Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy
Abstract
:1. Introduction
2. Materials and Methods
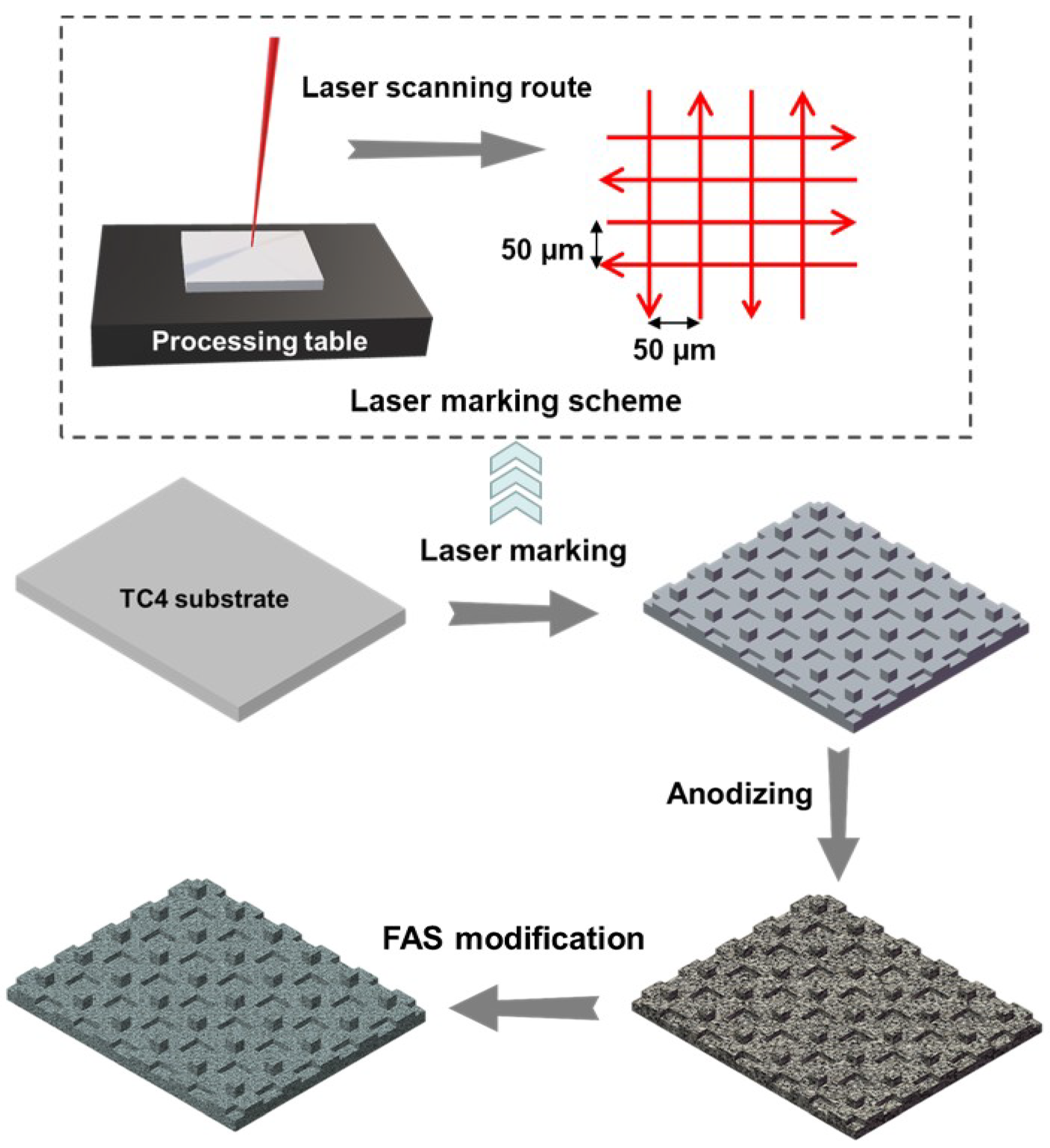
2.1. Preparation Procedures
2.2. Sample Characterization
3. Results and Discussion
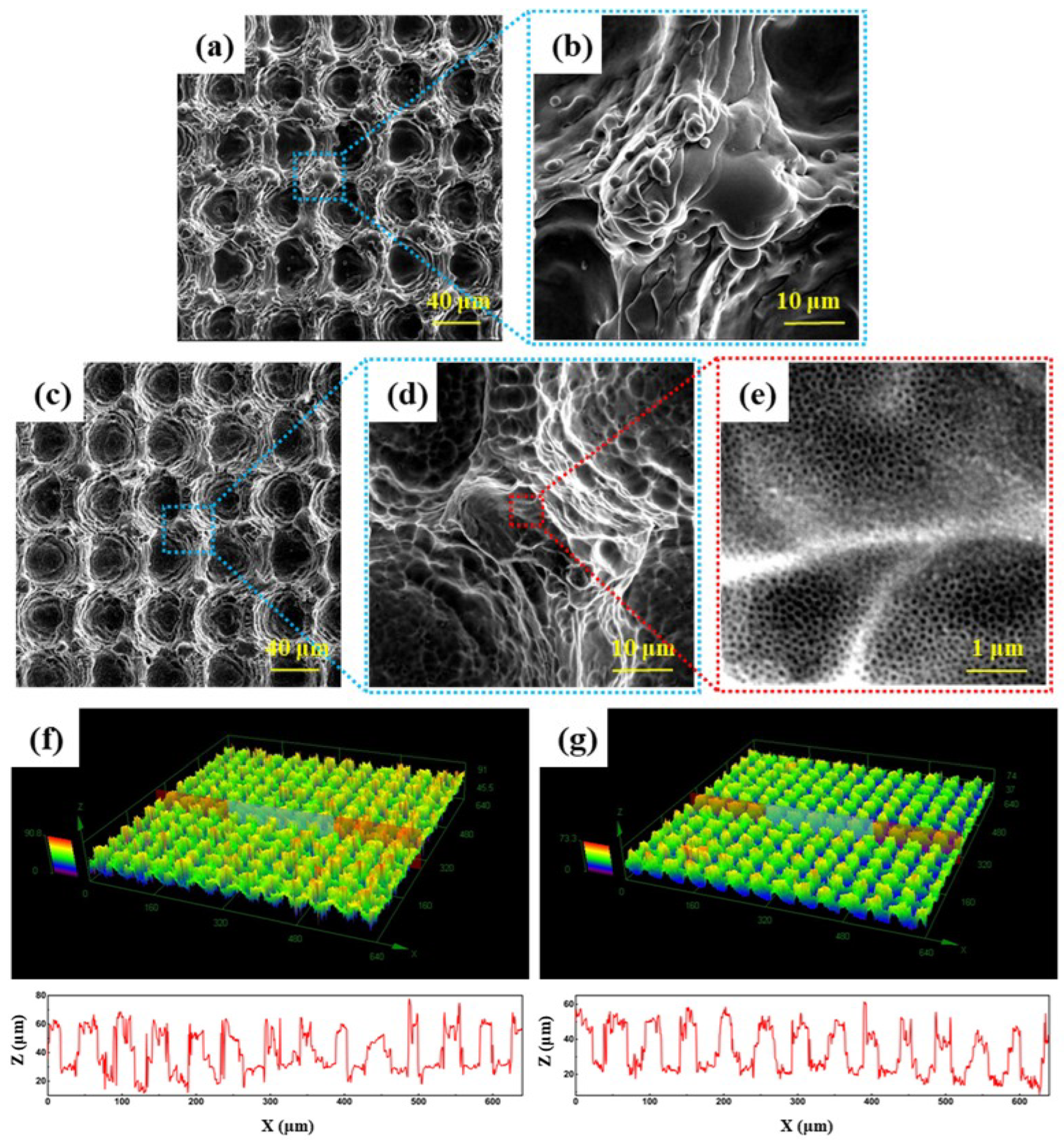
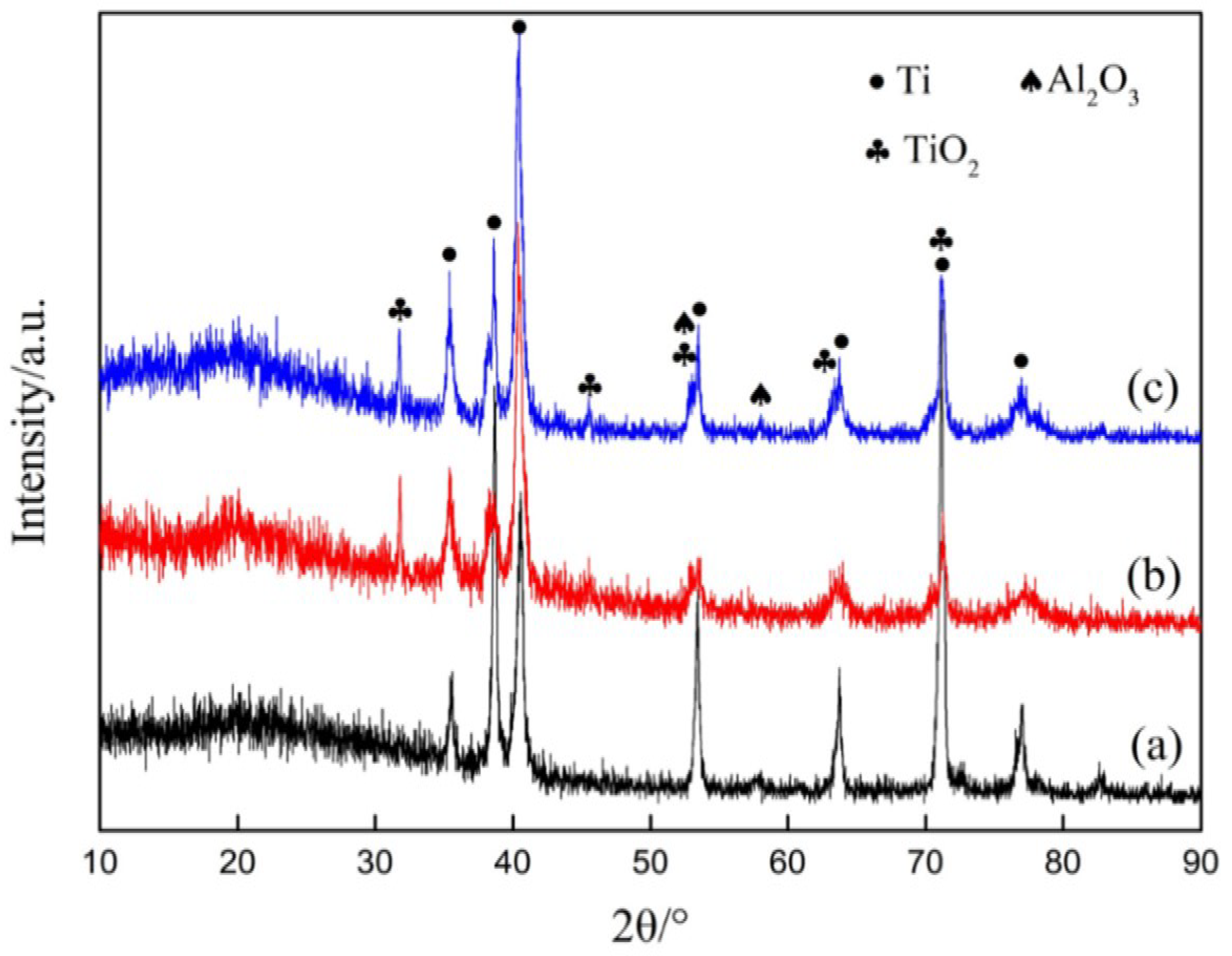
3.1. Microstructure and Phase Constituent
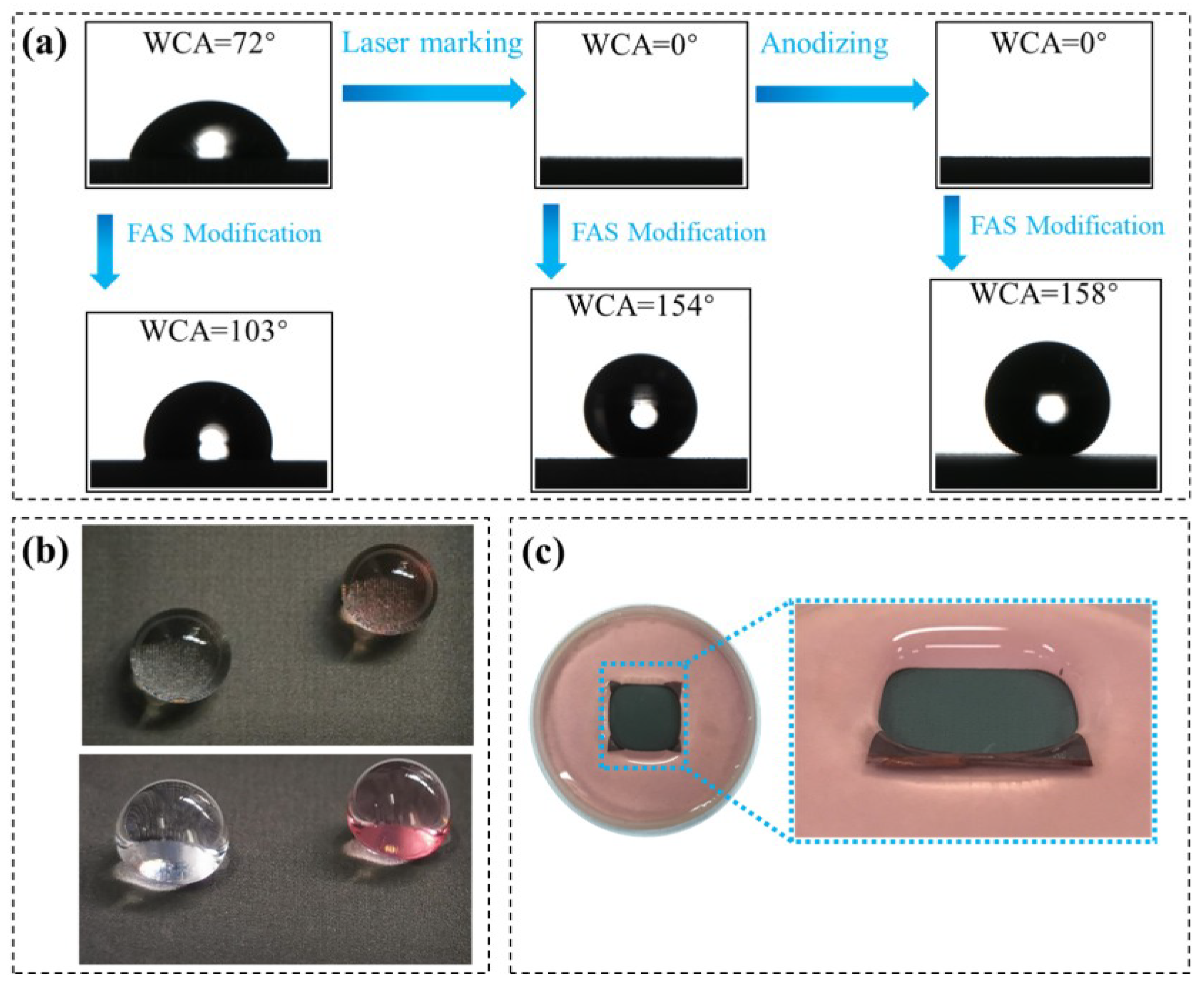
3.2. Wettability and Corrosion Behavior
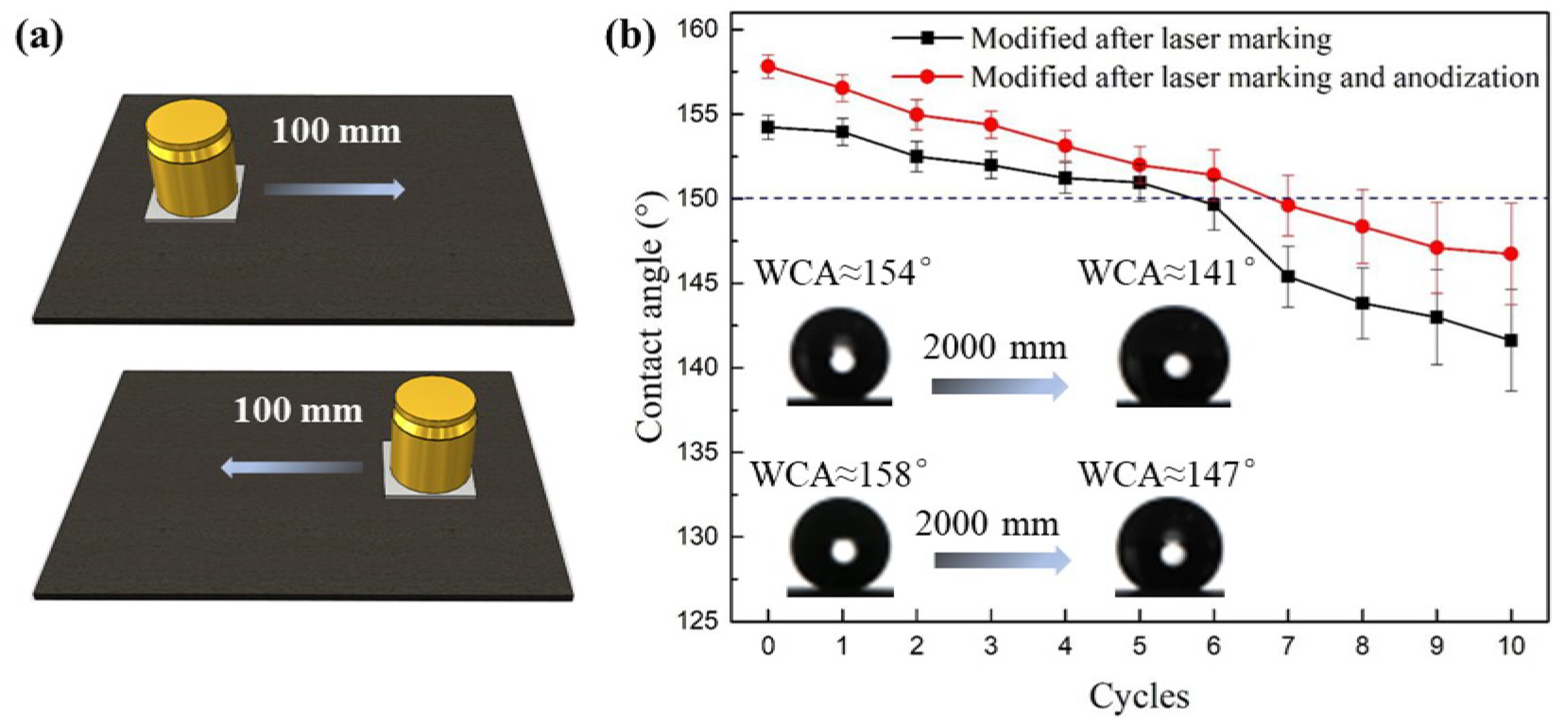
3.3. Superhydrophobic Surface Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, S.; Yang, Y.; Tan, J.; Chen, L.; Wang, Y.; He, Z. The hierarchical surface on AZ31 magnesium alloy: Preparation, properties, and performance. Int. J. Mod. Phys. B 2022, 36, 2240076. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, L.; He, Z.; Tan, J.; Singh, H.; Hayat, M.D.; Yao, C. Preparation and characterisation of AAO/Ni/Ni superhydrophobic coatings on aluminium alloys. Surf. Eng. 2021, 27, 1246–1254. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, eaaw9727. [Google Scholar] [CrossRef] [Green Version]
