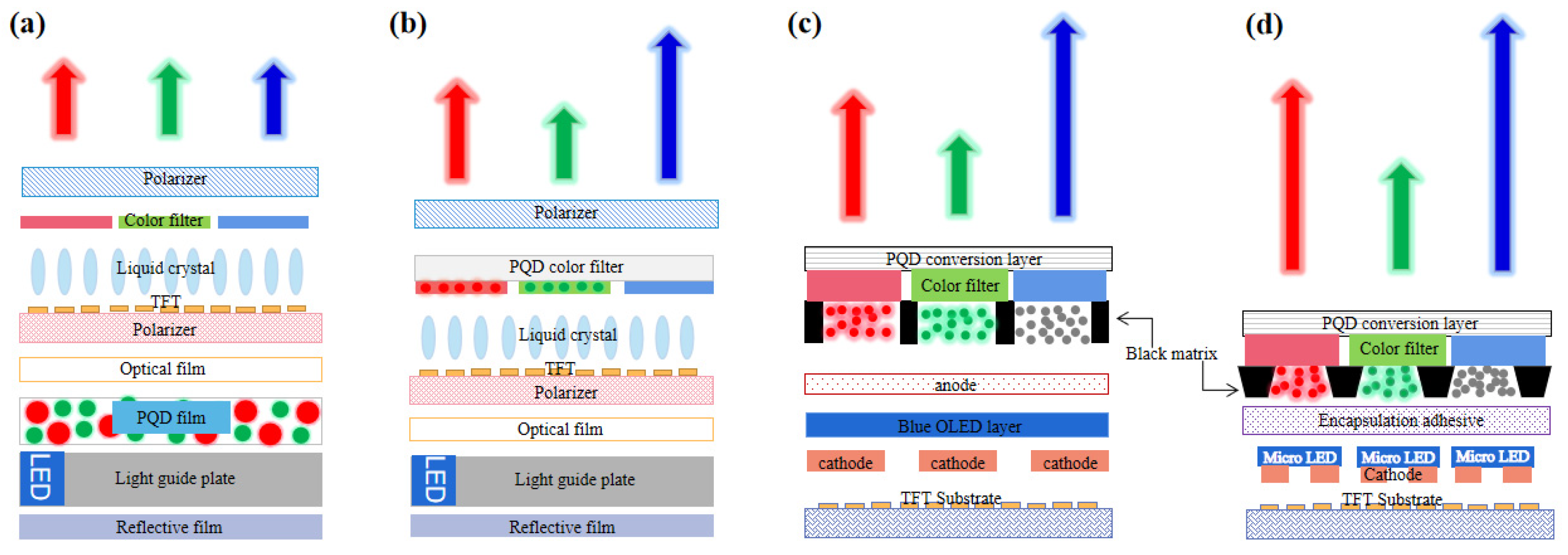
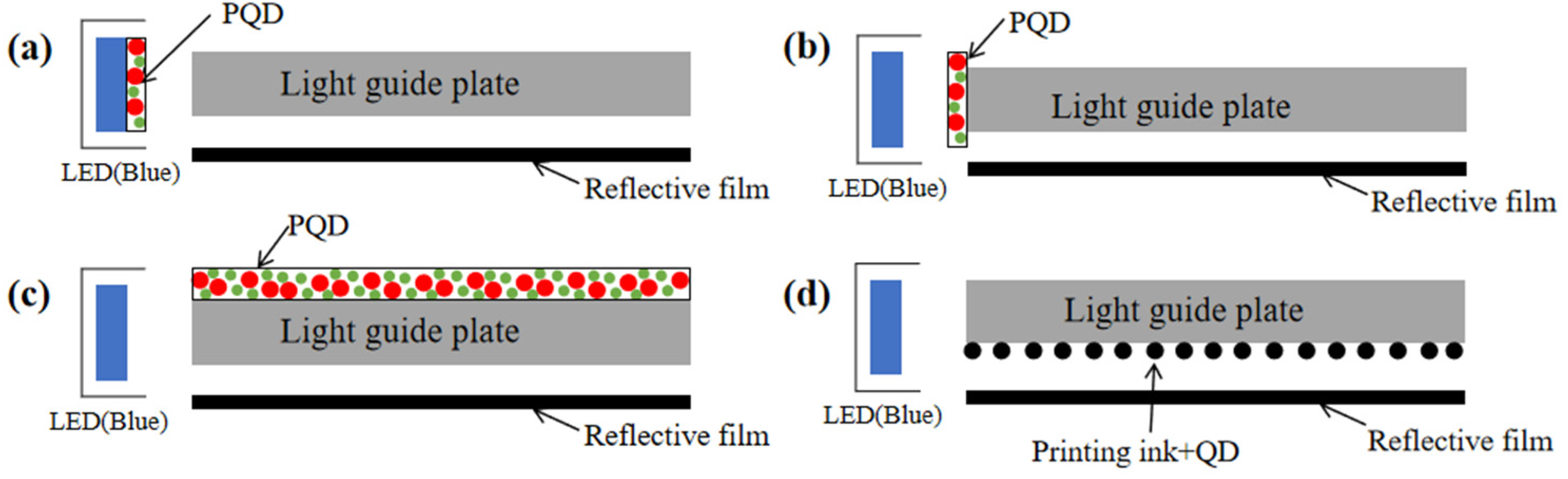
5.1.1. PQD Backlight
5.1.2. PQD Color Conversion Layer
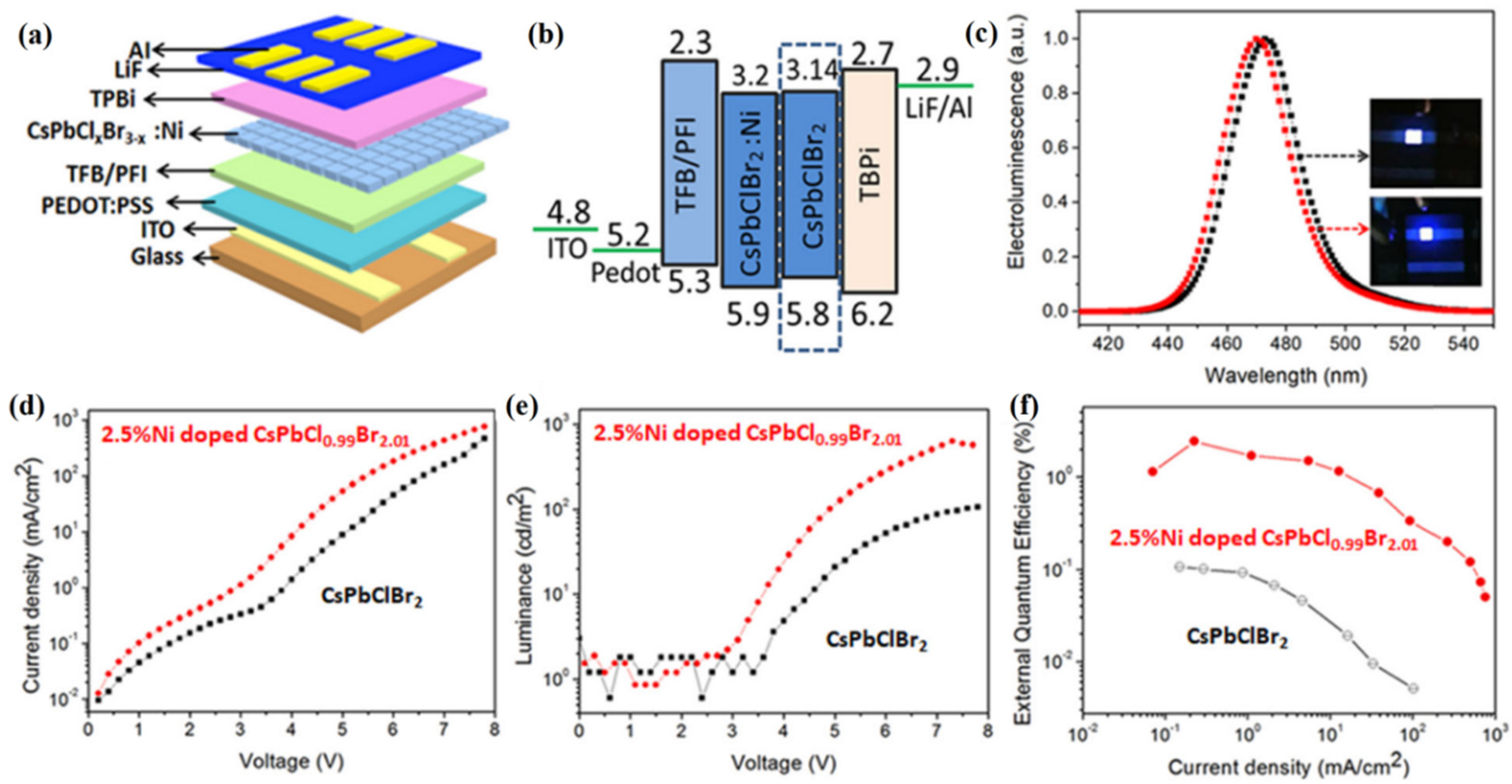
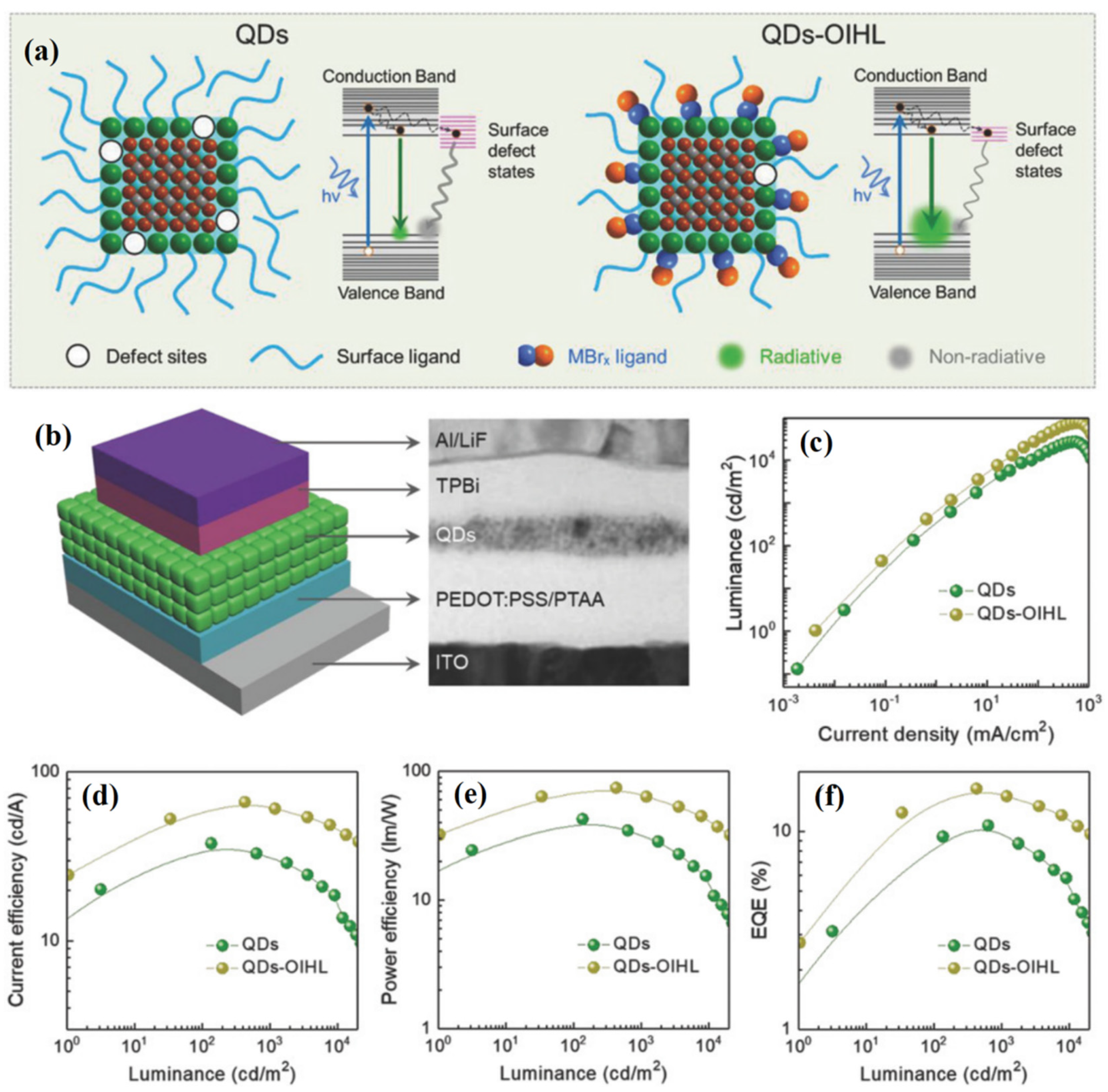
5.2. Display Applications Based on PQD Electroluminescence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Q.; Chen, S. Thermal assisted up-conversion electroluminescence in quantum dot light emitting diodes. Nat. Commun. 2022, 13, 369. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Yang, Q.; Wang, S.; Tian, H.; Ding, J.; Wang, L. Efficient narrowband red electroluminescence from a thermally activated delayed fluorescence polymer and quantum dot hybrid. Chem. Eng. J. 2022, 436, 135221. [Google Scholar] [CrossRef]
- Singh, R.K.; Chen, L.H.; Singh, A.; Jain, N.; Singh, J.; Lu, C.H. Progress of backlight devices: Emergence of halide perovskite quantum Dots/Nanomaterials. Front. Nanotechnol. 2022, 29, 863312. [Google Scholar] [CrossRef]
- Bornacelli, J.; Torres-Torres, C.; Silva-Pereyra, H.G.; Labrada-Delgado, G.J.; Crespo-Sosa, A.; Cheang-Wong, J.C.; Oliver, A. Superlinear photoluminescence by ultrafast laser pulses in dielectric matrices with metal nanoclusters. Sci. Rep. 2019, 9, 5699. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R.; Wang, Z.; Zhou, Y.; Shen, Q.; Ji, W.; Dang, D.; Meng, L.; Tang, B.Z. Recent advances in luminescent materials for super-resolution imaging via stimulated emission depletion nanoscopy. Chem. Soc. Rev. 2021, 50, 667–690. [Google Scholar] [CrossRef]
- Wang, Q.; Tong, Y.; Yang, M.; Ye, H.; Liang, X.; Wang, X.; Xiang, W. ZnO induced self-crystallization of CsPb(Br/I)3 nanocrystal glasses with improved stability for backlight display application. J. Mater. Sci. Technol. 2022, 121, 140–147. [Google Scholar] [CrossRef]
- Kang, C.; Tao, S.; Yang, F.; Yang, B. Aggregation and luminescence in carbonized polymer dots. Aggregate 2022, 3, e169. [Google Scholar] [CrossRef]
- Li, K.; Shen, C. White LED based on nano-YAG:Ce3+/YAG:Ce3+,Gd3+ hybrid phosphors. Optik 2012, 123, 621–623. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Wu, S.T. Recent Advances on Quantum-Dot-Enhanced Liquid-Crystal Displays. IEEE J. Sel. Top. Quant. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Tsai, Y.; Nguyen, H.; Lazarowska, A.; Mahlik, S.; Grinberg, M.; Liu, R. Improvement of the water resistance of a Narrow-Band Red-Emitting SrLiAl3N4:eu2+ phosphor synthesized under high isostatic pressure through coating with an organosilica layer. Angew. Chem. Int. Ed. 2016, 55, 9652–9656. [Google Scholar] [CrossRef]
- Wu, W.; Fang, M.; Zhou, W.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Brik, M.G.; Sheu, H.; Cheng, B.; Wang, J.; et al. High color rendering index of Rb2GeF6:mn4+ for Light-Emitting diodes. Chem. Mater. 2017, 29, 935–939. [Google Scholar] [CrossRef]
- Fang, M.; Wu, W.; Jin, Y.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Brik, M.G.; Srivastava, A.M.; Chiang, C.; Zhou, W.; et al. Control of luminescence by tuning of crystal symmetry and local structure in mn4+—Activated narrow band fluoride phosphors. Angew. Chem. Int. Ed. 2018, 57, 1797–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Wang, Q.; Li, D.; Yang, H.; Bai, X.; Li, S.; Liu, P.; Sun, X. Red and green quantum dot color filter for Full-Color Micro-LED arrays. Micromachines 2022, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Y.; Ye, W.; Shen, X.; Wen, Z.; Yuan, X.; Cao, Y.; Ma, C. Ligand-Free CsPbBr3 perovskite quantum dots in Silica-Aerogel composites with enhanced stability for w-LED and display by substituting pb2+ with pr3+ or gd3+ ions. Adv. Opt. Mater. 2022, 10, 2102200. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, M.; Wu, W.; Yang, X.; Sun, X.W.; Zhang, J.; Wang, H.; Liu, R.; Han, C.; Yang, H.; et al. Recent advances in quantum dot-based light-emitting devices: Challenges and possible solutions. Mater. Today 2019, 24, 69–93. [Google Scholar] [CrossRef]
