Redispersible Reduced Graphene Oxide Prepared in a Gradient Solvent System
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Graphite Oxide
2.3. Preparation of Reduced Graphene Oxide
2.4. Thermal Annealing
2.5. Characterization
3. Results and Discussion
3.1. Gradient Solvent Strategy
3.1.1. Determination of the Initial Solvent
3.1.2. Reduction of Graphene Oxide in the Gradient Solvent System
3.2. Effect of Different Reducing-Agent Dosages and Reducing the Temperature on the Degree of Reduction
3.3. Adsorption and Desorption
3.3.1. Effect of Different Dispersants on Electrical Conductivity
3.3.2. Thermal Extraction with Low-Boiling-Point Solvent
3.3.3. Thermal Desorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene Synthesis via Hydrogen Induced Low Temperature Exfoliation of Graphite Oxide. J. Mater. Chem. 2010, 20, 8467. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Sun, D.; Gao, Z.; Jiao, K.; Zhang, J. Graphene Oxide “Surfactant”-Directed Tunable Concentration of Graphene Dispersion. Small 2020, 16, 2003426. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Kim, H.J. Synthesis of High-Performance Nickel Hydroxide Nanosheets/Gadolinium Doped-α-MnO2 Composite Nanorods as Cathode and Fe3O4/GO Nanospheres as Anode for an All-Solid-State Asymmetric Supercapacitor. J. Energy Chem. 2022, 64, 475–484. [Google Scholar] [CrossRef]
- Poudel, M.B.; Ojha, G.P.; Kim, A.A.; Kim, H.J. Manganese-Doped Tungsten Disulfide Microcones as Binder-Free Electrode for High Performance Asymmetric Supercapacitor. J. Energy Storage 2022, 47, 103674. [Google Scholar] [CrossRef]
- Babu Poudel, M.; Shin, M.; Joo Kim, H. Interface Engineering of MIL-88 Derived MnFe-LDH and MnFe2O3 on Three-Dimensional Carbon Nanofibers for the Efficient Adsorption of Cr(VI), Pb(II), and As(III) Ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-Layered Double Hydroxide and α-Fe2O3 Nanorods on Hollow Porous Carbon Nanofibers: A Free-Standing Electrode for Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel Insight into the Adsorption of Cr(VI) and Pb(II) Ions by MOF Derived Co-Al Layered Double Hydroxide @hematite Nanorods on 3D Porous Carbon Nanofiber Network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical Functionalization of Graphene and Its Applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting Graphene Oxide—Years 1859 to 2019: A Review of Its Structure, Synthesis, Properties and Exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Xu, C.; Sun, J.; Gao, L. Facile One-Step Hydrazine-Assisted Solvothermal Synthesis of Nitrogen-Doped Reduced Graphene Oxide: Reduction Effect and Mechanisms. RSC Adv. 2013, 3, 1194–1200. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature Dependence of Graphene Oxide Reduced by Hydrazine Hydrate. Nanotechnology 2011, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (RGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Study on the Effect of Graphene and Glycerol Plasticizer on the Properties of Chitosan-Graphene Nanocomposites via in Situ Green Chemical Reduction of Graphene Oxide. Int. J. Biol. Macromol. 2018, 114, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct Reduction of Graphene Oxide Films into Highly Conductive and Flexible Graphene Films by Hydrohalic Acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical Methods for the Production of Graphenes. Nat. Nanotech 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. The Reduction of Graphene Oxide with Hydrazine: Elucidating Its Reductive Capability Based on a Reaction-Model Approach. Chem. Commun. 2016, 52, 72–75. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic Routes of the Reduced Graphene Oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Agharkar, M.; Kochrekar, S.; Hidouri, S.; Azeez, M.A. Trends in Green Reduction of Graphene Oxides, Issues and Challenges: A Review. Mater. Res. Bull. 2014, 59, 323–328. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical Reduction of Graphene Oxide Using Green Reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotech. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Li, X.; Huang, M.; Zhen, Z.; Zhong, Y.; Chen, Q.; Zhao, X.; He, Y.; Hu, R.; Yang, T.; et al. The Physics and Chemistry of Graphene-on-Surfaces. Chem. Soc. Rev. 2017, 46, 4417–4449. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Z.; Su, W.; Shang, Y.; Wang, Z.