Light-to-Heat Converting ECM-Mimetic Nanofiber Scaffolds for Neuronal Differentiation and Neurite Outgrowth Guidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Preparation of Nanofibrous Scaffolds
2.4. Synthesis of TNPs
2.5. Preparation of TNPs-Modified Nanofibers
2.6. Cell Culture
2.6.1. Rat Hippocampal Neurons
2.6.2. Human Neuroblastoma Cells SH-SY5Y
2.7. Cell Viability Assay
2.8. Neuronal Differentiation of SH-SY5Y Cells
2.9. Morphological Assay of Rat Hippocampal Neuron
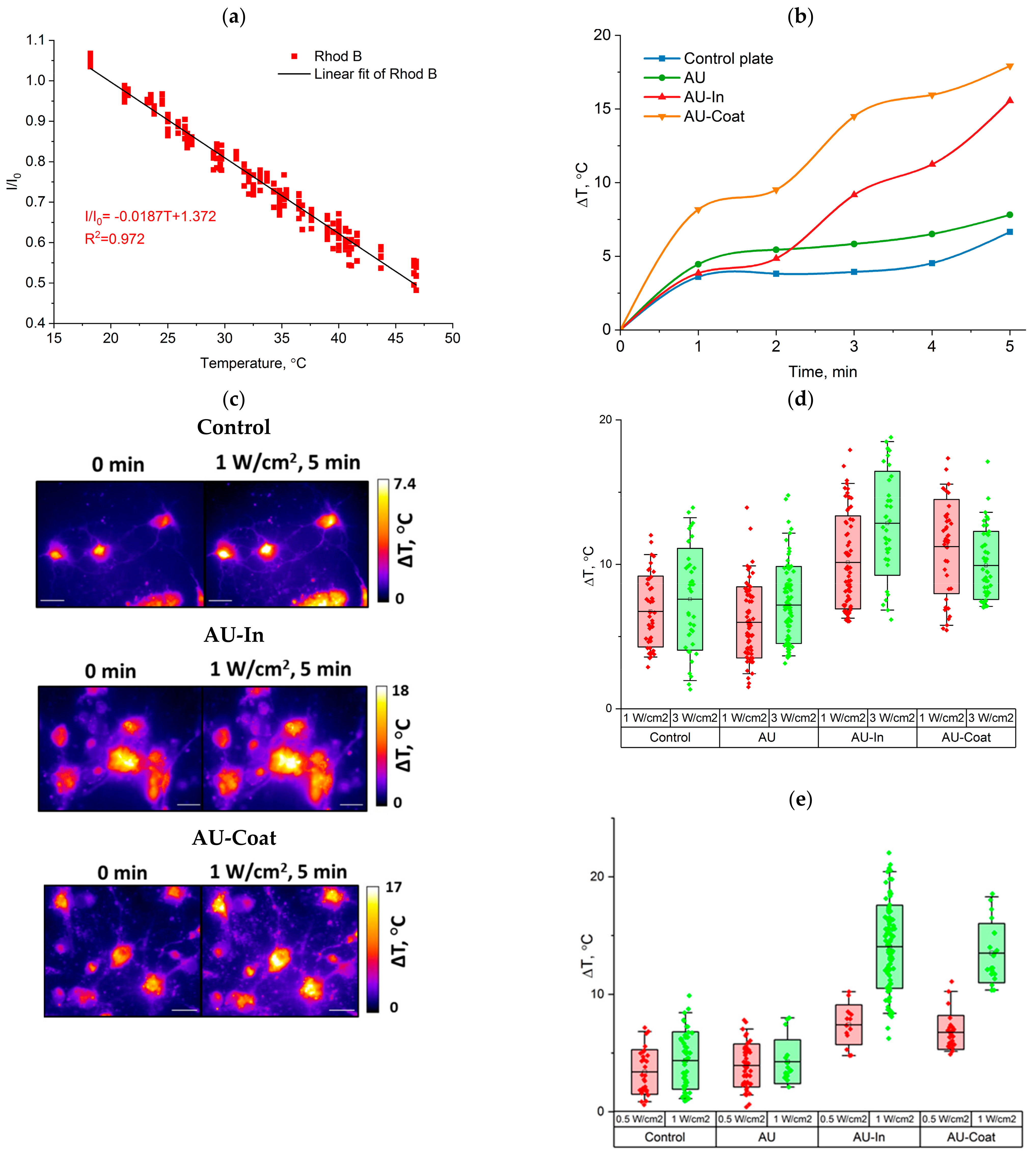
2.10. Photothermal Performance
2.10.1. Micro-Scale Measurement of Plasmonic Nanofiber Temperature during NIR Stimulation
2.10.2. Intracellular Temperature Detection
2.11. Statistics
3. Results
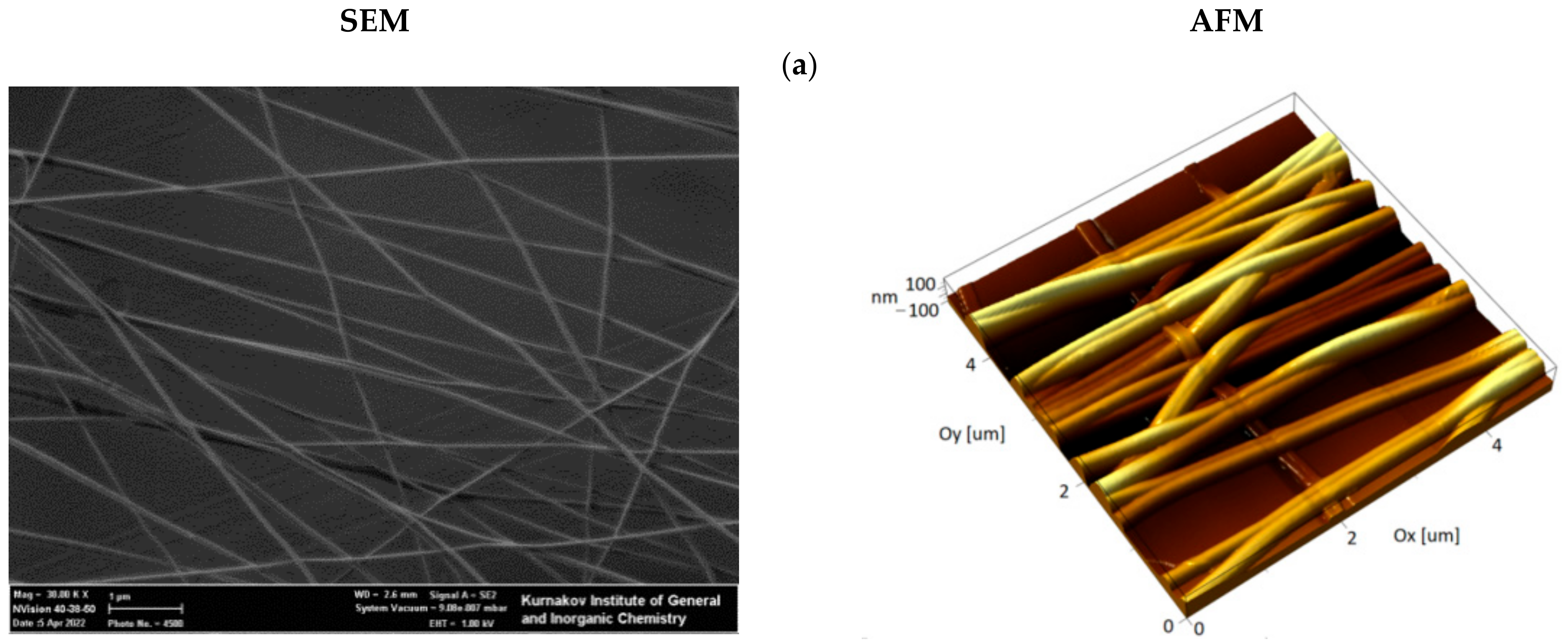
3.1. Preparation and Ultrastructural Analysis of the Hybrid Fibrous Materials
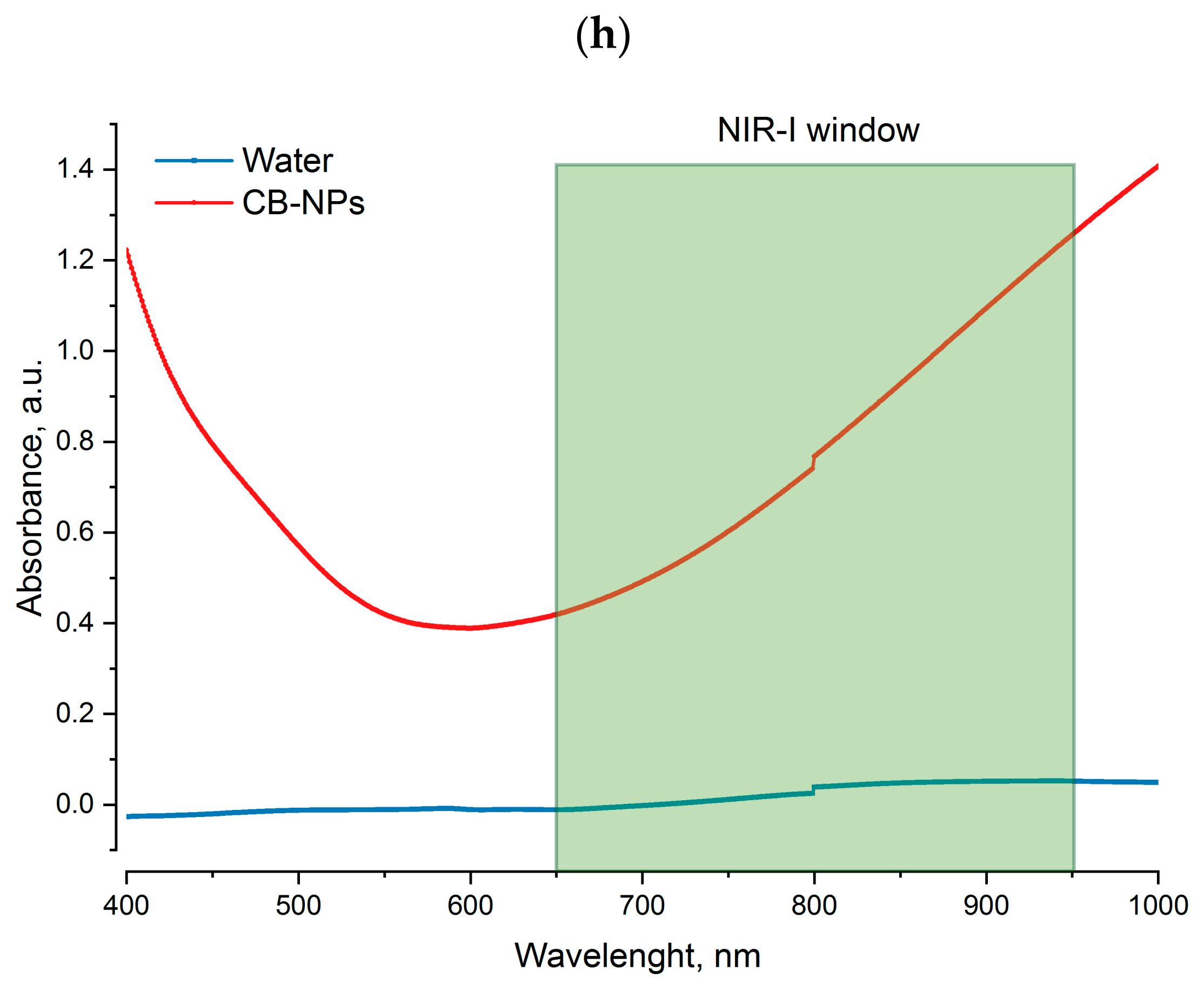
3.2. Photothermal Properties of Nanofibers
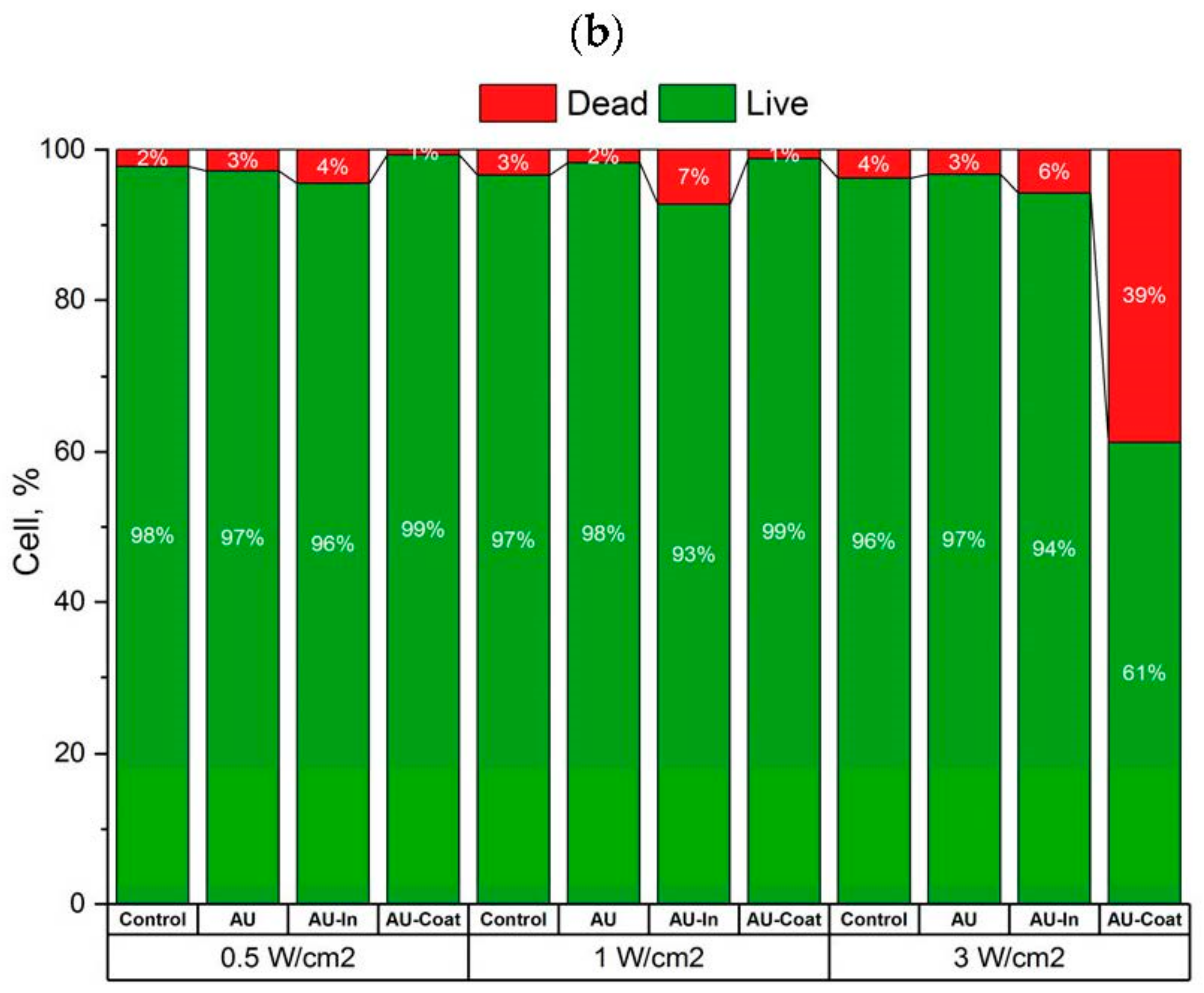
3.3. Effects of Photothermal Stimulation on Cell Viability of the SH-SY5Y Human Neuroblastome Cells
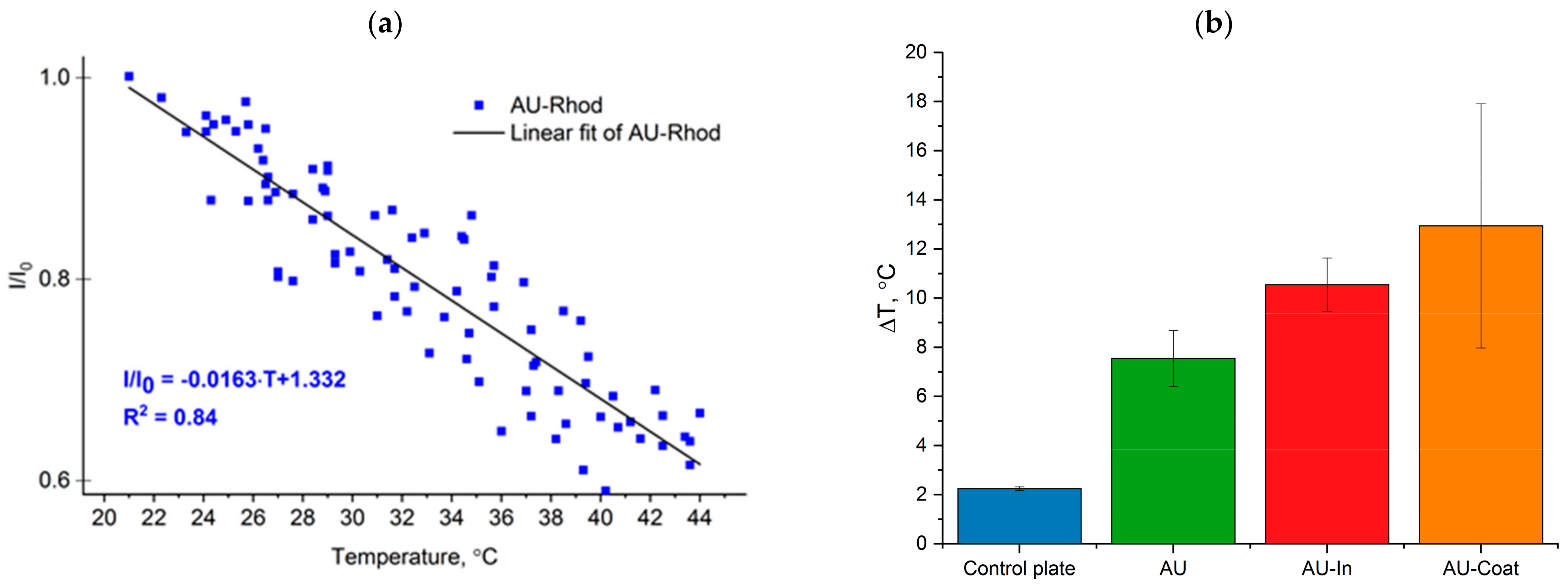
3.4. Heating Efficiency of Light-to-Heat Converting Scaffolds under NIR Irradiation and Measurement of Intracellular Temperature of Hippocampal Neurons
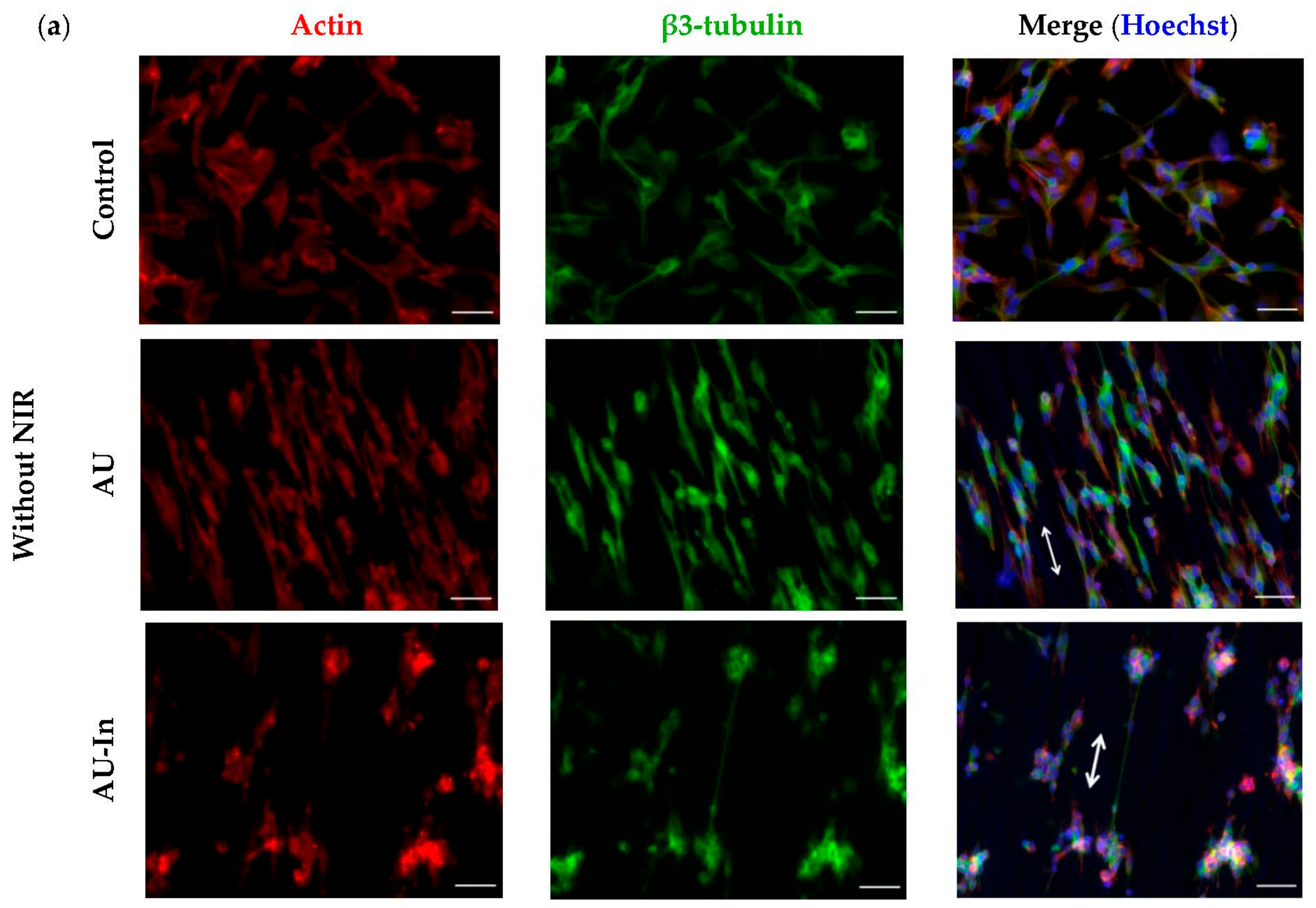
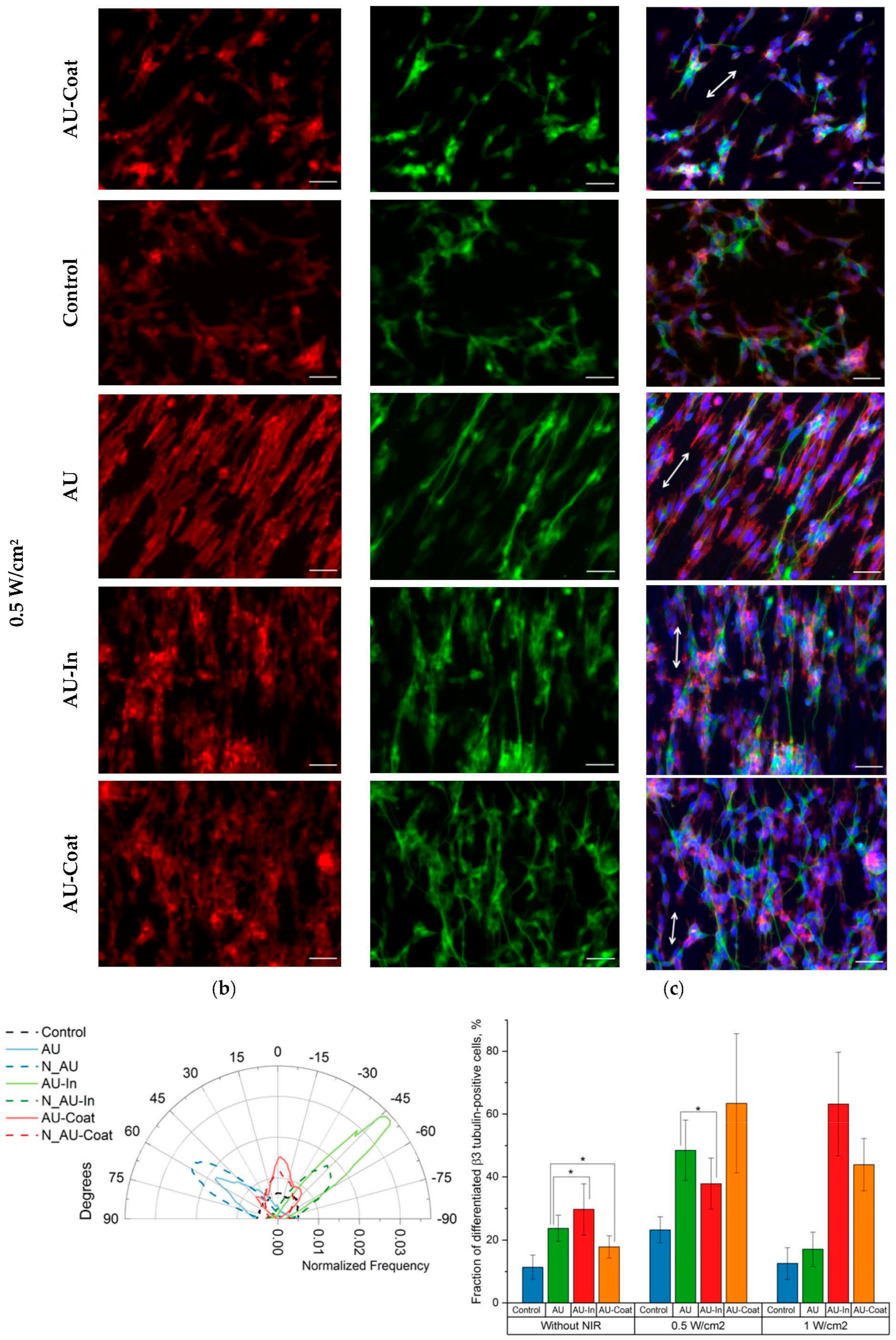
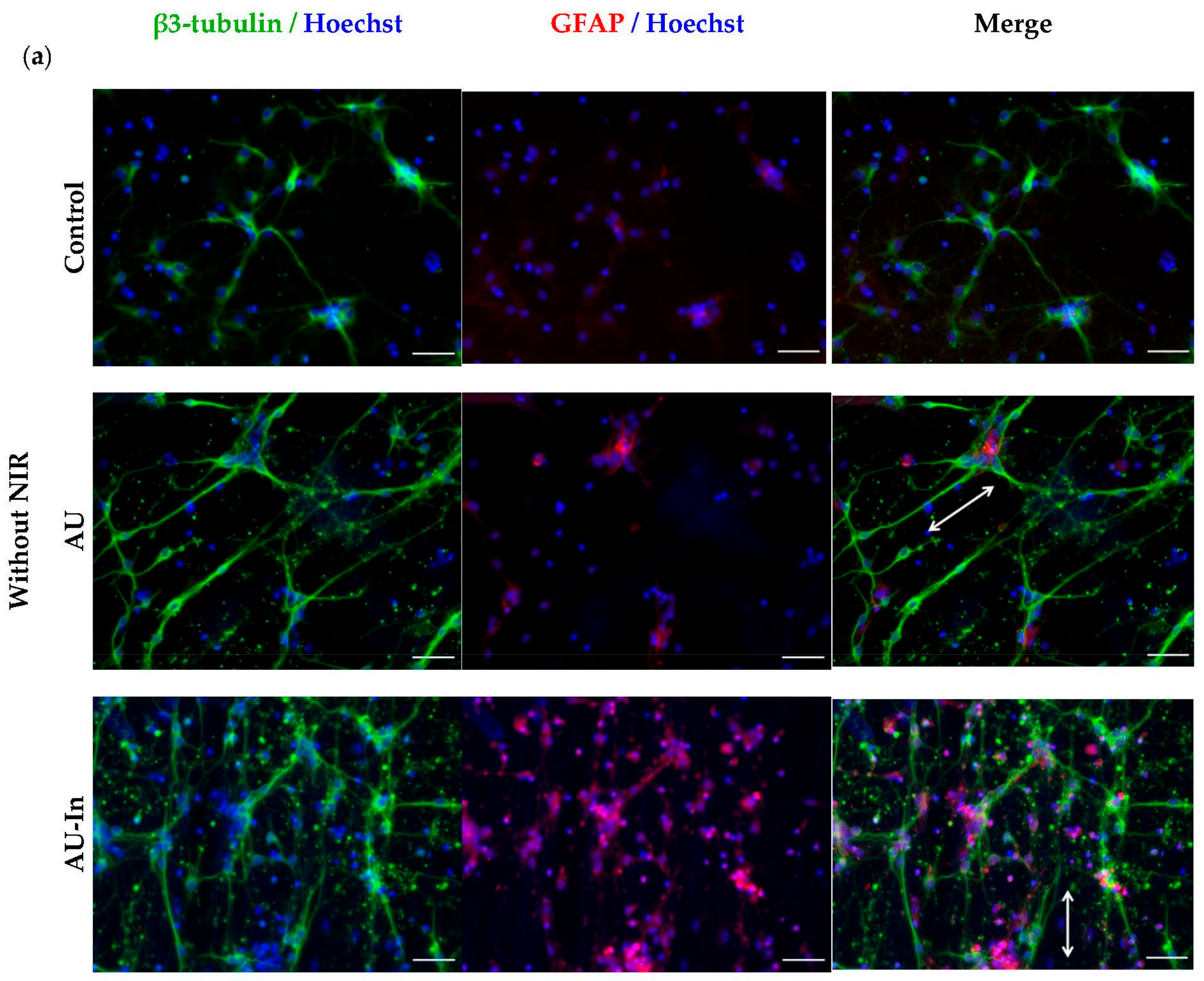
3.5. Effect of Photothermal Stimulation by Light-to-Heat Converting ECM-Mimetic Scaffolds on Neuronal Differentiation, Neurite Outgrowth and Elongation of Human Neuroblastoma Cell Line SH-SY5Y
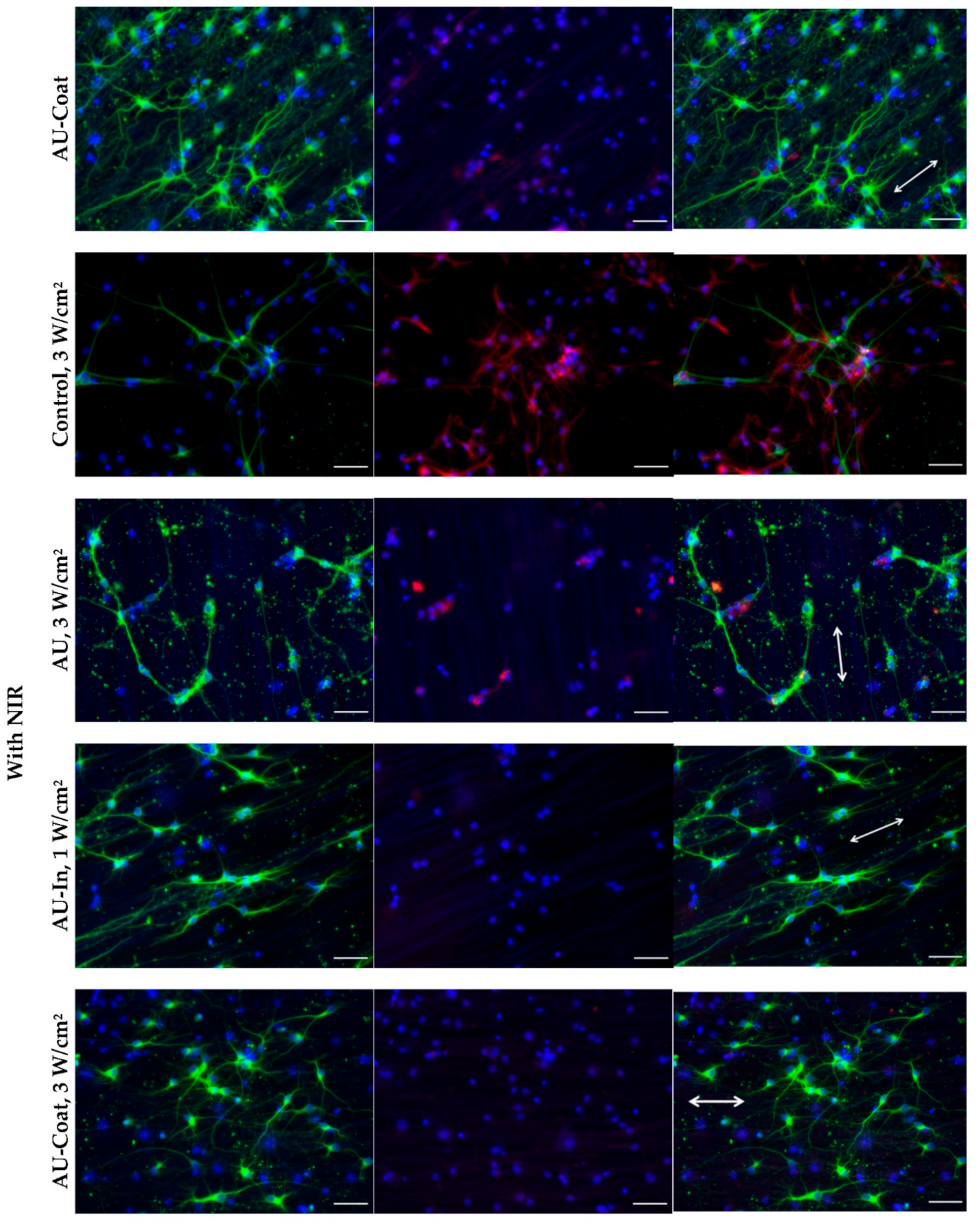
3.6. Effect of Photothermal Stimulation and Scaffolds Nanostructural Features on the Neurite elongation, Orientation and Branching of Hippocampal Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; SAbdalla, M.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, S.; Kowalewski, T.A.; Pierini, F. Fibrous polymer nanomaterials for biomedical applications and their transport by fluids: An overview. Soft Matter 2018, 14, 8421–8444. