Cr/13X Zeolite and Zn/13X Zeolite Nanocatalysts Used in Pyrolysis of Pretreated Residual Biomass to Produce Bio-Oil with Improved Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corn Cobs Biomass
2.2. Corn Cob Biomass Analysis
2.3. Preparation and Characterization of Nanocatalysts
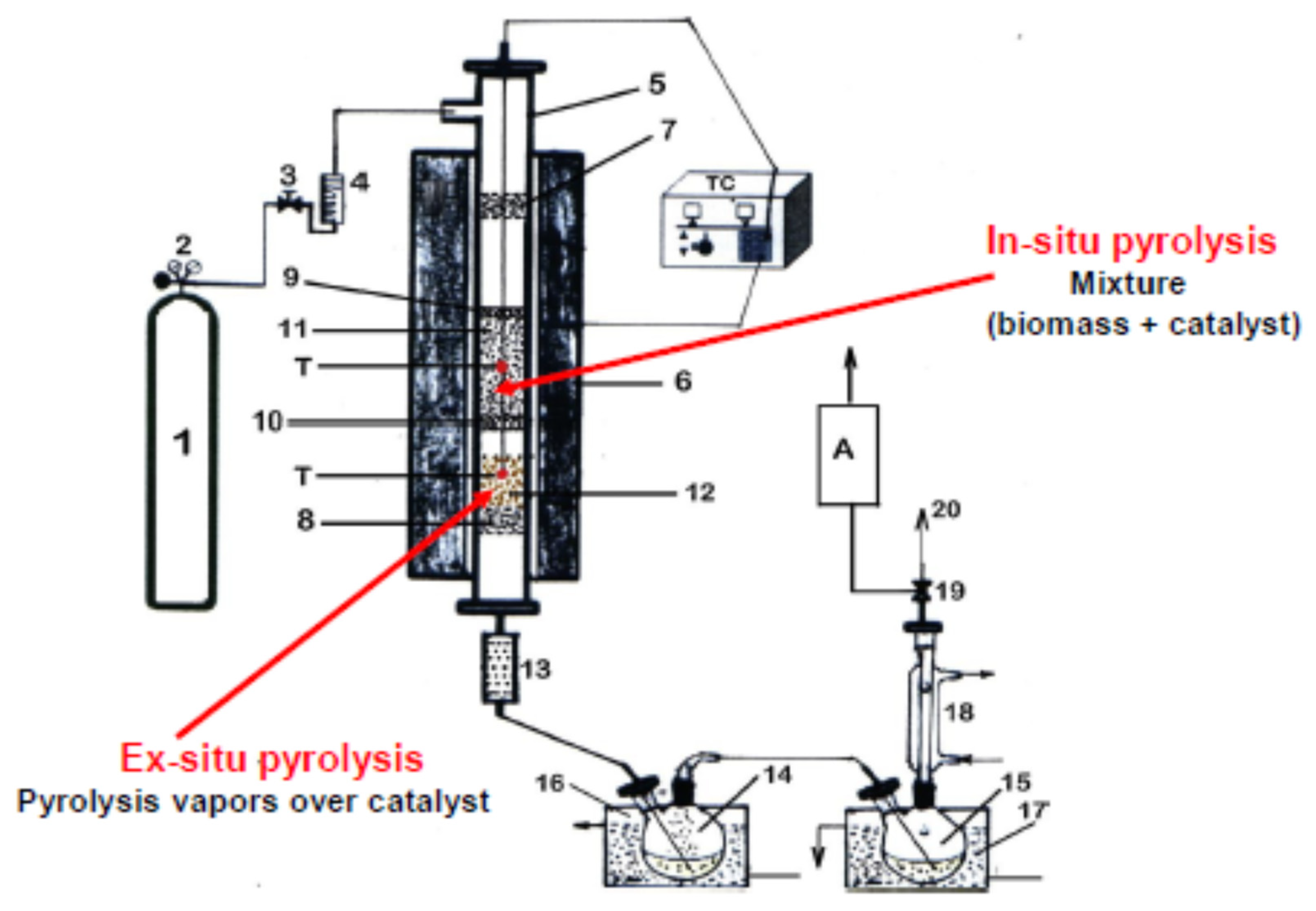
2.4. Pyrolysis Experiments
2.5. Coke Deposition on the Nanocatalysts
3. Results and Discussion
3.1. Influence of Acid Washing on Biomass Characteristics
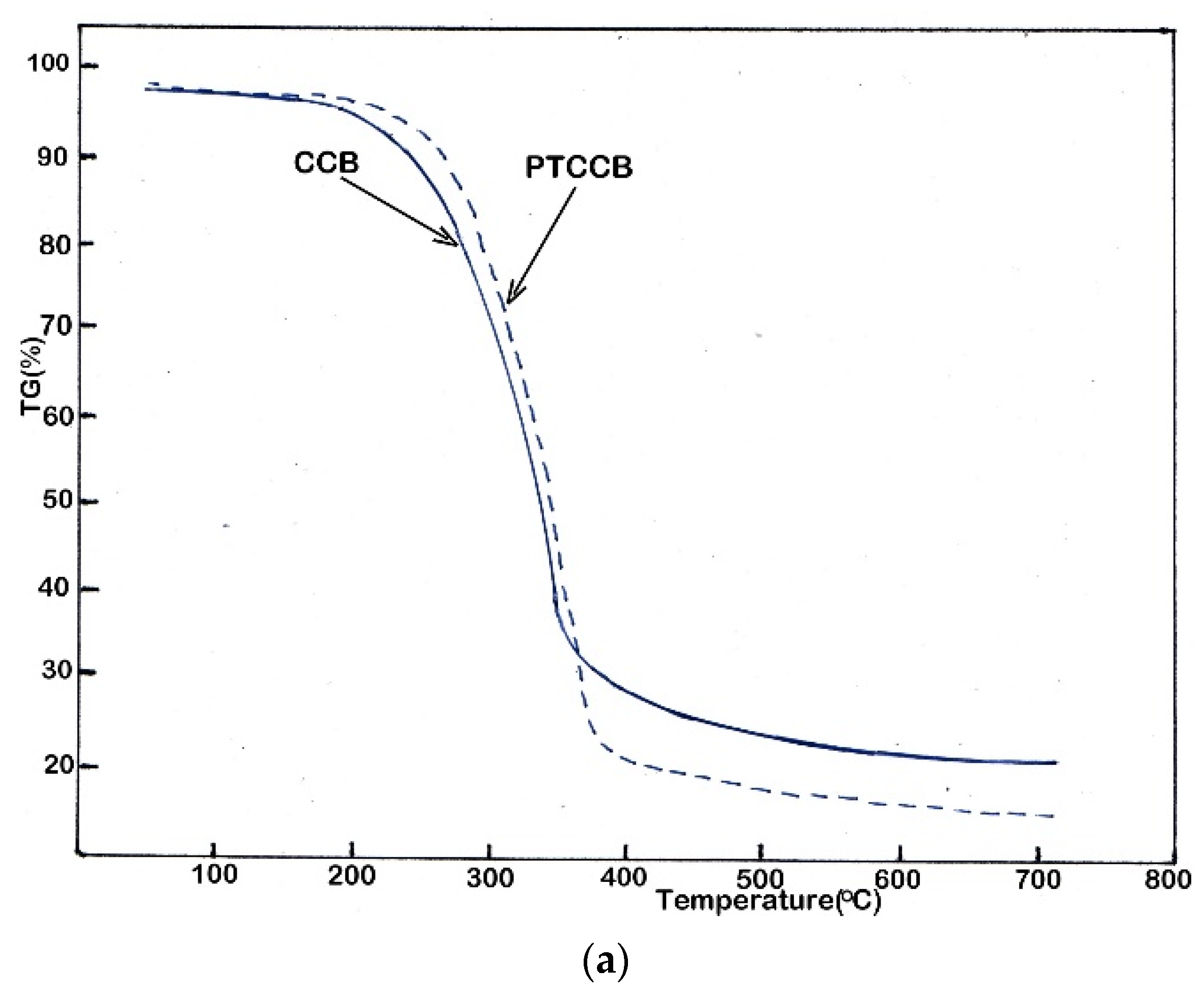
3.2. Thermal Degradation Analysis
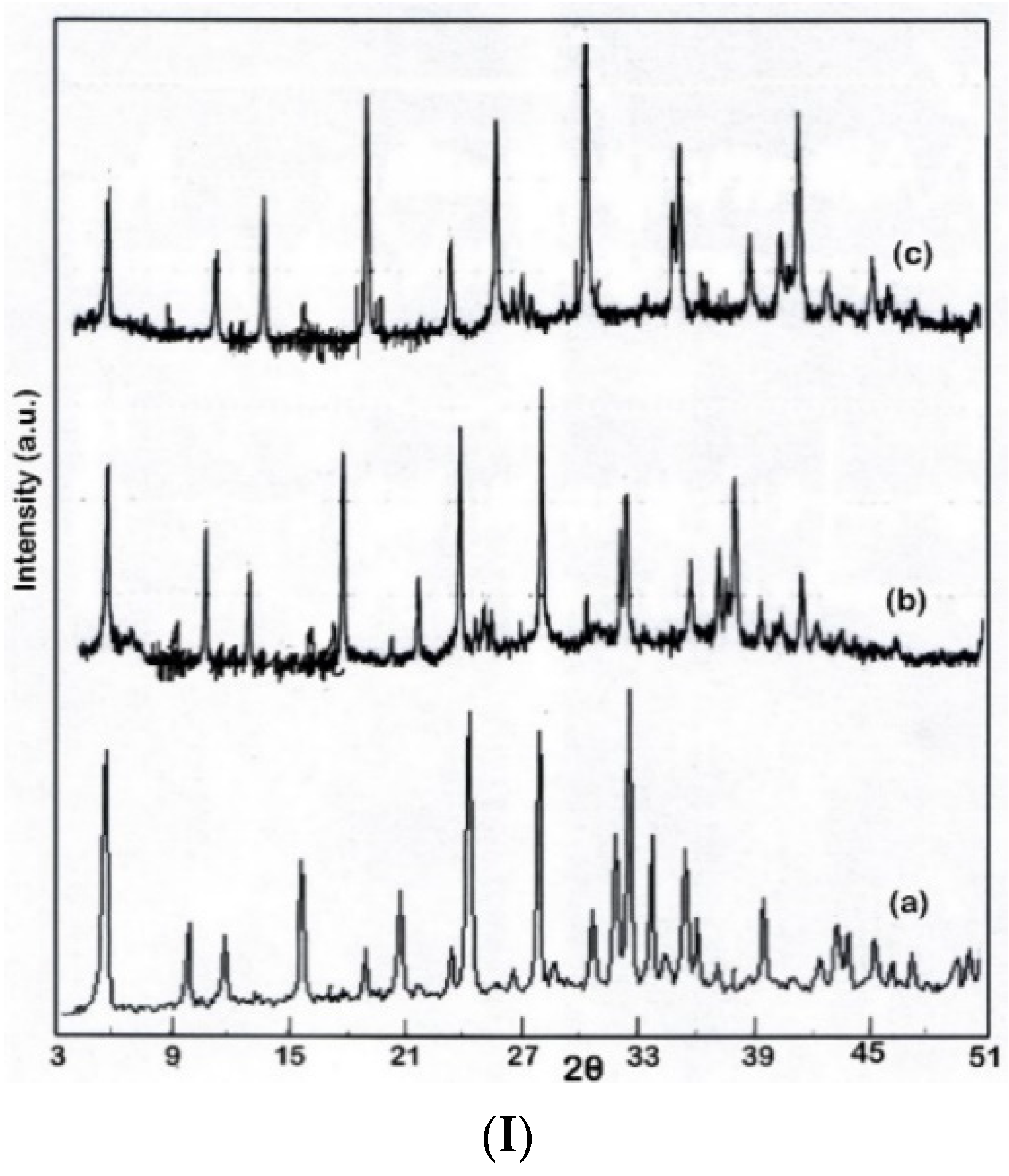
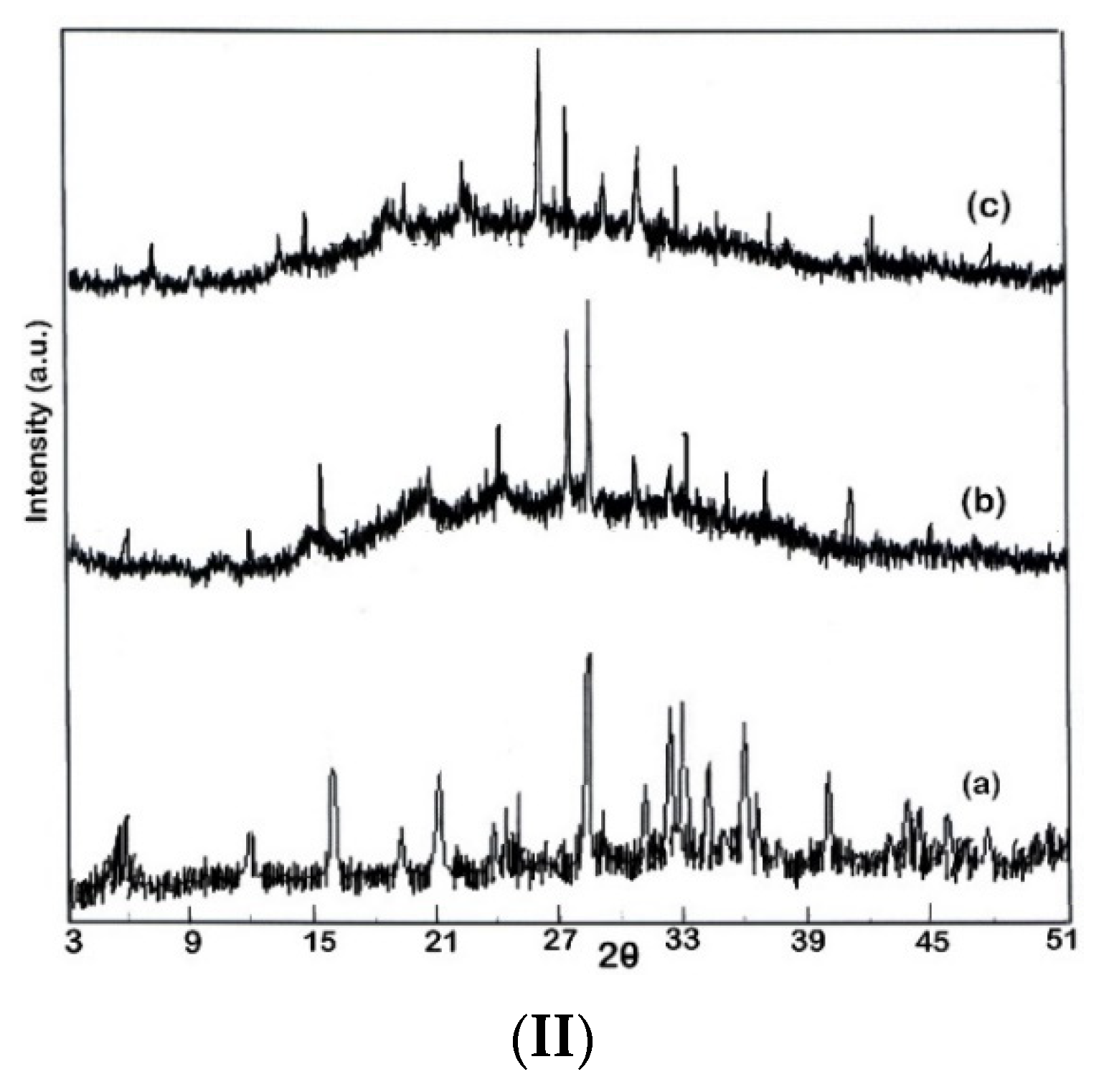
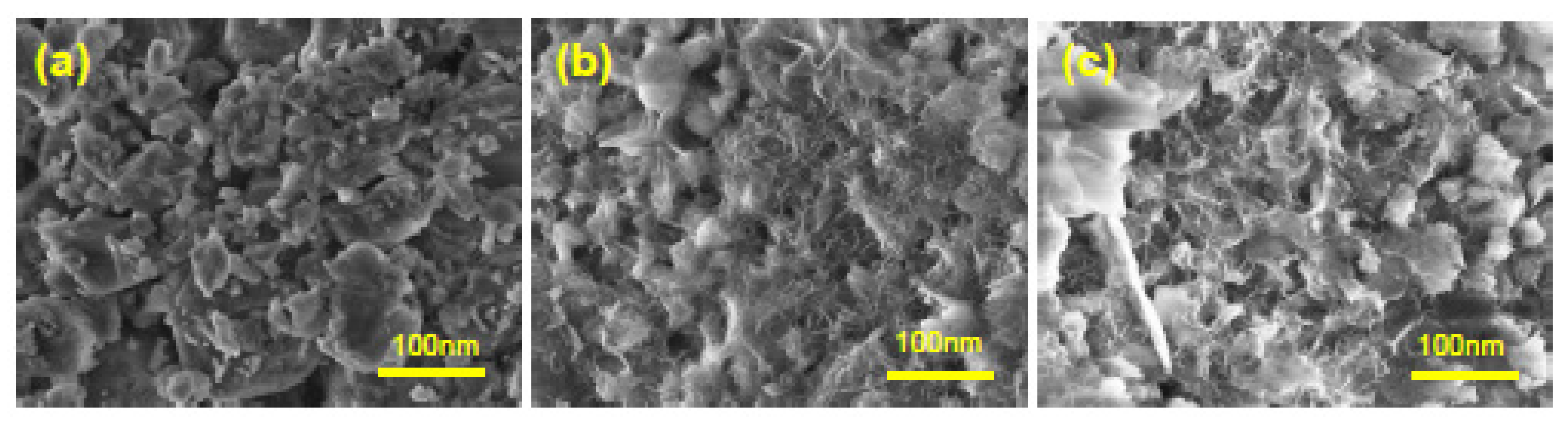
3.3. Nanocatalyst Characterization
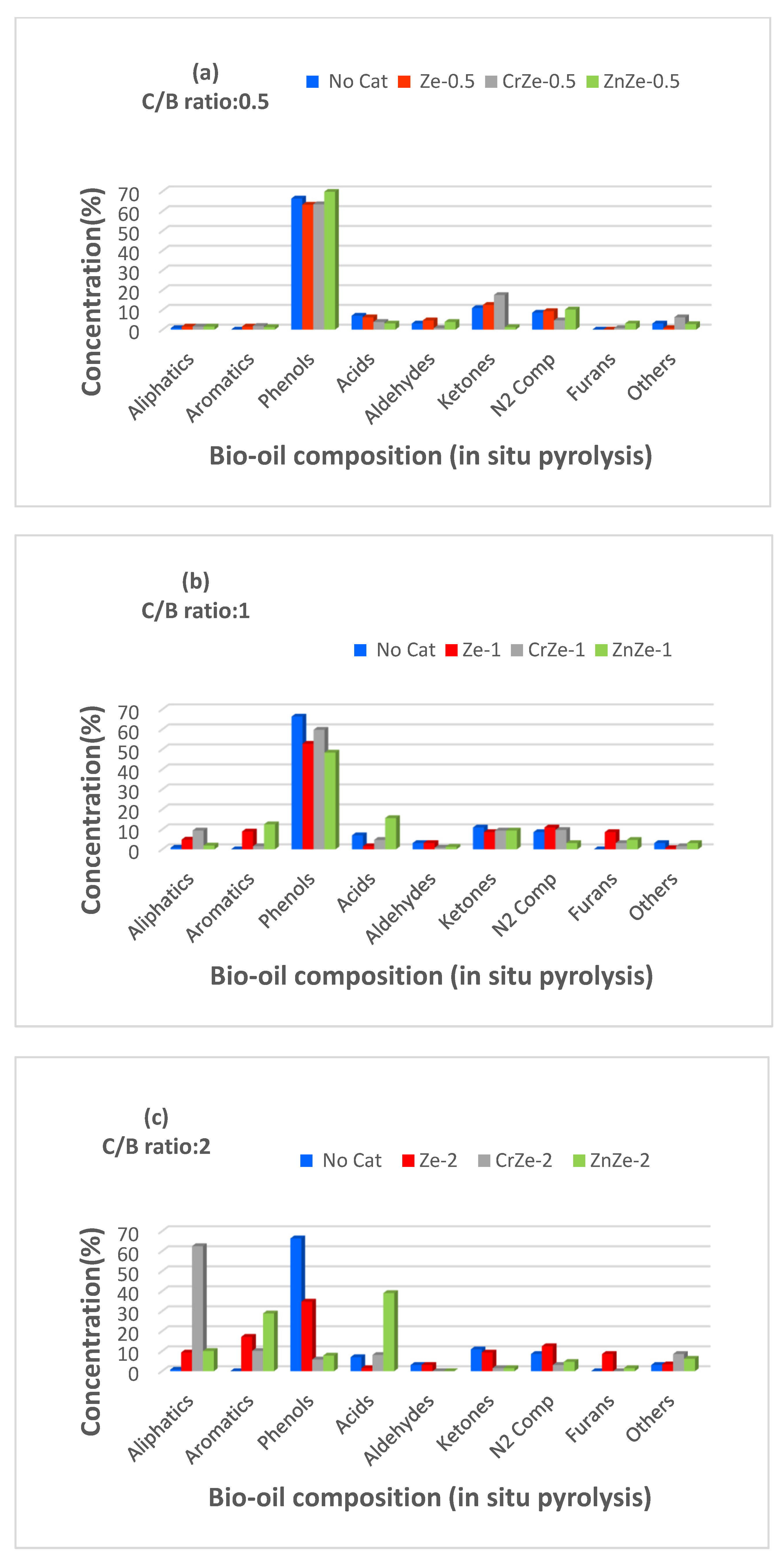
3.4. In Situ Pyrolysis of Corn Cobs Biomass
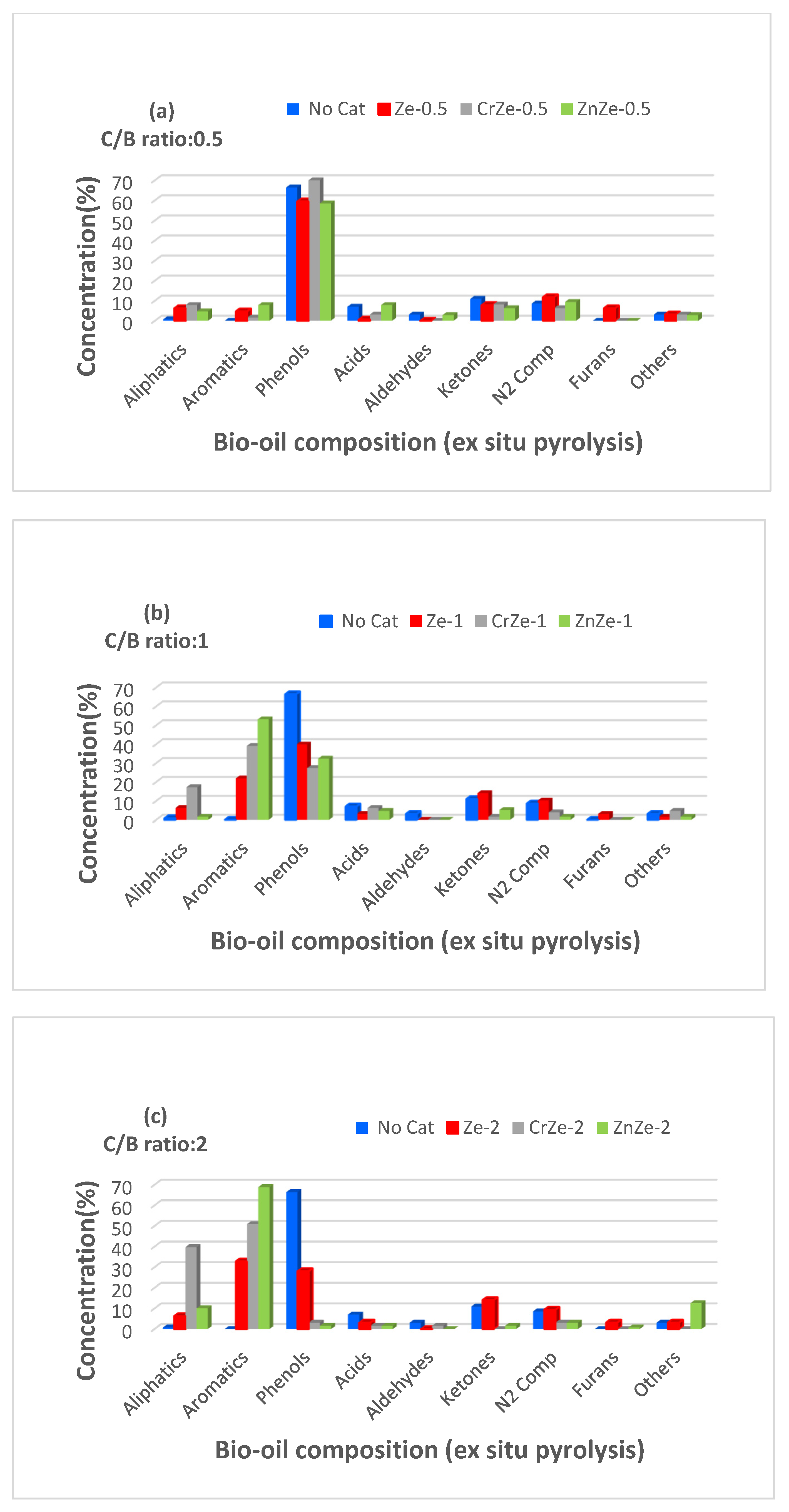
3.5. Ex Situ Pyrolysis of Corn Cobs Biomass
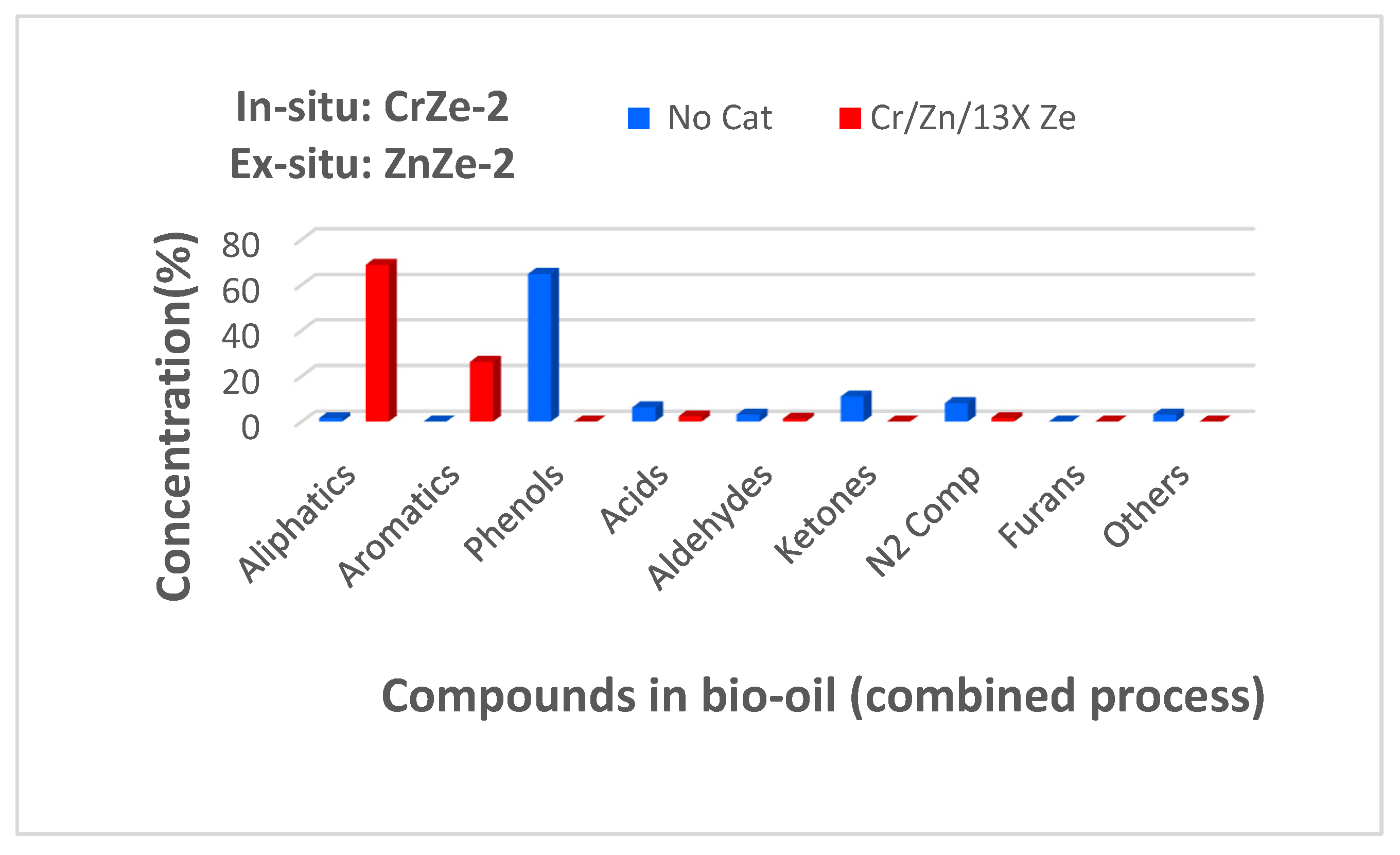
3.6. Combined In Situ and Ex Situ Pyrolysis of Corn Cobs Biomass
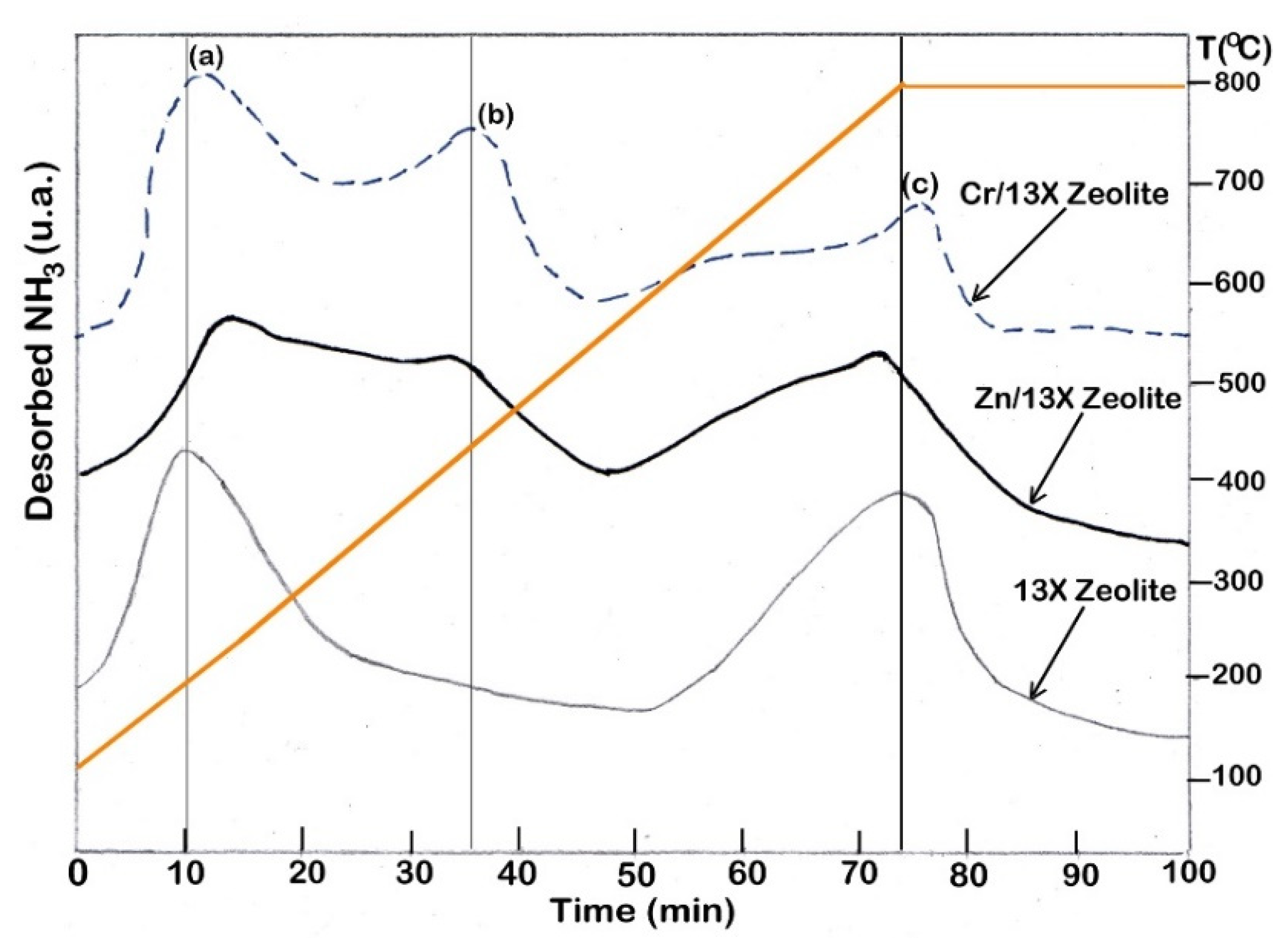
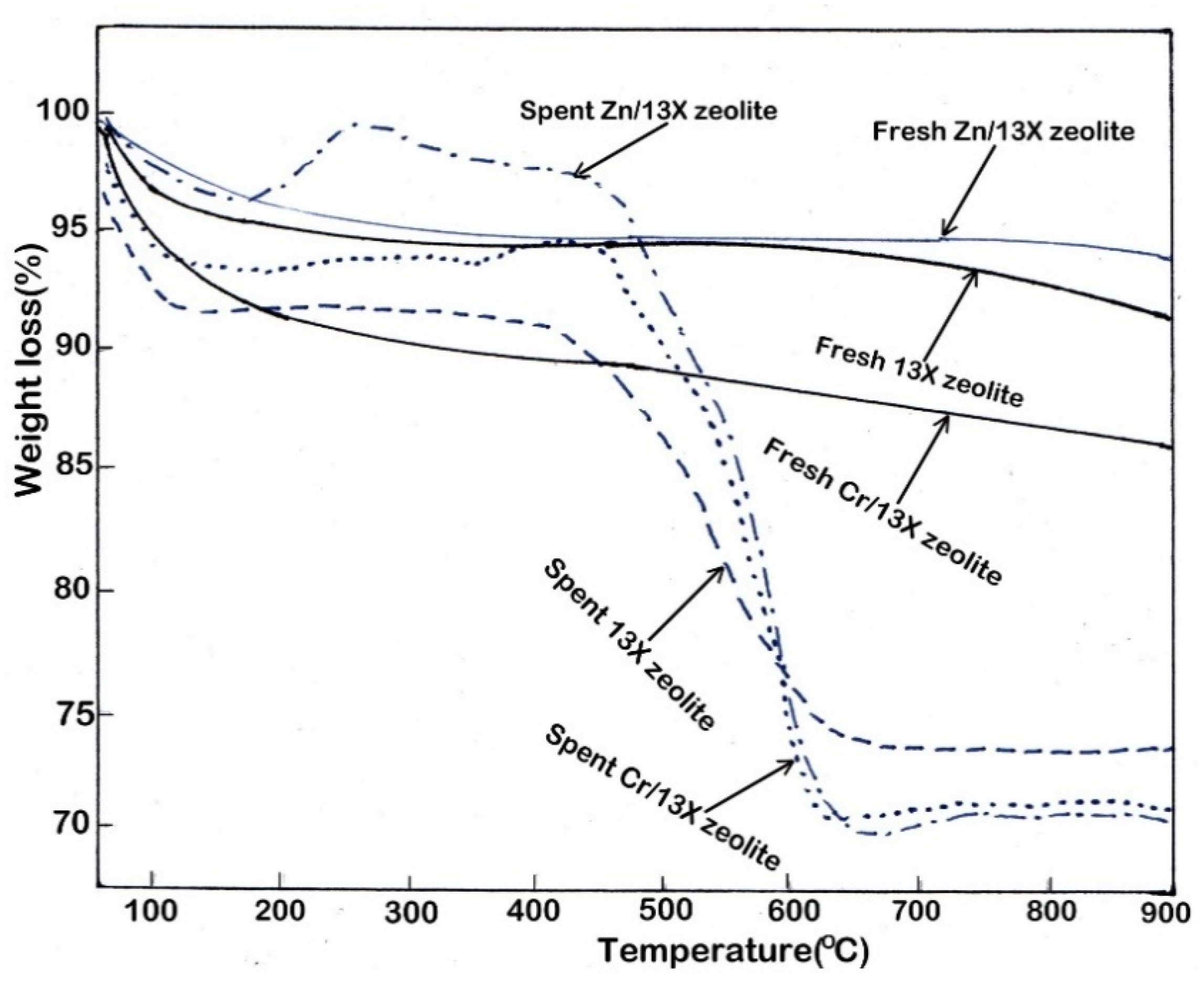
3.7. Evaluation of Coke Deposition on Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Firouzabadi, M.D.; Ghasemian, A.; de Wild, P.J.; Heeres, H.J. Sugarcane bagasse ex-situ catalytic fast pyrolysis for the production of Benzene, Toluene and Xylenes (BTX). J. Anal. Appl. Pyrolysis 2018, 131, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yaman, E.; Yargic, A.S.; Ozbay, N.; Uzun, B.B.; Kalogiannis, K.G.; Stefanidis, S.D.; Pachatouridou, E.P.; Iliopoulou, E.F.; Lappas, A.A. Catalytic upgrading of pyrolysis vapours: Effect of catalyst support and metal type on phenolic content of bio-oil. J. Clean. Prod. 2018, 185, 52–61. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Yang, J.; Hao, X.; Tsutsumi, A.; Abudula, A. Upgrading of bio-oil from biomass pyrolysis over Cu-modified β-zeolite catalyst with high selectivity and stability. Appl. Catal. B Environ. 2016, 186, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ma, J.; Xiao, Z.; Hector, S.B.; Liu, R.; Zuo, S.; Xie, X.; Zhang, A.; Wu, H.; Liu, Q. Catalytic cracking of Swida wilsoniana oil for hydrocarbon biofuel over Cu-modified ZSM-5 zeolite. Fuel 2018, 218, 59–66. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Peng, Y.; Ke, L.; Yang, Q.; Jiang, L.; Dai, L.; Liu, Y.; Ruan, R.; Xia, D.; et al. Microwave-assisted pyrolysis of waste cooking oil for hydrocarbon bio-oil over metal oxides and HZSM-5 catalysts. Energy Conv. Manag. 2020, 220, 113124. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Wenga, T.; Zhang, R.; Liu, B.; Chen, G.; Hou, L. Catalytic fast pyrolysis of Arundo donax in a two-stage fixed bed reactor over metal-modified HZSM-5 catalysts. Biomass Bioenergy 2021, 156, 106316. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.; Cheng, Y.W.; Xia, C.; Mahari, W.A.; Liew, R.K.; Peng, W.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach, Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Kuglarz, M.; Alvarado-Morales, M.; Dabkowska, K.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from rapeseed straw after dilute-acid pretreatment. Bioresour. Technol. 2018, 265, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.H.; Scott, J. Bio-oil upgrading with catalytic pyrolysis of biomass using Copper/zeolite-Nickel/zeolite and Copper-Nickel/zeolite catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.D.; Heracleous, E.; Patiaka, D.T.; Kalogiannis, K.G.; Michailof, C.M.; Lappas, A.A. Optimization of bio-oil yields by demineralization of low quality biomass. Biomass Bioenergy 2015, 83, 105–115. [Google Scholar] [CrossRef]
- Wang, H.; Srinivasan, R.; Yu, F.; Steele, P.; Li, Q.; Mitchell, B. Effect of acid, alkali, and steam explosion pretreatments on characteristics of bio-oil produced from pinewood. Energy Fuels 2011, 25, 3758–3764. [Google Scholar] [CrossRef]
- Chen, D.; Gao, D.; Capareda, S.C.; Huang, S.; Wang, Y. Effects of hydrochloric acid washing on the microstructure and pyrolysis bio-oil components of sweet sorghum bagasse. Bioresour. Technol. 2019, 277, 37–45. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31. [Google Scholar] [CrossRef]
- Cao, B.; Wang, S.; Hu, Y.; Abomohra, A.E.-F.; Qian, L.; He, Z.; Wang, Q.; Uzoejinwa, B.B.; Esakkimuthu, S. Effect of washing with diluted acids on Enteromorpha clathrata pyrolysis products: Towards enhanced bio-oil from seaweeds. Renew. Energy 2019, 138, 29–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Li, B.; Xie, G. Microwave-Assisted Pyrolysis of Biomass for Bio-Oil Production: A Review of the Operation Parameters. J. Energy Resour. Technol. 2018, 140, 040802. [Google Scholar] [CrossRef] [Green Version]