-L. Adsorption of Phenanthrene and 1-Naphthol to Graphene Oxide and L-Ascorbic-Acid-Reduced Graphene Oxide: Effects of PH and Surfactants. Env. Sci. Pollut. Res. 2019, 26, 11062–11073. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for Reduction of Graphene Oxide—A Comprehensive Review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Farmer, D.B.; Chiu, H.-Y.; Lin, Y.-M.; Jenkins, K.A.; Xia, F.; Avouris, P. Utilization of a Buffered Dielectric to Achieve High Field-Effect Carrier Mobility in Graphene Transistors. Nano Lett. 2009, 9, 4474–4478. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable Aqueous Dispersions of Graphene Nanosheets. Nat. Nanotech 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Du, W.; Wu, H.; Chen, H.; Xu, G.; Li, C. Graphene Oxide in Aqueous and Nonaqueous Media: Dispersion Behaviour and Solution Chemistry. Carbon 2020, 158, 568–579. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Murali, S.; Zhu, Y.; Ruoff, R.S.; Bielawski, C.W. Reduction of Graphite Oxide Using Alcohols. J. Mater. Chem. 2011, 21, 3443–3447. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion Behaviour of Graphene Oxide and Reduced Graphene Oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, C.; Li, X.; Wu, L.; Chen, Y. Aggregation Prevention: Reduction of Graphene Oxide in Mixed Medium of Alkylphenol Polyoxyethylene (7) Ether and 2-Methoxyethanol. RSC Adv. 2018, 8, 39140–39148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide-Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene Oxide as a Chemically Tunable Platform for Optical Applications. Nat. Chem 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Chung, J.S. Chemical Functionalization of Graphene Sheets by Solvothermal Reduction of a Graphene Oxide Suspension in N-Methyl-2-Pyrrolidone. J. Mater. Chem. 2011, 21, 3371–3377. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Yang, H.; Li, H.; Li, A.; Cheng, R. Flocculation Performance and Mechanism of Graphene Oxide for Removal of Various Contaminants from Water. Water Res. 2013, 47, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, P.; Xiao, H.; Zhang, Y.; Shi, X.; Lü, X.; Chen, X. Understanding Flocculation Mechanism of Graphene Oxide for Organic Dyes from Water: Experimental and Molecular Dynamics Simulation. AIP Adv. 2015, 5, 117151. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Shi, X.; Ji, A.; Shi, L.; Zhou, C.; Cui, Y. Fabrication and Characteristics of Reduced Graphene Oxide Produced with Different Green Reductants. PLoS ONE 2015, 10, e0144842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method To Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, X.; Lei, Y.; Luo, Y. Easy and Green Synthesis of Reduced Graphite Oxide-Based Hydrogels. Carbon 2011, 49, 4314–4321. [Google Scholar] [CrossRef]
- Park, H.; Lim, S.; Nguyen, D.D.; Suk, J.W. Electrical Measurements of Thermally Reduced Graphene Oxide Powders under Pressure. Nanomaterials 2019, 9, 1387. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced Graphene Oxide Film with Record-High Conductivity and Mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Fang, X.-Y.; Yu, X.-X.; Zheng, H.-M.; Jin, H.-B.; Wang, L.; Cao, M.-S. Temperature- and Thickness-Dependent Electrical Conductivity of Few-Layer Graphene and Graphene Nanosheets. Phys. Lett. A 2015, 379, 2245–2251. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical Reduction of Graphene Oxide: A Synthetic Chemistry Viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-Soluble Graphene Covalently Functionalized by Biocompatible Poly-L-Lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
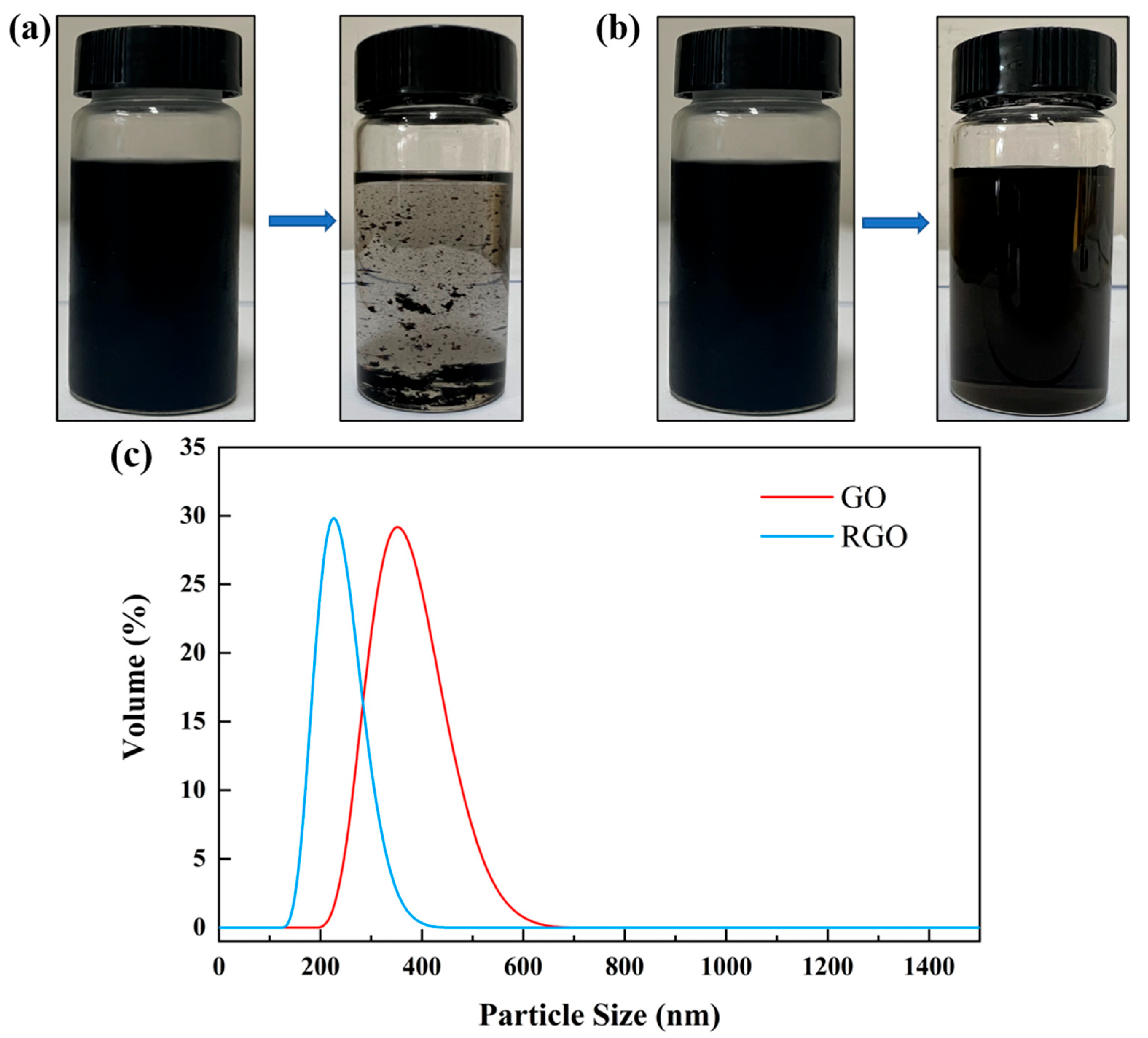




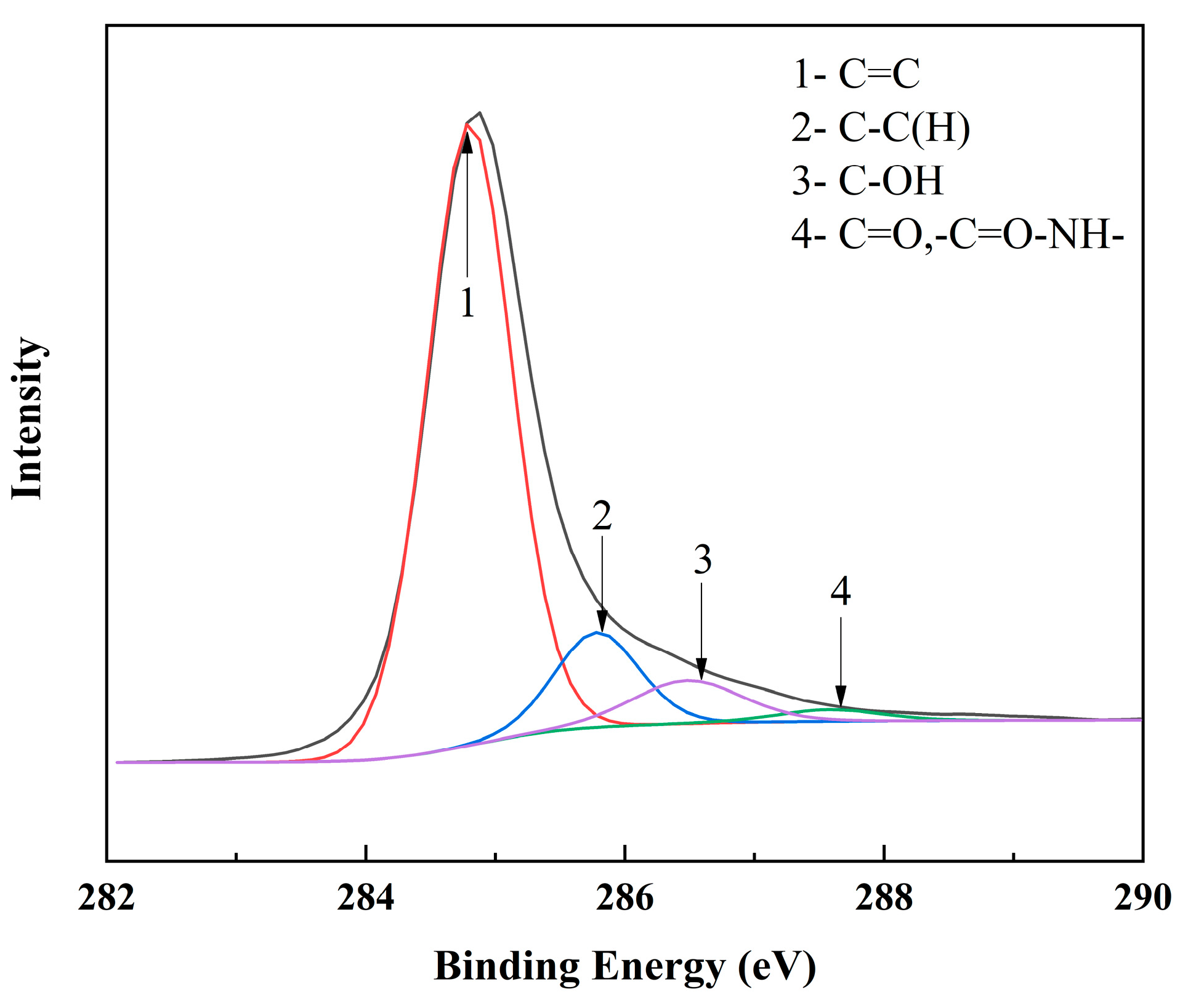


| H2O (mL) | EGM (mL) | NMP (mL) | Xylene (mL) | Average Particle Size (nm) |
|---|---|---|---|---|
| 14 | / | / | / | 278 ± 20 |
| / | 14 | / | / | 594 ± 20 |
| / | / | / | 14 | Non-diversified |
| / | 12 | / | 2 | 963 ± 20 |
| / | 10 | / | 4 | 1604 ± 20 |
| / | 8 | / | 6 | Flocculation |
| 1 | 12 | / | 2 | 628 ± 20 |
| Initial Solvent | Mid-Term Solvent | Post-Solvent | Dispersion | C/O Ratio | Electrical Conductivity (S m−1) | ||
|---|---|---|---|---|---|---|---|
| H2O (mL) | EGM (mL) | NMP (mL) | NMP (mL) | Xylene/mL | |||
| 10 | 90 | / | / | 40 | Uniform dispersion | 13.54 a | 4363 ± 300 |
| 10 | 60 | / | 80 | 20 | Slight aggregation | / | 3745 ± 300 |
| 10 | 60 | / | 60 | 40 | Uniform dispersion | 9.60 b | 4014 ± 300 |