- Barthwal, S.; Lim, S.-H. A durable, fluorine-free, and repairable superhydrophobic aluminum surface with hierarchical micro/nanostructures and its application for continuous oil-water separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Zhu, L.; Shen, L.; Zhang, Z.; Ren, T.; Zeng, Z.; Chen, T.; Xue, Q. Multifunctional superhydrophobic adsorbents by mixed-dimensional particles assembly for polymorphic and highly efficient oil-water separation. J. Hazard Mater. 2021, 407, 124374. [Google Scholar] [CrossRef]
- Gao, H.; Jian, Y.; Yan, Y. The effects of bio-inspired micro/nano scale structures on anti-icing properties. Soft Matter 2021, 17, 447–466. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Z.; Huang, W.; Zhang, J. Robust superhydrophobic surface for anti-icing and cooling performance: Application of fluorine-modified TiO2 and fumed SiO2. Appl. Surf. Sci. 2021, 538, 148131. [Google Scholar] [CrossRef]
- Lin, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and robust transparent superhydrophobic glass surfaces fabricated by a femtosecond laser with exceptional water repellency and thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, L.; He, Z.; Zhang, S.; Singh, H.; Hayat, M.D.; Yao, C. Influence of pretreatments on physicochemical properties of Ni-P coatings electrodeposited on aluminum alloy. Mater. Des. 2021, 197, 109233. [Google Scholar] [CrossRef]
