Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Garnet LLZTO Electrolytes
2.2. Preparation of the AlN Mixed Interlayer
2.3. The DFT Method
2.4. Assembly of Symmetric Cells and Hybrid Solid-State Full Cells
2.5. Characterizations
3. Results
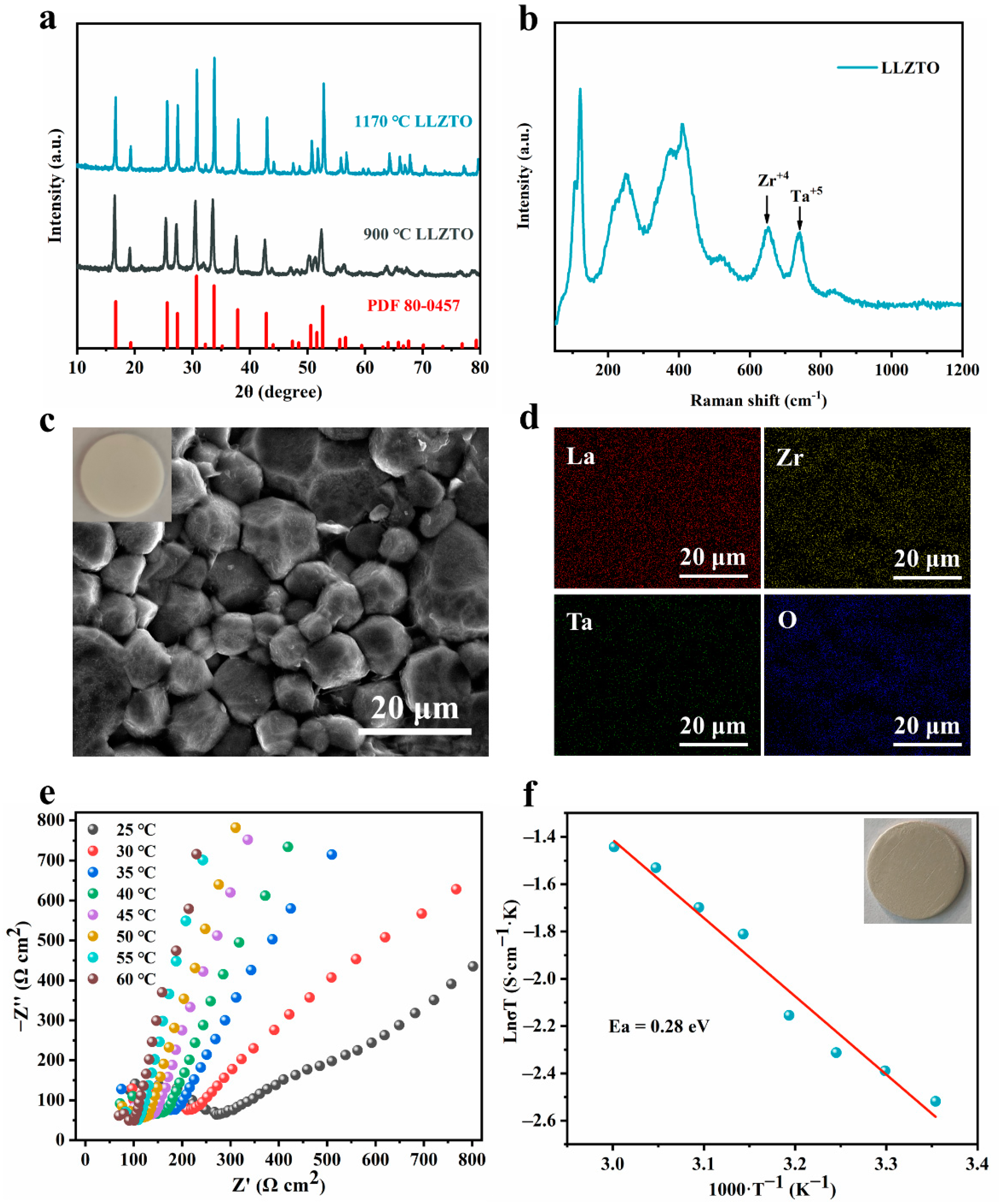
3.1. Characterization of LLZTO Solid Electrolyte Materials
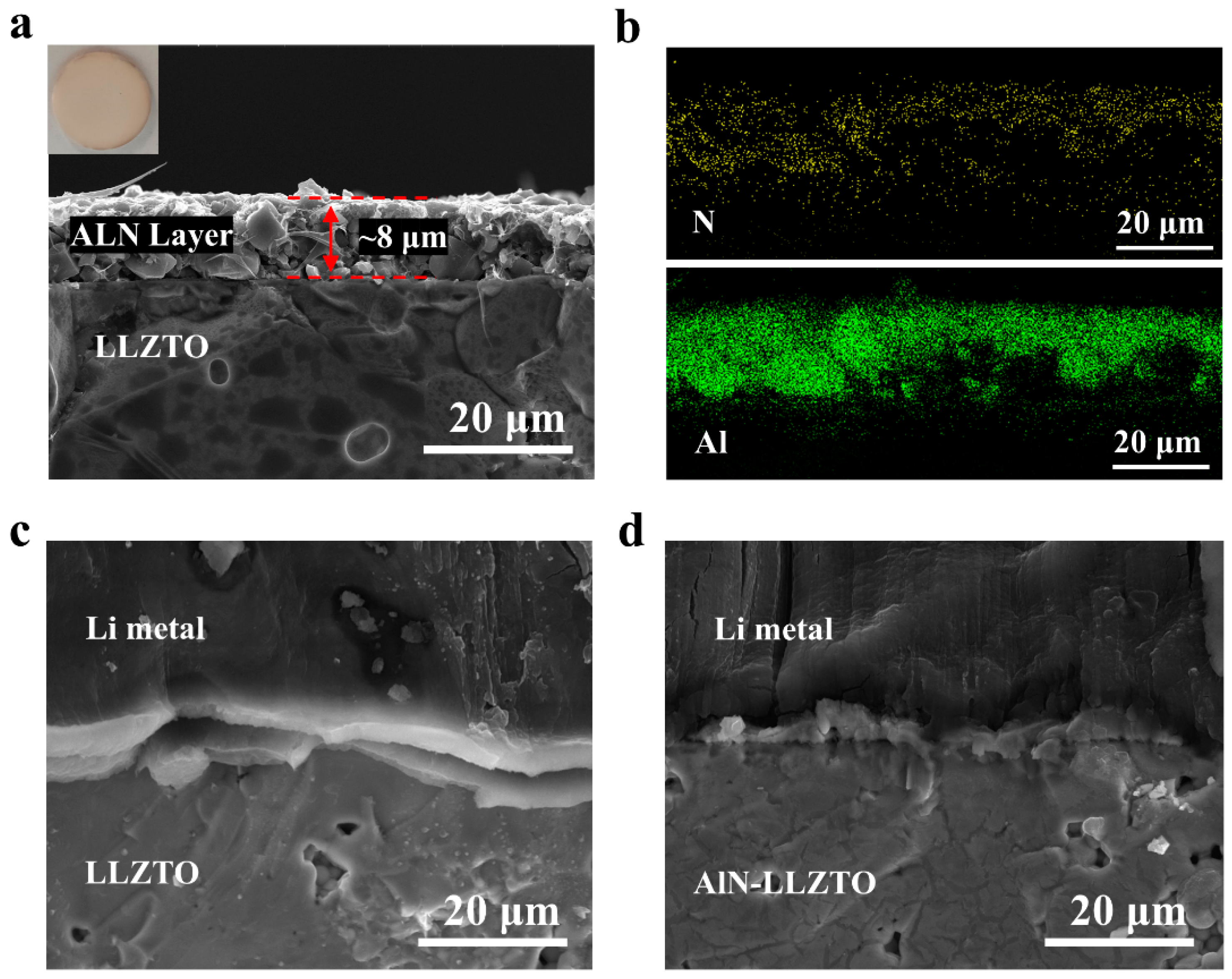
3.2. Characterization of AlN Modified LLZTO
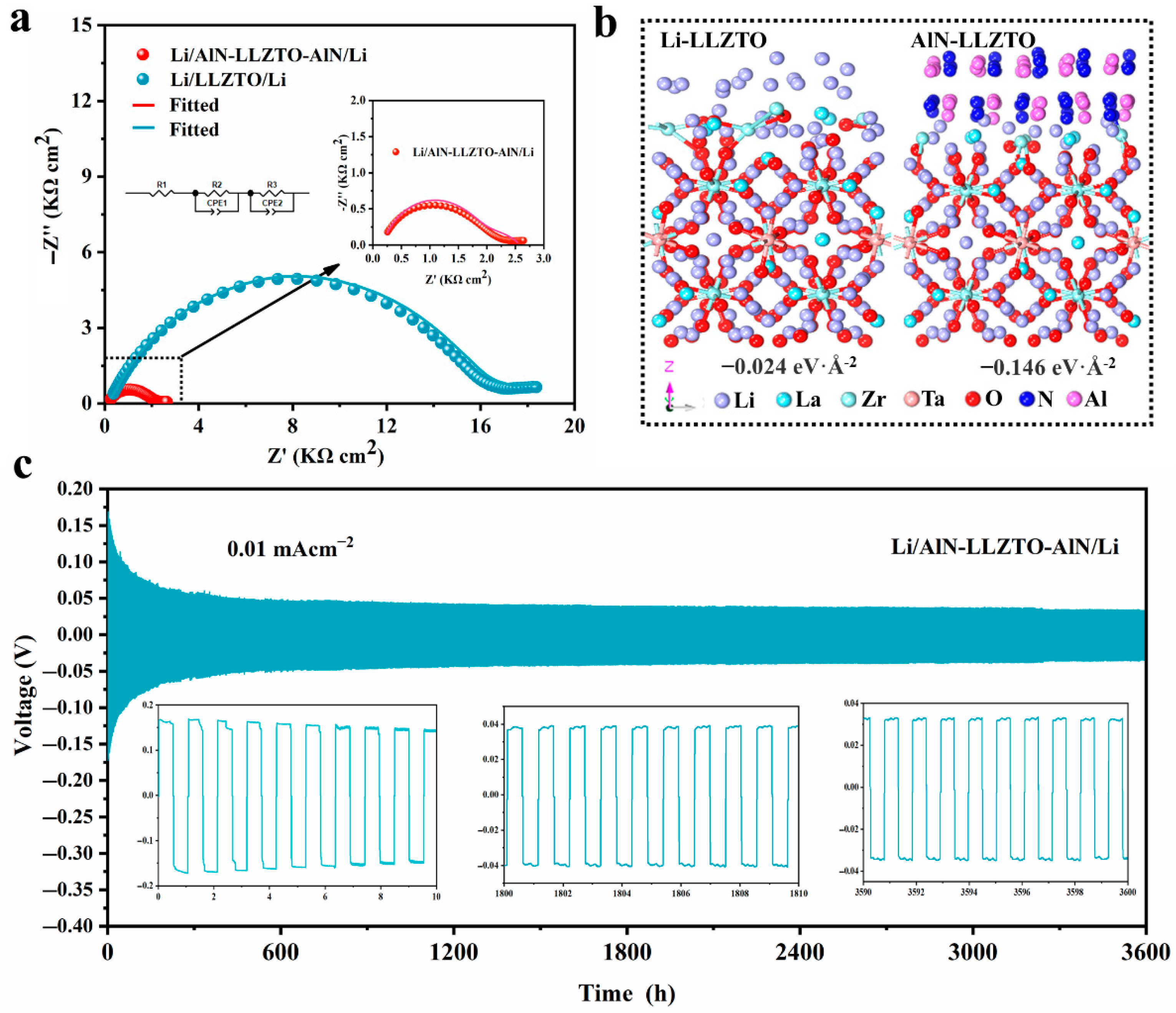
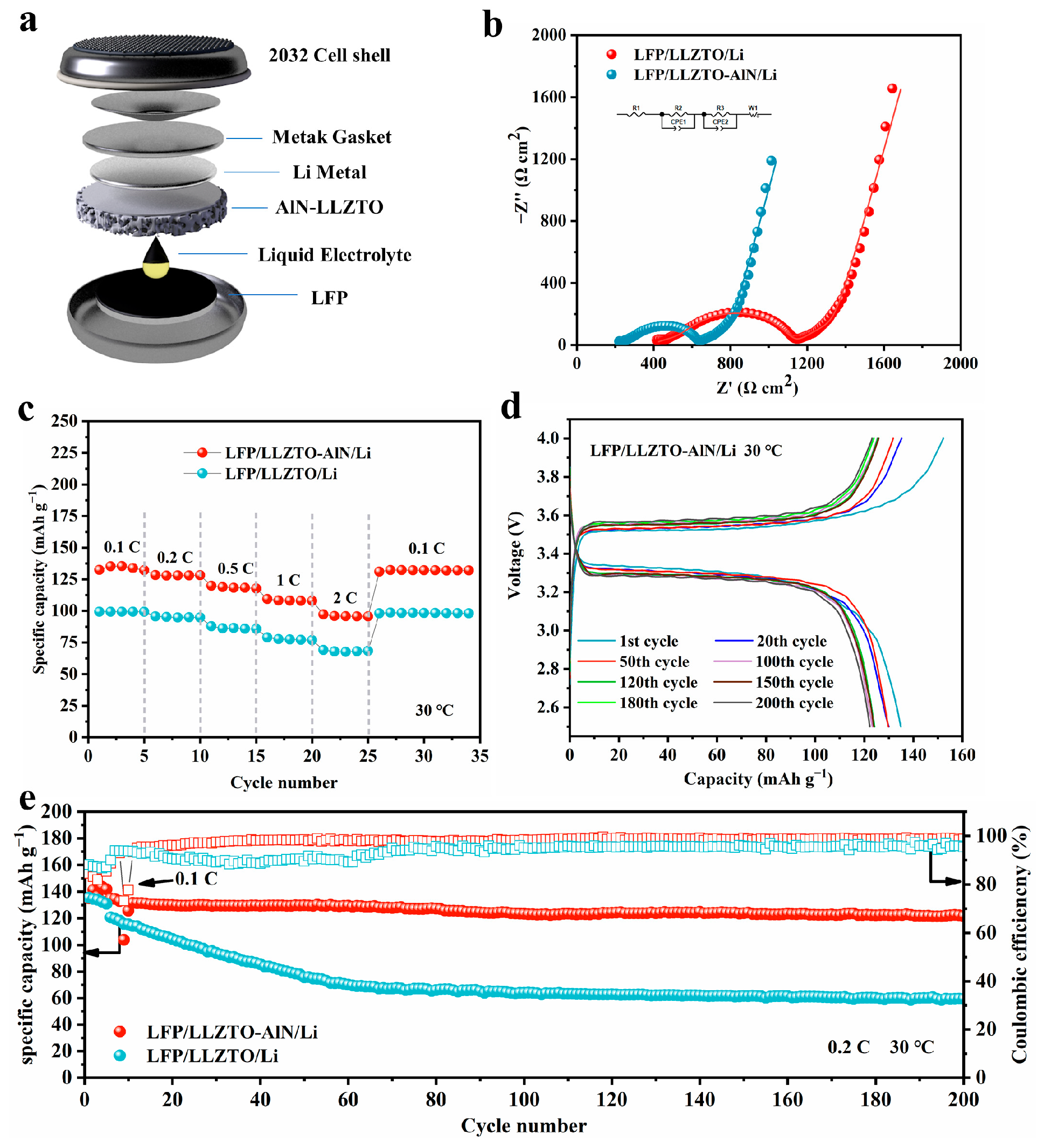
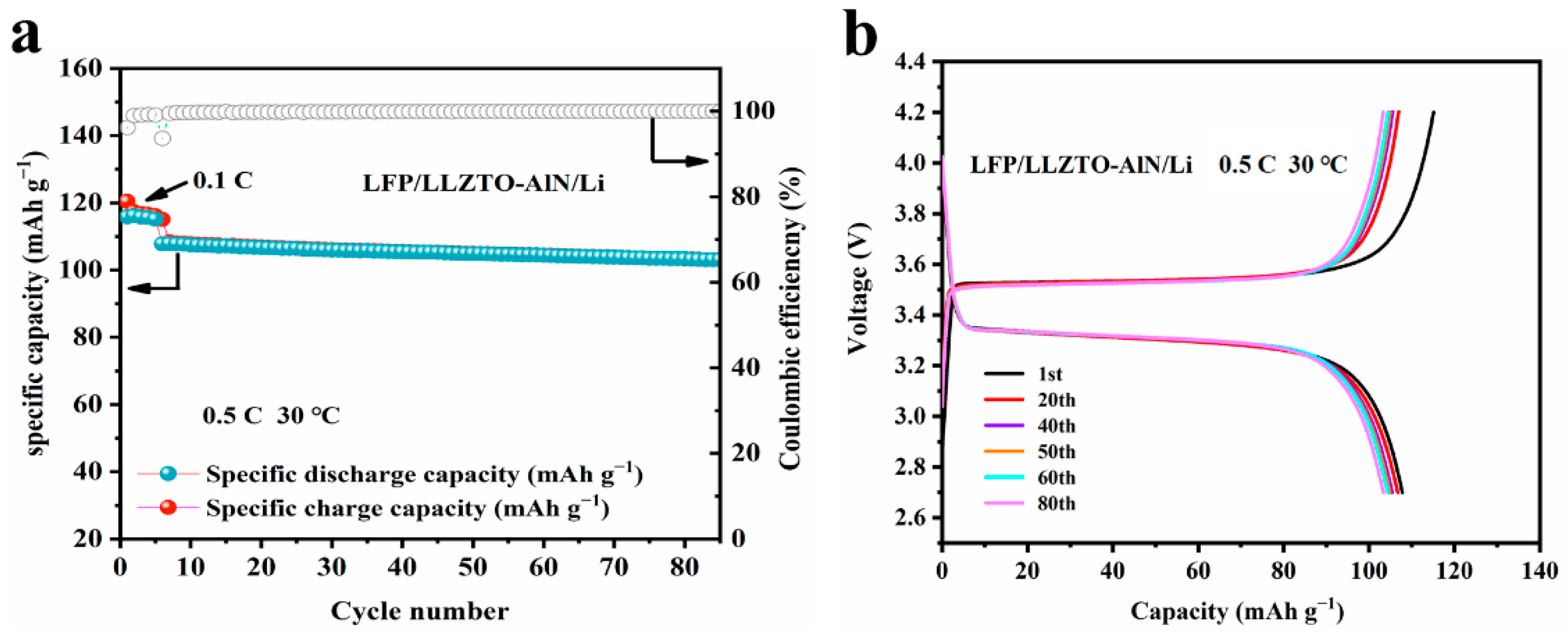
3.3. Electrochemical Analysis of AlN Modified LLZTO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid garnet batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy; Nature Publishing Group: Berlin, Germany, 2011; pp. 171–179. [Google Scholar]
- Grey, C.P.; Tarascon, J.M. Sustainability and in situ monitoring in battery development. Nat. Mater. 2016, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.; Ding, L.X.; Wang, S.; Wang, H. Enhancing interfacial contact in all solid-state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; Chen, Y.; He, X.; Xu, X.; Gao, X. Large-scale multifunctional electrochromic-energy storage device based on tungsten trioxide monohydrate nanosheets and prussian white. ACS Appl. Mater. Interfaces 2017, 9, 29872–29880. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Xie, H.; Pastel, G.; Dai, J.; Gong, Y.; Liu, B.; Wachsman, E.D.; Hu, L. Mixed ionic-electronic conductor enabled effective cathode-electrolyte interface in all solid-state batteries. Nano Energy 2018, 50, 393–400. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, N.; Bi, Z.; Fu, Z.; Xu, F.; Shi, C.; Guo, X. Polydopamine-coated garnet particles homogeneously distributed in poly (propylene carbonate) for the conductive and stable membrane electrolytes of solid lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 46162–46169. [Google Scholar] [CrossRef]
- Zhong, S.W.; Liang, T.X.; Yao, W.L.; Liu, X.L.; Lai, H.; Lei, C.; Li, D. Recent advancements in interface between cathode and garnet solid electrolyte for all solid-state Li-ion batteries. J. Inorg. Mater. 2019, 34, 694. [Google Scholar]
- Zeng, X.X.; Yin, Y.X.; Li, N.W.; Du, W.C.; Guo, Y.G.; Wan, L.J. Reshaping lithium plating/stripping behavior via bifunctional polymer electrolyte for room-temperature solid li metal batteries. J. Am. Chem. Soc. 2016, 138, 15825–15828. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2003, 104, 4303–4418. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Xie, J.; Xu, X.; Tu, J.; Zhang, P.; Zhao, X. Nonflammable quasi-solid-state electrolyte for stable lithium-metal batteries. RSC Adv. 2019, 9, 42183–42193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnam, P.R.; Wunder, S.L. Self-assembled janus-like multi-ionic lithium salts form nano-structured solid polymer electrolytes with high ionic conductivity and Li+ ion transference number. J. Mater. Chem. A 2013, 1, 1731–1739. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Subramanian, K.; Alexander, G.V.; Karthik, K.; Patra, S.; Indu, M.S.; Sreejith, O.V.; Viswanathan, R.; Narayanasamy, J.; Murugan, R. A brief review of recent advances in garnet structured solid electrolyte-based lithium metal batteries. J. Energy Storage 2021, 33, 102157. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium-ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer electrolytes for lithium-ion batteries: A critical study. Ionics 2017, 23, 497–540. [Google Scholar] [CrossRef]
- Liang, Y.F.; Xia, Y.; Zhang, S.Z.; Wang, X.L.; Xia, X.H.; Gu, C.D.; Wu, J.B.; Tu, J.P. A preeminent gel blending polymer electrolyte of poly (vinylidene fluoride-hexafluoropropylene) -poly (propylene carbonate) for solid-state lithium ion batteries. Electrochim. Acta 2019, 296, 1064–1069. [Google Scholar] [CrossRef]
- Langer, F.; Bardenhagen, I.; Glenneberg, J.; Kun, R. Microstructure and temperature dependent lithium-ion transport of ceramic-polymer composite electrolyte for solid-state lithium-ion batteries based on garnet-type Li7La3Zr2O12. Solid State Ion. 2016, 291, 8–13. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent advances in innovative polymer electrolytes based on polys (ionic liquid). Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Lacivita, V.; Westover, A.S.; Kercher, A.; Phillip, N.D.; Yang, G.; Veith, G.; Ceder, G.; Dudney, N.J. Resolving the amorphous structure of lithium phosphorus oxynitride (lipon). J. Am. Chem. Soc. 2018, 140, 11029–11038. [Google Scholar] [CrossRef]
- Sepúlveda, A.; Criscuolo, F.; Put, B.; Vereecken, P.M. Effect of high temperature LiPON electrolyte in all solid-state batteries. Solid State Ion. 2019, 337, 24–32. [Google Scholar] [CrossRef]
- Fang, H.; Jena, P. Li-rich antiperovskite superionic conductors based on cluster ions. Proc. Natl. Acad. Sci. USA 2017, 114, 11046–11051. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zheng, C.; Lou, J.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. Poly (ethylene oxide) reinforced Li6PS5Cl composite solid electrolyte for all-solid-state lithium battery: Enhanced electrochemical performance, mechanical property and interfacial stability. J. Power Source 2019, 412, 78–85. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Su, Y.; Falgenhauer, J.; Polity, A.; Leichtweiß, T.; Kronenberger, A.; Obel, J.; Zhou, S.; Schlettwein, D.; Janek, J.; Meyer, B.K. LiPON thin films with high nitrogen content for application in lithium batteries and electrochromic devices prepared by RF magnetron sputtering. Solid State Ion. 2015, 282, 63–69. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Sumner, J.; Sakamoto, J. Electrical and mechanical properties of hot-pressed versus sintered LiTi2 (PO4)3. Solid State Ion. 2009, 180, 961–967. [Google Scholar] [CrossRef]
