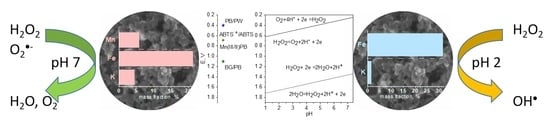
Intrinsic Multienzyme-like Activities of the Nanoparticles of Mn and Fe Cyano-Bridged Assemblies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nanoparticles
2.2.1. Synthesis of PB NCPs
2.2.2. Synthesis of Mn-PB NCPs
2.3. Characterization Methods
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. XRD, XPS, and ICP-OES Analysis
2.3.3. UV-Vis Spectroscopy
2.3.4. Electrochemical Characterization
2.4. Spectrophotometric Assay
2.4.1. Peroxidase Activity Assay
2.4.2. Peroxidase Kinetics Study
2.4.3. Peroxidase Kinetics Study with Horseradish Peroxidase
2.4.4. Oxidase-like Activity Assay
2.4.5. Catalase Assay
2.4.6. Superoxide Radical Anion Scavenging Activity
2.5. Preparation of the Glassy Carbon Electrode (GCE)
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wan, G.; Hu, N.; He, Z.; Shi, S.; Suo, Y.; Wang, K.; Xu, X.; Tang, Y.; Wang, G. Synthesis of Porous CoFe2O4 and Its Application as a Peroxidase Mimetic for Colorimetric Detection of H2O2 and Organic Pollutant Degradation. Nanomaterials 2018, 8, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, M.; Guan, X.; Wu, F.; Ma, L. CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis. Nanomaterials 2022, 12, 638. [Google Scholar] [CrossRef]
- Su, L.; Qin, W.; Zhang, H.; Rahman, Z.U.; Ren, C.; Ma, S.; Chen, X. The peroxidase/catalase-like activities of MFe2O4 (M=Mg, Ni, Cu) MNPs and their application in colorimetric biosensing of glucose. Biosens. Bioelectron. 2015, 63, 384–391. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2018, 48, 1004–1076. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual Enzyme-like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Cheon, H.; Nguyen, Q.; Kim, M. Highly Sensitive Fluorescent Detection of Acetylcholine Based on the Enhanced Peroxidase-Like Activity of Histidine Coated Magnetic Nanoparticles. Nanomaterials 2021, 11, 1207. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Adam, S.M.; Wijeratne, G.B.; Rogler, P.J.; Diaz, D.E.; Quist, D.A.; Liu, J.J.; Karlin, K.D. Synthetic Fe/Cu Complexes: Toward Understanding Heme-Copper Oxidase Structure and Function. Chem. Rev. 2018, 118, 10840–11022. [Google Scholar] [CrossRef]
- Mourzina, Y.G.; Offenhäusser, A. Electrochemical properties and biomimetic activity of water-soluble meso-substituted Mn(III) porphyrin complexes in the electrocatalytic reduction of hydrogen peroxide. J. Electroanal. Chem. 2020, 866, 114159. [Google Scholar] [CrossRef]
- Peng, R.; Offenhäusser, A.; Ermolenko, Y.; Mourzina, Y. Biomimetic sensor based on Mn(III) meso-tetra(N-methyl-4-pyridyl) porphyrin for non-enzymatic electrocatalytic determination of hydrogen peroxide and as an electrochemical transducer in oxidase biosensor for analysis of biological media. Sens. Actuators B Chem. 2020, 321, 128437. [Google Scholar] [CrossRef]
- Maroneze, C.M.; Gushikem, Y.; Kubota, L.T. Applications of MN4 Macrocyclic Metal Complexes in Electroanalysis. In Electrochemistry of N4 Macrocyclic Metal Complexes; Zagal, J., Bedioui, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 107–131. [Google Scholar] [CrossRef]
- Damos, F.S.; Sotomayor, M.D.P.T.; Kubota, L.T.; Tanaka, S.M.C.N.; Tanaka, A.A. Iron(iii) tetra-(N-methyl-4-pyridyl)-porphyrin as a biomimetic catalyst of horseradish peroxidase on the electrode surface: An amperometric sensor for phenolic compound determinations. Analyst 2003, 128, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme cofactor-resembling Fe–N single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 2020, 30, 1905410. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal–Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Huang, L.; Zhang, H.; Rong, K.; Zhang, H.; Dong, S. Prussian blue with intrinsic heme-like structure as peroxidase mimic. Nano Res. 2018, 11, 4905–4913. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Höfs, S.; Greiner, T.; Göbel, G.; Talke, A.; Lisdat, F. Activity determination of human monoamine oxidase B (Mao B) by selective capturing and amperometric hydrogen peroxide detection. Sens. Actuators B Chem. 2020, 328, 129020. [Google Scholar] [CrossRef]
- Zhao, P.; Jia, Y.; Liang, Y.; Zheng, J.; Yang, M.; Huo, D.; Wang, Y.; Hou, C. A Prussian blue-doped RGO/MXene composite aerogel with peroxidase-like activity for real-time monitoring of H2O2 secretion from living cells. Chem. Commun. 2021, 57, 9870–9873. [Google Scholar] [CrossRef]
- Sahar, S.; Zeb, A.; Ling, C.; Raja, A.; Wang, G.; Ullah, N.; Lin, X.-M.; Xu, A.-W. A Hybrid VOx Incorporated Hexacyanoferrate Nanostructured Hydrogel as a Multienzyme Mimetic via Cascade Reactions. ACS Nano 2020, 14, 3017–3031. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.-J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, J.; Gao, W.; Chen, J.; Hu, B.; Cai, X.; Zheng, Y. Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-κB signaling pathway. Theranostics 2021, 11, 3213–3228. [Google Scholar] [CrossRef]
- Zhang, K.; Tu, M.; Gao, W.; Cai, X.; Song, F.; Chen, Z.; Zhang, Q.; Wang, J.; Jin, C.; Shi, J.; et al. Hollow Prussian Blue Nanozymes Drive Neuroprotection against Ischemic Stroke via Attenuating Oxidative Stress, Counteracting Inflammation, and Suppressing Cell Apoptosis. Nano Lett. 2019, 19, 2812–2823. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Y.; Gu, N. Progress in Applications of Prussian Blue Nanoparticles in Biomedicine. Adv. Health Mater. 2018, 7, e1800347. [Google Scholar] [CrossRef] [PubMed]
- Noël, J.-M.; Médard, J.; Combellas, C.; Kanoufi, F. Prussian Blue Degradation during Hydrogen Peroxide Reduction: A Scanning Electrochemical Microscopy Study on the Role of the Hydroxide Ion and Hydroxyl Radical. ChemElectroChem 2016, 3, 1178–1184. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M. Prussian Blue: A Nanozyme with Versatile Catalytic Properties. Int. J. Mol. Sci. 2021, 22, 5993. [Google Scholar] [CrossRef]
