Polymethyl(1–Butyric acidyl)silane–Assisted Dispersion and Density Gradient Ultracentrifugation Separation of Single–Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
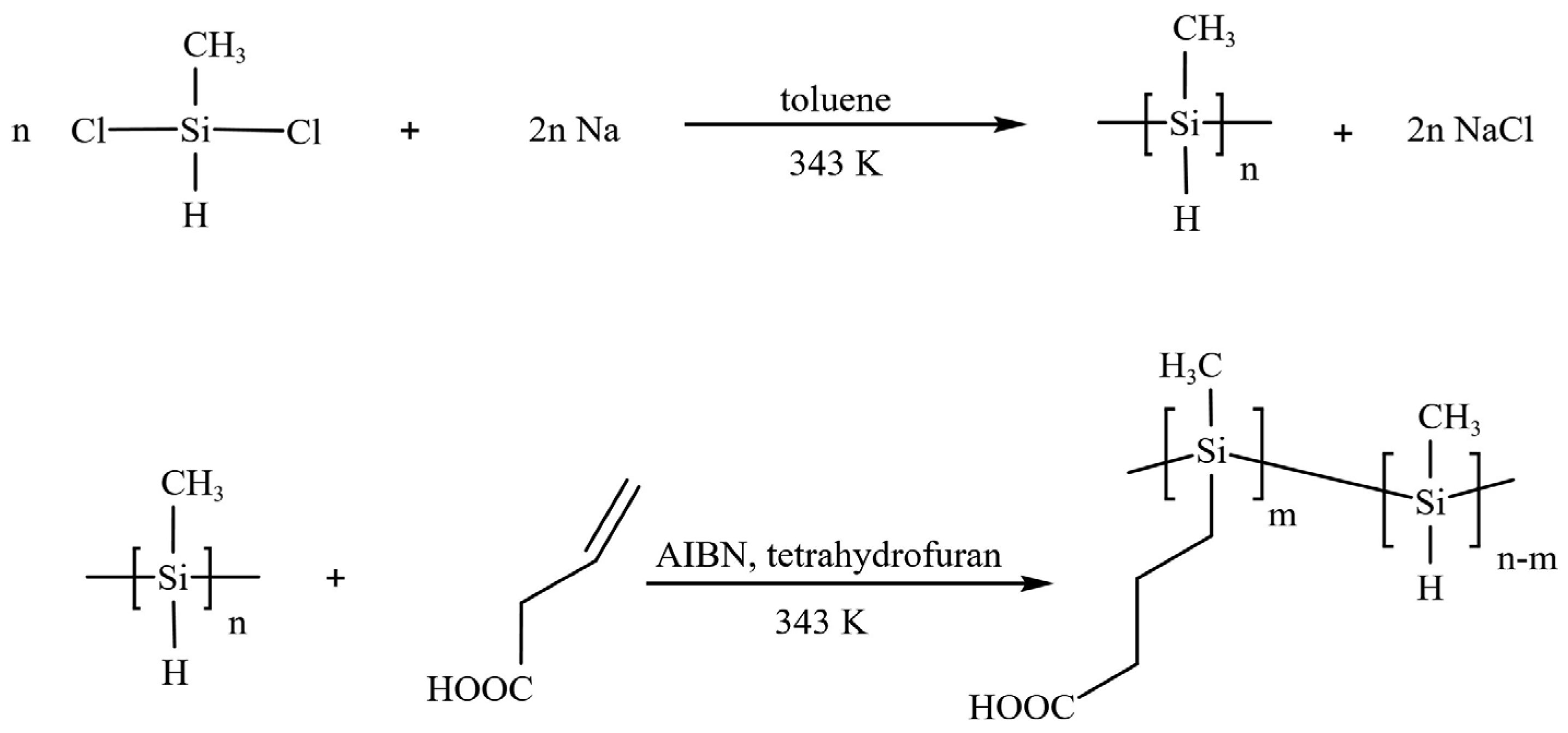
2.2. Synthesis of Water–Soluble BA–PMS
2.3. Preparation and Primary Purification of SWNTs
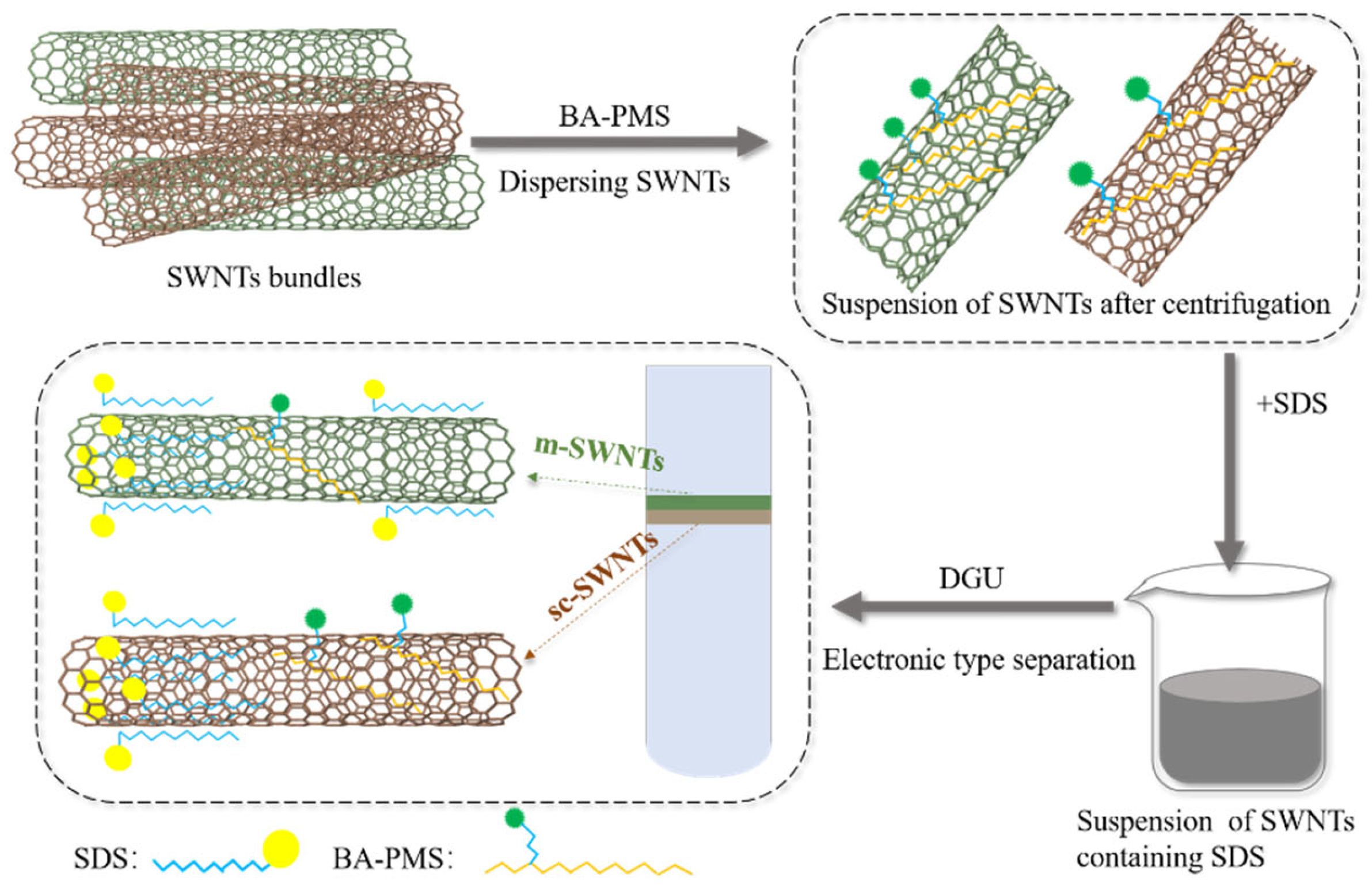
2.4. The Dispersion Procedure of the Primarily Purified SWNTs
2.5. Separation of BA–PMS–Dispersed SWNTs by Sucrose–DGU
2.6. Characterization Methods
3. Results
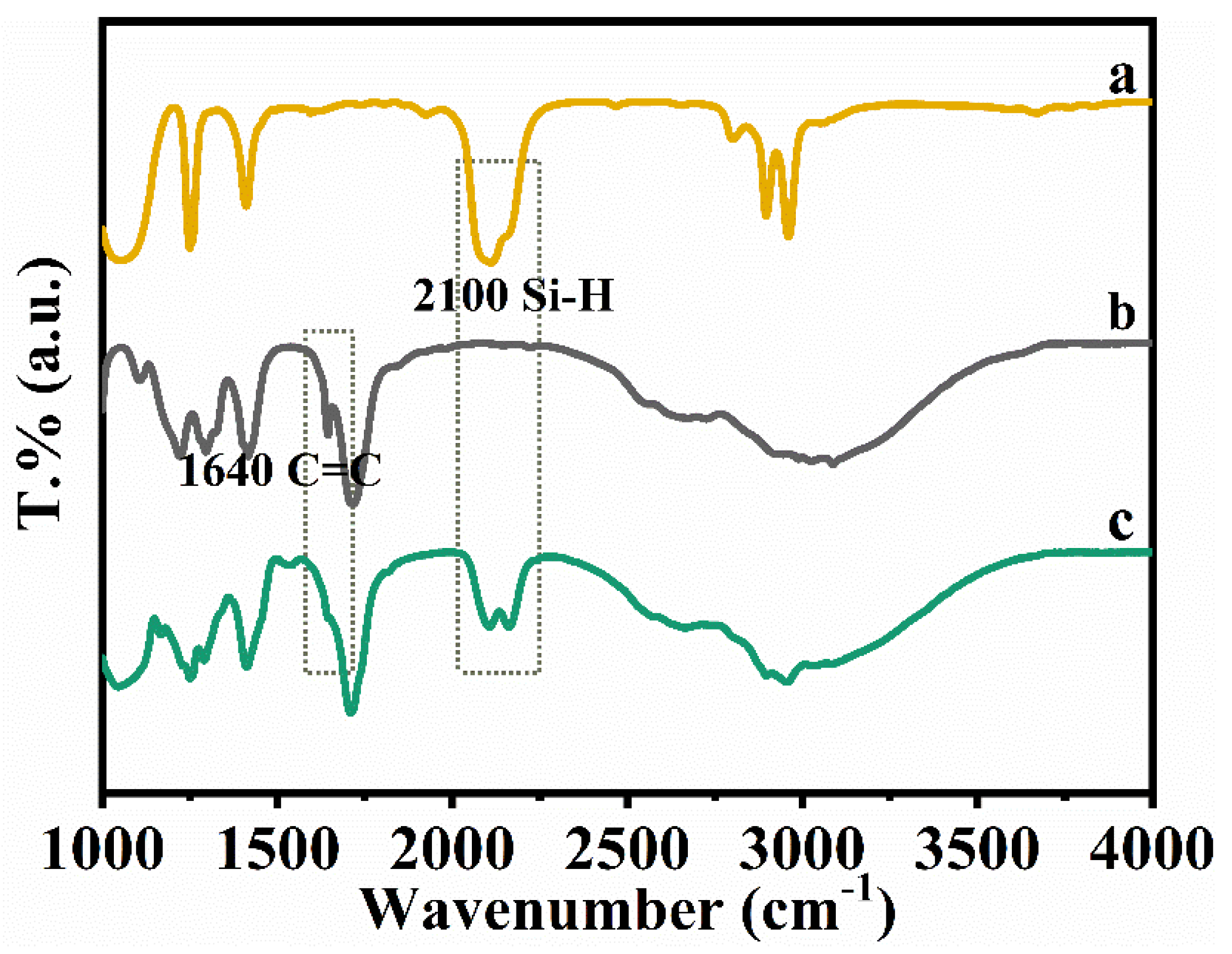
3.1. The Synthesis of BA–PMS
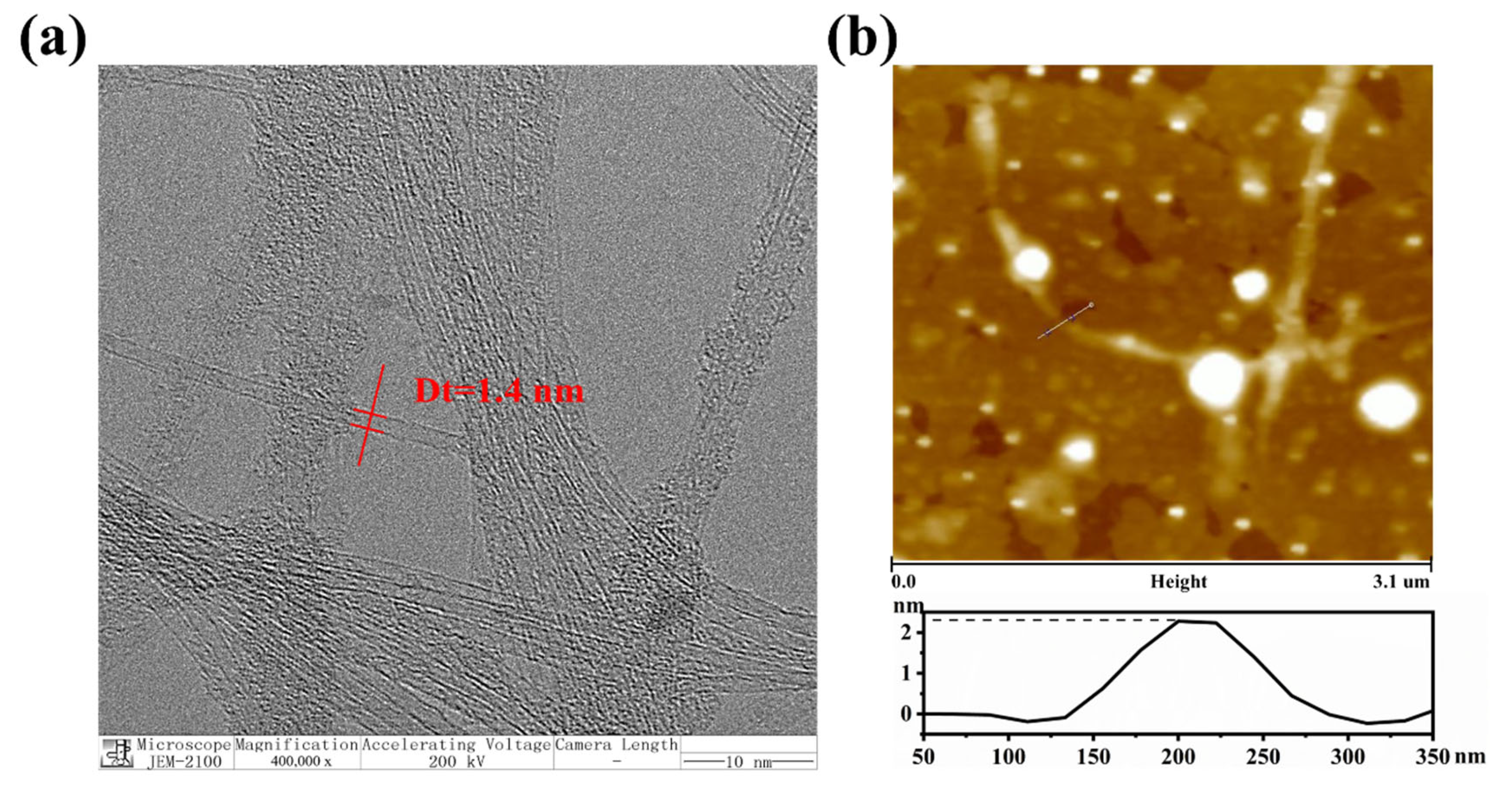
3.2. The Individual Dispersion of Arc–Discharged SWNTs with BA–PMS
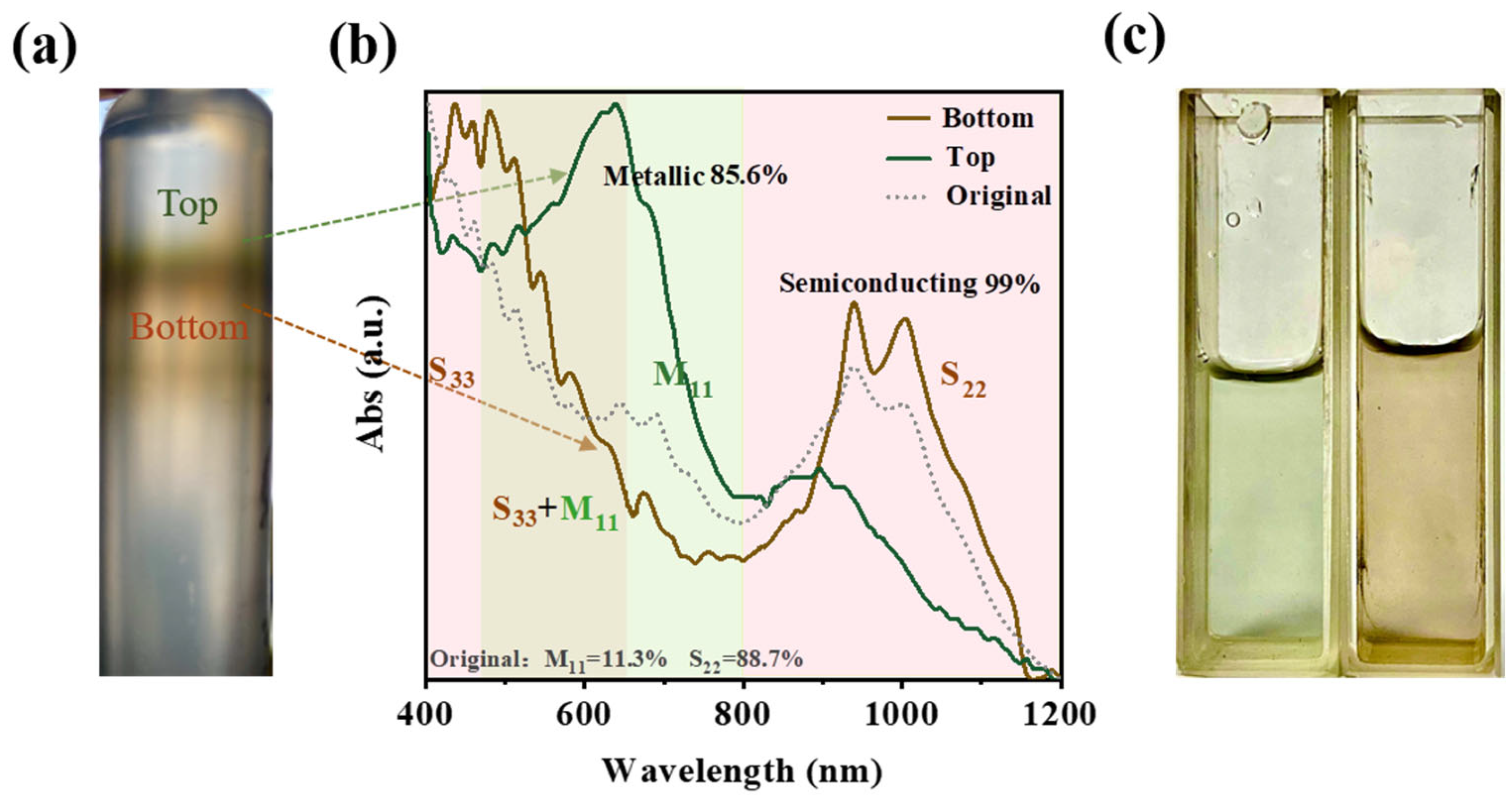
3.3. DGU Separation of the BA–PMS–Dispersed SWNTs
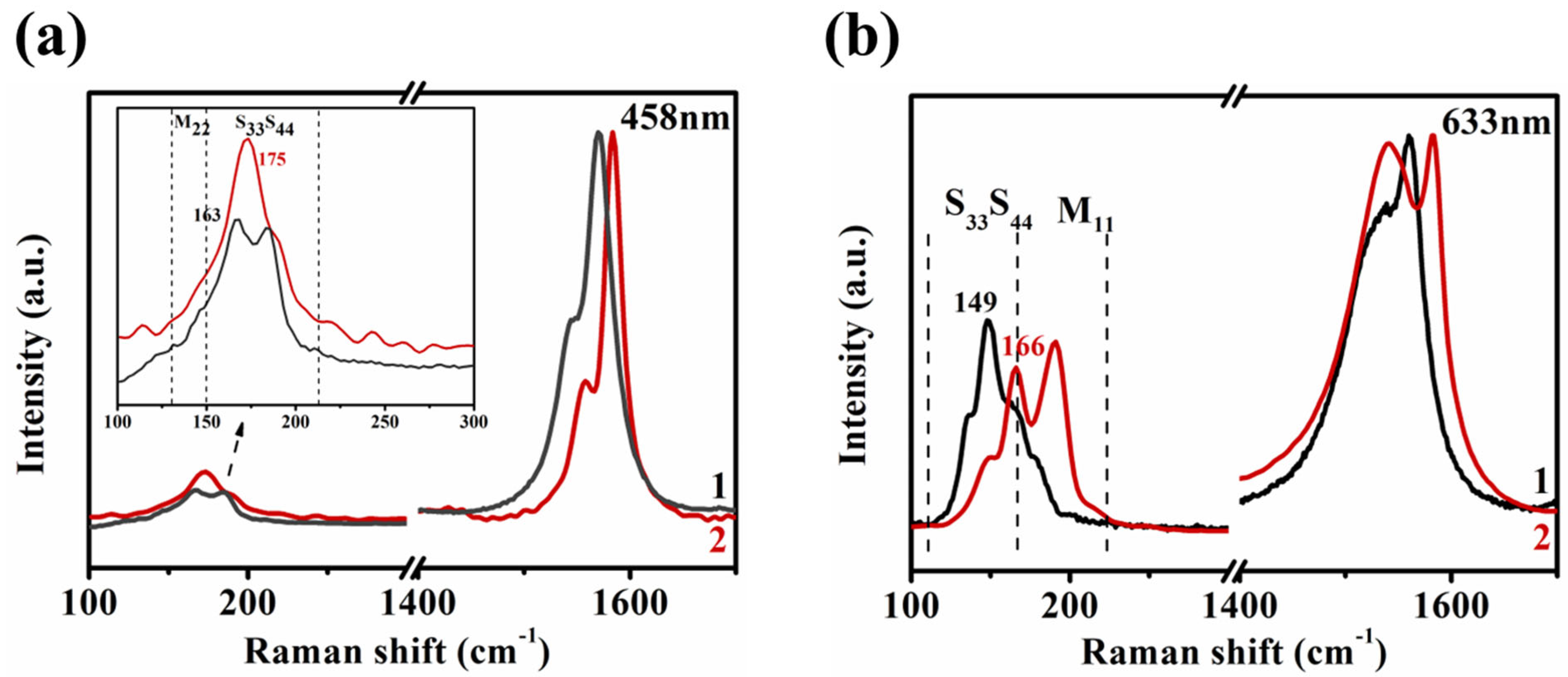
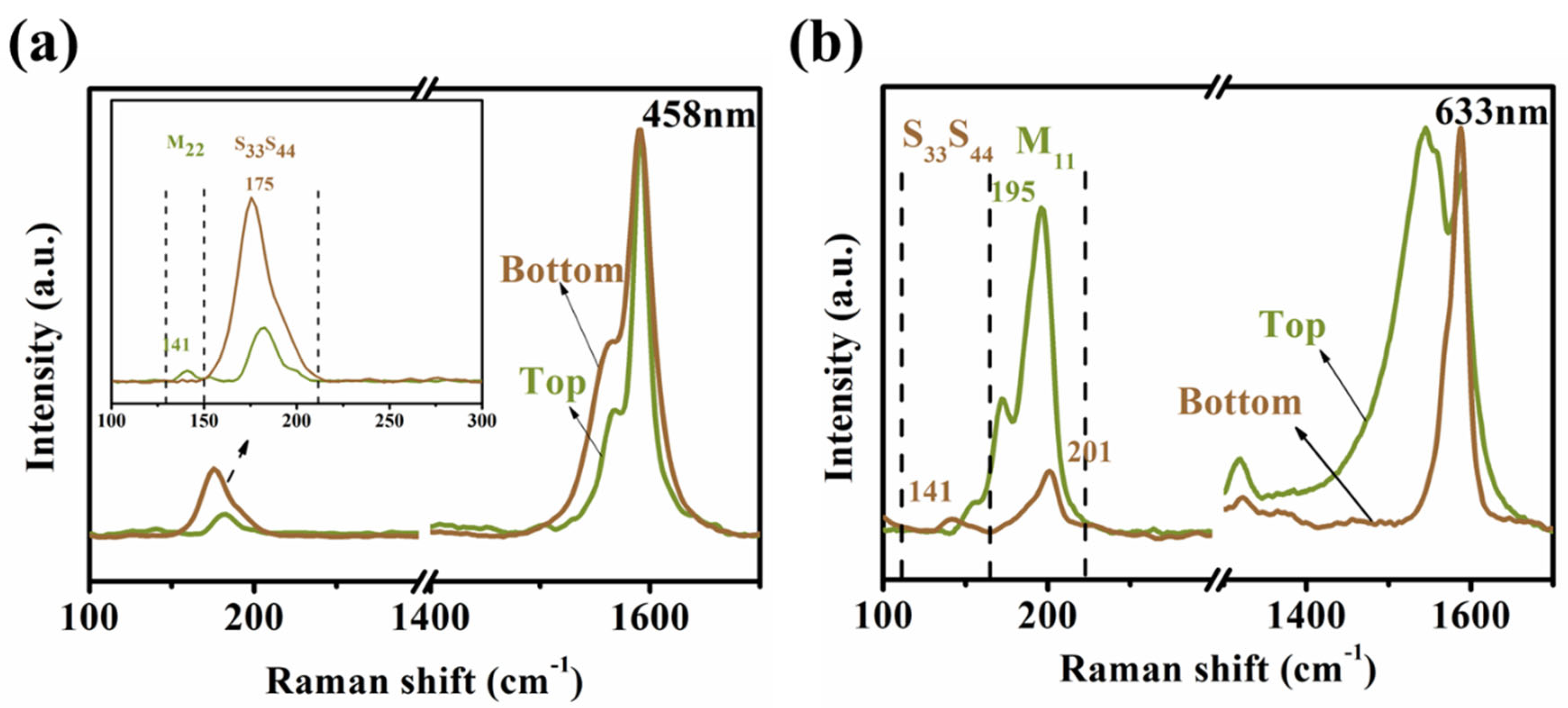
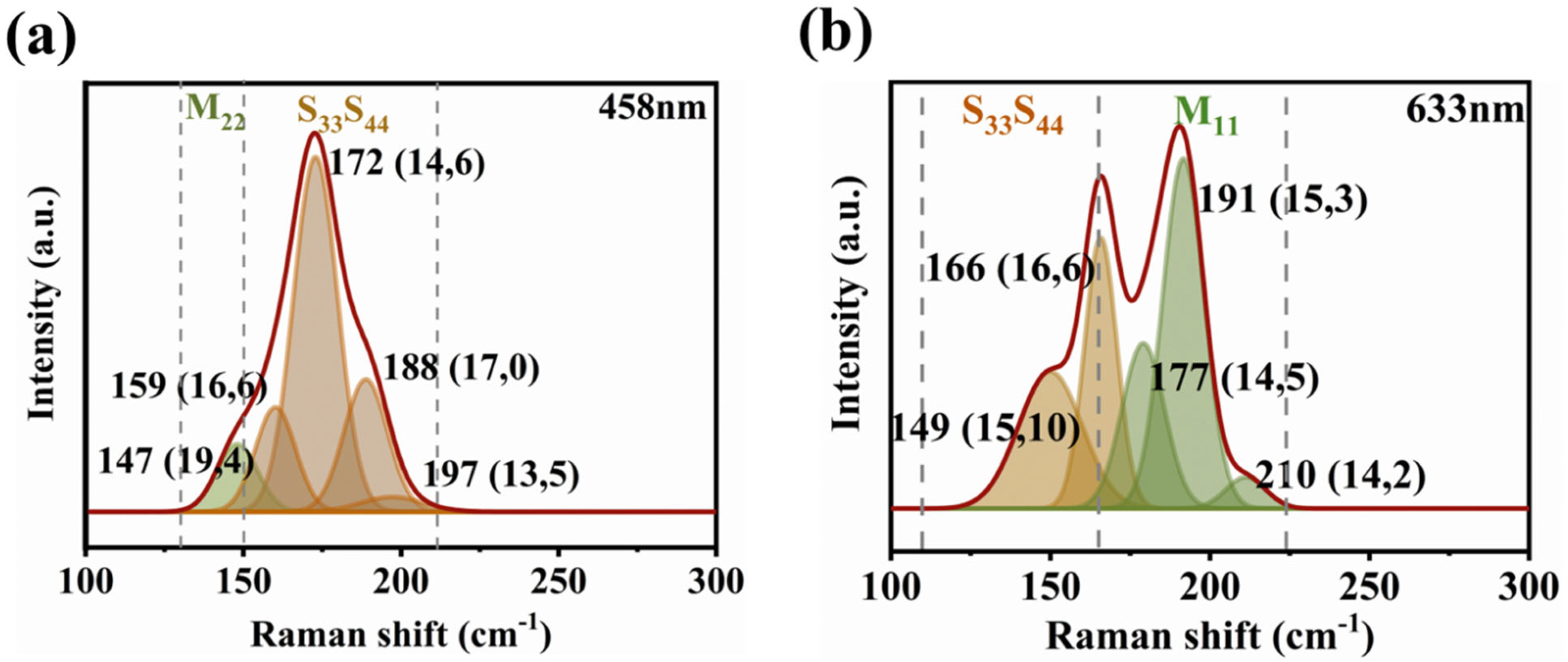
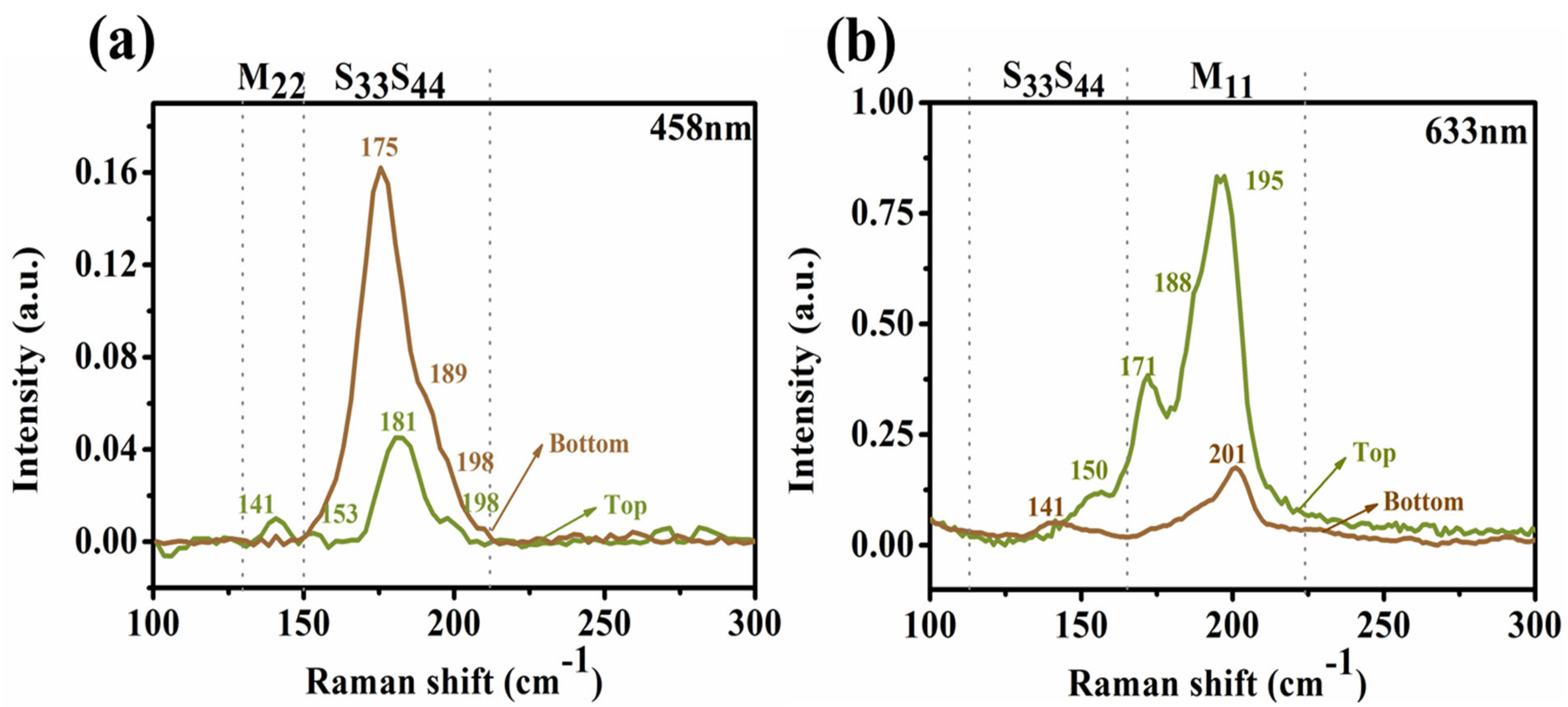
3.4. Assignment of the Chiral Indices (n,m) for the BA–PMS–Dispersed SWNTs before and after DGU
3.5. DGU Separation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zorn, N.F.; Berger, F.J.; Zaumseil, J. Charge transport in and electroluminescence from sp(3)–functionalized carbon nanotube networks. ACS Nano 2021, 15, 10451–10463. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.M.; Mohamed, M.G.; El–Mahdy, A.F.M.; Mansoure, T.H.; Wu, K.C.W.; Kuo, S.W. High–performance supercapacitor electrodes prepared from dispersions of tetrabenzonaphthalene–based conjugated microporous polymers and carbon nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 51906–51916. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Younse, H.; Hong, H.; Peterson, G.P. Improving mechanical properties of PVA based nano composite using aligned single–wall carbon nanotubes. Mater. Res. Express. 2019, 6, 10. [Google Scholar] [CrossRef]
- Liu, M.R.; Younes, H.; Hong, H.P.; Peterson, G.P. Polymer nanocomposites with improved mechanical and thermal properties by magnetically aligned carbon nanotubes. Polymer 2019, 166, 81–87. [Google Scholar] [CrossRef]