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, C.; Chen, X.; Xue, W.; Hutchins-Crawford, H.J.; Yu, Q.; Topham, P.D.; Wang, L. A review on electrospun magnetic nanomaterials: Methods, properties and applications. J. Mater. Chem. C 2021, 9, 9042–9082. [Google Scholar] [CrossRef]
- Azizan, A.; Samsudin, A.A.; Baharin, M.B.S.; Dzulkiflee, M.H.; Rosli, N.R.; Abu Bakar, N.F.; Adlim, M. Cellulosic fiber nanocomposite application review with zinc oxide antimicrobial agent nanoparticle: An opt for COVID-19 purpose. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Máková, V.; Holubová, B.; Krabicová, I.; Kulhánková, J.; Řezanka, M. Hybrid organosilane fibrous materials and their contribution to modern science. Polymer 2021, 228, 123862. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Wu, L.; Zheng, T.; Han, Q.; Liang, Y.; Zhang, L.; Li, G.; Yang, Y. The Influence of the Surface Topographical Cues of Biomaterials on Nerve Cells in Peripheral Nerve Regeneration: A Review. Stem Cells Int. 2021, 2021, 8124444. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional hybrid materials from poly(3-hydroxybutyrate), TiO2 nanoparticles, and chitosan oligomers by combining electrospinning/electrospraying and impregnation. Macromol. Biosci. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S. Electrospinning of continuous aligned polymer fibers. Appl. Phys. Lett. 2004, 84, 1222. [Google Scholar] [CrossRef]
- Montero, R.B.; Vial, X.; Nguyen, D.T.; Farhand, S.; Reardon, M.; Pham, S.M.; Tsechpenakis, G.; Andreopoulos, F.M. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012, 8, 1778–1791. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix—A review. Bioact. Mater. 2021, 15, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Behtaj, S.; Ekberg, J.A.K.; St John, J.A. Advances in Electrospun Nerve Guidance Conduits for Engineering Neural Regeneration. Pharmaceutics 2022, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Tiwari, A.P.; Chen, N.; Ramakrishna, S.; Yang, I.H. Development of an Axon-Guiding Aligned Nanofiber-Integrated Compartmentalized Microfluidic Neuron Culture System. ACS Appl. Bio Mater. 