- Catti, M. Local structure of the Li1/8La5/8TiO3 (LLTO) ionic conductor by theoretical simulations. J. Phys. Conf. Ser. 2008, 117, 012008. [Google Scholar] [CrossRef]
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. High lithium ion conducting glass-ceramics in the system Li2S–P2S5. Solid State Ion. 2006, 177, 2721–2725. [Google Scholar] [CrossRef]
- Saienga, J.; Martin, S.W. The comparative structure, properties, and ionic conductivity of LiI+Li2S+GeS2 glasses doped with Ga2S3 and La2S3. J. Non-Cryst. Solids 2008, 354, 1475–1486. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Gao, K.; He, M.; Li, Y.; Zhang, Y.; Gao, J.; Li, X.; Cui, Z.; Zhan, Z.; Zhang, T. Preparation of high-density garnet thin sheet electrolytes for all-solid-state Li-metal batteries by tape-casting technique. J. Alloys Compd. 2019, 791, 923–928. [Google Scholar] [CrossRef]
- Zhao, N.; Fang, R.; He, M.H.; Chen, C.; Li, Y.Q.; Bi, Z.J.; Guo, X.X. Cycle stability of lithium/garnet/lithium cells with different intermediate layers. Rare Met. 2018, 37, 473–479. [Google Scholar] [CrossRef]
- Flatscher, F.; Philipp, M.; Ganschow, S.; Wilkening, H.M.R.; Rettenwander, D. The natural critical current density limit for Li7La3Zr2O12 garnets. J. Mater. Chem. A 2020, 8, 15782–15788. [Google Scholar] [CrossRef] [Green Version]