- Nikolaev, K.; Ermakov, S.; Ermolenko, Y.; Averyaskina, E.; Offenhausser, A.; Mourzina, Y. A novel bioelectrochemical interface based on in situ synthesis of gold nanostructures on electrode surfaces and surface activation by Meerwein’s salt. A bioelectrochemical sensor for glucose determination. Bioelectrochemistry 2015, 105, 34–43. [Google Scholar] [CrossRef]
- Koposova, E.; Shumilova, G.; Ermolenko, Y.; Kisner, A.; Offenhäusser, A.; Mourzina, Y. Direct electrochemistry of cyt c and hydrogen peroxide biosensing on oleylamine- and citrate-stabilized gold nanostructures. Sens. Actuators B Chem. 2015, 207, 1045–1052. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn porphyrin-based redox-active drugs: Differential effects as cancer therapeutics and protectors of normal tissue against oxidative injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Mourzina, Y.G.; Ermolenko, Y.E.; Offenhäusser, A. Synthesizing Electrodes Into Electrochemical Sensor Systems. Front. Chem. 2021, 9, 641674. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Huang, Z.; Jiang, J.; Belikova, N.A.; Tyurin, V.; Epperly, M.W.; Greenberger, J.S.; Bayir, H.; Kagan, V.E. A manganese–porphyrin complex decomposes H2O2, inhibits apoptosis, and acts as a radiation mitigator in vivo. ACS Med. Chem. Lett. 2011, 2, 814–817. [Google Scholar] [CrossRef]
- Kubota, R.; Imamura, S.; Shimizu, T.; Asayama, S.; Kawakami, H. Synthesis of Water-Soluble Dinuclear Mn-Porphyrin with Multiple Antioxidative Activities. ACS Med. Chem. Lett. 2014, 5, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Pelluau, T.; Sene, S.; Garcia-Cirera, B.; Albela, B.; Bonneviot, L.; Larionova, J.; Guari, Y. Multifunctionalized Mesostructured Silica Nanoparticles Containing Mn2 Complex for Improved Catalase-Mimicking Activity in Water. Nanomaterials 2022, 12, 1136. [Google Scholar] [CrossRef]