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining near-unity luminescence in quantum dots with photothermal threshold quantum yield. Science 2019, 363, 1199–1202. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.; Fukumura, D.; Jain, R.K.; et al. Compact high-quality CdSe-CdS core-shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Won, Y.; Cho, O.; Kim, T.; Chung, D.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Kim, Y.; Ham, S.; Jang, H.; Min, J.H.; Chung, H.; Lee, J.; Kim, D.; Jang, E. Bright and uniform green light emitting InP/ZnSe/ZnS quantum dots for wide color gamut displays. ACS Appl. Nano Mater. 2019, 2, 1496–1504. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Xu, M.; Hong, J.; Tang, X.; Xu, L.; Wu, Y.; Zhu, R.; Chen, R.; Huang, W. Improving the stability of metal halide perovskite quantum dots by encapsulation. Adv. Mater. 2019, 31, 1900682. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Z.; Chang, Y.; Liu, R. Perovskite quantum dots for application in high color gamut backlighting display of Light-Emitting diodes. ACS Energy Lett. 2020, 5, 3374–3396. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Pazoki, M.; Philippe, B.; Dar, M.I.; Alsari, M.; Sadhanala, A.; Diyitini, G.; Imani, R.; Lilliu, S.; Kullgren, J.; et al. Dedoping of lead halide perovskites incorporating monovalent cations. ACS Nano 2018, 12, 7301–7311. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; García Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Gonzalez-Carrero, S.; Galian, R.E.; Perez-Prieto, J. Maximizing the emissive properties of CH3NH3PbBr3 perovskite nanoparticles. J. Mater. Chem. A 2015, 3, 9187–9193. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegas, A.; Gonzalez-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Minguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Perez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead tri-halide perovskites. Nat. Commun. 2014, 5, 3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graetzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range balanced electron- and Hole-Transport lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Motta, C.; El-Mellouhi, F.; Kais, S.; Tabet, N.; Alharbi, F.; Sanvito, S. Revealing the role of organic cations in hybrid halide perovskite CH3NH3PbI3. Nat. Commun. 2015, 6, 7026. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Ponseca, C.S., Jr.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A.; et al. Organometal halide perovskite solar cell materials rationalized: Ultrafast charge generation, high and Microsecond-Long balanced mobilities, and slow recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef]
- Zhang, D.W. Preparation, Properties and Application of Perovskite Quantum Dots and Metal—Organic Framework Composites; University of Science & Technology Beijing: Beijing, China, 2019. [Google Scholar]
- Bai, Y.; Hao, M.; Ding, S.; Chen, P.; Wang, L. Surface Chemistry Engineering of Perovskite Quantum Dots: Strategies, Applications, and Perspectives. Adv. Mater. 2022, 34, 2105958. [Google Scholar] [CrossRef]
- Byun, J.; Cho, H.; Wolf, C.; Jang, M.; Sadhanala, A.; Friend, R.H.; Yang, H.; Lee, T.-W. Efficient Visible Quasi-2D Perovskite Light-Emitting Diodes. Adv. Mater. 2016, 28, 7515–7520. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly Luminescent Colloidal Nanoplates of Perovskite Cesium Lead Halide and Their Oriented Assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yuan, S.; Wang, X.; Xu, T.; Wang, X.; Li, H.; Zheng, W.; Fan, P.; Li, Y.; Sun, L.; et al. Vapor Growth and Tunable Lasing of Band Gap Engineered Cesium Lead Halide Perovskite Micro/Nanorods with Triangular Cross Section. ACS Nano 2017, 11, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.B.; Lai, M.; Eaton, S.W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P. Growth and Anion Exchange Conversion of CH3NH3PbX3 Nanorod Arrays for Light-Emitting Diodes. Nano Lett. 2015, 15, 5519–5524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Yuan, X.; Fan, J.; Sun, B.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Jana, A.; Mittal, M.; Singla, A.; Sapra, S. Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. 2017, 53, 3046–3049. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazon, F.; El Ajjouri, Y.; Sebastia-Luna, P.; Lauciello, S.; Manna, L.; Bolink, H.J. Mechanochemical synthesis of inorganic halide perovskites: Evolution of phase-purity, morphology, and photoluminescence. J. Mater. Chem. C 2019, 7, 11406–11410. [Google Scholar] [CrossRef]
