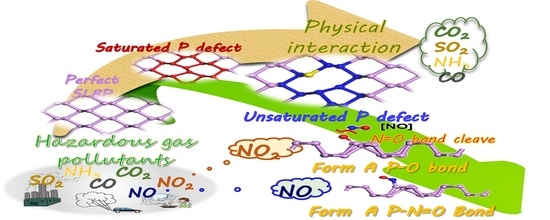
Unique Interaction between Layered Black Phosphorus and Nitrogen Dioxide
Abstract
:1. Introduction
2. Computational Methods
2.1. Adsorbent Model
2.2. Adsorption Complex Models
2.3. Computational Methods
2.4. Experimental Methods
3. Results and Discussion
3.1. Adsorption of Gas Molecules on Perfect LBP
3.2. Adsorption of Gas Molecules on Defects in LBP
3.3. Interaction Mechanisms of Defective LBP and Gas Molecules
3.4. Orbital Analysis on the Nature of Interaction
3.5. Experimental Verification of Oxidation of LBP by NO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resitoglu, I.A.; Altinisik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2014, 17, 15–27. [Google Scholar] [CrossRef]
- Raoa, S.; Klimont, Z.; Steven, J.S.; Van Dingenen, R.; Dentener, F.; Bouwman, L.; Riahi, K.; Amann, M.; Bodirsky, B.L.; van Vuuren, D.P.; et al. Future air pollution in the shared socio-economic pathways. Glob. Environ. Chang. 2016, 42, 346–358. [Google Scholar] [CrossRef]
- Shen, R.; Schafer, K.; Shao, L.; Schnelle-Kreis, J.; Wang, Y.; Li, F.; Liu, Z.; Emeis, S.; Schmid, H.P. Chemical characteristics of PM2.5 during haze episodes in spring 2013 in Beijing. Urban Clim. 2017, 22, 51–63. [Google Scholar] [CrossRef]
- International Energy Agency. Energy and Air Pollution 2016—World Energy Outlook Special Report; International Energy Agency: Paris, France, 2016. [Google Scholar]
- Lu, C.; Norback, D.; Li, Y.; Deng, Q. Early-life exposure to air pollution and childhood allergic diseases: An update on the link and its implications. Expert Rev. Clin. Immunol. 2020, 16, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Siakavelas, G.I.; Georgiadis, A.G.; Charisiou, N.D. Cost-effective adsorption of oxidative coupling-derived ethylene using a molecular sieve. Chem. Eng. Technol. 2021, 44, 2041–2048. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Zhao, Y. Ultra-highly porous carbon from wasted soybean residue with tailored porosity and doped structure as renewable multi-purpose absorbent for efficient CO2, toluene and water vapor capture. J. Clean. Prod. 2022, 337, 130283. [Google Scholar] [CrossRef]
- Pangh, A.; Esrafili, M.D.; Nejad, M.R. A DFT investigation of CO and NO adsorption on Cu5Sc and Cu6Sc+ metallic clusters. Comput. Theor. Chem. 2022, 1210, 113657. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, X. Development of graphene aerogels with high strength and ultrahigh adsorption capacity for gas purification. Mater. Des. 2021, 208, 109903. [Google Scholar] [CrossRef]
- Ngoc, H.V.; Pham, K.D. First-principles study on N2, H2, O2, NO, NO2, CO, CO2, and SO2 gas adsorption properties of the Sc2CF2 monolayer. Physica E 2022, 141, 115162. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Hsu, H.C.; Shown, I.; Wei, H.Y.; Chang, Y.C.; Du, H.Y.; Lin, Y.G.; Tseng, C.A.; Wang, C.H. Graphene oxide as A promising photocatalyst for CO2 to methanol conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Z.; Luo, Z.; Xu, X.F.; Tomanek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with A high hole mobility. ACS Nano. 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ma, W.; Pan, B.; Zhang, Q.; Zhang, X.; Zhang, S.; Xing, B. Wrinkle-induced high sorption makes few-layered black phosphorus: A superior adsorbent for ionic organic compounds. Environ. Sci. Nano. 2018, 5, 1454–1465. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.; Koh, H.J.; Jung, H.; Kim, J.S.; Yoo, H.W.; Kim, J.; Jung, H.T. Superior chemical sensing performance of black phosphorus: Comparison with MoS2 and graphene. Adv. Mater. 2016, 28, 7020–7028. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Abbas, B.L.; Liang, C.; Ma, Y.Q.; Cong, S.; Zhou, C. Black phosphorus gas sensors. ACS Nano. 2015, 9, 5618–5624. [Google Scholar]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for In Vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Shin, Y.C.; Lee, H.U.; Kim, B.; Han, D.W.; Lim, D. Dose- and time-dependent cytotoxicity of layered black phosphorus in fibroblastic cells. Nanomaterials 2018, 8, 408. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Lei, L.; Yu, X.F.; Chen, J.; Ma, C.; Wu, F.; Zhao, Q.; Xing, B. pH-dependent degradation of layered black phosphorus: Essential role of hydroxide ions. Angew. Chem. Int. Edit. 2019, 58, 467–471. [Google Scholar] [CrossRef]
- Sibari, A.; Kerrami, Z.; Benaissa, M.; Kara, A. Coverage-dependent adsorption of small gas molecules on black phosphorene: A DFT study. Surf. Sci. 2021, 710, 121860. [Google Scholar] [CrossRef]
- Shinde, P.V.; Kumar, A.; Late, D.J.; Rout, C.S. Recent advances in 2D black phosphorus based materials for gas sensing applications. J. Mater. Chem. C 2021, 9, 3773–3794. [Google Scholar] [CrossRef]
- Donarelli, M.; Giancaterini, L.; Fioravanti, G.; Perrozzi, F.; Cantalini, C. Exfoliated black phosphorus gas sensing properties at room temperature. 2D Mater. 2016, 3, 025002. [Google Scholar] [CrossRef]
- Han, D.; Han, X.M.; Liu, L.L.; Li, D.H.; Liu, Y.; Liu, Z.H.; Liu, D.M.; Chen, Y.; Zhuo, K.; Sang, S.B. Sub-ppb-Level Detection of Nitrogen Dioxide Based on High-Quality Black Phosphorus. ACS Appl. Mater. Interfaces 2022, 14, 13942–13951. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Duan, Z.; Xu, J.; Zhu, Y.; Xu, Q.; Wang, H.; Tai, H.; Weng, J.; Zhao, Y. The art of integrated functionalization: Super stable black phosphorus achieved through metal-organic framework coating. Adv. Funct. Mater. 2020, 30, 2002232. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, Y.; Wang, Y.; Zhu, X.; Gao, C.; Guo, Y. Improving room-temperature trace NO2 sensing of black phosphorus nanosheets by incorporating benzyl viologen. Sens. Actuators B Chem. 2020, 321, 128520. [Google Scholar] [CrossRef]
- Zhang, H.; Kou, L.; Jiao, Y.; Du, A.; Tang, Y.; Ni, Y. Strain engineering of selective chemical adsorption on monolayer black phosphorous. Appl. Surf. Sci. 2020, 503, 144033. [Google Scholar] [CrossRef]
- Yu, Q.H.; Jiang, Y.; Zhang, W.; Wu, B.Z.; Yin, J.R.; Zhang, P.; Ding, Y.H. Noble metal atoms doped phosphorene: Electronic properties and gas adsorption ability. Mater. Res. Express 2017, 4, 045703. [Google Scholar] [CrossRef]
- Tit, N.; Said, K.; Yamani, Z. Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: Role of intrinsic defects on gas sensing. Appl. Surf. Sci. 2017, 394, 219–230. [Google Scholar] [CrossRef]
- Lei, S.Y.; Luan, S.; Yu, H. Co-doped phosphorene: Enhanced sensitivity of CO gas sensing. Int. J. Mod. Phys. B 2018, 32, 1850068. [Google Scholar] [CrossRef]