2021, 4, 8424–8432. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Mattiassi, S.; Goh, E.L.; Yim, E.K.F. Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation. Neural Regen. Res. 2020, 15, 573–585. [Google Scholar] [CrossRef]
- Dubey, S.; Shivahare, R.; Sharma, G.T. Nanomaterials, Neural Stem Cells, and the Path to Neural Tissue Engineering. In Engineered Nanomaterials for Innovative Therapies and Biomedicine; Sarma, H., Gupta, S., Narayan, M., Prasad, R., Krishnan, A., Eds.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Sarma, H.; Gupta, S.; Narayan, M.; Prasad, R.; Krishnan, A. Engineered Nanomaterials for Innovative Therapies and Biomedicine; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Hwang, T.I.; Oh, J.M.; Maharjan, B.; Chun, S.; Kim, B.S.; Joshi, M.K.; Park, C.H.; Kim, C.S. pH/NIR-Responsive Polypyrrole-Functionalized Fibrous Localized Drug-Delivery Platform for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 20256–20270. [Google Scholar] [CrossRef]
- Demir, U.S.; Shahbazi, R.; Calamak, S.; Ozturk, S.; Gultekinoglu, M.; Ulubayram, K. Gold nano-decorated aligned polyurethane nanofibers for enhancement of neurite outgrowth and elongation. J. Biomed. Mater. Res. A 2018, 106, 1604–1613. [Google Scholar] [CrossRef]
- Khan, F.A.; Almohazey, D.; Alomari, M.; Almofty, S.A. Impact of nanoparticles on neuron biology: Current research trends. Int. J. Nanomed. 2018, 13, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Polak, P.; Shefi, O. Nanometric agents in the service of neuroscience: Manipulation of neuronal growth and activity using nanoparticles. Nanomedicine 2015, 11, 1467–1479. [Google Scholar] [CrossRef]
- Paviolo, C.; Thompson, A.C.; Yong, J.; Brown, W.G.; Stoddart, P.R. Nanoparticle-enhanced infrared neural stimulation. J. Neural. Eng. 2014, 11, 065002. [Google Scholar] [CrossRef]