- Guo, P.; Hossain, M.K.; Shen, X.; Sun, H.; Yang, W.; Liu, C.; Ho, C.Y.; Kwok, C.K.; Tsang, S.; Luo, Y.; et al. Room-temperature red-green-blue whispering-gallery mode lasing and white-light emission from cesium lead halide perovskite (CsPbX3, X = Cl, Br, I) microstructures. Adv. Opt. Mater. 2018, 6, 1700993. [Google Scholar] [CrossRef]
- Lignos, I.; Maceiczyk, R.; de Mello, A.J. Microfluidic Technology: Uncovering the Mechanisms of Nanocrystal Nucleation and Growth. Acc. Chem. Res. 2017, 50, 1248–1257. [Google Scholar] [CrossRef]
- Lignos, I.; Morad, V.; Shynkarenko, Y.; Bernasconi, C.; Maceiczyk, R.M.; Protesescu, L.; Bertolotti, F.; Kumar, S.; Ochsenbein, S.T.; Masciocchi, N.; et al. Exploration of Near-Infrared-Emissive Colloidal Multinary Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform. ACS Nano 2018, 12, 5504–5517. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All inorganic halide perovskites nanosystem: Synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhou, L.; Shen, W.; Li, M.; He, R. Highly luminescent and stable quasi-2D perovskite quantum dots by introducing large organic cations. Nanoscale Adv. 2021, 3, 5393–5398. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, F.; Shen, Q.; Tao, S. The role of sodium in stabilizing tin-lead (Sn-Pb) alloyed perovskite quantum dots. J. Mater. Chem. A 2021, 9, 12087–12098. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, G.; Wang, R.; Shao, H.; Wang, H.; Xu, W.; Cui, H.; Song, H. Considerably enhanced exciton emission of CsPbCl3 perovskite quantum dots by the introduction of potassium and lanthanide ions. Nanoscale 2018, 10, 14067–14072. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, Q.; Li, Z.; Shan, A.; Li, L. Postsynthesis Potassium-Modification method to improve stability of CsPbBr3 perovskite nanocrystals. Adv. Opt. Mater. 2018, 6, 1701106. [Google Scholar] [CrossRef]
- Baek, S.; Kim, S.; Noh, J.Y.; Heo, J.H.; Im, S.H.; Hong, K.-H.; Kim, S.-W. Development of Mixed-Cation CsxRb1–xPbX3 Perovskite Quantum Dots and Their Full-Color Film with High Stability and Wide Color Gamut. Adv. Opt. Mater. 2018, 6, 1800295. [Google Scholar] [CrossRef]
- Shao, H.; Bai, X.; Cui, H.; Pan, G.; Jing, P.; Qu, S.; Zhu, J.; Zhai, Y.; Dong, B.; Song, H. White light emission in Bi3+/Mn2+ ion co-doped CsPbCl3 perovskite nanocrystals. Nanoscale 2018, 10, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yin, J.; Cai, B.; Wang, Z.; Dong, Y.; Xu, X.; Zeng, H. Lead-free, stable, high-efficiency (52%) blue luminescent FA3Bi2Br9 perovskite quantum dots. Nanoscale Horiz. 2020, 5, 580–585. [Google Scholar] [PubMed]
- Luo, C.; Li, W.; Fu, J.; Yang, W. Constructing gradient energy levels to promote exciton energy transfer for photoluminescence controllability of All-Inorganic perovskites and application in Single-Component WLEDs. Chem. Mater. 2019, 31, 5616–5624. [Google Scholar]
- Bi, C.; Wang, S.; Li, Q.; Kershaw, S.V.; Tian, J.; Rogach, A.L. Thermally stable Copper(II)-Doped cesium lead halide perovskite quantum dots with strong blue emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef]
- Xu, H.; Liang, J.; Zhang, Z.; Deng, Z.; Qiu, Y.; He, M.; Wang, J.; Yang, Y.; Chen, C. Lead-free bright blue light-emitting cesium halide nanocrystals by zinc doping. RSC Adv. 2021, 11, 2437–2445. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Yang, C.; Ji, L.; Wang, L. Fe2+ doped in CsPbCl3 perovskite nanocrystals: Impact on the luminescence and magnetic properties. RSC Adv. 2019, 9, 33017–33022. [Google Scholar]
- Zou, S.; Yang, G.; Yang, T.; Zhao, D.; Gan, Z.; Chen, W.; Zhong, H.; Wen, X.; Jia, B.; Zou, B. Template-Free synthesis of High-Yield Fe-Doped cesium lead halide perovskite ultralong microwires with enhanced Two-Photon absorption. J. Phys. Chem. Lett. 2018, 9, 4878–4885. [Google Scholar]
