Potassium Chloroaurate-Mediated In Vitro Synthesis of Gold Nanoparticles Improved Root Growth by Crosstalk with Sucrose and Nutrient-Dependent Auxin Homeostasis in Arabidopsis thaliana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Quantification of the Morphological Traits
2.3. Transmission Electron Microscopy (TEM)
2.4. UV–Vis Spectroscopy
2.5. Reporter Gene Assay
2.6. qRT-PCR Analysis
2.7. Statistical Analysis
3. Results and Discussion
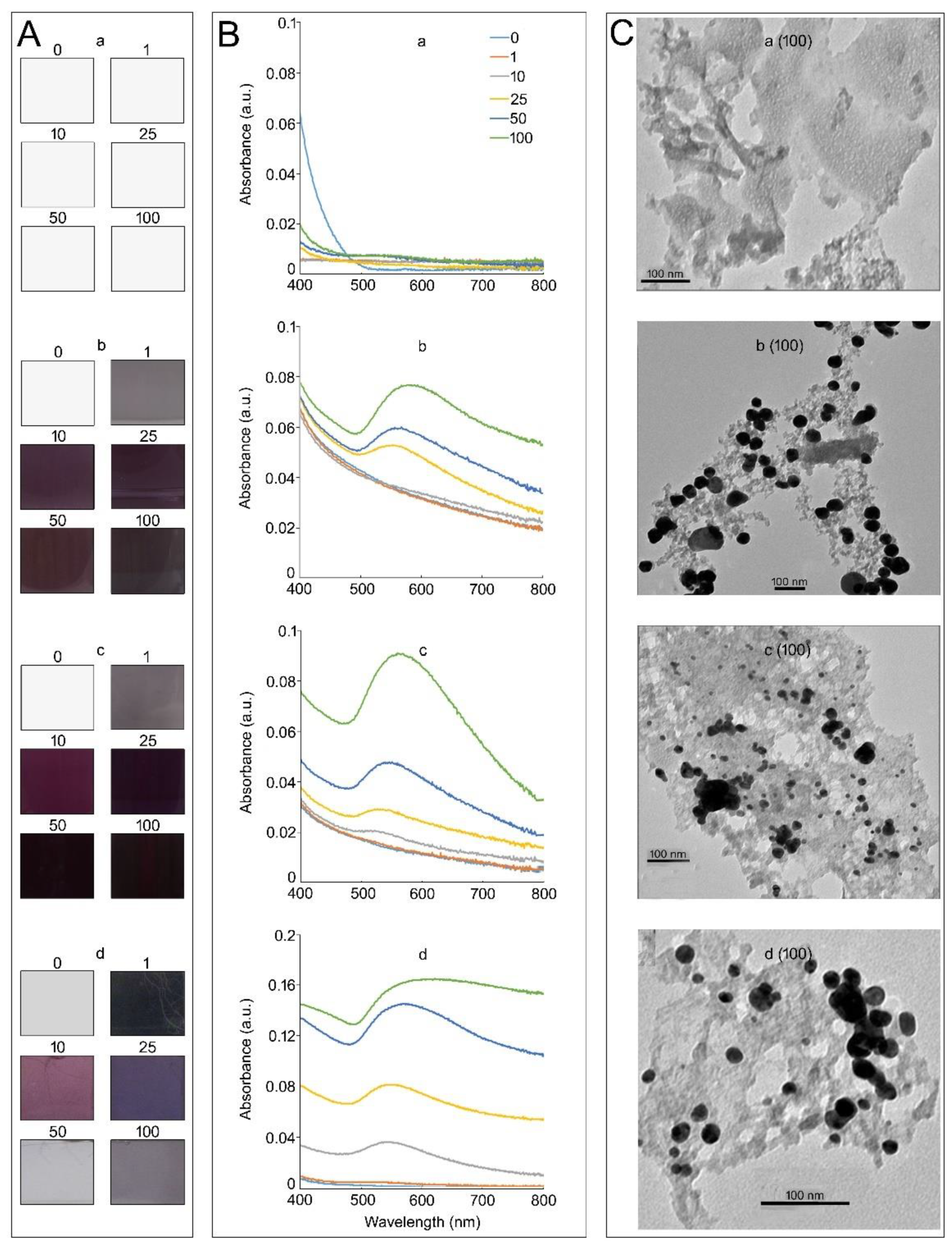
3.1. Medium Composition Affects the Properties of KAuCl4-Mediated In Vitro Synthesis of AuNPs in a Hydroponic System
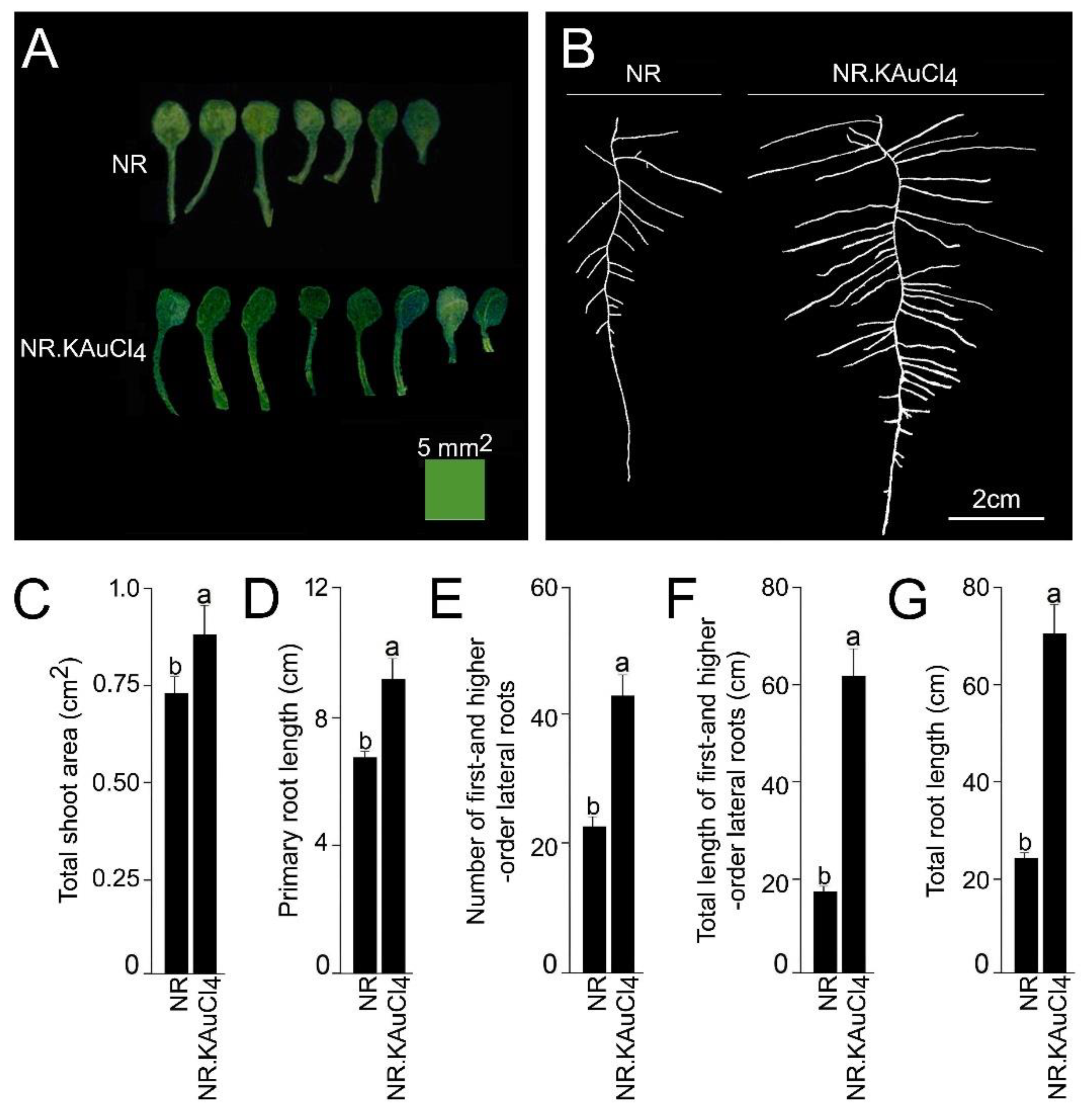
3.2. KAuCl4 Triggers a Dosage-Dependent Augmented Growth Response
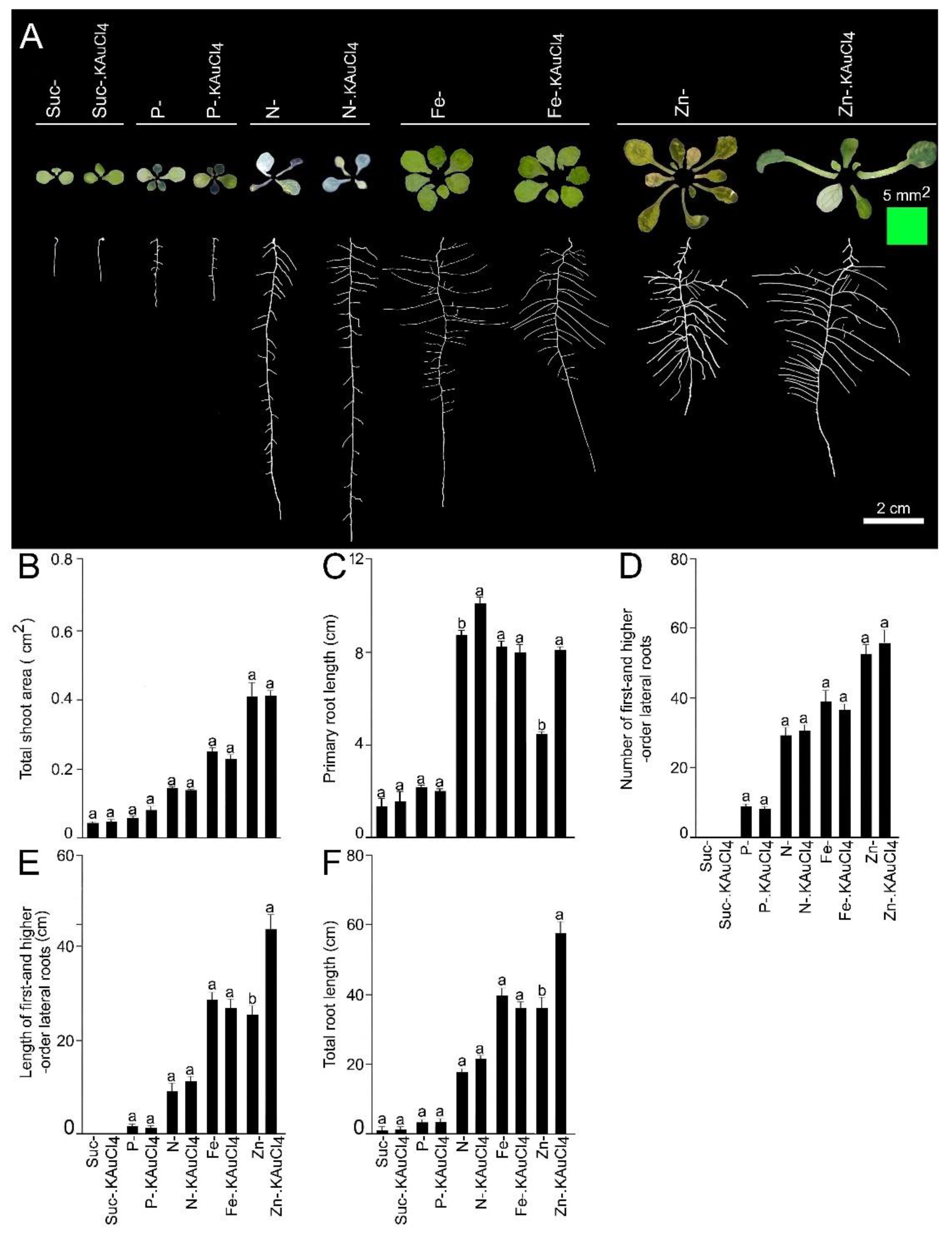
3.3. KAuCl4-Mediated Augmented Growth Response Is Dependent on Suc and Nutrients
3.4. Differential Efficacy of KAuCl4 in Recuperating Natural and Synthetic Auxin-Mediated Modulation in RSA
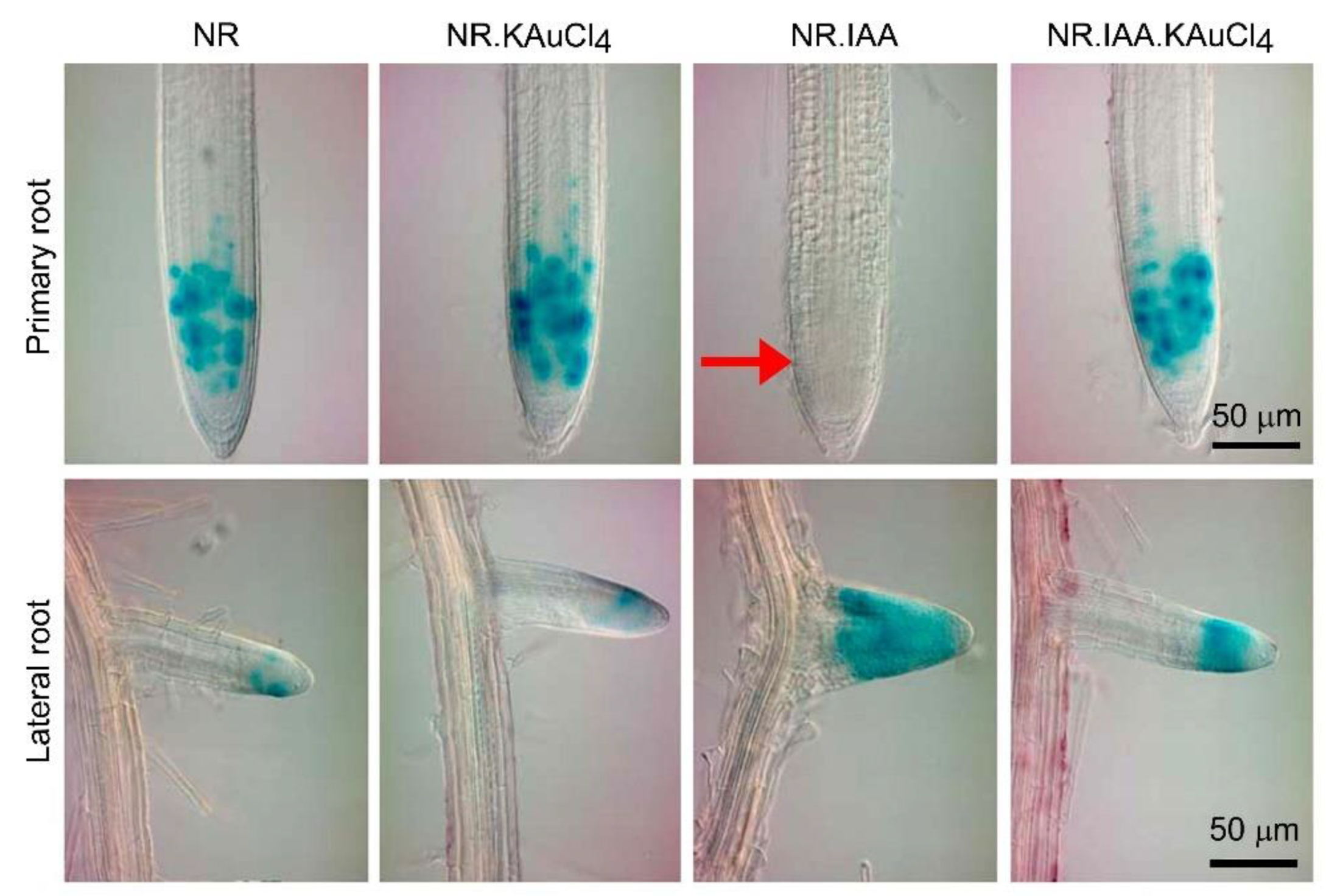
3.5. KAuCl4 Recuperates the IAA-Mediated Inhibitory Effect on Primary Root Growth
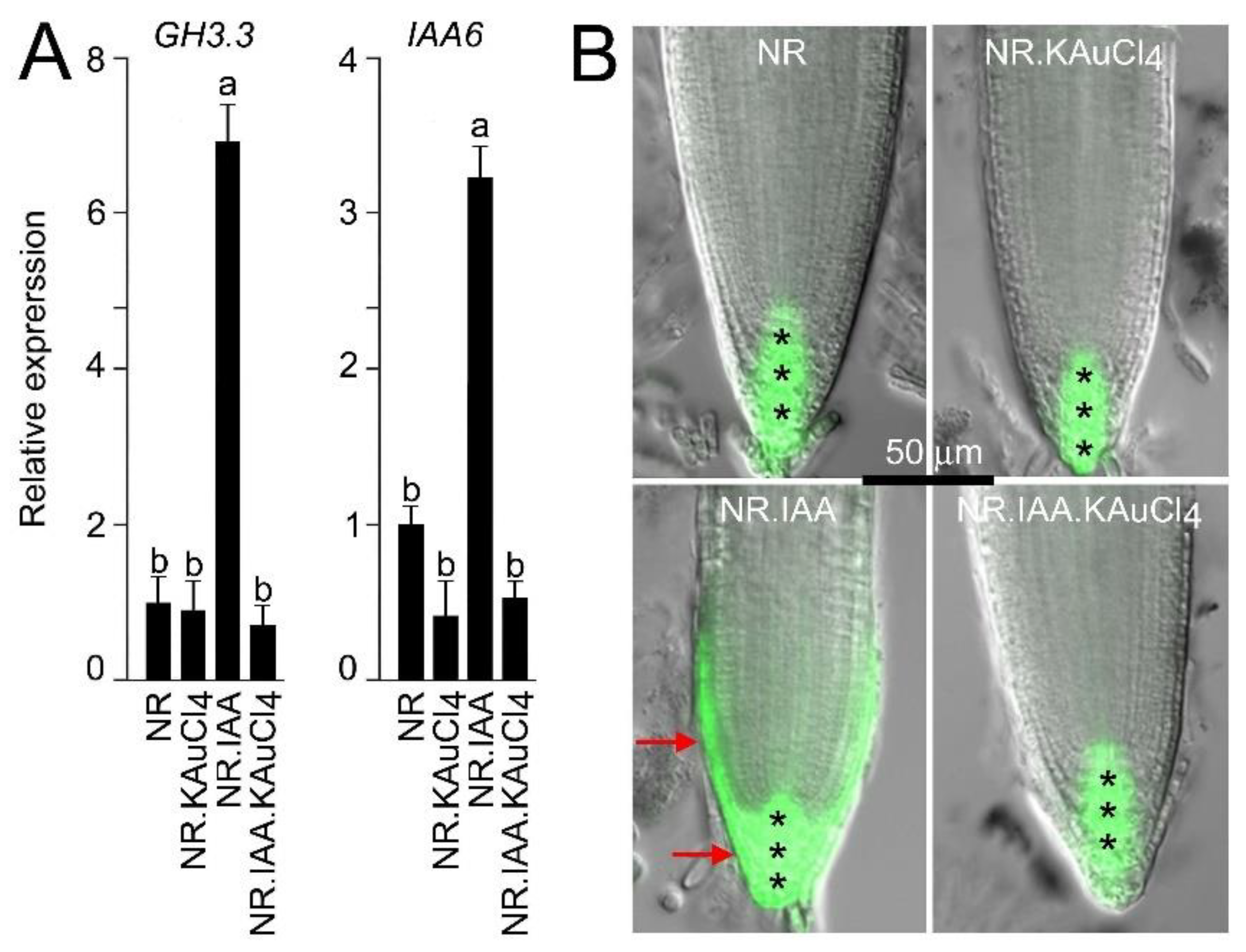
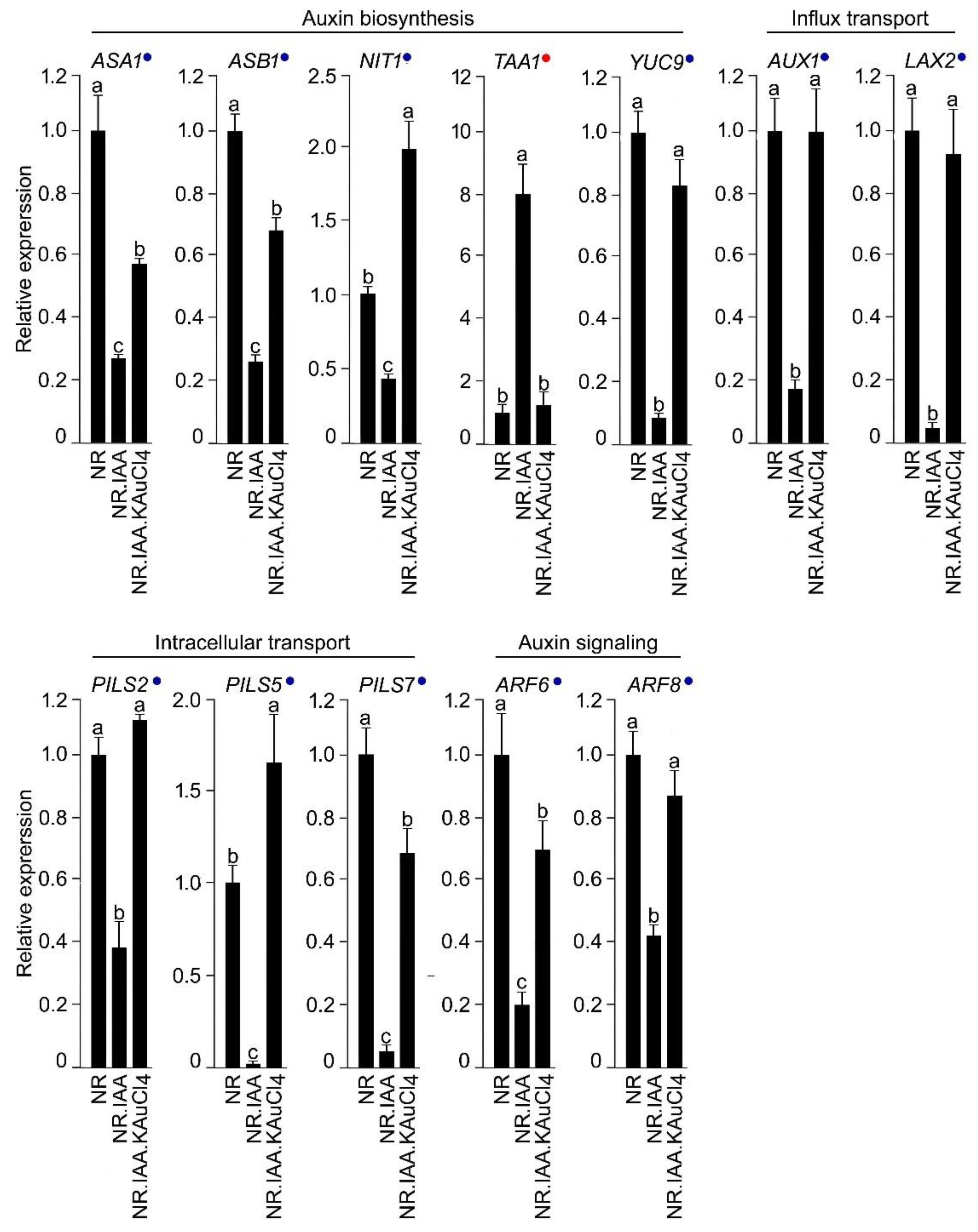
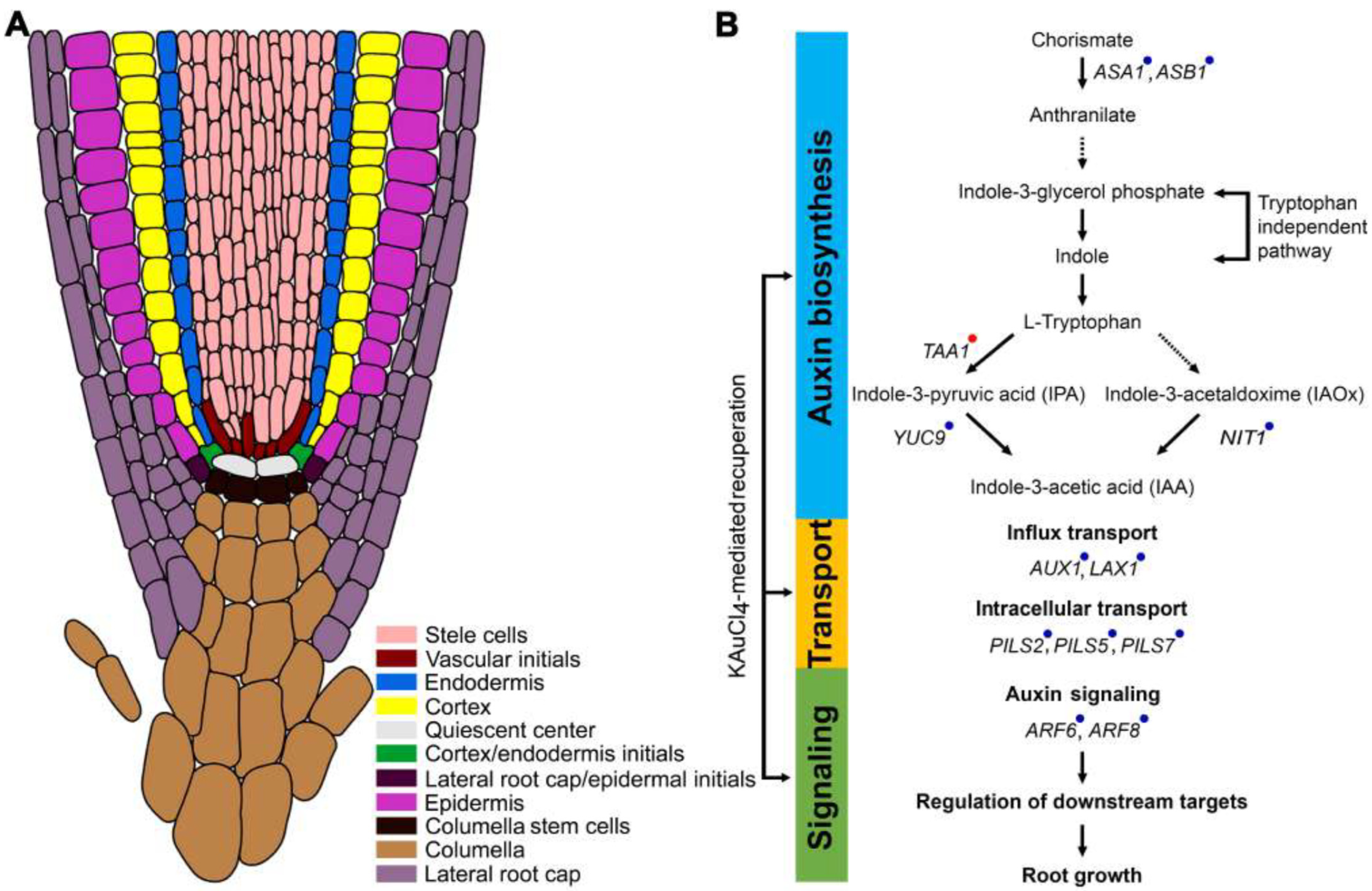
3.6. KAuCl4 Affects Root Growth by Modulating the Components of the Auxin Response Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Lednický, T.; Bonyár, A. Large scale fabrication of ordered gold nanoparticle–epoxy surface nanocomposites and their application as label-free plasmonic DNA biosensors. ACS Appl. Mater. Interfaces 2020, 12, 4804–4814. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Sharma, N.; Tiwari, P.; Singh, S.R.; Sahi, S.V. Fabrication of innocuous gold nanoparticles using plant cells in culture. Sci. Rep. 2019, 9, 12040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, T.; Das, D.; Borges e Soares, G.A.; Chakrabarti, P.; Ai, Z.; Chopra, H.; Hasan, M.A.; Simona Cavalu, S. Novel green approaches for the preparation of gold nanoparticles and their promising potential in oncology. Processes 2022, 10, 426. [Google Scholar] [CrossRef]