- Baibarac, M.; Arzumanyan, G.; Daescu, M.; Udrescu, A.; Mamatkulov, K. Anisotropic photoluminescence of poly(3–hexyl thiophene) and their composites with single–walled carbon nanotubes highly separated in metallic and semiconducting tubes. Molecules 2021, 26, 294. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Bao, Z.N. Conjugated polymer sorting of semiconducting carbon nanotubes and their electronic applications. Nano Today 2015, 10, 737–758. [Google Scholar] [CrossRef] [Green Version]
- Baeg, K.J.; Jeong, H.J.; Jeong, S.Y.; Han, J.T.; Lee, G.W. Enhanced ambipolar charge transport in staggered carbon nanotube field–effect transistors for printed complementary–like circuits. Curr. Appl. Phys. 2017, 17, 541–547. [Google Scholar] [CrossRef]
- Bronikowski, M.J.; Willis, P.A.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas–phase production of carbon single–walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vac. Sci. Technol. A 2001, 19, 1800–1805. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.Y.; Zhai, J.L.; Zhang, Y.J.; Tian, L.; Zhang, Z.P. Integrating brucine with carbon nanotubes toward electrochemical sensing of hydroxylamine. Microchim. Acta 2020, 187, 343. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, Y.X.; Jimenez–Oses, G.; Liu, P.; Fang, Y.; Zhang, J.; Lai, Y.C.; Park, S.; Chen, L.W.; Houk, K.N.; et al. N–type conjugated polymer–enabled selective dispersion of semiconducting carbon nanotubes for flexible CMOS–like circuits. Adv. Funct. Mater. 2015, 25, 1837–1844. [Google Scholar] [CrossRef]
- Campo, T.; Pinilla, S.; Galvez, S.; Maria Sanz, J.; Marquez, F.; Morant, C. Synthesis procedure of highly densely packed carbon nanotube forests on TiN. Nanomaterials 2019, 9, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, Y.; Hou, X.; Xiong, B. Bioelectronic nose based on single–stranded DNA and single–walled carbon nanotube to identify a major plant volatile organic compound (p–Ethylphenol) released by phytophthora cactorum infected strawberries. Nanomaterials 2020, 10, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, W.E.; Pompeo, F.; Herrera, J.E.; Balzano, L.; Resasco, D.E. Characterization of single–walled carbon nanotubes (SWNTs) produced by CO disproportionation on Co–Mo Catalysts. Chem. Mater. 2002, 14, 1853–1858. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Chirality dependence of the density–of–states singularities in carbon nanotubes. Phys. Rev. B 2000, 62, 4273–4276. [Google Scholar] [CrossRef] [Green Version]