- Chen, D.; Zhou, S.; Tian, F.; Ke, H.; Jiang, N.; Wang, S.; Peng, Y.; Liu, Y. Halogen-hot-injection synthesis of Mn-doped CsPb(Cl/Br)3 nanocrystals with blue/orange dual-color luminescence and high photoluminescence quantum yield. Adv. Opt. Mater. 2019, 7, 1901082. [Google Scholar] [CrossRef]
- Yang, H.; Yin, W.; Dong, W.; Gao, L.; Tan, C.; Li, W.; Zhang, X.; Zhang, J. Enhancing light-emitting performance and stability in CsPbBr3 perovskite quantum dots via simultaneous doping and surface passivation. J. Mater. Chem. C 2020, 8, 14439–14445. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Macias-Pinilla, D.F.; Masi, S.; Echeverría-Arrondo, C.; Agouram, S.; Muñoz-Sanjosé, V.; Rodríguez-Pereira, J.; Macak, J.M.; Mora-Seró, I. Engineering Sr-doping for enabling long-term stable FAPb1-xSrxI3 quantum dots with 100% photoluminescence quantum yield. J. Mater. Chem. C 2021, 9, 1555–1566. [Google Scholar] [CrossRef]
- Sun, M.-J.; Zheng, C.; Gao, Y.; Johnston, A.; Najarian, A.M.; Wang, P.-X.; Voznyy, O.; Hoogland, S.; Sargent, E.H. Linear Electro-Optic Modulation in Highly Polarizable Organic Perovskites. Adv. Mater. 2021, 33, 2006368. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cao, M.; Xu, Y.; Li, P.; Zhang, Y.; Hu, H.; Yang, D.; Xu, Y.; Wang, L.; Li, Y.; et al. L-Type Ligand-Assisted Acid-Free Synthesis of CsPbBr3 Nanocrystals with Near-Unity Photoluminescence Quantum Yield and High Stability. Nano Lett. 2019, 19, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Wang, W.; Tsai, H.-Y.; Wang, H.-C.; Chen, S.; Liu, R.-S. Photo-/electro-luminescence enhancement of CsPbX3 (X = Cl, Br, or I) perovskite quantum dots via thiocyanate surface modification. J. Mater. Chem. C 2020, 8, 1065–1071. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, A.-y.; Yu, J.C.; Nam, Y.S.; Choi, Y.; Park, J.; Song, M.H. Surface Ligand Engineering for Efficient Perovskite Nanocrystal-Based Light-Emitting Diodes. ACS Appl. Mater. 2019, 11, 8428–8435. [Google Scholar] [CrossRef]
- Huang, Y.; Luan, W.; Liu, M.; Turyanska, L. DDAB-assisted synthesis of iodine-rich CsPbI3 perovskite nanocrystals with improved stability in multiple environments. J. Mater. Chem. C 2020, 8, 2381–2387. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Guan, H.; Zhao, S.; Cao, S.; Chen, C.; Liu, M.; Zang, Z. Highly stable CsPbBr3 quantum dots by silica-coating and ligand modification for white light-emitting diodes and visible light communication. Chem. Eng. J. 2021, 419, 129551. [Google Scholar] [CrossRef]
- Yang, L.; Fu, B.; Li, X.; Chen, H.; Li, L. Poly(vinylidene fluoride)-passivated CsPbBr3 perovskite quantum dots with near-unity photoluminescence quantum yield and superior stability. J. Mater. Chem. C 2021, 9, 1983–1991. [Google Scholar] [CrossRef]
- Song, J.; Fang, T.; Li, J.; Xu, L.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Organic–Inorganic Hybrid Passivation Enables Perovskite QLEDs with an EQE of 16.48%. Adv. Mater. 2018, 30, 1805409. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.; Zhang, R.; Liu, X.; Zhang, W.; Zhang, J.; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Jang, J.; Kim, Y.-H.; Park, S.; Yoo, D.; Cho, H.; Jang, J.; Jeong, H.B.; Lee, H.; Yuk, J.M.; Park, C.B.; et al. Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years. Adv. Mater. 2021, 33, 2005255. [Google Scholar] [CrossRef] [PubMed]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and Properties of Biocompatible Water-Soluble Silica-Coated CdSe/ZnS Semiconductor Quantum Dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Chen, E.; Ye, Y.; Xu, S.; Guo, T. Highly Stabilized Gradient Alloy Quantum Dots and Silica Hybrid Nanospheres by Core Double Shells for Photoluminescence Devices. J. Phys. Chem. Lett. 2020, 11, 1428–1434. [Google Scholar] [CrossRef]