- Fodah, A.E.M.; Ghosal, M.K.; Behera, D. Bio-oil and biochar from microwave-assisted catalytic pyrolysis of corn stover using sodium carbonate catalyst. J. Energy Inst. 2020, 94, 242–251. [Google Scholar] [CrossRef]
- Tirapanampai, C.; Phetwarotai, W.; Phusunti, N. Effect of temperature and the content of Na2CO3 as a catalyst on the characteristics of bio-oil obtained from the pyrolysis of microalgae. J. Anal. Appl. Pyrolysis 2019, 142, 104644. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Saha, B. A comparative production and characterisation of fast pyrolysis bio-oil from Populus and Spruce woods. Energy 2020, 214, 118930. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Cheah, W.Y.; Kamaludin, N.H.; Ibrahim, T.N.B.T.; Sonne, C.; Peng, W.; Show, P.-L.; Lam, S.S. Progress in waste valorization using advanced pyrolysis techniques for hydrogen and gaseous fuel production. Bioresour. Technol. 2020, 320, 124299. [Google Scholar] [CrossRef]
- Wei, X.; Xue, X.; Wu, L.; Yu, H.; Liang, J.; Sun, Y. High-grade bio-oil produced from coconut shell: A comparative study of microwave reactor and core-shell catalyst. Energy 2020, 212, 118692. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Huo, X.; Xiao, J.; Song, M.; Zhu, L. Comparison between in-situ and ex-situ catalytic pyrolysis of sawdust for gas production. J. Anal. Appl. Pyrolysis 2018, 135, 189–198. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. In situ fast pyrolysis of biomass with zeolite catalysts for bioaromatics/gasoline production: A review. Energy Convers. Manag. 2015, 105, 338–354. [Google Scholar] [CrossRef]
- Zhao, C.; Lercher, J.A. Upgrading Pyrolysis Oil over Ni/HZSM-5 by Cascade Reactions. Angew. Chem. 2012, 124, 6037–6042. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; López, J.M.; Callén, M.S.; Solsona, B.; García, T. Promoting Deoxygenation of Bio-Oil by Metal-Loaded Hierarchical ZSM-5 Zeolites. ACS Sustain. Chem. Eng. 2015, 4, 1653–1660. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Chen, Y.; Chen, W.; Lei, T.; Zhang, W.; Chen, H. Catalytic fast pyrolysis of biomass to produce furfural using heterogeneous catalysts. J. Anal. Appl. Pyrolysis 2017, 127, 292–298. [Google Scholar] [CrossRef]