- Lalitha, M.; Nataraj, Y.; Lakshmipathi, S. Calcium decorated and doped phosphorene for gas adsorption. Appl. Surf. Sci. 2016, 377, 311–323. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as A superior gas sensor: Selective adsorption and distinct I-V response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.P.; Meng, R.S.; Jiang, J.-K.; Liang, Q.H.; Tan, C.J.; Cai, M.; Sun, X.; Yang, D.G.; Ren, T.L. First-principles study of sulfur dioxide sensor based on phosphorenes. IEEE Electron Device Lett. 2016, 37, 660–662. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, L.; Liu, X.; Zhou, W.; Song, X.; Zhang, S. First-principles study of SO2 sensors based on phosphorene and its isoelectronic counterparts: GeS, GeSe, SnS, SnSe. Chem. Phys. Lett. 2017, 686, 83–87. [Google Scholar] [CrossRef]
- Kaewmaraya, T.; Ngamwongwan, L.; Moontragoon, P.; Karton, A.; Hussain, T. Drastic improvement in gas-sensing characteristics of phosphorene nanosheets under vacancy defects and elemental functionalization. J. Phys. Chem. C 2018, 122, 20186–20193. [Google Scholar] [CrossRef]
- Hiroshi, K.; Keiji, M.; Kenichi, F.; Teijiro, Y. The electronic structures of NO2, NO2+ and NO2−. Bull. Chem. Soc. Jpn. 1964, 37, 11. [Google Scholar]
- Fourre, I.; Silvi, B. What can we learn from two-center three-electron bonding with the topological analysis of ELF? Heteroatom. Chem. 2007, 18, 135–160. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, Q.; Zhang, G.; Zhang, Y.W. Energetics, charge transfer, and magnetism of small molecules physisorbed on phosphorene. J. Phys. Chem. C 2015, 119, 3102–3110. [Google Scholar] [CrossRef]
- Frenking, G.; Loschen, C.; Krapp, A.; Fau, S.; Strauss, S.H. Electronic structure of CO—An exercise in modern chemical bonding theory. J. Comput. Chem. 2007, 28, 117–126. [Google Scholar] [CrossRef]
- Wei, H.; Yang, J.Y. Defects in phosphorene. J. Phys. Chem. C 2015, 119, 20474–20480. [Google Scholar]
- Su, Y.; Zheng, X.; Wang, X.; Zhang, X.; Sui, Y.; Wang, X. Two stable phosphorus-containing four-membered ring radical cations with inverse spin density distributions. J. Am. Chem. Soc. 2014, 136, 6251–6254. [Google Scholar] [CrossRef]
- Mahabal, M.S.; Deshpande, M.D.; Hussain, T.; Ahuja, R. Sensing characteristics of phosphorene monolayers toward PH3 and AsH3 gases upon the introduction of vacancy defects. J. Phys. Chem. C 2016, 120, 20428–20436. [Google Scholar] [CrossRef]
- Sun, S.; Hussain, T.; Zhang, W. Blue phosphorene monolayers as potential nano sensors for volatile organic compounds under point defects. Appl. Surf. Sci. 2019, 486, 52–57. [Google Scholar] [CrossRef]
- Banhart, F.; Arkady, V.K.; Kotakoski, J. Structural defects in graphene. ACS Nano. 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Hashmi, A.; Hong, J. Anisotropic bias dependent transport property of defective phosphorene layer. Sci. Rep. 2015, 5, 12482. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- John, P.; Perdew, K.B.; Matthias, E. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 4. [Google Scholar]
- Giannozzi, P.; Car, R.; Scoles, G. Oxygen adsorption on graphite and nanotubes. J. Chem. Phys. 2003, 118, 1003–1006. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with A long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Menga, R.S.; Chen, X.P. Adsorption of gas molecules on graphene-like InN monolayer: A first-principle study. Appl. Surf. Sci 2017, 404, 291–299. [Google Scholar] [CrossRef]