- Xie, H.; Chen, E.; Ye, Y.; Xu, S.; Guo, T. Interfacial optimization of quantum dot and silica hybrid nanocomposite for simultaneous enhancement of fluorescence retention and stability. Appl. Phys. Lett. 2020, 117, 171101. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ziegler, J.; Xu, S.; Kucur, E.; Meister, F.; Batentschuk, M.; Gindele, F.; Nann, T. Silica-Coated InP/ZnS nanocrystals as converter material in white LEDs. Adv. Mater. 2008, 20, 4068–4073. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Z.; Bian, Y.; Li, S.; Tang, X.; Du, J.; Zang, Z.; Zhou, M.; Hu, W.; Tian, Y.; et al. Enhanced Two-Photon-Pumped emission from in situ synthesized nonblinking CsPbBr3/SiO2 nanocrystals with excellent stability. Adv. Opt. Mater. 2018, 6, 1700997. [Google Scholar] [CrossRef]
- Chen, W.; Hao, J.; Hu, W.; Zang, Z.; Tang, X.; Fang, L.; Niu, T.; Zhou, M. Enhanced stability and tunable photoluminescence in perovskite CsPbX3/ZnS quantum dot heterostructure. Small 2017, 13, 1604085. [Google Scholar] [CrossRef]
- Li, Z.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.; Wu, L.; Zheng, W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 Core/Shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Cai, J.; Lin, J.; Chen, Y.; Xu, S.; Ye, Y.; Chen, E.; Guo, T. Stacked Encapsulation Structure for Discretely Distributed Quantum Dot Array. IEEE Photonics J. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Lin, J.; Hu, X.; Wang, C.; Chen, E.; Sun, J.; Yan, Q.; Guo, T. Quantum-dot array with a random rough interface encapsulated by atomic layer deposition. Opt. Lett. 2022, 47, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhou, B.; Cao, K.; Wen, Y.; Li, Y.; Wang, Z.; Jiang, C.; Shan, B.; Chen, R. Bottom up Stabilization of CsPbBr3 Quantum Dots-Silica Sphere with Selective Surface Passivation via Atomic Layer Deposition. Chem. Mater. 2018, 30, 8486–8494. [Google Scholar] [CrossRef]
- Wang, H.; Lin, S.; Tang, A.; Singh, B.P.; Tong, H.; Chen, C.; Lee, Y.; Tsai, T.; Liu, R. Mesoporous silica particles integrated with All-Inorganic CsPbBr3 perovskite Quantum-Dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing Defect-Tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef]
- Malgras, V.; Henzie, J.; Takei, T.; Yamauchi, Y. Stable blue luminescent CsPbBr3 perovskite nanocrystals confined in mesoporous thin films. Angew. Chem. 2018, 130, 9019–9023. [Google Scholar] [CrossRef]
- Yang, G.; Fan, Q.; Chen, B.; Zhou, Q.; Zhong, H. Reprecipitation synthesis of luminescent CH3NH3PbBr3/NaNO3 nanocomposites with enhanced stability. J. Mater. Chem. C 2016, 4, 11387–11391. [Google Scholar] [CrossRef]
- Dirin, D.N.; Benin, B.M.; Yakunin, S.; Krumeich, F.; Raino, G.; Frison, R.; Kovalenko, M.V. Microcarrier-Assisted inorganic shelling of lead halide perovskite nanocrystals. ACS Nano 2019, 13, 11642–11652. [Google Scholar] [CrossRef]
- Wei, Y.; Xiao, H.; Xie, Z.; Liang, S.; Liang, S.; Cai, X.; Huang, S.; Al Kheraif, A.A.; Jang, H.S.; Cheng, Z.; et al. Highly luminescent lead halide perovskite quantum dots in hierarchical CaF2 matrices with enhanced stability as phosphors for white Light-Emitting diodes. Adv. Opt. Mater. 2018, 6, 1701343. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Zhu, Y.; Gong, Y.; Li, C. A polymer-coated template-confinement CsPbBr3 perovskite quantum dot composite. Nanoscale 2021, 13, 6586–6591. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Wang, L.; Xie, R.-J. A Facile Synthesis of Water-Resistant CsPbBr3 Perovskite Quantum Dots Loaded Poly(methyl methacrylate) Composite Microspheres Based on In Situ Polymerization. Adv. Opt. Mater. 2019, 7, 1901075. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Zhang, R.; Huang, F.; Chen, D. Research progresses in preparation and applications of CsPbX 3 (X= Cl, Br, I) perovskite quantum dots-embedded glass. Chin. J. Lumin. 2021, 42, 1331–1344. [Google Scholar] [CrossRef]
- Hou, J.; Chen, P.; Shukla, A.; Krajnc, A.; Wang, T.; Li, X.; Doasa, R.; Tizei, L.H.G.; Chan, B.; Johnstone, D.N.; et al. Liquid-phase sintering of lead halide perovskites and metal-organic framework glasses. Science 2021, 374, 621–625. [Google Scholar] [CrossRef]
- Chen, E.; Xie, H.; Huang, J.; Miu, H.; Shao, G.; Li, Y.; Guo, T.; Xu, S.; Ye, Y. Flexible/curved backlight module with quantum-dots microstructure array for liquid crystal displays. Opt. Express 2018, 26, 3466–3482. [Google Scholar] [CrossRef]