- Paviolo, C.; Stoddart, P.R. Metallic nanoparticles for peripheral nerve regeneration: Is it a feasible approach? Neural Regen. Res. 2015, 10, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Needham, K.; Brown, W.G.A.; Yong, J.; Stoddart, P.R. Stimulation of Primary Auditory Neurons Mediated by Near-Infrared Excitation of Gold Nanorods. In Use of Nanoparticles in Neuroscience. Neuromethods; Santamaria, F., Peralta, X., Eds.; Humana Press: New York, NY, USA, 2018; p. 135. [Google Scholar] [CrossRef]
- Migliori, B.; Di Ventra, M.; Kristan, W., Jr. Photoactivation of neurons by laser-generated local heating. AIP Adv. 2012, 2, 032154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Lu, K.; Zheng, Y.; Huang, C.; Wang, G.; Zhang, Y.; Yu, Q. Photothermal scaffolds/surfaces for regulation of cell behaviors. Bioact. Mater. 2021, 8, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Han, Y.; Wang, X.; Qin, C.; Zhai, D.; Yi, Z.; Chang, J.; Xiao, Y.; Wu, C. Copper silicate hollow microspheres-incorporated scaffolds for chemo-photothermal therapy of melanoma and tissue healing. ACS Nano 2018, 12, 2695–2707. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Thang, D.C.; Wang, Z.; Lu, X.; Xing, B. Precise cell behaviors manipulation through light-responsive nano-regulators: Recent advance and perspective. Theranostics 2019, 9, 3308–3340. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, H.; Nam, Y. Thermo-plasmonic gold nanofilms for simple and mass-producible photothermal neural interfaces. Nanoscale 2018, 10, 9226–9235. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, H.; Cho, H.H.; Lee, J.H.; Nam, Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials 2018, 153, 59–69. [Google Scholar] [CrossRef]
- Kang, H.; Lee, G.H.; Jung, H.; Lee, J.W.; Nam, Y. Inkjet-Printed Biofunctional Thermo-Plasmonic Interfaces for Patterned Neuromodulation. ACS Nano 2018, 12, 1128–1138. [Google Scholar] [CrossRef]