- Ebrahimian, J.; Khayatkashani, M.; Soltani, N.; Yousif, Q.A.; Salavati-Niasari, M. Catechin mediated green synthesis of au nanoparticles: Experimental and theoretical approaches to the determination HOMO-LUMO energy gap and reactivity indexes for the (+)-epicatechin (2S, 3S). Arab. J. Chem. 2022, 15, 103758. [Google Scholar] [CrossRef]
- Starnes, D.L.; Jain, A.; Sahi, S.V. In planta engineering of gold nanoparticles of desirable geometries by modulating growth conditions: An environment-friendly approach. Environ. Sci. Technol. 2010, 44, 7110–7115. [Google Scholar] [CrossRef]
- Parsons, J.G.; Aldrich, M.V.; Gardea-Torresdey, J.L. Environmental and biological applications of extended x-ray absorption fine structure (EXAFS) and X-ray absorption near edge structure (XANES) spectroscopies. Appl. Spectrosc. Rev. 2002, 37, 187–222. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V.; Nath, S.; Parsons, J.G.; Gardea-Torresdey, J.L.; Pal, T. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ. Sci. Technol. 2007, 41, 5137–5142. [Google Scholar] [CrossRef] [Green Version]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [Green Version]
- O’Malley, R.C.; Ecker, J.R. Linking genotype to phenotype using the Arabidopsis unimutant collection. Plant J. 2010, 61, 928–940. [Google Scholar] [CrossRef]
- Koornneef, M.; Meinke, D. The development of Arabidopsis as a model plant. Plant J. 2010, 61, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sinilal, B.; Starnes, D.L.; Sanagala, R.; Krishnamurthy, S.; Sahi, S.V. Role of Fe-responsive genes in bioreduction and transport of ionic gold to roots of Arabidopsis thaliana during synthesis of gold nanoparticles. Plant Physiol. Biochem. 