- Lin, S.; Tan, G.; Yu, J.; Chen, E.; Weng, Y.; Zhou, X.; Xu, S.; Ye, Y.; Yan, Q.F.; Guo, T. Multi-primary-color quantum-dot down-converting films for display applications. Opt. Express 2019, 27, 28480–28493. [Google Scholar] [CrossRef]
- Chen, E.; Lin, J.; Yang, T.; Chen, Y.; Zhang, X.; Ye, Y.; Sun, J.; Yan, Q.; Guo, T. Asymmetric Quantum-Dot Pixelation for Color-Converted White Balance. ACS Photonics 2021, 8, 2158–2165. [Google Scholar] [CrossRef]
- Huang, B.; Chen, E.; Sun, L.; Guo, T. Quantum-dot color conversion film patterned by screen printing and overprinting process for display backlights. Opt. Laser Technol. 2022, 145, 107486. [Google Scholar] [CrossRef]
- Huang, B.L.; Guo, T.L.; Xu, S.; Ye, Y.; Chen, E.G.; Lin, Z.X. Color Converting Film With Quantum-Dots for the Liquid Crystal Displays Based on Inkjet Printing. IEEE Photonics J. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Yin, Y.; Meng, H. Progress of quantum dots and perovskite as color conversion materials for full-color display. Chin. J. Lumin. 2021, 42, 419–447. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Yang, T.; Cai, J.; Ye, Y.; Chen, E.; Xu, S.; Ye, Y.; Sun, J.; Yan, Q.; et al. Tripling Light Conversion Efficiency of μLED Displays by Light Recycling Black Matrix. IEEE Photonics J. 2022, 14, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Cai, J.; Ye, Y.; Hu, X.; Zhong, L.; Xie, H.; Chen, E.; Ye, Y.; Xu, S.; Sun, J.; et al. Full-visible-spectrum perovskite quantum dots by anion exchange resin assisted synthesis. Nanophotonics 2022, 11, 1355–1366. [Google Scholar] [CrossRef]
- Nick, R.J.; Breen, C.A.; Denton, C.M.; Sadasivan, S.; Linton, J.R.; QD Vision Inc. Method of making components including quantum dots, methods, and products. U.S. Patent Application 20150049491A1, 19 February 2015. [Google Scholar]
- Ji, H.; Ye, D.; Xu, H.; Chen, E.; Ge, Z. Multi-layer co-extruded quantum-dot diffuser plate for ultra-large TV backlights. Opt. Mater. Express 2022, 12, 1648–1656. [Google Scholar] [CrossRef]
- Chen, E.; Guo, J.; Jiang, Z.; Shen, Q.; Ye, Y.; Xu, S.; Sun, J.; Yan, Q.; Guo, T. Edge/direct-lit hybrid mini-LED backlight with U-grooved light guiding plates for local dimming. Opt. Express 2021, 29, 12179–12194. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Xu, S.; Kang, D.; Lin, Z.; Chen, E.; Guo, T.; Yang, L. Fabrication and properties of quantum-dots backlight light guide plate. Chin. J. Lumin. 2017, 38, 91–96. [Google Scholar] [CrossRef]
- Chen, E.; Lin, S.; Jiang, Z.; Guo, Q.; Xu, S.; Ye, Y.; Yan, Q.F.; Guo, T. Analytic design of light extraction array for light guide plate based on extended sources. Opt. Express 2019, 27, 34907–34920. [Google Scholar] [CrossRef]
- Yang, H.S.; Noh, S.H.; Suh, E.H.; Jung, J.; Oh, J.G.; Lee, K.H.; Jang, J. Enhanced stabilities and production yields of MAPbBr3 quantum dots and their applications as stretchable and Self-Healable color filters. ACS Appl. Mater. Interfaces 2021, 13, 4374–4384. [Google Scholar] [CrossRef]
- Cai, J.; Wang, C.; Hu, X.; Ye, Y.; Zhong, L.; Chen, E.; Ye, Y.; Xu, S.; Sun, J.; Yan, Q.; et al. Water-driven photoluminescence reversibility in CsPbBr3/PDMS-PUa composite. Nano Res. 2022. [Google Scholar] [CrossRef]
- Yin, Y.; Ali, M.U.; Liu, M.; Miao, J.; Peng, W.; Li, D.; Chen, S.; Lee, C.; Meng, H. Vacuum-drying processed micrometer-thick stable CsPbBr3 perovskite films with efficient blue-to-green photoconversion. Small 2019, 15, 1901954. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Ali, M.U.; Peng, W.; Zhang, S.; Li, D.; Zou, T.; Li, Y.; Jiao, S.; Chen, S.J.; et al. Inkjet printed uniform quantum dots as color conversion layers for full-color OLED displays. Nanoscale 2020, 12, 2103–2110. [Google Scholar] [CrossRef]