- Liu, A.-h.; Xu, J.-l. Preparation and corrosion resistance of superhydrophobic coatings on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 2287–2293. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Bi, X.; Wang, L.; Zang, J.; Zhao, Y.; Wang, B. Preparation of durable, self-cleaning and photocatalytic superhydrophobic Ni3S2 coating on 304 stainless steel surface against contaminations. J. Mater. Sci. 2021, 56, 6719–6731. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Xu, X.; Yao, J.; Cai, J.; Luo, K. High-performance integrated additive manufacturing with laser shock peening -induced microstructural evolution and improvement in mechanical properties of Ti6Al4V alloy components. Int. J. Mach. Tools Manuf. 2020, 148, 103475. [Google Scholar] [CrossRef]
- Abd El-Kader, M.F.H.; Elabbasy, M.T.; Adeboye, A.A.; Zeariya, M.G.M.; Menazea, A.A. Morphological, structural and antibacterial behavior of eco-friendly of ZnO/TiO2 nanocomposite synthesized via Hibiscus rosa-sinensis extract. J. Mater. Res. Technol. 2021, 15, 2213–2220. [Google Scholar] [CrossRef]
- Jianbing, M.; Xiaojuan, D.; Yugang, Z.; Rufeng, X.; Xue, B.; Haian, Z. Fabrication of a Low Adhesive Superhydrophobic Surface on Ti6Al4V Alloys Using TiO₂/Ni Composite Electrodeposition. Micromachines 2019, 10, 121. [Google Scholar]
- Ge He, S.L.; Wenguo, X.; Tianlong, Y.; Jingyan, L.; Tanlong, D. Durable superhydrophobic Zn ZnO TiO2 surfaces on Ti6V4V substrate with self-cleaning property and switchable wettability. Ceram. Int. 2018, 44, 638–647. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Parkin, I.P. Repellent materials. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, J.; Tao, H.; Chen, S.; Pan, L.; Wang, T. Nanostructures in superhydrophobic Ti6Al4V hierarchical surfaces control wetting state transitions. Soft Matter 2015, 11, 3806–3811. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Z.; Cao, L.; Weng, Z.; Wang, L.; Li, L.; Saeed, S.; Lian, Z.; Wang, Z. Fabrication of biomimetic superhydrophobic and anti-icing Ti6Al4V alloy surfaces by direct laser interference lithography and hydrothermal treatment. Appl. Surf. Sci. 2020, 534, 147576. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Guo, D. Facile fabrication of superhydrophobic surfaces with low roughness on Ti–6Al–4V substrates via anodization. Appl. Surf. Sci. 2014, 314, 754–759. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, W.; Liu, A.; Zhang, L.; Dong, G.; Bo, W.; Ling, X. Preparation and Properties of Superhydrophobic Titanium Alloy with Hierarchical Structure. Rare Met. Mater. Eng. 2018, 47, 3748–3753. [Google Scholar]
- Sun, J.; Chen, C.; Song, J.; Liu, J.; Yang, X.; Liu, J.; Liu, X.; Lu, Y. A universal method to create surface patterns with extreme wettability on metal substrates. J. Colloid Interface Sci. 2019, 535, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Aldalbahi, A.; El-Naggar, M.E.; Ahmed, M.K.; Periyasami, G.; Rahaman, M.; Menazea, A.A. Core–shell Au@Se nanoparticles embedded in cellulose acetate/polyvinylidene fluoride scaffold for wound healing. J. Mater. Res. Technol. 2020, 9, 15045–15056. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Ke, C.; Yu, J.; Wang, R. Nanosecond laser fabrication of superhydrophobic Ti6Al4V surfaces assisted with different liquids. Colloid Interface Sci. Commun. 2020, 35, 100256. [Google Scholar] [CrossRef]
- Xin, G.; Wu, C.; Cao, H.; Liu, W.; Li, B.; Huang, Y.; Rong, Y.; Zhang, G. Superhydrophobic TC4 alloy surface fabricated by laser micro-scanning to reduce adhesion and drag resistance. Surf. Coat. Technol. 2020, 391, 125707. [Google Scholar] [CrossRef]