- Agboola, A.E.; Pike, R.W.; Hertwig, T.A.; Lou, H.H. Conceptual design of carbon nanotube processes. Clean Technol. Environ. Policy 2007, 9, 289–311. [Google Scholar] [CrossRef]
- Koh, B.; Park, J.B.; Hou, X.; Cheng, W. Comparative dispersion studies of single–walled carbon nanotubes in aqueous solution. J. Phys. Chem. B. 2011, 115, 2627–2633. [Google Scholar] [CrossRef]
- Zhang, S.C.; Wang, X.; Yao, F.R.; He, M.S.; Lin, D.W.; Ma, H.; Sun, Y.Y.; Zhao, Q.C.; Liu, K.H.; Ding, F.; et al. Controllable growth of (n, n–1) family of semiconducting carbon nanotubes. Chem 2019, 5, 1182–1193. [Google Scholar] [CrossRef]
- Qin, X.; Peng, F.; Yang, F.; He, X.; Huang, H.; Luo, D.; Yang, J.; Wang, S.; Liu, H.; Peng, L.; et al. Growth of semiconducting single–walled carbon nanotubes by using ceria as catalyst supports. Nano Lett. 2014, 14, 512–517. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.Q.; Xu, Z.; Peng, F.; et al. Chirality–specific growth of single–walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- Gillen, A.J.; Boghossian, A.A. Non–covalent methods of engineering optical sensors based on single–walled carbon nanotubes. Front Chem. 2019, 7, 612. [Google Scholar] [CrossRef]
- Mulvey, J.J.; Feinberg, E.N.; Alidori, S.; McDevitt, M.R.; Heller, D.A.; Scheinberg, D.A. Synthesis, pharmacokinetics, and biological use of lysine–modified single–walled carbon nanotubes. Int. J. Nanomed. 2014, 9, 4245–4255. [Google Scholar]
- Zhang, C.; Wang, P.; Barnes, B.; Fortner, J.; Wang, Y. Cleanly removable surfactant for carbon nanotubes. Chem. Mater. 2021, 33, 4551–4557. [Google Scholar] [CrossRef]
- Liu, L.; Wei, X.; Yao, Z.; Li, X.; Wang, W.; Wang, Y.; Zhou, W.; Xiong, F.; Kataura, H.; Xie, S.; et al. Separation of metallic and semiconducting single–wall carbon nanotubes using sodium hyodeoxycholate surfactant. J. Phys. Chem. C 2022, 126, 3787–3795. [Google Scholar] [CrossRef]