- Yan, F.; Xing, J.; Xing, G.; Quan, L.; Tan, S.T.; Zhao, J.; Su, R.; Zhang, L.; Chen, S.; Zhao, Y.; et al. Highly efficient visible colloidal Lead-Halide perovskite nanocrystal Light-Emitting diodes. Nano Lett. 2018, 18, 3157–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient Light-Emitting diodes based on in situ fabricated FAPbBr3 nanocrystals: The enhancing role of the Ligand-Assisted reprecipitation process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion synthesis of Size-Tunable CH3NH3PbBr3 quantum dots: An alternative route toward efficient Light-Emitting diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yan, F.; Zhao, Y.; Chen, S.; Yu, H.; Zhang, Q.; Zeng, R.; Demir, H.V.; Sun, X.; Huan, A.; et al. High-Efficiency Light-Emitting diodes of organometal halide perovskite amorphous nanoparticles. ACS Nano 2016, 10, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeong, S.; Park, M.; Kim, Y.; Wolf, C.; Lee, C.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Wang, W.; Zhang, J.; Xu, B.; Karen, K.L.; Zheng, Y.; Liu, S.; Chen, S.; Wang, K.; et al. Hybrid perovskite Light-Emitting diodes based on perovskite nanocrystals with Organic-Inorganic mixed cations. Adv. Mater. 2017, 29, 1606405. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Wang, C.; Muhammad, I.; Zhang, F.; Shmshad, A.; Xue, X.; Ji, W.; Chang, S.; Zhong, H. Highly efficient light emitting diodes based on in situ fabricated FAPbI3 nanocrystals: Solvent effects of on-chip crystallization. Adv. Opt. Mater. 2019, 7, 1900774. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Xu, W.; Chen, X.; Zhai, Y.; Zhu, J.; Shao, H.; Ding, N.; Xu, L.; Dong, B.; et al. Bright blue light emission of Ni2+ ion-doped CsPbClxBr3-x perovskite quantum dots enabling efficient light-emitting devices. ACS Appl. Mater. Interfaces 2020, 12, 14195–14202. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, G.; Kim, Y.; Wolf, C.; Yun, H.J.; Kwon, W.; Park, C.G.; Lee, T.W. High efficiency perovskite light-emitting diodes of ligand-engineered colloidal formamidinium lead bromide nanoparticles. Nano Energy 2017, 38, 51–58. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Yang, Q.; Wang, S.; Ding, J.; Wang, L. Heterogeneous post-passivation of inorganic cesium lead halide perovskite quantum dots for efficient electroluminescent devices. J. Mater. Chem. C 2021, 9, 3978–3986. [Google Scholar] [CrossRef]
- Khan, Q.; Subramanian, A.; Yu, G.; Maaz, K.; Li, D.; Sagar, R.U.R.; Chen, K.; Lei, W.; Shabbir, B.; Zhang, Y. Structure optimization of perovskite quantum dot light-emitting diodes. Nanoscale 2019, 11, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.; Chen, B.; Chekini, M.; Baek, S.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Shrestha, S.; Vilá, R.A.; Huang, W.; Liu, C.; Hou, C.; Huang, H.; Wen, X.; Li, M.; Wiederrecht, G.; et al. Bright and stable light-emitting diodes made with perovskite nanocrystals stabilized in metal–organic frameworks. Nat. Photonics 2021, 15, 843–849. [Google Scholar] [CrossRef]


| Methods | Principle | Results | Drawbacks | Reference |
|---|---|---|---|---|
| Hot injection | High temperature | High yield, good properties, suitable for ion doping and ligand modification, widely used | Complex process | [26] |
| Anion exchange | Doping | Full-spectrum luminescence, easy X-position doping | Multi-step process | [47] |
| Room-temperature reprecipitation | Different solubility | Easy operation, high repeatability, suitable for ligand modification | Uneven size | [48] |
| Ultrasonic method | Ultrasonic treatment | Easy operation, suitable for ligand modification | High cost | [49] |
| Microwave-assisted synthesis | Microwave treatment | Easy operation, high repeatability, suitable for ligand modification | High cost | [50] |
| Solvothermal synthesis | Mixed high temperature | Easy to synthesize, controllable morphology | System temperature unevenness, not suitable for ion doping and ligand modification | [51] |
| Mechanochemical synthesis | Mixed grinding | High yield, easy to synthesize | Not applicable to ligand modification | [52] |
| Wet ball milling | Mixed grinding | Easy to synthesize | Low synthetic efficiency | [53] |
| Dry ball milling | Mixed grinding, solvent-free | Fast, high synthetic purity | Easy to generate surface defects | [54] |
| Chemical vapor deposition | Chemical reaction, deposition | Excellent performance | Large size, precise equipment | [55] |
| Microfluidic platform | Carrier spacing reaction | Automatic, homogeneity | Immature | [57] |