- Zhang, J.; Fidalgo, B.; Kolios, A.; Shen, D.; Gu, S. Mechanism of deoxygenation in anisole decomposition over single-metal loaded HZSM-5: Experimental study. Chem. Eng. J. 2018, 336, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Gamliel, D.P.; Du, S.; Bollas, G.; Valla, J.A. Investigation of in situ and ex situ catalytic pyrolysis of miscanthus × giganteus using a PyGC–MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour. Technol. 2015, 191, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Xiao, R.; Zhang, H. Ex-situ catalytic fast pyrolysis of biomass over HZSM-5 in a two-stage fluidized-bed/fixed-bed combination reactor. Bioresour. Technol. 2017, 243, 1133–1140. [Google Scholar] [CrossRef]
- Stefanidis, S.; Kalogiannis, K.; Iliopoulou, E.; Lappas, A.; Pilavachi, P. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef]
- Wang, K.; Johnston, P.A.; Brown, R.C. Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Bioresour. Technol. 2014, 173, 124–131. [Google Scholar] [CrossRef]
- Iisa, K.; French, R.J.; Orton, K.A.; Yung, M.M.; Johnson, D.K.; Dam, J.T.; Watson, M.J.; Nimlos, M.R. In Situ and ex Situ Catalytic Pyrolysis of Pine in a Bench-Scale Fluidized Bed Reactor System. Energy Fuels 2016, 30, 2144–2157. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Cao, J.-P.; Zhao, X.-Y.; Yang, Z.; Liu, T.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Catalytic upgrading of pyrolysis vapors from lignite over mono/bimetal-loaded mesoporous HZSM-5. Fuel 2018, 218, 33–40. [Google Scholar] [CrossRef]
- Hernando, H.; Hernández-Giménez, A.M.; Ochoa-Hernández, C.; Bruijnincx, P.C.A.; Houben, K.; Baldus, M.; Pizarro, P.; Coronado, J.M.; Fermoso, J.; Čejka, J.; et al. Engineering the acidity and accessibility of the zeolite ZSM-5 for efficient bio-oil upgrading in catalytic pyrolysis of lignocellulose. Green Chem. 2018, 20, 3499–3511. [Google Scholar] [CrossRef]
- Dai, G.; Wang, S.; Huang, S.; Zou, Q. Enhancement of aromatics production from catalytic pyrolysis of biomass over HZSM-5 modified by chemical liquid deposition. J. Anal. Appl. Pyrolysis 2018, 134, 439–445. [Google Scholar] [CrossRef]
- Carreon, M.A. Molecular sieve membranes for N2/CH4 separation. J. Mater. Res. 2018, 33, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Carreon, M.A. Porous crystals as membranes. Science 2020, 367, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, F.; Xie, K.; Singh, R.; Zhao, J.; Xiao, P.; Webley, P.A. Synthesis of a novel hybrid adsorbent which combines activated carbon and zeolite NaUSY for CO2 capture by electric swing adsorption (ESA). Chem. Eng. J. 2017, 336, 659–668. [Google Scholar] [CrossRef]