- Fu, K.K.; Gong, Y.; Fu, Z.; Xie, H.; Yao, Y.; Liu, B.; Carter, M.; Wachsman, E.; Hu, L. Transient behavior of the metal interface in lithium metal-garnet batteries. Angew. Chem. Int. Ed. Engl. 2017, 56, 14942–14947. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.; Zhu, Y.; Li, Y.; Yao, Y.; Zhang, Y.; Fu, K.K.; Pastel, G.; Lin, C.F.; Mo, Y.; et al. Reducing interfacial resistance between garnet-structured solid-state electrolyte and li-metal anode by a germanium layer. Adv. Mater. 2017, 29, 1606042. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, H.; Zhang, L.; Gong, Y.; Pastel, G.; Dai, J.; Liu, B.; Wachsman, E.D.; Hu, L. Universal soldering of lithium and sodium alloys on various substrates for batteries. Adv. Energy Mater. 2017, 8, 1701963. [Google Scholar] [CrossRef]
- Chengwei, W.; Gong, Y.; Liu, B.; Fu, K.; Yao, Y.; Hitz, E.; Li, Y.; Dai, J.; Xu, S.; Luo, W.; et al. Conformal, Nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett. 2017, 17, 565–571. [Google Scholar]
- Chen, Y.; He, M.; Zhao, N.; Fu, J.; Huo, H.; Zhang, T.; Li, Y.; Xu, F.; Guo, X. Nanocomposite intermediate layers formed by conversion reaction of SnO2 for Li/garnet/Li cycle stability. J. Power Source 2019, 420, 15–21. [Google Scholar] [CrossRef]
- Sudo, R.; Nakata, Y.; Ishiguro, K.; Matsui, M.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Interface behavior between garnet-type lithium-conducting solid electrolyte and lithium metal. Solid State Ion. 2014, 262, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef]