- Jung, S.; Harris, N.; Niyonshuti, I.I.; Jenkins, S.V.; Hayar, A.M.; Watanabe, F.; Jamshidi-Parsian, A.; Chen, J.; Borrelli, M.J.; Griffin, R.J. Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation. Nanomaterials 2021, 11, 1216. [Google Scholar] [CrossRef]
- Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E.; et al. Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 35782–35798. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Garg, R.; Scopelliti, M.G.; Pinto, B.I.; Hartung, J.E.; Kim, S.; Murphey, C.G.E.; Johnson, N.; San Roman, D.; Bezanilla, F.; et al. Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. Proc. Natl. Acad. Sci. USA 2020, 117, 13339–13349. [Google Scholar] [CrossRef] [PubMed]
- Zare, I.; Yaraki, M.T.; Speranza, G.; Najafabadi, A.H.; Shourangiz-Haghighi, A.; Nik, A.B.; Manshian, B.B.; Saraiva, C.; Soenen, S.J.; Kogan, M.J.; et al. Gold nanostructures: Synthesis, properties, and neurological applications. Chem. Soc. Rev. 2022, 51, 2601–2680. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jiang, X.; Wei, Y.; Wang, Q.; Cui, K.; Xu, X.; Wang, F.; Zhang, L. Application of phototherapeutic-based nanoparticles in colorectal cancer. Int. J. Biol. Sci. 2021, 17, 1361–1381. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, Y.; Ke, H.; Wang, Q.; Lv, X.; Wu, H.; Tang, Y.; Yang, X.; Chen, C.; Zhao, Y.; et al. Protein-Nanoreactor-Assisted Synthesis of Semiconductor Nanocrystals for Efficient Cancer Theranostics. Adv. Mater. 2016, 28, 5923–5930. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, J.; Liu, Q.; Chen, L.; Shi, K.; Han, R.; Yang, Q.; Zhong, L.; Zha, R.; Qu, Y.; et al. Ultrasmall CuS@BSA nanoparticles with mild photothermal conversion synergistically induce MSCs-differentiated fibroblast and improve skin regeneration. Theranostics 2020, 10, 1500–1513. [Google Scholar] [CrossRef]
- Poudel, K.; Gautam, M.; Jin, S.G.; Choi, H.G.; Yong, C.S.; Kim, J.O. Copper sulfide: An emerging adaptable nanoplatform in cancer theranostics. Int. J. Pharm. 2019, 562, 135–150. [Google Scholar] [CrossRef]