2014, 84, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Krishnamurthy, S.; Sahi, S.V. Genome wide transcriptome analysis reveals ABA mediated response in Arabidopsis during gold (AuCl−4) treatment. Front. Plant Sci. 2014, 5, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, M.; Krishnamurthy, S.; Shukla, D.; Kiiskila, J.; Jain, A.; Datta, R.; Sharma, N.; Sahi, S.V. Comparative transcriptome and proteome analysis to reveal the biosynthesis of gold nanoparticles in Arabidopsis. Sci. Rep. 2016, 6, 21733. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Venkatachalam, V.; Penarrubia, L.; Sahi, S.V. COPT2, a plasma membrane located copper transporter, is involved in the uptake of Au in Arabidopsis. Sci. Rep. 2017, 7, 11430. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, E.; Barbero, F.; Busquets-Fité, M.; Franz-Wachtel, M.; Köhler, H.-R.; Puntes, V.; Kemmerling, B. Growth-promoting gold nanoparticles decrease stress responses in Arabidopsis seedlings. Nanomaterials 2021, 11, 3161. [Google Scholar] [CrossRef]
- Malamy, J.E.; Ryan, K.S. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol. 2001, 127, 899–909. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Jain, A.; Poling, M.D.; Karthikeyan, A.S.; Blakeslee, J.J.; Peer, W.A.; Titapiwatanakun, B.; Murphy, A.S.; Raghothama, K.G. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 2007, 144, 232–247. [Google Scholar] [CrossRef] [Green Version]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Kellermeier, F.; Armengaud, P.; Seditas, T.J.; Danku, J.; Salt, D.E.; Amtmann, A. Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell 2014, 26, 1480–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, V.; Sanagala, R.; Sinilal, B.; Yadav, S.; Sarkar, A.K.; Dantu, P.K.; Jain, A. Iron availability affects phosphate deficiency-mediated responses, and evidence of crosstalk with auxin and zinc in Arabidopsis. Plant Cell Physiol. 