- Yamamoto, T.; Miyauchi, Y.; Motoyanagi, J.; Fukushima, T.; Aida, T.; Kato, M.; Maruyama, S. Improved bath sonication method for dispersion of individual single–walled carbon nanotubes using new triphenylene–based surfactant. Jpn. J. Appl. Phys. 2008, 47, 2000–2004. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chu, H.; Li, Y. Why single–walled carbon nanotubes can be dispersed in imidazolium–based ionic liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.H.; Lian, Y.F. Dispersion of arc–discharged single–walled carbon nanotubes using the natural alpha–amino acid derivative N–dodecanoyl leucinate. RSC Adv. 2020, 10, 21643–21649. [Google Scholar] [CrossRef]
- Ao, G.; Streit, J.K.; Fagan, J.A.; Zheng, M. Differentiating left– and right–handed carbon nanotubes by DNA. J. Am. Chem. Soc. 2016, 138, 16677–16685. [Google Scholar] [CrossRef]
- Li, K.; Kardelis, V.; Adronov, A. “Click” generation of a conjugated polymer library for SWNT dispersion. J. Polym. Sci. Pol. Chem. 2018, 56, 2053–2058. [Google Scholar] [CrossRef]
- Xu, L.; Valasek, M.; Hennrich, F.; Sedghamiz, E.; Penaloza–Amion, M.; Haussinger, D.; Wenzel, W.; Kappes, M.M.; Mayor, M. Enantiomeric separation of semiconducting single–walled carbon nanotubes by acid cleavable chiral polyfluorene. ACS Nano 2021, 15, 4699–4709. [Google Scholar] [CrossRef]
- Bodnaryk, W.J.; Fong, D.; Adronov, A. Enrichment of metallic carbon nanotubes using a two–polymer extraction method. ACS Omega 2018, 3, 16238–16245. [Google Scholar] [CrossRef]
- Fukumaru, T.; Toshimitsu, F.; Fujigaya, T.; Nakashima, N. Effects of the chemical structure of polyfluorene on selective extraction of semiconducting single–walled carbon nanotubes. Nanoscale 2014, 6, 5879–5886. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, J.; Qian, L.; Han, X.; Wu, L.; Wu, W.; Song, M.; Zhou, L.; Su, W.; Wang, C.; et al. Sorting of large–diameter semiconducting carbon nanotube and printed flexible driving circuit for organic light emitting diode (OLED). Nanoscale 2014, 6, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, Z.; Lefebvre, J.; Cheng, F.; Dubey, G.; Zou, S.; Finnie, P.; Hrdina, A.; Scoles, L.; Lopinski, G.P.; et al. Enrichment of large–diameter semiconducting SWCNTs by polyfluorene extraction for high network density thin film transistors. Nanoscale 2014, 6, 2328–2339. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Yoon, Y.; Park, S.; Oh, J.H.; Hong, S.; Liyanage, L.S.; Wang, H.; Morishita, S.; Patil, N.; Park, Y.J.; et al. Selective dispersion of high purity semiconducting single–walled carbon nanotubes with regioregular poly(3–alkylthiophene)s. Nat. Commun. 2011, 2, 541. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Pochorovski, I.; Bao, Z. Separation of semiconducting carbon nanotubes for flexible and stretchable electronics using polymer removable method. Acc. Chem. Res. 2017, 50, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Gomulya, W.; Costanzo, G.D.; de Carvalho, E.J.; Bisri, S.Z.; Derenskyi, V.; Fritsch, M.; Frohlich, N.; Allard, S.; Gordiichuk, P.; Herrmann, A.; et al. Semiconducting single–walled carbon nanotubes on demand by polymer wrapping. Adv. Mater. 2013, 25, 2948–2956. [Google Scholar] [CrossRef]
- Chen, F.; Wang, B.; Chen, Y.; Li, L.J. Toward the extraction of single species of single–walled carbon nanotubes using fluorene–based polymers. Nano Lett. 2007, 7, 3013–3017. [Google Scholar] [CrossRef]
- Chung, W.; Nobusawa, K.; Kamikubo, H.; Kataoka, M.; Fujiki, M.; Naito, M. Time–resolved observation of chiral–index–selective wrapping on single–walled carbon nanotube with non–aromatic polysilane. J. Am. Chem. Soc. 2013, 135, 2374–2383. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Y.; Lian, Y. Selective extraction of metallic arc–discharged single–walled carbon nanotubes by a water soluble polymethylsilane derivative. RSC Adv. 2015, 5, 102238–102246. [Google Scholar] [CrossRef]
- Li, H.P.; Zhou, L.L.; Wu, T. Sodium dodecyl benzene sulfonate for single–walled carbon nanotubes separation in gel chromatography. Diamond Relat. Mater. 2018, 88, 189–192. [Google Scholar] [CrossRef]
