Abstract
The monitoring of benzene and other carcinogenic aromatic volatile compounds at the ppb level requires boosting both the selectivity and sensitivity of the corresponding sensors. A workable solution is the introduction in the devices of preconcentrator units containing molecular receptors. In particular, quinoxaline cavitands (QxCav) resulted in very efficient preconcentrator materials for the BTEX in air to the point that they have been successfully implemented in a commercial sensor. In this work, we report a highly efficient quinoxaline-based preconcentrator material, in which the intrinsic adsorption capacity of the QxCav has been maximized. The new material consists of silica particles covalently coated with a suitable functionalized QxCav derivative (QxCav@SiO2). In this way, all the cavities are exposed to the analyte flux, boosting the performance of the resulting preconcentration cartridge well above that of the pure QxCav. It is noteworthy that the preconcentrator adsorption capacity is independent of the relative humidity of the incoming air.
1. Introduction
Environmental monitoring represents one of the greatest challenges for the chemical community due to the stringent sensor requirements in terms of sensitivity and selectivity. Preconcentrators have found wide acceptance as an effective solution to impart both sensitivity and selectivity to sensor devices [1]. Their use is particularly appealing for the real-time detection of benzene, the carcinogenic component [2] of benzene, toluene, ethylbenzene and xylenes (BTEX) at low ppb levels in air [3]. The issue of achieving molecular-level selectivity and low-ppb sensitivity at the same time can be addressed by disconnecting the recognition element from the detection unit by using molecular receptors as preconcentrators. In this context, our group has pioneered the use of cavitands as selective preconcentrators in indoor and outdoor sensors for BTEX [4]. The outcome of this activity is a commercially available stand-alone device [5] capable of monitoring the concentration of benzene in air below the current EU limit value of 5 µg m−3 [6]. In its most recent configuration, the device is composed of a micro-electro-mechanical system (MEMS) cartridge packed with a solid cavitand receptor to selectively trap the BTEX during sampling at room temperature. The separation of the different aromatic compounds released by the cavitand preconcentrator after thermal desorption is obtained by interfacing the preconcentrator with a GC-MEMS column [7]. The separated analytes were then individually channeled to a photoionization detector (PID), achieving a detection limit in the sub-ppb range.
The tetraquinoxaline cavitand QxCav (Scheme 1) is a molecular receptor featuring a preorganized cavity of nanosize dimensions [4]. It has been shown to be selective for the uptake of aromatic analytes in air in the presence of larger amounts of aliphatic hydrocarbons [8,9]. Moreover, QxCav is insensitive to air humidity and to the inorganic pollutants present in air such as CO2, NOx and SOx. Its remarkable selectivity at the gas–solid interface is attributed to CH–π interactions between the analytes and the inner cavity aromatic surfaces [10,11]. In the present device configuration, QxCav is loaded in the MEMS cartridge as amorphous powder with particles 180–250 µm in size [7]. This particle size distribution has been optimized to allow optimal air flux through the preconcentrator and to be easily retained by an MEMS mechanical filter. This solution has the advantage of synthetic simplicity, but it brings in some undesired issues: (1) only cavitands on the particles surface are active in BTEX uptake as most of them are buried in the bulk solid; (2) cavitands are randomly oriented, and only those with the mouth of the cavity exposed can trap the analytes; and (3) the cavitand particles tend to become smaller and smaller by mechanical stress due to flow pumping while cycling at high temperature, causing a progressive increase in MEMS impedance and a consequent loss of performance. These issues prompted us to consider the grafting of QxCav on solid supports as a workable solution [12,13].
Scheme 1.
Structure of tetraquinoxaline cavitand (QxCav).
Here, we report a structurally designed, highly efficient preconcentrator material for BTEX adsorption, in which the QxCav is covalently coated on the surface of silica particles. The resulting material overcomes the two undesired issues reported above related to the use of pure cavitand powder as a preconcentrator. The new preconcentrator is used in the final device to test its performances under real working conditions in comparison with pure cavitand particles and bare silica.
2. Materials and Methods
The experimental details, including the materials and apparatus, are listed in the Supplementary Materials. The precursor QxCav with four ω-decenyl feet was prepared by following a previously published procedure [14]. Mono-hydrosilylated QxCav was synthetized by adapting a previously published procedure [15].