2015, 56, 1107–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dijk, J.R.; Kranchev, M.; Blust, R.; Cuypers, A.; Vissenberg, K. Arabidopsis root growth and development under metal exposure presented in an adverse outcome pathway framework. Plant Cell Environ. 2022, 45, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.N.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef] [Green Version]
- Konstantinova, N.; Korbei, B.; Luschnig, C. Auxin and root gravitropism: Addressing basic cellular processes by exploiting a defined growth response. Int. J. Mol. Sci. 2021, 22, 2749. [Google Scholar] [CrossRef]
- Hauser, M.-T.; Bauer, E. Histochemical analysis of root meristem activity in Arabidopsis thaliana using a cyclin:GUS (β-glucuronidase) marker line. Plant Soil 2000, 226, 1–10. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- She, W.; Lin, W.; Zhu, Y.; Chen, Y.; Jin, W.; Yang, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. The gypsy insulator of Drosophila melanogaster, together with its binding protein Suppressor of Hairy-wing, facilitate high and precise expression of transgenes in Arabidopsis thaliana. Genetics 2010, 185, 1141–1150. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Poling, M.D.; Smith, A.P.; Nagarajan, V.K.; Lahner, B.; Meagher, R.B.; Raghothama, K.G. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 2009, 150, 1033–1049. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Collins, T.J. ImageJ for microscopy. BioTechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV-vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef] [Green Version]
- Mönchgesang, S.; Strehmel, N.; Schmidt, S.; Westphal, L.; Taruttis, F.; Müller, E.; Herklotz, S.; Neumann, S.; Scheel, D. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Sci. Rep. 2016, 6, 29033. [Google Scholar] [CrossRef]