- Nagarajan, S.; Balme, S.; Kalkura, N.S.; Miele, P.; Bohatier, C.P.; Bechelany, M. Various Techniques to Functionalize Nanofibers. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Antonova, O.Y.; Kochetkova, O.Y.; Shlyapnikov, Y.M. ECM-Mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials 2021, 11, 516. [Google Scholar] [CrossRef]
- Antonova, O.Y.; Kochetkova, O.Y.; Kanev, I.L.; Shlyapnikova, E.A.; Shlyapnikov, Y.M. Rapid Generation of Neurospheres from Hippocampal Neurons Using Extracellular-Matrix-Mimetic Scaffolds. ACS Chem. Neurosci. 2021, 12, 2838–2850. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.Y.; Zhang, X.; Yu, C.; Zhao, Y.; Sun, S.K. BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo. Dalton Trans. 2015, 44, 13112–13118. [Google Scholar] [CrossRef]
- Salazar, I.L.; Mele, M.; Caldeira, M.V.; Costa, R.O.; Correia, B.; Frisari, S.; Duarte, C.B. Preparation of Primary Cultures of Embryonic Rat Hippocampal and Cerebrocortical Neurons. Bio-Protocol 2017, 7, e2551. [Google Scholar] [CrossRef] [PubMed]
- Kitazono, S.; Takiguchi, Y.; Ashinuma, H.; Saito-Kitazono, M.; Kitamura, A.; Chiba, T.; Sakaida, E.; Sekine, I.; Tada, Y.; Kurosu, K.; et al. Effect of metformin on residual cells after chemotherapy in a human lung adenocarcinoma cell line. Int. J. Oncol. 2013, 43, 1846–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, D.; Lefort, C.; Burke, R.; Leveque, P.; O’Connor, R.P. Rhodamine B as an optical thermometer in cells focally exposed to infrared laser light or nanosecond pulsed electric fields. Biomed. Opt. Express 2015, 6, 4105–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced Near-Infrared Light for Monitoring and Modulating the Spatiotemporal Dynamics of Cell Functions in Living Systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef] [Green Version]
- Yamano, N.; Kawasaki, N.; Ida, S.; Nakayama, Y.; Nakayama, A. Biodegradation of polyamide 4 in vivo. Polym. Degrad. Stab. 2017, 137, 281–288. [Google Scholar] [CrossRef]