2.1. Monosilylated QxCav
300 mg (194 µmol, 1 eq) of QxCav with four ω-decenyl feet was dissolved in 30 mL of dry toluene. Four freeze-pump-thaw cycles were performed to degas the solution, and 226 µL of triethoxysilane (95%, 116 mmol, 6 eq) and 75 µL of Karstedt’s catalyst (xylene solution, Pt~2%) were added under nitrogen atmosphere. The mixture was stirred for 48 h at room temperature. Then, the reaction was quenched into 250 mL of methanol and the white solid was filtered and washed with methanol. The product became pale brown after drying (235 mg, 65% yield) (see Figures S2–S6 for characterization).
2.2. Silica Grafting Procedure
500 mg of silica (180–250 µm size) were placed in a two-necked round-bottom flask and dried by heating under vacuum. Monosilylated QxCav (250 mg) was dissolved in the minimum amount of dry toluene (15 mL) and added to the reaction flask. The suspension was kept at 100 °C overnight under gentle stirring. After cooling to 25 °C, the solid was filtered on a sintered glass. The solid residue was abundantly washed first with dichloromethane and then with methanol to remove the unreacted cavitand. The functionalized silica appeared to be brownish, and the weight percentage of the organic layer deposited was quantified as 15% by performing TGA from 30 to 900 °C at 10 °C/min in air (Figure 1).
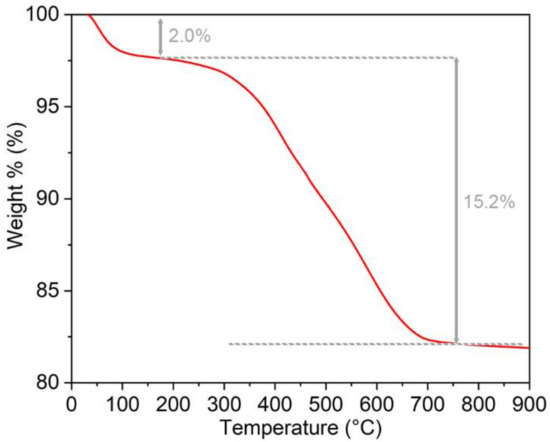
Figure 1.
Thermogravimetric analysis (TGA) of QxCav@SiO2.
2.3. Preconcentrator Testing Protocol
The QxCav@SiO2 preconcentrator was tested in the commercial mini-GC system PyxisGC BTEX, equipped with a MiniPID 2 ppb by ION Science (UK) [5]. The same measurements were conducted on bare silica and pure QxCav as control experiments. Each MEMS cartridge (Figure S1) was filled with 50 mm3 of bare silica, QxCav and QxCav@SiO2, respectively, corresponding to roughly 100 mg of material for silica and QxCav@SiO2 and 50 mg of QxCav. The sampled air contained a mixture of BTEX from a certified cylinder, each of them at a 3-ppb concentration, except for the p-xylene present at a 6-ppb concentration. The air was humidified at 30, 50 and 80% RH to mimic real ambient conditions. A flow of 250 sccm of the humid sampled air was pumped into the preconcentrator for 300 s at 25 °C. At the end of preconcentration, the embedded 3-way mini-valve was switched, connecting the preconcentration unit to the GC pump. The temperature of the MEMS was raised up to 110 °C to promote the desorption of the analytes with an inverted air flux of clean indoor air. The desorbed analytes were injected into the MEMS GC separation column for 40 s. The mini-GC column operated in a temperature ramp from 50 to 185 °C in 140 s in order to maximize the separation of peaks. A typical chromatogram is reported in Figure 2. The detection of each analyte was provided by a photo ionization detector (PID) positioned at the end of the MEMS column. At the end of injection, the preconcentrator was cleaned for 2 min at 110 °C. After cooling the entire apparatus to 25 °C in 120 s, new air sampling began. A single measurement takes 10 min to be completed, enabling a benzene detection limit of 0.8 µg m−3. Longer cycle times allowed for even higher sensitivity by increasing the sampling time (Figure 3a). As shown in Figure 2, all analytes were separated well under these experimental conditions.
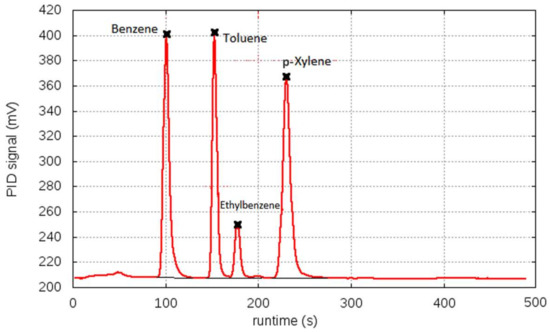
Figure 2.