| 10 | 60 | / | 40 | 60 | Uniform dispersion | / | 3948 ± 300 |
| 10 | 60 | / | 20 | 80 | Slight aggregation | / | 3508 ± 300 |
| 10 | / | 90 | / | 40 | Uniform dispersion | 7.92 c | 5236 ± 300 |
| 0 | 100 | / | / | 0 | Aggregation | 3.09 d | 124 ± 300 |
| Sample Number | Temperature (°C) | Vc Dosage (g) | C:O Ratio a | Electrical Conductivity (S m−1) | RGO Dispersions |
|---|---|---|---|---|---|
| RGO80 | 80 | 0.4 | 7.92 | 5236 | Uniform dispersion |
| RGO90 | 90 | 0.4 | 7.20 | 3190 | Slight aggregation |
| RGO120 | 120 | 0.4 | 6.15 | — | Aggregation |
| RGOVC10 | 80 | 10 | 7.58 | 1284 | Uniform dispersion, stable |
| RGOVC20 | 80 | 20 | 7.39 | 1268 | Uniform dispersion, stable |
| Dispersant | OP-7 | AEO-3 | DEP | PVP30 | PS |
|---|---|---|---|---|---|
| Conductivity (S m−1) | 4679 | 5236 | 1000 | 125 | 898 |
| Sample Number a | Conductivity (S m−1) | ||
|---|---|---|---|
| Vacuum Filtration | First Thermal Reflux | Second Thermal Reflux | |
| RGOT70 | 2100 | 3029 | 6800 |
| RGOT150 | 5236 | 6209 | 6580 |
| RGOT300 | 9024 | 9130 | 9200 |
| Heat Treatment Temperature (°C) | Conductivity (S m−1) | |||
|---|---|---|---|---|
| AEO-3 | OP-7 | DEP | / | |
| 70 | 2100 | 1056 | 260 | 700 |
| 150 | 5236 | 4083 | 1000 | 2401 |
| 300 | 9024 | 8771 | 2034 | 8425 |
| 500 | 18,000 | 12,000 | 2984 | 8425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Zhou, Y.; Tang, C.; Cheng, X.; Zhang, C. Redispersible Reduced Graphene Oxide Prepared in a Gradient Solvent System. Nanomaterials 2022, 12, 1982. https://doi.org/10.3390/nano12121982
Sheng Y, Zhou Y, Tang C, Cheng X, Zhang C. Redispersible Reduced Graphene Oxide Prepared in a Gradient Solvent System. Nanomaterials. 2022; 12(12):1982. https://doi.org/10.3390/nano12121982
Chicago/Turabian StyleSheng, Yitian, Youliang Zhou, Changwei Tang, Xiangnan Cheng, and Chaocan Zhang. 2022. "Redispersible Reduced Graphene Oxide Prepared in a Gradient Solvent System" Nanomaterials 12, no. 12: 1982. https://doi.org/10.3390/nano12121982
APA StyleSheng, Y., Zhou, Y., Tang, C., Cheng, X., & Zhang, C. (2022). Redispersible Reduced Graphene Oxide Prepared in a Gradient Solvent System. Nanomaterials, 12(12), 1982. https://doi.org/10.3390/nano12121982