- Lee, A.; Kang, W.; Choi, J.-S. Highly Enhanced Enzymatic Activity of Mn-Induced Carbon Dots and Their Application as Colorimetric Sensor Probes. Nanomaterials 2021, 11, 3046. [Google Scholar] [CrossRef]
- Ali, L.M.; Mathlouthi, E.; Kajdan, M.; Daurat, M.; Long, J.; Sidi-Boulenouar, R.; Cardoso, M.; Goze-Bac, C.; Amdouni, N.; Guari, Y.; et al. Multifunctional manganese-doped Prussian blue nanoparticles for two-photon photothermal therapy and magnetic resonance imaging. Photodiagn. Photodyn. Ther. 2018, 22, 65–69. [Google Scholar] [CrossRef]
- Vojtech, J.M.; Cano-Mejia, J.; Dumont, M.F.; Sze, R.W.; Fernandes, R. Biofunctionalized Prussian Blue Nanoparticles for Multimodal Molecular Imaging Applications. J. Vis. Exp. 2015, 98, e52621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of one electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobisethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Hornok, V.; Dékány, I. Synthesis and stabilization of Prussian blue nanoparticles and application for sensors. J. Colloid Interface Sci. 2007, 309, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Faulkner, K.; Liochev, S.; Fridovich, I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994, 269, 23471–23476. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef] [Green Version]
- Gallati, H. Peroxidase aus Meerrettich: Kinetische studien sowie optimierung der aktivitätsbestimmung mit den substraten H2O2 und 2,2′-Azino-di-(3-ethyl-benzthiazolinsulfonsäure-(6)) (ABTS). J. Clin. Chem. Clin. Biochem. 1979, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine Meets Fenton Chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Walling, C.; Goosen, A. Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J. Am. Chem. Soc. 1973, 95, 2987–2991. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Branchi, B.; Galli, C.; Gentili, P. Kinetics of oxidation of benzyl alcohols by the dication and radical cation of ABTS. Comparison with laccase–ABTS oxidations: An apparent paradox. Org. Biomol. Chem. 2005, 3, 2604–2614. [Google Scholar] [CrossRef]
- Panov, A. Perhydroxyl Radical (HO2•) as Inducer of the Isoprostane Lipid Peroxidation in Mitochondria. Mol. Biol. 2018, 52, 295–305. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974; p. 644. [Google Scholar]
- Pegis, M.L.; Wise, C.F.; Martin, D.J.; Mayer, J.M. Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. [Google Scholar] [CrossRef]
- Sawada, Y.; Yamazaki, I. One-electron transfer reactions in biochemical systems. VIII. Kinetic study of superoxide dismutase. Biochim. Biophys. Acta (BBA)-Enzym. 1973, 327, 257–265. [Google Scholar] [CrossRef]
- Butler, J.; Koppenol, W.; Margoliash, E. Kinetics and mechanism of the reduction of ferricytochrome c by the superoxide anion. J. Biol. Chem. 1982, 257, 10747–10750. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S. Oxidative Stress at the Single Cell Level. In Electrochemical Methods for Neuroscience; Michael, A.C., Borland, L.M., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Y.; Cheng, T.; Lai, W.-Y.; Huang, W. Uniform manganese hexacyanoferrate hydrate nanocubes featuring superior performance for low-cost supercapacitors and nonenzymatic electrochemical sensors. Nanoscale 2015, 7, 16012–16019. [Google Scholar] [CrossRef]
- Ciogli, L.; Zumpano, R.; Poloznikov, A.A.; Hushpulian, D.M.; Tishkov, V.I.; Andreu, R.; Gorton, L.; Mazzei, F.; Favero, G.; Bollella, P. Highly Sensitive Hydrogen Peroxide Biosensor Based on Tobacco Peroxidase Immobilized on p-Phenylenediamine Diazonium Cation Grafted Carbon Nanotubes: Preventing Fenton-like Inactivation at Negative Potential. ChemElectroChem 2021, 8, 2495–2504. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, D.; Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 2014, 120, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Chen, Y.; Li, S.; Gu, N.; Hu, S.; Sun, Y.; Chen, X.; Quan, L. Prussian Blue modified ferritin as peroxidase mimetics and its applications in biological detection. J. Nanosci. Nanotechnol. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cai, Y.; Gao, L.; Liang, M.; Miao, B.; Wu, H.; Liu, Y.; Xie, N.; Tang, A.; Fan, K.; et al. Exosome-like nanozyme vesicles for H2O2-responsive catalytic photoacoustic imaging of xenograft nasopharyngeal carcinoma. Nano Lett. 2019, 19, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zakharzhevskii, M.; Drozdov, A.S.; Kolchanov, D.S.; Shkodenko, L.; Vinogradov, V.V. Test-System for Bacteria Sensing Based on Peroxidase-Like Activity of Inkjet-Printed Magnetite Nanoparticles. Nanomaterials 2020, 10, 313. [Google Scholar] [CrossRef] [Green Version]
- Ponlakhet, K.; Amatatongchai, M.; Sroysee, W.; Jarujamrus, P.; Chairam, S. Development of sensitive and selective glucose colorimetric assay using glucose oxidase immobilized on magnetite–gold–folate nanoparticles. Anal. Methods 2016, 8, 8288–8298. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, F. Substrate-specific modifications on magnetic iron oxide nanoparticles as an artificial peroxidase for improving sensitivity in glucose detection. Nanotechnology 2011, 22, 145704. [Google Scholar] [CrossRef]
- Su, L.; Xiong, Y.; Yang, H.; Zhang, P.; Ye, F. Prussian blue nanoparticles encapsulated inside a metal–organic framework via in situ growth as promising peroxidase mimetics for enzyme inhibitor screening. J. Mater. Chem. B 2016, 4, 128–134. [Google Scholar] [CrossRef]
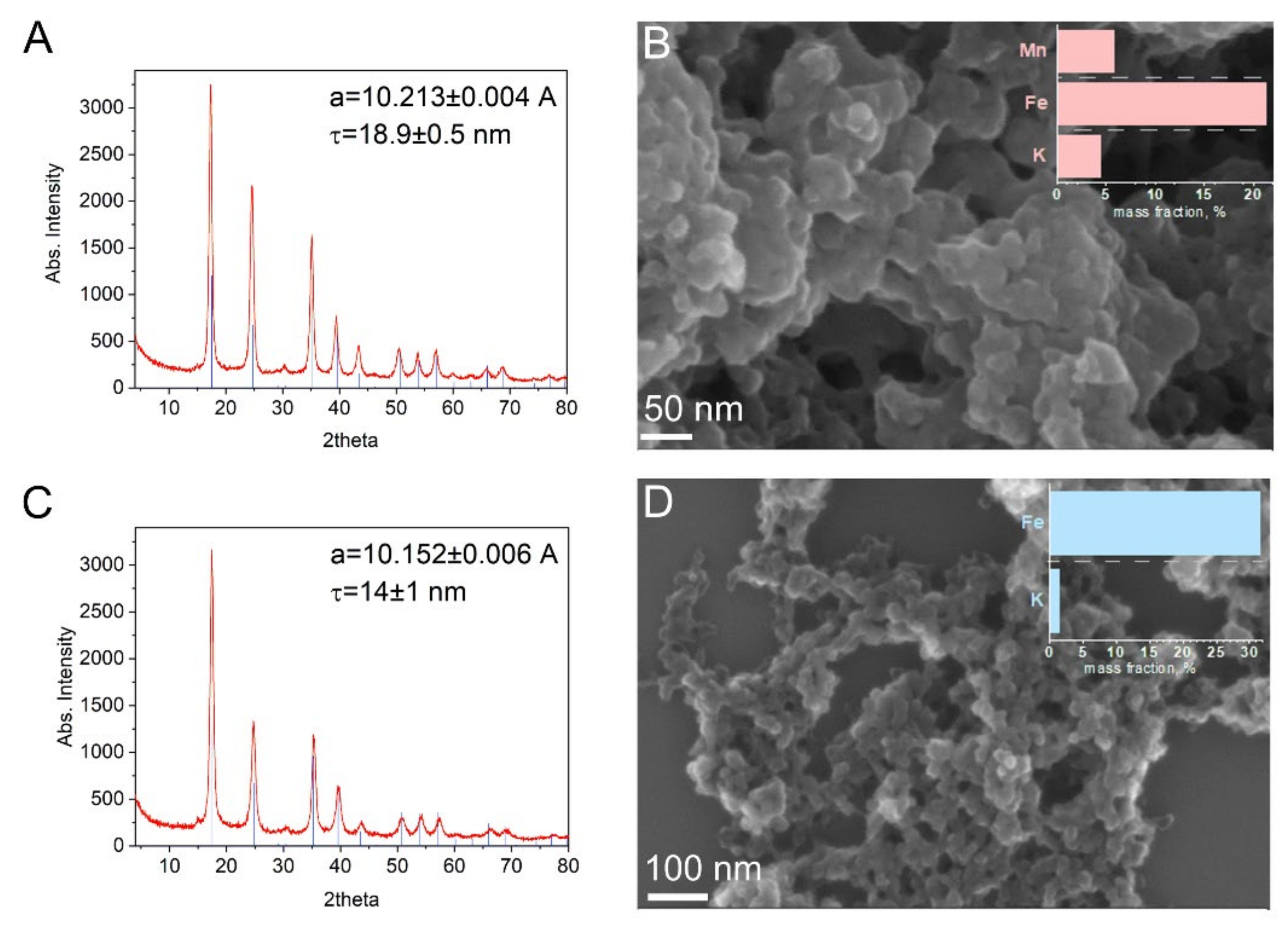
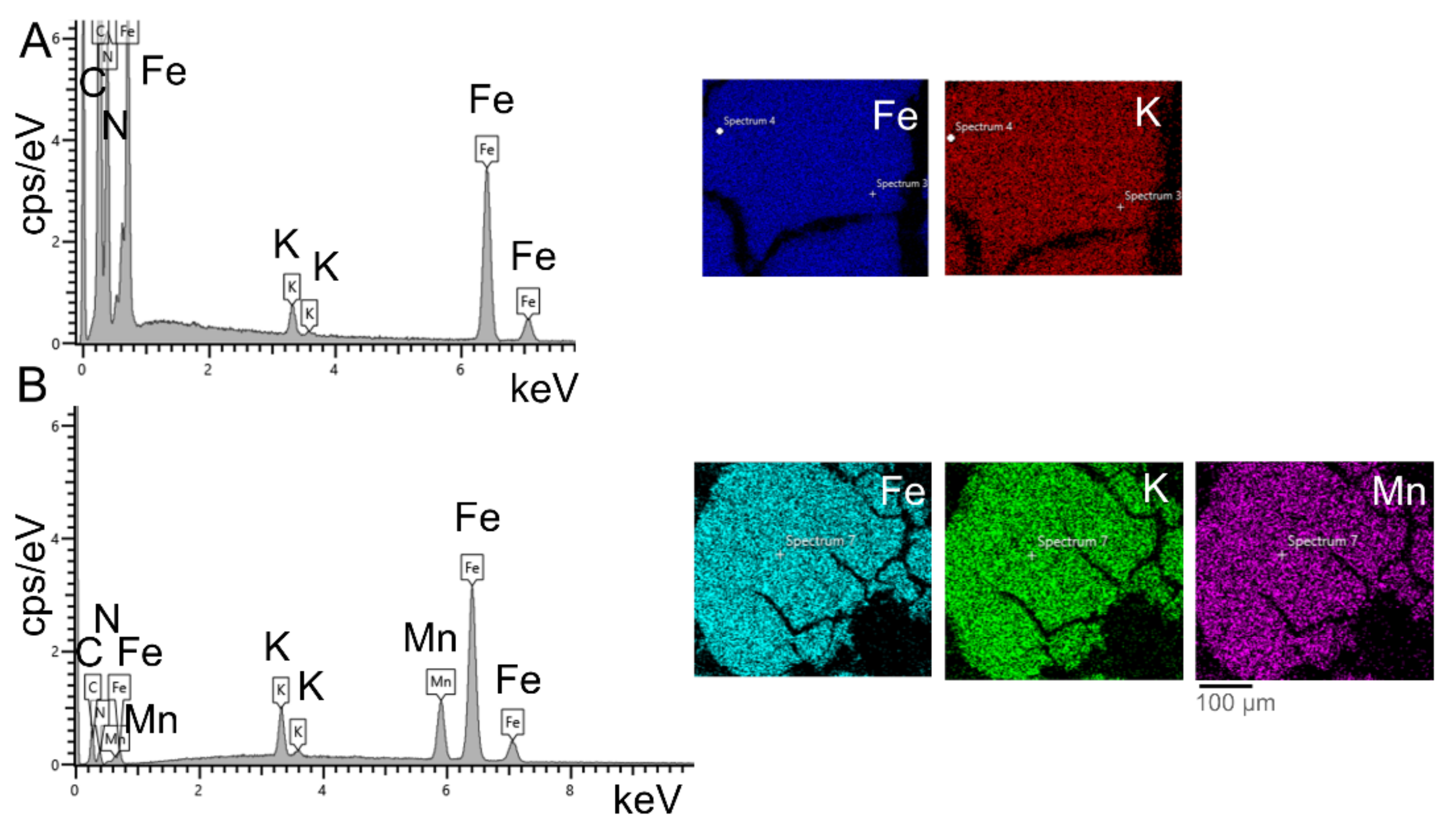
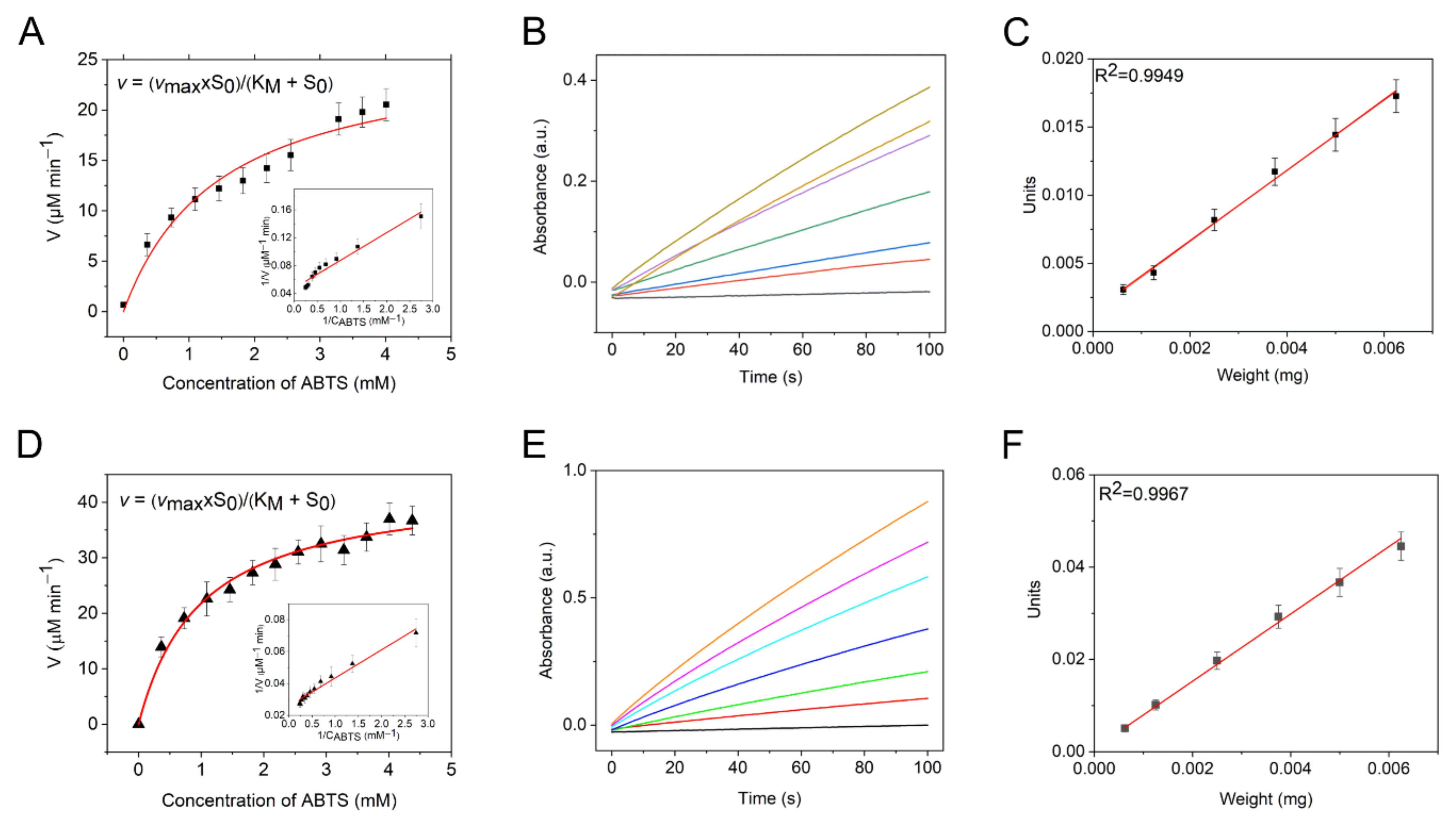
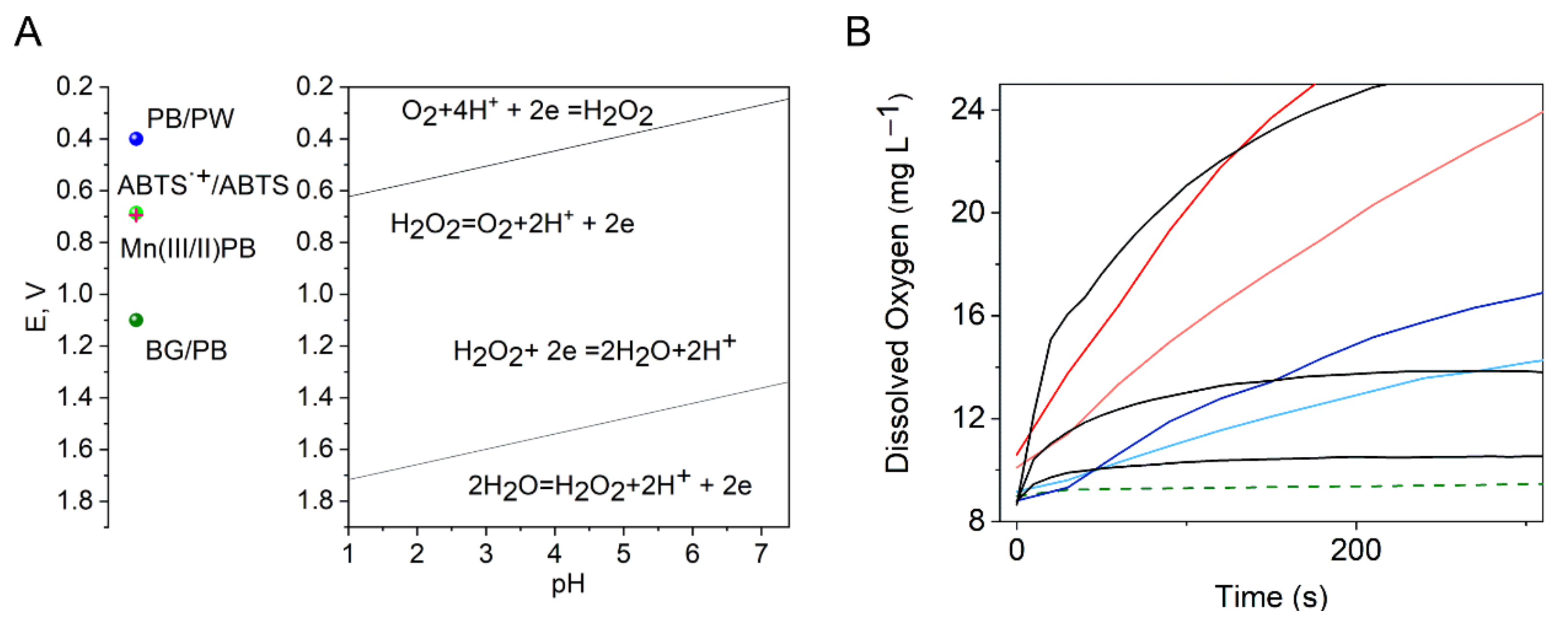