- Ohayon, D.; Le Van Mao, R.; Ciaravino, D.; Hazel, H.; Cochennec, A.; Rolland, N. Methods for pore size engineering in ZSM-5 zeolite. Appl. Catal. A Gen. 2001, 217, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of Novel Catalysts for Fischer-Tropsch Synthesis: Tuning the Product Selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Jun, K.-W.; Roh, H.-S.; Kim, K.-S.; Ryu, J.-S.; Lee, K.-W. Catalytic investigation for Fischer–Tropsch synthesis from bio-mass derived syngas. Appl. Catal. A Gen. 2004, 259, 221–226. [Google Scholar] [CrossRef]
- Fernando Morales, B.M.W. Promotion Effects in Co-based Fischer-Trosch Catalysis. Catalysis 2006, 19, 1–40. [Google Scholar]
- Sharma, P.; Elder, T.; Groom, L.H.; Spivey, J.J. Effect of Structural Promoters on Fe-Based Fischer–Tropsch Synthesis of Biomass Derived Syngas. Top. Catal. 2013, 57, 526–537. [Google Scholar] [CrossRef]
- Li, S.; Li, A.; Krishnamoorthy, S.; Iglesia, E. Effects of Zn, Cu and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Catal. Lett. 2001, 77, 197–205. [Google Scholar] [CrossRef]
- Ning, W.; Koizumi, N.; Yamada, M. Researching Fe Catalyst Suitable for CO2-Containing Syngas for Fischer−Tropsch Synthesis. Energy Fuels 2009, 23, 4696–4700. [Google Scholar] [CrossRef]
- Chen, X.; Dong, M.; Niu, X.; Wang, K.; Chen, G.; Fan, W.; Wang, J.; Qin, Z. Influence of Zn species in HZSM-5 on ethylene aromatization. Chin. J. Catal. 2015, 35, 880–888. [Google Scholar] [CrossRef]
- Coqueblin, H.; Richard, A.; Uzio, D.; Pinard, L.; Pouilloux, Y.; Epron, F. Effect of the metal promoter on the performances of H- ZSM5 in ethylene aromatization. Catal. Today 2017, 289, 62–69. [Google Scholar] [CrossRef]
- Hagen, A.; Roessner, F. Ethane to aromatic hydrocarbons—Past, present, future. Catal. Rev. 2000, 42, 403–437. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Lin, L.; Zhang, X.; He, N.; Liu, C.; Guo, H. Enhanced dehydrogenative aromatization of propane by incorporating Fe and Pt into the Zn/HZSM-5 catalyst. Ind. Eng. Chem. Res. 2018, 57, 16246–16256. [Google Scholar] [CrossRef]