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Chikramane, P.S.; Suresh, A.K.; Kane, S.G.; Bellare, J.R. Metal nanoparticle induced hormetic activation: A novel mechanism of homeopathic medicines. Homeopathy 2017, 106, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application: What is missing? Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Giehl, R.F.H.; Meyer, R.C.; Altmann, T.; von Wirén, N. Natural variation of BSK3 tunes brassinosteroid signaling to regulate root foraging under low nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef] [Green Version]
- Grillet, L.; Schmidt, W. Iron acquisition strategies in land plants: Not so different after all. New Phytol. 2019, 224, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Piñeros, M.A.; Li, X.; Yang, H.; Liu, Y.; Murphy, A.S.; Kochian, L.V.; Liu, D. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol. Plant 2017, 10, 244–259. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of deficiency and excess of zinc on morphophysiological traits and spatiotemporal regulation of zinc-responsive genes reveal incidence of cross talk between micro-and macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Tsednee, M.; Yeh, K.-C. ZINC TOLERANCE INDUCED BY IRON1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J. 2012, 69, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, C.P.; Grundstad, M.L.; Evanoff, M.A.; Keith, J.D.; Lentz, D.S.; Wagner, S.L.; Culler, A.H.; Cohen, J.D. Auxin-induced leaf blade expansion in Arabidopsis requires both wounding and detachment. Plant Signal. Behav. 2011, 6, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Kalve, S.; Sizani, B.L.; Markakis, M.N.; Helsmoortel, C.; Vandeweyer, G.; Laukens, K.; Sommen, M.; Naulaerts, S.; Vissenberg, K.; Prinsen, E.; et al. Osmotic stress inhibits leaf growth of Arabidopsis thaliana by enhancing ARF-mediated auxin responses. New Phytol. 2020, 226, 1766–1780. [Google Scholar] [CrossRef]