- Pu, Z.; Zhang, D.; Jing, X.; Yang, Z.; Yang, C.; Ehmann, K.F. Fabrication of super-hydrophobic and highly oleophobic Ti-6Al-4 V surfaces by a hybrid method. Mater. Res. Bull. 2020, 130, 110915. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Wu, Y.; Liu, G.; Wu, X. Micro/nano-structures transition and electrochemical response of Ti-6Al-4V alloy in simulated seawater. Surf. Topogr. Metrol. Prop. 2018, 6, 034009. [Google Scholar] [CrossRef]
- Plummer, D.M.; Göke, S.; Rauber, R.M.; Di Girolamo, L. Discrimination of Mixed- versus Ice-Phase Clouds Using Dual-Polarization Radar with Application to Detection of Aircraft Icing Regions. J. Appl. Meteorol. Climatol. 2010, 49, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Politovich, M.K. Aircraft Icing Caused by Large Supercooled Droplets. J. Appl. Meteorol. 1989, 28, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Xiang, G.-X.; Li, S.-Y.; Song, H.; Nan, Y.-G. Fabrication of modifier-free superhydrophobic surfaces with anti-icing and self-cleaning properties on Ti substrate by anodization method. Microelectron. Eng. 2020, 233, 111430. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, H.; Chen, S.; Xie, Y.; Zhou, T.; Wang, T.; Tao, J. Water repellency of hierarchical superhydrophobic Ti6Al4V surfaces improved by secondary nanostructures. Appl. Surf. Sci. 2014, 321, 469–474. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wu, Y.; Lian, J.; Wang, Y.; Sun, J.; He, Z.; Gu, Z. Potentiostatic electrodeposition of self-supported Ni S electrocatalyst supported on Ni foam for efficient hydrogen evolution. Mater. Des. 2021, 198, 109316. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, Y.; He, Z.; Gu, Z.; You, S. Potentiostatic electrodeposited of Ni–Fe–Sn on Ni foam served as an excellent electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 26930–26939. [Google Scholar] [CrossRef]
- Wu, H.; Xie, L.; Zhang, R.; Tian, Y.; Liu, S.; He, M.; Huang, C.; Tian, W. A novel method to fabricate organic-free superhydrophobic surface on titanium substrates by removal of surface hydroxyl groups. Appl. Surf. Sci. 2019, 479, 1089–1097. [Google Scholar] [CrossRef]

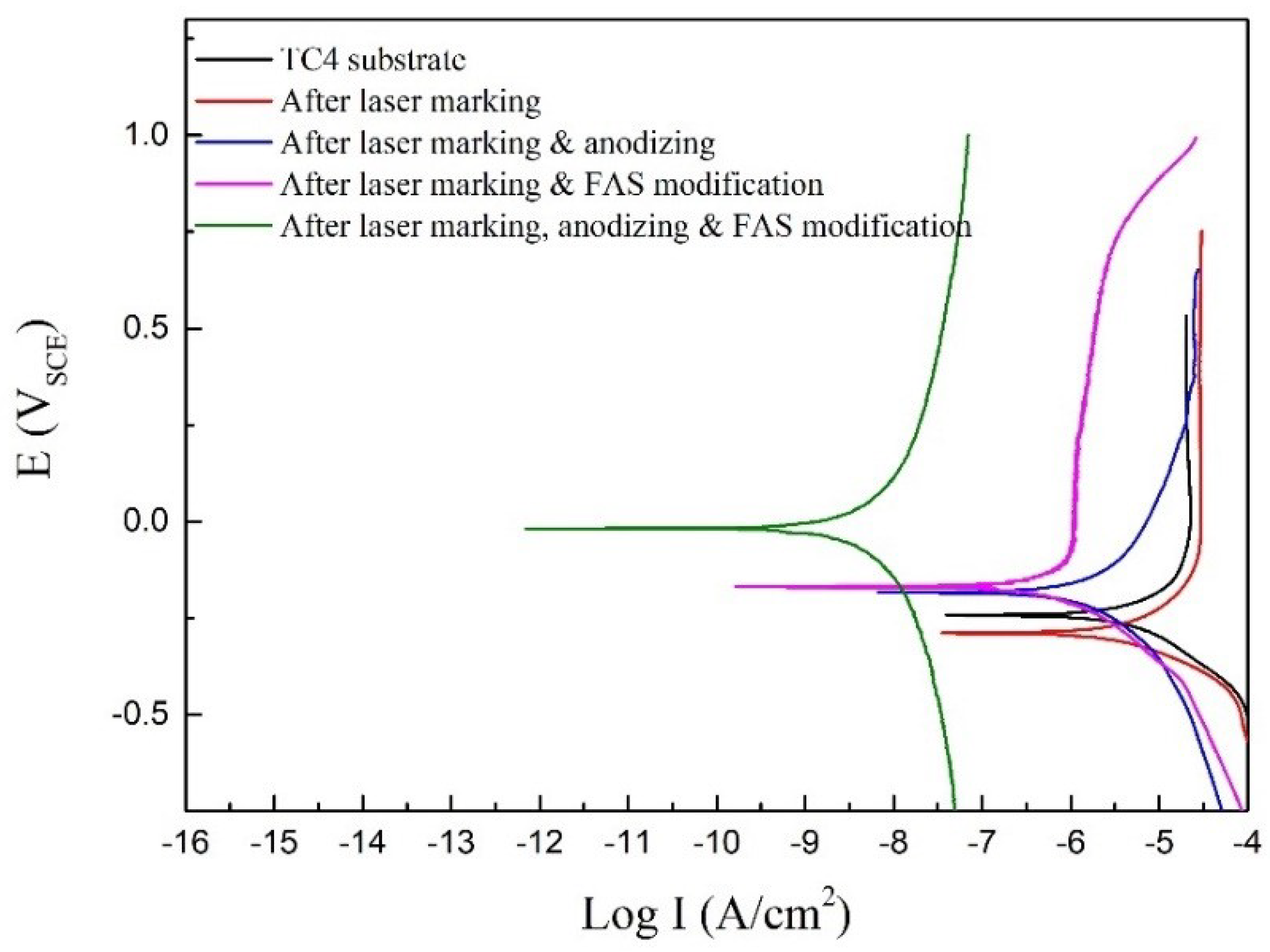

| Sample | Ecorr (VSCE) | icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| TC4 substrate | −0.243 | 1.37 × 10−5 | 0.161 |