- Alferov, K.A.; Belov, G.P.; Meng, Y. Chromium catalyst for selective ethylene oligomerization to 1-hexane and 1-octene: Recent results. Appl. Catal. 2017, 542, 71–85. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Brito, J.L.F.; Dieguez, L.C. Characterization of chromium species in catalysts for dehydrogenation and polymerization. J. Mol. Catal. A Chem. 2003, 203, 251–266. [Google Scholar] [CrossRef]
- Cherian, M.; Rao, M.S.; Hirt, A.M.; Wachs, I.E.; Deo, G. Oxidative Dehydrogenation of Propane over Supported Chromia Catalysts: Influence of Oxide Supports and Chromia Loading. J. Catal. 2002, 211, 482–495. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Iliopoulou, E.; Stefanidis, S.; Kalogiannis, K.; Delimitis, A.; Lappas, A.; Triantafyllidis, K. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Bai, X.; Yi, W.; Fu, P. Catalytic pyrolysis of lignin in a cascade dual-catalyst system of modified red mud and HZSM-5 for aromatic hydrocarbon production. Bioresour. Technol. 2019, 278, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, M.; Wang, X.S.; Shen, J.; Rhodes, M.J.; Tian, F.; Lee, W.-J.; Wu, A.H.; Li, C.-Z. Fast Pyrolysis of Oil Mallee Woody Biomass: Effect of Temperature on the Yield and Quality of Pyrolysis Products. Ind. Eng. Chem. Res. 2008, 47, 1846–1854. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Kinetic Parameters Determination of Biomass Pyrolysis Fuels Using TGA and DTA Techniques. Waste Biomass Valorizat. 2015, 6, 401–415. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y.; Liu, X.; Zhu, S. Effects of water washing and torrefaction pretreatments on rice husk pyrolysis by microwave heating. Bioresour. Technol. 2015, 193, 442–448. [Google Scholar] [CrossRef]
- Mei, Y.; Che, Q.; Yang, Q.; Draper, C.; Yang, H.; Zhang, S.; Chen, H. Torrefaction of different parts from a corn stalk and its effect on the characterization of products. Ind. Crops Prod. 2016, 92, 26–33. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; Li, X.; Wang, X.; Huang, Z.; He, F.; Li, H. Effect of hydrothermal pretreatment on properties of bio-oil produced from fast pyrolysis of eucalyptus wood in a fluidized bed reactor. Bioresour. Technol. 2013, 138, 321–328. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Chen, F.; Ma, Z.; Zhou, J.; Li, M. Are the typical organic components in biomass pyrolyzed bio-oil available for leaching of alkali and alkaline earth metallic species (AAEMs) from biomass? Fuel 2020, 260, 116347. [Google Scholar] [CrossRef]