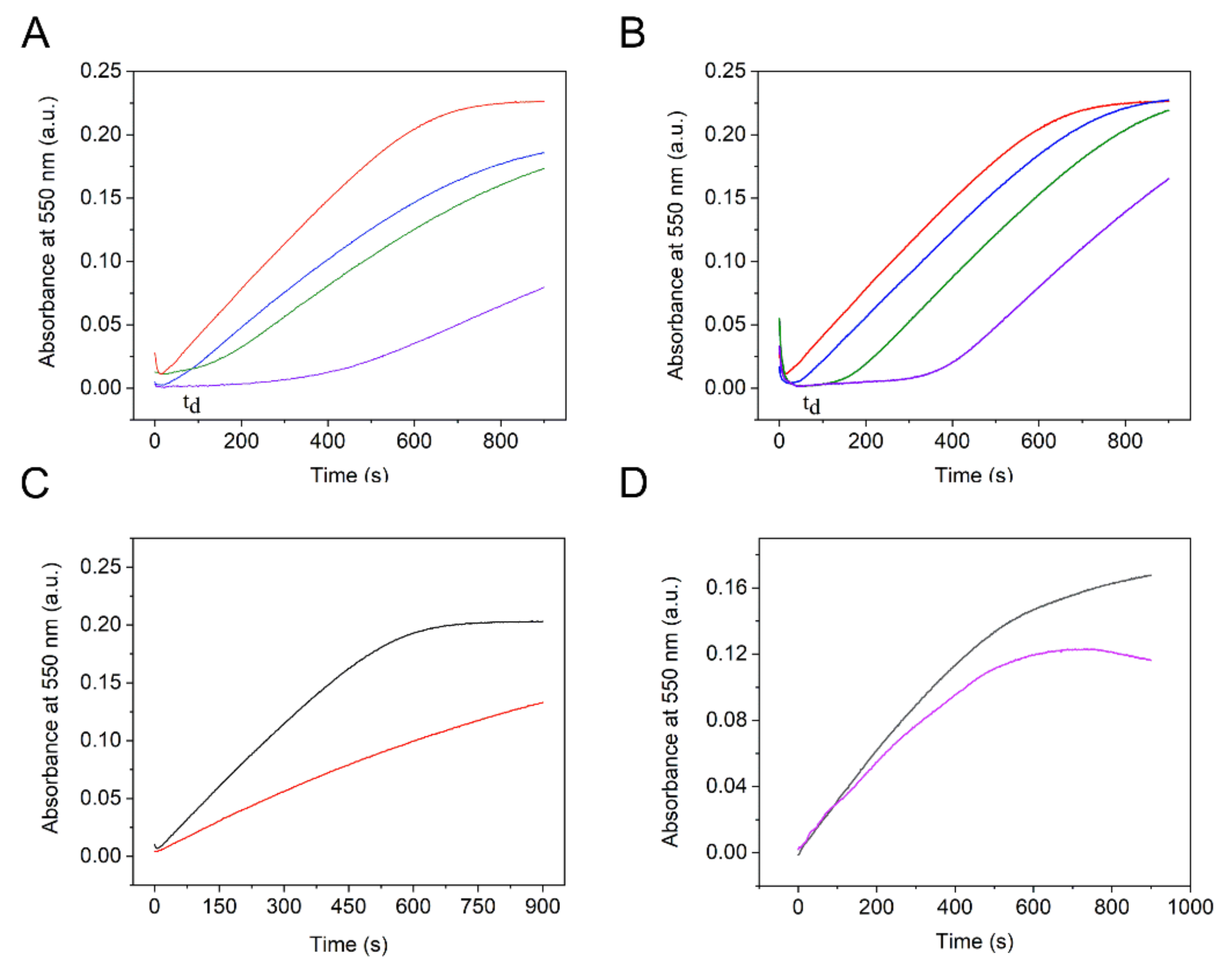
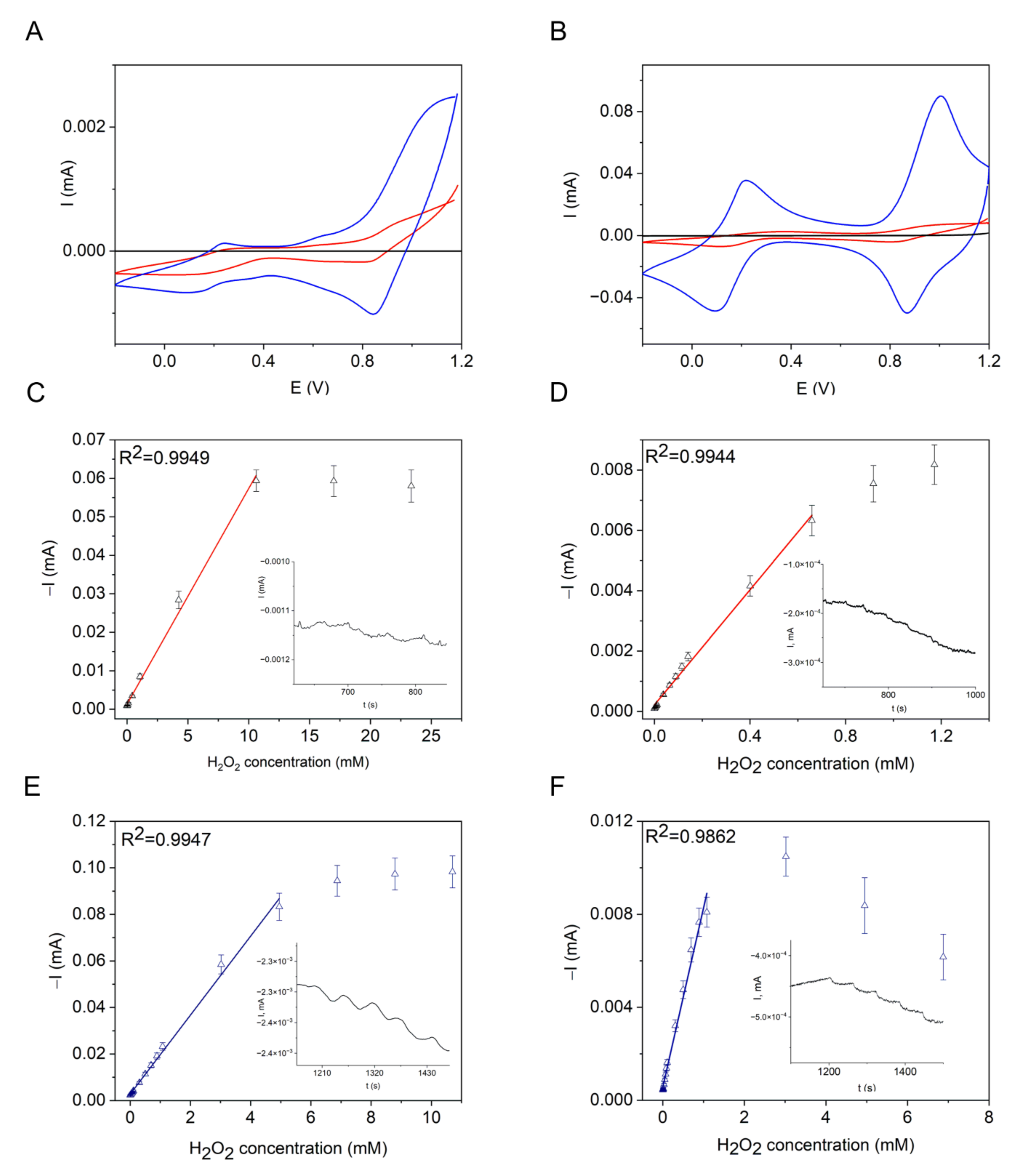
| Peroxidase-like Activity | Catalase Activity | O2●− Scavenging Activity | ||||
|---|---|---|---|---|---|---|
| a 1, U mg−1 | KMABTS, mM | VmaxABTS, µM min−1 | kcat, s−1 mL µg−1 | td, s | VNCP/V0 | |
| Mn-PB | 2.6 | 0.8 | 21 | 0.017 | 300 | 0.40 |
| PB | 7.2 | 0.68 | 38 | 0.006 | 320 | 0.83 |
| HRP | - | 0.48 2 | 23 2 | - | - | - |
| PB/PW 1 | PY, BG/PB 1 | |||||
|---|---|---|---|---|---|---|
| Ea,V | Ec,V | ΔE, V | Ic/Ia | Ea, V | Ec, V | |
| PB | 0.216 | 0.105 | 0.111 | 1 | 1002 | 0.870 |
| Mn-PB | 0.238 0.330 2 | 0.116 0.638 2 | 0.122 | 1 | 1072 | 0.843 |
| LDLH2O2, µM | S A/(M⋅cm2) | WCR, mM | ||||
|---|---|---|---|---|---|---|
| pH 3 | pH 7.4 | 3 | 7.4 | 3 | 7.4 | |
| PB | 1 | 3 | 0.23 | 0.11 | 1 × 10−3–5 | 3 × 10−3–1 |
| Mn-PB | 2 | 3 | 0.12 | 0.14 | 2 × 10−3–10 | 3 × 10−3–0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kudriashov, D.; Pershina, L.; Offenhäusser, A.; Mourzina, Y. Intrinsic Multienzyme-like Activities of the Nanoparticles of Mn and Fe Cyano-Bridged Assemblies. Nanomaterials 2022, 12, 2095. https://doi.org/10.3390/nano12122095
Zhang Y, Kudriashov D, Pershina L, Offenhäusser A, Mourzina Y. Intrinsic Multienzyme-like Activities of the Nanoparticles of Mn and Fe Cyano-Bridged Assemblies. Nanomaterials. 2022; 12(12):2095. https://doi.org/10.3390/nano12122095
Chicago/Turabian StyleZhang, Yunong, David Kudriashov, Liubov Pershina, Andreas Offenhäusser, and Yulia Mourzina. 2022. "Intrinsic Multienzyme-like Activities of the Nanoparticles of Mn and Fe Cyano-Bridged Assemblies" Nanomaterials 12, no. 12: 2095. https://doi.org/10.3390/nano12122095
APA StyleZhang, Y., Kudriashov, D., Pershina, L., Offenhäusser, A., & Mourzina, Y. (2022). Intrinsic Multienzyme-like Activities of the Nanoparticles of Mn and Fe Cyano-Bridged Assemblies. Nanomaterials, 12(12), 2095. https://doi.org/10.3390/nano12122095