- Eyer, L.; Vain, T.; Pařízková, B.; Oklestkova, J.; Barbez, E.; Kozubíková, H.; Pospíšil, T.; Wierzbicka, R.; Kleine-Vehn, J.; Fránek, M.; et al. 2,4-D and IAA amino acid conjugates show distinct metabolism in Arabidopsis. PLoS ONE 2016, 11, e0159269. [Google Scholar] [CrossRef] [Green Version]
- Delbarre, A.; Muller, P.; Imhoff, V.; Guern, J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 1996, 198, 532–541. [Google Scholar] [CrossRef]
- Campanoni, P.; Nick, P. Auxin-dependent cell division and cell elongation. 1-naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol. 2005, 137, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Torres-Martínez, H.H.; Hernández-Herrera, P.; Corkidi, G.; Dubrovsky, J.G. From one cell to many: Morphogenetic field of lateral root founder cells in Arabidopsis thaliana is built by gradual recruitment. Proc. Natl. Acad. Sci. USA 2020, 117, 20943–20949. [Google Scholar] [CrossRef]
- Dewitte, W.; Murray, J.A.H. The plant cell cycle. Annu. Rev. Plant Biol. 2003, 54, 235–264. [Google Scholar] [CrossRef] [Green Version]
- Glotzer, M.; Murray, A.W.; Kirschner, M.W. Cyclin is degraded by the ubiquitin pathway. Nature 1991, 349, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Doerner, P.; Jørgensen, J.-E.; You, R.; Steppuhn, J.; Lamb, C. Control of root growth and development by cyclin expression. Nature 1996, 380, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Colón-Carmona, A.; You, R.; Haimovitch-Gal, T.; Doerner, P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999, 20, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Kuo, W.-T.; Chuang, Y.-T.; Chen, C.-Y.; Lin, C.