- Yomogida, Y.; Tanaka, T.; Zhang, M.; Yudasaka, M.; Wei, X.; Kataura, H. Industrial–scale separation of high–purity single–chirality single–wall carbon nanotubes for biological imaging. Nat. Commun. 2016, 7, 12056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Gordeev, G.; Garrity, O.; Peyyety, N.A.; Selvasundaram, P.B.; Dehm, S.; Krupke, R.; Cambre, S.; Wenseleers, W.; Reich, S.; et al. Separation of specific single–enantiomer single–wall carbon nanotubes in the large–diameter regime. ACS Nano 2020, 14, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Turek, E.; Wasiak, T.; Stando, G.; Janas, D. Probing the mechanics of aqueous two–phase extraction using large diameter single–walled carbon nanotubes. Nanotechnology 2018, 29, 40. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.T.; Schmidt, C.F.; Ros, A. Length–selective dielectrophoretic manipulation of single–walled carbon nanotubes. Anal. Chem. 2020, 92, 8901–8908. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.T.; Sonker, M.; Ros, A. Carbon nanotube dielectrophoresis: Theory and applications. Electrophoresis 2020, 41, 1893–1914. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, D.C.; Zhao, H.; Li, Y. Separation of single–walled carbon nanotubes using the amino acid surfactant N–cocoyl sarcosinate. ACS Appl. Nano Mater. 2019, 2, 5440–5449. [Google Scholar] [CrossRef]
- Arnold, M.S.; Stupp, S.I.; Hersam, M.C. Enrichment of single–walled carbon nanotubes by diameter in density gradients. Nano Lett. 2005, 5, 713–718. [Google Scholar] [CrossRef]
- Chernov, A.I.; Obraztsova, E.D. Metallic single–wall carbon nanotubes separated by density gradient ultracentrifugation. Phys. Status Solidi B 2009, 246, 2477–2481. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Hasan, T.; Tan, P.H.; Sciascia, C.; Privitera, G.; Di Marco, G.; Gucciardi, P.G.; Ferrari, A.C. Density gradient ultracentrifugation of nanotubes: Interplay of bundling and surfactants encapsulation. J. Phys. Chem. C 2010, 114, 17267–17285. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Einarsson, E.; Lagoudas, G.; Shiomi, J.; Chiashi, S.; Maruyama, S. Tunable separation of single–walled carbon nanotubes by dual–surfactant density gradient ultracentrifugation. Nano Res. 2011, 4, 623–634. [Google Scholar] [CrossRef]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting carbon nanotubes by electronic structure using density differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Seo, J.W.T.; Green, A.A.; Hersam, M.C. Sorting single–walled carbon nanotubes by electronic type using nonionic, biocompatible block copolymers. ACS Nano 2010, 4, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.R.; West, R. Photoinitiation of vinyl polymerization by polysilanes. Appl. Organomet. Chem. 1987, 1, 7–14. [Google Scholar] [CrossRef]
- Pu, W.; Li, X.; Li, G.; Hu, T. Phenol substituted polymethylsilane: A soluble conducting polymer with low cross–linking density. Polym. Bull. 2015, 72, 779–790. [Google Scholar] [CrossRef]
- Kawabe, T.; Naito, M.; Fujiki, M. Multiblock polysilane copolymers: one–pot wurtz synthesis, fluoride anion–induced block–selective scission experiments, and spectroscopic characterization. Macromolecules 2008, 41, 1952–1960. [Google Scholar] [CrossRef]
- Naito, M.; Fujiki, M. Polysilanes on surfaces. Soft Matter. 2008, 4, 211–223. [Google Scholar] [CrossRef]
- Itkis, M.E.; Perea, D.E.; Niyogi, S.; Rickard, S.M.; Hamon, M.A.; Hu, H.; Zhao, B.; Haddon, R.C. Purity evaluation of as–prepared single–walled carbon nanotube soot by use of solution–phase Near–IR spectroscopy. Nano Lett. 2003, 3, 309–314. [Google Scholar] [CrossRef]
- Qiu, H.; Maeda, Y.; Akasaka, T. Facile and scalable route for highly efficient enrichment of semiconducting single–walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 16529–16533. [Google Scholar] [CrossRef]
- Mistry, K.S.; Larsen, B.A.; Blackburn, J.L. High–yield dispersions of large–diameter semiconducting single–walled carbon nanotubes with tunable narrow chirality distributions. ACS Nano 2013, 7, 2231–2239. [Google Scholar] [CrossRef]
- Seo, J.-W.T.; Yoder, N.L.; Shastry, T.A.; Humes, J.J.; Johns, J.E.; Green, A.A.; Hersam, M.C. Diameter refinement of semiconducting arc discharge single–Walled carbon nanotubes via density gradient ultracentrifugation. J. Phys. Chem. Lett. 2013, 4, 2805–2810. [Google Scholar] [CrossRef]