- Kornev, A.N.; Galperin, V.E.; Panova, Y.S.; Arapova, A.V.; Baranov, E.V.; Fukin, G.K.; Abakumov, G.A. Structural variability of R2C adducts of 3a,6a-Diaza-1,4-diphosphapentalene: Tuning the N-P bonding. Z. Anorg. Allg. Chem. 2017, 643, 1208–1214. [Google Scholar] [CrossRef]
- Veinot, A.J.; Blair, A.D.; Masuda, J.D. Crystal structure of 2-azido-1,3-bis-(2,6-diiso-propyl-phen-yl)-1,3,2-di-aza-phospho-lidine. Crystallogr. Commun. 2017, 73, 905–907. [Google Scholar] [CrossRef]
- Ziletti, A.; Carvalho, A.; Trevisanutto, P.E.; Campbell, D.K.; Coker, D.F.; Castro Neto, A.H. Phosphorene oxides: Bandgap engineering of phosphorene by oxidation. Phys. Rev. B 2015, 91, 085407. [Google Scholar] [CrossRef]
- Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Castro Neto, A.H. Oxygen defects in phosphorene. Phys. Rev. Lett. 2015, 114, 046801. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Steckel, J.A. Density Fuctional Theory: A Practical Introduction; John Wiley & Sons: Hobocen, NJ, USA, 2009; pp. 179–192. [Google Scholar]
- You, Y.; Deng, J.; Tan, X.; Gorjizadeh, N.; Yoshimura, M.; Smith, S.C.; Sahajwalla, V.; Joshi, R.K. On the mechanism of gas adsorption for pristine, defective and functionalized graphene. Phys. Chem. Chem. Phys. 2017, 19, 6051–6056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ye, Z. DFT study on interaction of NO2 with the vacancy-defected WO3 nanowires for gas-sensing. Sens. Actuators B Chem. 2016, 222, 499–507. [Google Scholar] [CrossRef]
- Muhammad, A.; Nacir, T. Adsorption of NO and NO2 molecules on defected-graphene and ozone-treated graphene: First-principles analysis. Surf. Sci 2019, 684, 28–36. [Google Scholar]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S.; Hersam, M.C. Solvent exfoliation of electronic-grade, two-dimensional black phosphorus. ACS Nano. 2015, 9, 3596–3604. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.F. In-plane black phosphorus/dicobalt phosphide heterostructure for efficient electrocatalysis. Angew. Chem. 2018, 57, 2600–2604. [Google Scholar] [CrossRef]






| Gases | Orientation [a] | dshortest [b] (Å) | Ead [c] (eV) | EvdW (eV) | EvdW/Ead [d] | Δq [e] (e) |
|---|---|---|---|---|---|---|
| NO2 | 2Ovz | 2.91 | −0.225 | −0.160 | 71% | −0.14 |
| SO2 | pz | 2.91 | −0.310 | −0.195 | 63% | −0.11 |
| Svz | 3.07 | −0.309 | −0.175 | 57% | −0.09 | |
| CO2 | pa | 3.44 | −0.156 | −0.124 | 79% | −0.01 |
| NO | pa | 2.47 | −0.200 | −0.142 | 71% | −0.15 |
| CO | pa | 3.37 | −0.138 | −0.113 | 82% | −0.01 |
| pa | 3.41 | −0.138 | −0.110 | 80% | −0.02 | |
| NH3 | Nva | 3.17 | −0.207 | −0.148 | 72% | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, X.; Zhao, Q.; Yu, X.-F.; Zhang, S.; Xing, B. Unique Interaction between Layered Black Phosphorus and Nitrogen Dioxide. Nanomaterials 2022, 12, 2011. https://doi.org/10.3390/nano12122011
Zhao J, Zhang X, Zhao Q, Yu X-F, Zhang S, Xing B. Unique Interaction between Layered Black Phosphorus and Nitrogen Dioxide. Nanomaterials. 2022; 12(12):2011. https://doi.org/10.3390/nano12122011
Chicago/Turabian StyleZhao, Jingjing, Xuejiao Zhang, Qing Zhao, Xue-Feng Yu, Siyu Zhang, and Baoshan Xing. 2022. "Unique Interaction between Layered Black Phosphorus and Nitrogen Dioxide" Nanomaterials 12, no. 12: 2011. https://doi.org/10.3390/nano12122011
APA StyleZhao, J., Zhang, X., Zhao, Q., Yu, X.-F., Zhang, S., & Xing, B. (2022). Unique Interaction between Layered Black Phosphorus and Nitrogen Dioxide. Nanomaterials, 12(12), 2011. https://doi.org/10.3390/nano12122011