- Motoyama, M.; Tanaka, Y.; Yamamoto, T.; Tsuchimine, N.; Kobayashi, S.; Iriyama, Y. The Active Interface of Ta-doped Li7La3Zr2O12 for Li plating/stripping revealed by acid aqueous etching. ACS Appl. Energy Mater. 2019, 2, 6720–6731. [Google Scholar] [CrossRef]
- Ma, C.; Rangasamy, E.; Liang, C.; Sakamoto, J.; More, K.L.; Chi, M. Excellent stability of a lithium-ion-conducting solid electrolyte upon reversible Li+/H+ exchange in aqueous solutions. Angew. Chem. Int. Ed. Engl. 2015, 54, 129–133. [Google Scholar] [CrossRef]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Rettenwander, D. One step closer to realizing solid-state batteries with cubic Li7La3Zr2O12 garnets. Chem 2019, 5, 1695–1696. [Google Scholar] [CrossRef]
- Peng, Z.; Ren, F.; Yang, S.; Wang, M.; Sun, J.; Wang, D.; Xu, W.; Zhang, J.G. A highly stable host for lithium metal anode enabled by Li9Al4-Li3N-AlN structure. Nano Energy 2019, 59, 110–119. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Tietz, F.; Wegener, T.; Gerhards, M.T.; Giarola, M.; Mariotto, G. Synthesis and raman micro-spectroscopy investigation of Li7La3Zr2O12. Solid State Ion. 2013, 230, 77–82. [Google Scholar] [CrossRef]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419–11428. [Google Scholar] [CrossRef] [Green Version]