-C. Cyclin B1 destruction box-mediated protein instability: The enhanced sensitivity of fluorescent-protein-based reporter gene system. BioMed Res. Int. 2013, 2013, 732307. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L.; Bertell, G.; Bolander, E. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol. 1989, 91, 310–314. [Google Scholar] [CrossRef]
- Hu, Y.; Omary, M.; Hu, Y.; Doron, O.; Hoermayer, L.; Chen, Q.; Megides, O.; Chekli, O.; Ding, Z.; Friml, J.; et al. Cell kinetics of auxin transport and activity in Arabidopsis root growth and skewing. Nat. Commun. 2021, 12, 1657. [Google Scholar] [CrossRef]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef] [Green Version]
- Di, D.-W.; Li, G.; Sun, L.; Wu, J.; Wang, M.; Kronzucker, H.J.; Fang, S.; Chu, J.; Shi, W. High ammonium inhibits root growth in Arabidopsis thaliana by promoting auxin conjugation rather than inhibiting auxin biosynthesis. J. Plant Physiol. 2021, 261, 153415. [Google Scholar] [CrossRef]
- Walter, A.; Caputi, L.; O’Connor, S.; van Pée, K.-H.; Ludwig-Müller, J. Chlorinated auxins-how does Arabidopsis thaliana deal with them? Int. J. Mol. Sci. 2020, 21, 2567. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Ma, W.; Yang, H.; Ma, G.; Mantyla, J.J.; Benning, C. The Arabidopsis WRINKLED1 transcription factor affects auxin homeostasis in roots. J. Exp. Bot. 2017, 68, 4627–4634. [Google Scholar] [CrossRef] [Green Version]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Q.; Uhlir, N.J.; Reed, J.W. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 2002, 14, 301–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [PubMed] [Green Version]
- Peer, W.A.; Blakeslee, J.J.; Yang, H.; Murphy, A.S. Seven things we think we know about auxin transport. Mol. Plant 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Zhou, J.J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [Green Version]