Example of MEMS mini-GC chromatogram, showing PID signal versus elution time. Sampling performed at 50% RH.
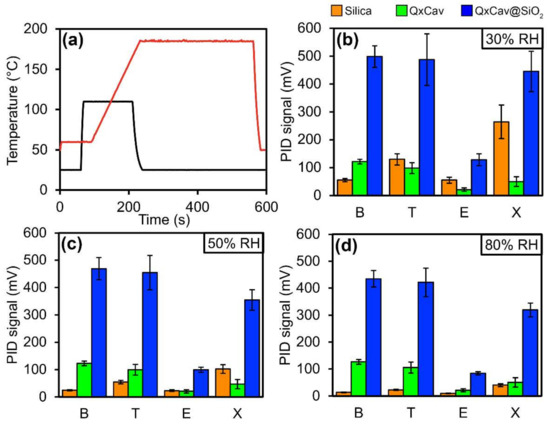
Figure 3.
(a) Temperature profile of preconcentrator (blue) and gas chromatography column (orange). Histograms of PID signals at 30 (b), 50 and (c) 80 (d) relative humidity (RH) for benzene (B, 3 ppb), toluene (T, 3 ppb), ethylbenzene (E, 3 ppb) and p-xylene (X, 6 ppb) with the three absorption phases. Each value is the average of 20 individual measurements.
The elution time was constant for every analysis for all analytes. At least 20 measurements were collected for each combination of experimental parameters and stationary phases. The resulting histograms reported in Figure 3 are the averages of 20 measurements, comparing the heights of the GC peaks for each preconcentration material tested.
3. Results and Discussion
3.1. Preparation and Characterization of the Preconcentrator Material
For the covalent grafting of QxCav on the silica surface, monosilylated QxCav was prepared by adapting previously reported procedures [15,16]. Karstedt’s catalyzed hydrosilylation between the ω-decenyl-footed QxCav and an excess of triethoxysilane afforded a mixture of differently functionalized cavitands (Scheme 2). Its composition was assessed by 1D and 2D nuclear magnetic resonance spectroscopy (NMR, Figures S2–S5), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF, Figure S6) and FT-IR spectroscopy (Figure S7). Although no residual signals ascribable to a terminal double bond were observed in 1H NMR, only mono- and di-functionalized products were observed in the mass spectrum as a consequence of olefin isomerization side reaction in the hydrosilylation protocol. An average of 1.3 silyl groups per cavitand was estimated by 1H NMR signal integration. Surface decoration was performed by reacting the as-obtained mixture in boiling toluene with pre-meshed silica (180–250 µm, Scheme 2). The ratio between the cavitand and silica particles was 1:2 w/w. The weight percentage of the deposited organic layer was quantified to be 15% by TGA (Figure 1). The initial 2% loss observed in the TGA thermogram was ascribable to the release of the cavity-included and surface physisorbed toluene, which was used as a solvent in the silica grafting procedure.
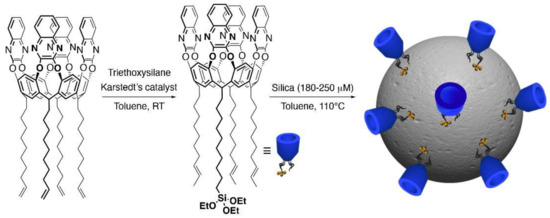
Scheme 2.
Preparation of QxCav@SiO2.
3.2. Uptake and Release Capacity of the Material
The adsorption performances of the three preconcentration materials, namely bare silica, pure QxCav and functionalized silica (QxCav@SiO2), are summarized in the histograms of Figure 3. Each histogram reports the analyses performed at different percentages of relative humidity (RH). In all cases, the QxCav@SiO2 outmatched both the bare silica and pure QxCav in BTEX adsorption by far. The formation of a QxCav monolayer on the silica, with all cavities available for complexation, increased the preconcentrator uptake ability dramatically independent (within the error range) of the relative humidity of the incoming air. This is in sharp contrast with the behavior of the bare silica, whose BTEX physisorption was highly dependent on the relative humidity. The reduced silica retention at a high RH could be attributed to its hydrophilicity; water competes with organic molecules for surface physisorption. Despite the much larger amount of loaded cavitand (50 mg of pure QxCav versus ≈15 mg of QxCav coating the silica, based on TGA analysis), the QxCav preconcentrator was able to adsorb a much lower amount of BTEX. In the QxCav powder, the number of accessible cavities at the air–solid interface was limited, and the cavity orientation at the interface was random. Its RH response was similar to that of QxCav@SiO2 since they both presented hydrophobic cavities.