- Wang, X.; Lv, F.; Li, T.; Han, Y.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Electrospun Micropatterned Nanocomposites Incorporated with Cu2S Nanoflowers for Skin Tumor Therapy and Wound Healing. ACS Nano 2017, 11, 11337–11349. [Google Scholar] [CrossRef]
- Shao, J.; Xie, H.; Wang, H.; Zhou, W.; Luo, Q.; Yu, X.F.; Chu, P.K. 2D Material-Based Nanofibrous Membrane for Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1155–1163. [Google Scholar] [CrossRef]
- Zhong, Z.; Wingert, M.C.; Strzalka, J.; Wang, H.H.; Sun, T.; Wang, J.; Chen, R.; Jiang, Z. Structure-induced enhancement of thermal conductivities in electrospun polymer nanofibers. Nanoscale 2014, 6, 8283–8291. [Google Scholar] [CrossRef]
- Yang, C.; Park, S. Nanomaterials-assisted thermally induced neuromodulation. Biomed. Eng. Lett. 2021, 11, 163–170. [Google Scholar] [CrossRef]
- Okabe, K.; Sakaguchi, R.; Shi, B.; Kiyonaka, S. Intracellular thermometry with fluorescent sensors for thermal biology. Pflugers Arch-Eur. J. Physiol. 2018, 470, 717–731. [Google Scholar] [CrossRef]
- Chrétien, D.; Bénit, P.; Ha, H.H.; Keipert, S.; El-Khoury, R.; Chang, Y.T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Yi, N.; Park, J.; Kim, D. Thermal conductivity of single biological cells and relation with cell viability. Appl. Phys. Lett. 2013, 102, 203702. [Google Scholar] [CrossRef] [Green Version]
- ElAfandy, R.T.; AbuElela, A.F.; Mishra, P.; Janjua, B.; Oubei, H.M.; Büttner, U.; Majid, M.A.; Ng, T.K.; Merzaban, J.S.; Ooi, B.S. Nanomembrane-Based, Thermal-Transport Biosensor for Living Cells. Small 2017, 13, 1603080. [Google Scholar] [CrossRef] [Green Version]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; Stoddart, P.R.; McArthur, S.L. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 2013, 110, 2277–2291. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A. Near infrared laser stimulation of human neural stem cells into neurons on graphene nanomesh semiconductors. Colloids Surf. B Biointerfaces 2015, 126, 313–321. [Google Scholar] [CrossRef]