| After laser marking | −0.289 | 9.88 × 10−6 | 0.116 |
| After laser marking & anodizing | −0.184 | 4.72 × 10−6 | 0.055 |
| After laser marking & FAS modification | −0.168 | 4.34 × 10−8 | 5.096 × 10−4 |
| After laser marking, anodizing & FAS modification | −0.01 | 7.66 × 10−9 | 8.994 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, J.; Yang, Y.; Liu, Z.; Wang, H.; He, Z. Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy. Nanomaterials 2022, 12, 2086. https://doi.org/10.3390/nano12122086
Wang Y, Chen J, Yang Y, Liu Z, Wang H, He Z. Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy. Nanomaterials. 2022; 12(12):2086. https://doi.org/10.3390/nano12122086
Chicago/Turabian StyleWang, Yuxin, Jiahuan Chen, Yifan Yang, Zihan Liu, Hao Wang, and Zhen He. 2022. "Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy" Nanomaterials 12, no. 12: 2086. https://doi.org/10.3390/nano12122086
APA StyleWang, Y., Chen, J., Yang, Y., Liu, Z., Wang, H., & He, Z. (2022). Nanostructured Superhydrophobic Titanium-Based Materials: A Novel Preparation Pathway to Attain Superhydrophobicity on TC4 Alloy. Nanomaterials, 12(12), 2086. https://doi.org/10.3390/nano12122086