- Ghosh, S.; Bachilo, S.M.; Weisman, R.B. Advanced sorting of single–Walled carbon nanotubes by nonlinear density–gradient ultracentrifugation. Nat. Nanotechnol. 2010, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, K.; Iitsuka, T.; Fujii, S.; Kataura, H. Separations of metallic and semiconducting carbon nanotubes by using sucrose as a gradient medium. J. Phys. Chem. C 2008, 112, 18889–18894. [Google Scholar] [CrossRef]
- Homenick, C.M.; Rousina Webb, A.; Cheng, F.; Jakubinek, M.B.; Malenfant, P.R.L.; Simard, B. High–yield, single–step separation of metallic and semiconducting SWCNTs using block copolymers at low temperatures. J. Phys. Chem. C 2014, 118, 16156–16164. [Google Scholar] [CrossRef] [Green Version]
- Yanagi, K.; Miyata, Y.; Kataura, H. Optical and conductive characteristics of metallic single–wall carbon nanotubes with three basic colors; cyan, magenta, and yellow. Appl. Phys. Express. 2008, 1, 3. [Google Scholar] [CrossRef]
- Nair, N.; Kim, W.J.; Braatz, R.D.; Strano, M.S. Dynamics of surfactant–suspended single–walled carbon nanotubes in a centrifugal field. Langmuir 2008, 24, 1790–1795. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhou, Q.; Lian, Y. Polymethyl(1–Butyric acidyl)silane–Assisted Dispersion and Density Gradient Ultracentrifugation Separation of Single–Walled Carbon Nanotubes. Nanomaterials 2022, 12, 2094. https://doi.org/10.3390/nano12122094
Liu H, Zhou Q, Lian Y. Polymethyl(1–Butyric acidyl)silane–Assisted Dispersion and Density Gradient Ultracentrifugation Separation of Single–Walled Carbon Nanotubes. Nanomaterials. 2022; 12(12):2094. https://doi.org/10.3390/nano12122094
Chicago/Turabian StyleLiu, Hongming, Qin Zhou, and Yongfu Lian. 2022. "Polymethyl(1–Butyric acidyl)silane–Assisted Dispersion and Density Gradient Ultracentrifugation Separation of Single–Walled Carbon Nanotubes" Nanomaterials 12, no. 12: 2094. https://doi.org/10.3390/nano12122094
APA StyleLiu, H., Zhou, Q., & Lian, Y. (2022). Polymethyl(1–Butyric acidyl)silane–Assisted Dispersion and Density Gradient Ultracentrifugation Separation of Single–Walled Carbon Nanotubes. Nanomaterials, 12(12), 2094. https://doi.org/10.3390/nano12122094