It is also worth noticing the cavitand bias toward benzene and toluene with respect to ethylbenzene and p-xylene, as evidenced by the chromatogram in Figure 2 and by the histograms in Figure 3b–d [17]. This selectivity was the result of the interaction mode of the aromatic hydrocarbons with the cavity, which was mainly driven by CH–π interactions between the guests’ hydrogens and the cavity’s aromatic walls. Two of them were with the lower part of the cavity, as demonstrated by the molecular mechanic calculations based on the cavitand complexes’ crystal structures [18]. Therefore, the presence of two methyl groups, as in p-xylene, destabilized the complexation. On the other hand, substituents larger than methyl, as in the case of ethylbenzene, introduced steric hindrance at the mouth of the cavity, thus jeopardizing the complexation. The preferential inclusion of B and T was pivotal for quantitative detection of carcinogenic benzene in air.
Regarding the particle size shrinking, no impedance increase was detected during the entire QxCav@SiO2 preconcentrator set of experiments, thus supporting the dimensional stability of the coated silica particles under service conditions.
4. Conclusions
In summary, grafting the QxCav onto precisely sized silica particles via hydrosilylation allowed the full exposure of the receptor cavities to the analyte flow. The remarkable selectivity exhibited by the QxCav cavity allowed the preferential uptake of benzene and toluene in the presence of overwhelming amounts of water in air. The combined effect of complete cavity exposure to the gas phase and its intrinsic selectivity led to a remarkable increase in BTEX uptake. The resulting QxCav@SiO2 preconcentrator showed superior properties in benzene detection with respect to the preconcentrator filled with the pure QxCav powder and the unfunctionalized silica particles. These unique properties of QxCav@SiO2 make it the preconcentrator of choice for benzene sensing in outdoor and indoor air. Furthermore, this miniaturized MEMS preconcentration unit is amenable to be interfaced with other transducers, like metal oxide semiconductors (MOSs) [19] or ion mobility spectrometers (IMSs) [20,21].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano12132204/s1: general experimental methods comprising MEMS device geometries (Figure S1), the NMR spectra (Figures S2–S5) and MALDI-TOF spectrum (Figure S6) of monosilylated QxCav, the IR spectra (Figure S7) and scanning electron microscopy (SEM) images (Figures S8 and S9) of tested materials and the preconcentrator testing conditions.
Author Contributions
Conceptualization, R.P. and E.D.; cavitands synthesis, A.R.; validation, A.P. and R.P.; sensor measurements, E.C.; MEMS production, I.E.; experimental set-up, S.Z.; data curation, A.R. and S.Z.; writing—original draft preparation, A.R. and A.P.; writing—review and editing, R.P. and E.D.; visualization, A.R. and A.P.; supervision, E.D.; funding acquisition, R.P. and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge Regione Emilia Romagna for financial support through the POR FSE Emilia Romagna 2014/2020 Bando Alte Competenze project (Air Quality Monitoring). This work benefited from the equipment and framework of the COMP-HUB Initiative, funded by the “Departments of Excellence” program of the Italian Ministry for Education, University and Research (MIUR, 2018–2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Centro Interfacoltà di Misure “G. Casnati” of the University of Parma for the use of NMR facilities. Davide Orsi is warmly acknowledged for the SEM analyses.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Lahlou, H.; Vilanova, X.; Correig, X. Gas phase micro-preconcentrators for benzene monitoring: A review. Actuators B Chem. 2013, 176, 198–210. [Google Scholar] [CrossRef]
- Stenehjem, J.S.; Kjærheim, K.; Bråtveit, M.; Samulesen, S.O.; Barone-Adesi, F.; Rothman, N.; Lan, Q.; Grimsrud, T.H. Benzene exposure and risk of lymphohaematopoietic cancers in 25 000 offshore oil industry workers. Br. J. Cancer 2015, 112, 1603–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Environmental gas sensing with cavitands. Chem. Eur. J. 2018, 24, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pollution.it/en/?product=pyxis-gc-btex&noredirect=en-US (accessed on 20 April 2022).
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Available online: https://ec.europa.eu/environment/air/quality/existing_leg.htm (accessed on 25 May 2022).