- David, I.N.; Thompson, T.; Wolfenstine, J.; Allen, J.L.; Sakamoto, J.; Viyas, B. Microstructure and Li-ion conductivity of hot-pressed cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 1209–1214. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Dolocan, A.; Cui, Z.; Xin, S.; Xue, L.; Xu, H.; Park, K.; Goodenough, J.B. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 2018, 140, 6448–6455. [Google Scholar] [CrossRef]
- Ruan, Y.; Lu, Y.; Huang, X.; Su, J.; Sun, C.; Jin, J.; Wen, Z. Acid induced conversion towards a robust and lithiophilic interface for Li–Li7La3Zr2O12 solid-state batteries. J. Mater. Chem. A 2019, 7, 14565–14574. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer. Nanomaterials 2022, 12, 2023. https://doi.org/10.3390/nano12122023
Jiang W, Dong L, Liu S, Ai B, Zhao S, Zhang W, Pan K, Zhang L. Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer. Nanomaterials. 2022; 12(12):2023. https://doi.org/10.3390/nano12122023
Chicago/Turabian StyleJiang, Wen, Lingling Dong, Shuanghui Liu, Bing Ai, Shuangshuang Zhao, Weimin Zhang, Kefeng Pan, and Lipeng Zhang. 2022. "Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer" Nanomaterials 12, no. 12: 2023. https://doi.org/10.3390/nano12122023
APA StyleJiang, W., Dong, L., Liu, S., Ai, B., Zhao, S., Zhang, W., Pan, K., & Zhang, L. (2022). Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer. Nanomaterials, 12(12), 2023. https://doi.org/10.3390/nano12122023