- Alghazali, K.; Hamzah, R.; Nima, Z.; Steiner, R.; Dhar, M.; Anderson, D.; Hayar, A.; Griffin, R.; Biris, A. Plasmonic Nanofactors as Switchable Devices to Promote or Inhibit Neuronal Activity and Function. Nanomaterials 2019, 9, 1029. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, H.; Wei, H.; Cheng, H.; Cai, J.; Chen, X.; Xia, L.; Wang, H.; Chai, R. Scaffolds with anisotropic structure for neural tissue engineering. Eng. Regen. 2022, 3, 154–162. [Google Scholar] [CrossRef]
- Jing, W.; Ao, Q.; Wang, L.; Huang, Z.; Cai, Q.; Chen, G.; Yang, X.; Zhong, W. Constructing conductive conduit with conductive fibrous infilling for peripheral nerve regeneration. Chem. Eng. J. 2018, 345, 566–577. [Google Scholar] [CrossRef]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef]
- Oyama, K.; Zeeb, V.; Kawamura, Y.; Arai, T.; Gotoh, M.; Itoh, H.; Itabashi, T.; Suzuki, M.; Ishiwata, S. Triggering of high-speed neurite outgrowth using an optical microheater. Sci. Rep. 2015, 5, 16611. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, O.Y.; Kochetkova, O.Y.; Kanev, I.L. Light-to-Heat Converting ECM-Mimetic Nanofiber Scaffolds for Neuronal Differentiation and Neurite Outgrowth Guidance. Nanomaterials 2022, 12, 2166. https://doi.org/10.3390/nano12132166
Antonova OY, Kochetkova OY, Kanev IL. Light-to-Heat Converting ECM-Mimetic Nanofiber Scaffolds for Neuronal Differentiation and Neurite Outgrowth Guidance. Nanomaterials. 2022; 12(13):2166. https://doi.org/10.3390/nano12132166
Chicago/Turabian StyleAntonova, Olga Yu., Olga Yu. Kochetkova, and Igor L. Kanev. 2022. "Light-to-Heat Converting ECM-Mimetic Nanofiber Scaffolds for Neuronal Differentiation and Neurite Outgrowth Guidance" Nanomaterials 12, no. 13: 2166. https://doi.org/10.3390/nano12132166
APA StyleAntonova, O. Y., Kochetkova, O. Y., & Kanev, I. L. (2022). Light-to-Heat Converting ECM-Mimetic Nanofiber Scaffolds for Neuronal Differentiation and Neurite Outgrowth Guidance. Nanomaterials, 12(13), 2166. https://doi.org/10.3390/nano12132166