- Zampolli, S.; Elmi, I.; Mancarella, F.; Betti, P.; Dalcanale, E.; Cardinali, G.C.; Severi, M. Real-time monitoring of sub-ppb concentrations of aromatic volatiles with a MEMS-enabled miniaturized gas-chromatograph. Actuators B Chem. 2009, 141, 322–328. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Motta, A.; Favazza, M.; Gurrieri, E.; Betti, P.; Dalcanale, E. Molecular recognition of halogen-tagged aromatic VOCs at the air–silicon interface. Chem. Commun. 2010, 46, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Tudisco, C.; Fragalà, M.E.; Giuffrida, A.E.; Bertani, F.; Pinalli, R.; Dalcanale, E.; Compagnini, G.; Condorelli, G.G. Hierarchical Route for the Fabrication of Cavitand-Modified Nanostructured ZnO Fibers for Volatile Organic Compound Detection. J. Phys. Chem. C 2016, 120, 12611–12617. [Google Scholar] [CrossRef]
- Soncini, P.; Bonsignore, S.; Dalcanale, E.; Ugozzoli, F. Cavitands as Versatile Molecular Receptors. J. Org. Chem. 1992, 57, 4608–4612. [Google Scholar] [CrossRef]
- Vincenti, M.; Dalcanale, E. Host-guest Complexation in the Gas Phase. Investigation of the Mechanism of Interaction between Cavitands and Neutral Guest Molecules. J. Chem. Soc. Perkin Trans. 1995, 2, 1069–1076. [Google Scholar] [CrossRef]
- Fanizza, E.; Depalo, N.; Fedorenko, S.; Iacobazzi, R.M.; Mukhametshina, A.; Zairov, R.; Salatino, A.; Vischio, F.; Panniello, A.; Laquintana, V.; et al. Green Fluorescent Terbium (III) Complex Doped Silica Nanoparticles for TSPO Targeting. Int. J. Mol. Sci. 2019, 20, 3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone Calix[4]arene Derivatives—A New Type of Versatile Ligands for Metal Complexes and Nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Motta, A.; Favazza, M.; Fragalà, I.L.; Busi, M.; Menozzi, E.; Dalcanale, E.; Cristofolini, L. Grafting Cavitands on the Si(100) Surface. Langmuir 2006, 22, 11126–11133. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Pinalli, R.; Ugozzoli, F.; Spera, S.; Careri, M.; Dalcanale, E. Cavitands as superior sorbents for benzene detection at trace level. New J. Chem. 2003, 27, 502–509. [Google Scholar] [CrossRef]
- Bianchi, F.; Mattarozzi, M.; Betti, P.; Bisceglie, F.; Careri, M.; Mangia, A.; Sidisky, L.; Ongarato, S.; Dalcanale, E. Innovative cavitand-based sol-gel coatings for the environmental monitoring of benzene and chlorobenzenes via solid-phase microextraction. Anal. Chem. 2008, 80, 6423–6430. [Google Scholar] [CrossRef]
- The PID Response Factors for the Four Aromatic Analytes Tested are Comparable; Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg MD, USA, 2008; p. 20899. Available online: http://webbook.nist.gov (accessed on 25 May 2022).
- Trzciński, W.; Pinalli, R.; Riboni, N.; Pedrini, A.; Bianchi, F.; Zampolli, S.; Elmi, I.; Massera, C.; Ugozzoli, F.; Dalcanale, E. In Search of the Ultimate Benzene Sensor: The EtQxBox Solution. ACS Sens. 2017, 2, 590–598. [Google Scholar] [CrossRef] [PubMed]
- El Mohajir, A.; Castro-Gutiérrez, J.; Sehn Canevesi, R.L.; Bezverkhyy, I.; Weber, G.; Bellat, J.-P.; Berger, F.; Celzard, A.; Fierro, V.; Sanchez, J.-B. Novel Porous Carbon Material for the Detection of Traces of Volatile Organic Compounds in Indoor Air. ACS Appl. Mater. Interfaces 2021, 13, 40088–40097. [Google Scholar] [CrossRef]
- Martin, M.; Crain, M.; Walsh, K.; McGill, R.A.; Houser, E.; Stepnowski, J.; Stepnowski, S.; Wu, H.-D.; Ross, S. Microfabricated vapor preconcentrator for portable ion mobility spectroscopy. Actuators B Chem. 2007, 126, 447–454. [Google Scholar] [CrossRef]
- Liedtke, S.; Zampolli, S.; Elmi, I.; Masini, L.; Barboza, T.; Dalcanale, E.; Pinalli, R.; Pählerd, M.; Drees, C.; Vautz, W. Hyphenation of a MEMS based pre-concentrator and GC-IMS. Talanta 2019, 191, 141–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).