- Vieten, A.; Vanneste, S.; Wisniewska, J.; Benkova, E.; Benjamins, R.; Beeckman, T.; Lusching, C.; Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005, 132, 4521–4531. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Ric, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In Situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Yugandhar, P.; Alavilli, H.; Raliya, R.; Singh, A.; Sahi, S.V.; Sarkar, A.K.; Jain, A. Potassium Chloroaurate-Mediated In Vitro Synthesis of Gold Nanoparticles Improved Root Growth by Crosstalk with Sucrose and Nutrient-Dependent Auxin Homeostasis in Arabidopsis thaliana. Nanomaterials 2022, 12, 2099. https://doi.org/10.3390/nano12122099
Yadav S, Yugandhar P, Alavilli H, Raliya R, Singh A, Sahi SV, Sarkar AK, Jain A. Potassium Chloroaurate-Mediated In Vitro Synthesis of Gold Nanoparticles Improved Root Growth by Crosstalk with Sucrose and Nutrient-Dependent Auxin Homeostasis in Arabidopsis thaliana. Nanomaterials. 2022; 12(12):2099. https://doi.org/10.3390/nano12122099
Chicago/Turabian StyleYadav, Sandeep, Poli Yugandhar, Hemasundar Alavilli, Ramesh Raliya, Archita Singh, Shivendra V. Sahi, Ananda K. Sarkar, and Ajay Jain. 2022. "Potassium Chloroaurate-Mediated In Vitro Synthesis of Gold Nanoparticles Improved Root Growth by Crosstalk with Sucrose and Nutrient-Dependent Auxin Homeostasis in Arabidopsis thaliana" Nanomaterials 12, no. 12: 2099. https://doi.org/10.3390/nano12122099
APA StyleYadav, S., Yugandhar, P., Alavilli, H., Raliya, R., Singh, A., Sahi, S. V., Sarkar, A. K., & Jain, A. (2022). Potassium Chloroaurate-Mediated In Vitro Synthesis of Gold Nanoparticles Improved Root Growth by Crosstalk with Sucrose and Nutrient-Dependent Auxin Homeostasis in Arabidopsis thaliana. Nanomaterials, 12(12), 2099. https://doi.org/10.3390/nano12122099