- Mourant, D.; Wang, Z.H.; He, M.; Wang, X.S.; Garcia-Perez, M.; Ling, K.; Li, C.-Z. Mallee wood fast pyrolysis: Effects of alkali and alkaline earth metallic species on the yield and composition of bio-oil. Fuel 2011, 90, 2915–2922. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Chen, H.; Chen, X.; Yu, G. Study on reactivity characteristics and synergy behaviours of rice straw and bituminous coal co-gasification. Bioresour. Technol. 2016, 220, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]



| Nanocatalyst | Abbreviation | Nanocatalyst (g) | Biomass (g) | Ratio C/B |
|---|---|---|---|---|
| In-situ/Ex-situ pyrolysis process | ||||
| 13X zeolite | Ze-0.5 | 10 | 20 | 0.5 |
| 13X zeolite | Ze-1 | 20 | 20 | 1.0 |
| 13X zeolite | Ze-2 | 40 | 20 | 2.0 |
| Zn/13X zeolite | ZnZe-0.5 | 10 | 20 | 0.5 |
| Zn/13X zeolite | ZnZe-1 | 20 | 20 | 1.0 |
| Zn/13X zeolite | ZnZe-2 | 40 | 20 | 2.0 |
| Cr/13X zeolite | CrZe-0.5 | 10 | 20 | 0.5 |
| Cr/13X zeolite | CrZe-1 | 20 | 20 | 1.0 |
| Cr/13X zeolite | CrZe-2 | 40 | 20 | 2.0 |
| Combined pyrolysis process (in-situ + ex-situ) | ||||
| Zn/13X zeolite (in situ pyrolysis) | ZnZe-2 | 40 | 20 | 2.0 |
| Cr/13X zeolite (ex situ pyrolysis) | CrZe-2 | 40 | 20 | 2.0 |
| Characteristics | CCB | PTCCB |
|---|---|---|
| Proximate analysis (wt.%, db) | ||
| Moisture | 8.27 ± 0.12 | 6.14 ± 0.25 |
| Volatile matter | 76.05 ± 1.42 | 80.52 ± 1.25 |
| Fixed carbon | 12.22 ± 0.27 | 11.19 ± 0.65 |
| Ash content | 3.46 ± 0.26 | 2.15 ± 0.35 |
| Ultimate analysis (wt.%, db) | ||
| Carbon | 44.81 ± 0.24 | 48.96 ± 0.62 |
| Hydrogen | 5.83 ± 0.26 | 6.82 ± 0.15 |
| Nitrogen | 0.36 ± 003 | 0.42 ± 0.05 |
| Sulfur | 0.17 ± 0.01 | 0.26 ± 0.01 |
| a Oxygen | 48.62 ± 0.27 | 43.19 ± 0.55 |
| H/C molar ratio | 1.56 | 1.67 |
| O/C molar ratio | 0.81 | 0.66 |
| Component analysis (wt.%, db) | ||
| Cellulose | 31.74 ± 0.2 | 43.54 ± 0.2 |
| Hemicellulose | 32.42 ± 0.2 | 36.65 ± 0.2 |
| Lignin | 12.84 ± 0.2 | 16.76 ± 0.2 |
| Empirical formula | CH1.56 O0.81 N0.007 S0.001; | CH1.67 O0.66 N0.007 S0.002 |
| pH | 5.46 | 5.06 |
| b GCVc (MJ/kg) | 16.75 | 17.35 |
| Metals Content (ppm) | Na | K | Ca | Mg | Fe | Al | Cu | Zn |
|---|---|---|---|---|---|---|---|---|