| Doping | Excitation (nm) | PL (nm) | FWHM (nm) | PLQY (%) | τ (ns) | Stability | Advantages | Reference |
|---|---|---|---|---|---|---|---|---|
| A—site doping | ||||||||
| BA+ | − | − | − | 49.44 | 24.58 | Stable (50 days, 80% RH) | Reduced dimensionality | [59] |
| K+ | 365 | 408 | 12.7 | 10.3 | 13.6 | − | Greatly improved PLQY | [61] |
| Rb+ | 365 | 505–515 | 18–20 | 93 | 5.32 | 30% (100 °C, 24 h) | Increased exciton binding energy | [63] |
| B—site doping | ||||||||
| Eu3+ | 365 | 408 | 11.3 | 31.2 | 15.24 | − | Greatly improved PLQY | [61] |
| Bi3+ | 365 | 420–520 | − | 52 | 9.5 | 70% (30 days, air) | Lead-free PQDs | [64] |
| Tm3+ | 365 | − | − | 54 | 4.8–5 | Stable (80 °C, 24 h) | Introduction of new energy level | [66] |
| Cu2+ | 365 | 450–460 | 15–26 | >80% | 2.3–5 | 90% (30 days, 60% RH, 25 ℃) | Eliminating halide vacancies | [67] |
| Zn2+ | 365/380 | 395–550 | 47 | 79.05 | − | 63.77% PLQY (50 days, air) | Lead-free PQDs | [68] |
| Fe2+ | − | 401–403 | 13.8–14.6 | 6.2 | 14.6 | − | Size homogeneity improvement | [69] |
| Mn2+ | 365 | − | − | 65 | − | − | Toxic ions reduction and PLQY improvement | [71] |
| Co2+ | 365 | 516 | 18–20 | 89 | 17.93 | 90% (50 days) | Defect passivation | [72] |
| Sr | − | 589,583,530 | − | 100 | − | Stable for 8 months (40–50% RH, 6.5 months) | Defect passivation | [73] |
| X—site doping | ||||||||
| BF4− | − | 515 | − | − | − | − | Increased hole space of perovskite | [74] |
| Multiple ion doping | ||||||||
| Bi3+, Mn2+ | 365 | 420–520 | − | 52 | 9.5 | 70% (30 days, air) | Wide range of CCT | [64] |
| Tm3+, Mn2+ | 365 | − | − | 54 | 4.8–5 | Stable (80 °C, 24 h) | Promotion of exciton energy transfer | [66] |
| Wrapping | Excitation (nm) | PL (nm) | FWHM (nm) | PLQY(%) | τ (ns) | Stability | Advantages | References |
|---|---|---|---|---|---|---|---|---|
| CsPbBr3/SiO2 | 350 | 533 | 18 | − | 48.3 | 73.8% (75% RH, air, 12 h); 36.4% (60 °C, 15 h) | Anion exchange prevention and stability improvement | [90] |
| CsPbX3/ZnS | 365 | − | − | 70 | − | − | More stable, tunable | [91] |
| CsPbBr3/TiO2 | 405 | 518 | 32 | − | 2.1 | Stable for 12 weeks (water); ≈75% (UV, 24 h) | Suppress anion exchange and photodegradation | [92] |
| CsPbBr3/Al2O3 | 365 | 516 | 23 | 65 | 36.57 | PL stable (96 h, water); 80% (450 nm, 200 mW/cm2, 40 h) | Defect passivation | [95] |
| CsPbBr3/Mesoporous silica | 365 | 457–698 | 13–35 | − | − | 80% (365 nm, 6 W UV, 96 h) | Prevent ion exchange and increase stability | [96] |
| MAPbBr3/NaNO3 | 365 | 525–526 | 24 | 42 | 155.5 | 30% (100 °C, 5 h); 80% (365 nm/6 W UV, 14 h) | Improved stability | [99] |
| CsPbBr3@SiO2/Poly-CLA | 365 | 511 | 20 | 79.16 | 218.11 | 77% (water, 1 week) | Improved stability | [102] |
| CsPbBr3@PMMA | 395 | 514 | 26 | 32.8 | 122.2 | 91% (water, 7 days); stable (water, 1 month) | Improved water resistance and storage stability | [103] |
| CsPbI3/ZIF glass | − | − | 42 | >65% | 17.6 (average) | 80% (water, 10,000 h) no CsPbI3 phases change (air condition) active phase preserved (after 77 K) Over 80% (after 100 °C in air or 80 °C in air for 1000 cycles) 90% (57 mW/cm2 over 5000 s) | Improved stability | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhang, X.; Xie, H.; Cai, J.; Wang, C.; Chen, E.; Xu, S.; Ye, Y.; Sun, J.; Yan, Q.; et al. Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives. Nanomaterials 2022, 12, 2243. https://doi.org/10.3390/nano12132243
Ren X, Zhang X, Xie H, Cai J, Wang C, Chen E, Xu S, Ye Y, Sun J, Yan Q, et al. Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives. Nanomaterials. 2022; 12(13):2243. https://doi.org/10.3390/nano12132243
Chicago/Turabian StyleRen, Xinxin, Xiang Zhang, Hongxing Xie, Junhu Cai, Chenhui Wang, Enguo Chen, Sheng Xu, Yun Ye, Jie Sun, Qun Yan, and et al. 2022. "Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives" Nanomaterials 12, no. 13: 2243. https://doi.org/10.3390/nano12132243
APA StyleRen, X., Zhang, X., Xie, H., Cai, J., Wang, C., Chen, E., Xu, S., Ye, Y., Sun, J., Yan, Q., & Guo, T. (2022). Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives. Nanomaterials, 12(13), 2243. https://doi.org/10.3390/nano12132243