| CCB | 55 ± 0.45 | 2720 ± 2.5 | 780 ± 10.5 | 3300 ± 3.45 | 980 ± 9.5 | 50 ± 0.5 | 60 ± 0.05 | 40 ± 0.3 |
| PTCCB | 10 ± 0.2 | 250 ± 0.2 | 12 ± 0.2 | 70 ± 5 | 47 ± 3.5 | 14 ± 0.2 | 3.65 ± 0.05 | 3 ± 0.05 |
| Removal rate (%) | 81.82 | 90.81 | 98.46 | 97.87 | 95.21 | 72.00 | 93.92 | 92.5 |
| Material | SBET (m2/g) | Aps (nm) | Vtp (cm3/g) | SMC (nm) |
|---|---|---|---|---|
| 13X zeolite | 682 | 6.12 | 0.678 | - |
| Cr/13X zeolite | 286 | 6.26 | 0.546 | 8.2 |
| Zn/13X zeolite | 218 | 6.85 | 0.507 | 28.5 |
| Material | Relative Acidity (μmol/g) | Temperature | Total Acidity (μmol/g) | ||||
|---|---|---|---|---|---|---|---|
| a | b | c | (°C) | a | b | c | |
| 13X zeolite | 26 | - | 35 | 125 | - | 750 | 62 |
| Cr/13X zeolite | 38 | 28 | 26 | 150 | 385 | 780 | 91 |
| Zn/13X zeolite | - | 41 | 30 | - | 250 | 685 | 72 |
| Compound | Formula | Retention Time (min) | Area% |
|---|---|---|---|
| 2,4-methylhexane | C8H18 | 3.85 | 3.26 |
| 3-methylheptane | C7H16 | 3.88 | 1.51 |
| 1,4-dimethylcyclohexane | C8H16 | 3.94 | 5.03 |
| Toluene | C7H8 | 4.31 | 0.68 |
| 2,6-dimethylheptane | C9H20 | 4.61 | 2.32 |
| Furfural | C5H4O2 | 6.42 | 2.45 |
| p-Xylene | C8H10 | 7.43 | 0.67 |
| Styrene | C8H8 | 8.05 | 1.43 |
| 5-methyldecane | C11H24 | 8.36 | 4.83 |
| Benzene,1,2-diethyl | C10H14 | 10.74 | 1.03 |
| Phenol | C6H6O | 10.81 | 12.92 |
| Benzofuran | C8H6O | 11.32 | 2.85 |
| Indane | C9H10 | 12.82 | 7.85 |
| 2-methyl phenol | C7H8O | 13.14 | 4.81 |
| 4-methyl phenol | C7H8O | 13.82 | 7.62 |
| Undecane | C11H24 | 14.51 | 1.12 |
| 2/3/4-ethyl phenol | C8H10O | 14.79/16.55 | 2.19 |
| 2,3/4/6-dimethyl phenol | C8H10O | 14.81/16.88 | 0.21 |
| Benzene, hexyl | C12H18 | 15.51 | 0.67 |
| 3,4-dimethyl phenol | C8H10O | 16.91 | 0.16 |
| Naphthalene | C10H8 | 17.22 | 7.66 |
| 4-ethyl-2-methoxy phenol | C9H12O2 | 19.71 | 0.45 |
| Naphthalene, 1-methyl | C11H10 | 20.38 | 3.78 |
| Pentadecane | C15H32 | 20.56 | 0.81 |
| Naphthalene, 2-methyl | C11H10 | 20.78 | 3.04 |
| Biphenyl | C12H10 | 22.66 | 0.76 |
| Naphthalene, 1-ethyl | C12H12 | 23.05 | 0.43 |
| Acenaphthylene | C12H8 | 24.49 | 3.62 |
| Acenaphthene | C12H10 | 25.33 | 0.74 |
| Fluorene | C13H10 | 27.83 | 3.18 |
| Anthracene | C14H10 | 32. 42 | 2.23 |
| Phenanthrene | C14H10 | 32.71 | 1.04 |
| 2,6-Dimethoxy-phenol | C8H10O3 | 33.11 | 0.37 |
| 3-Methyl-1H-Indole | C9H9N | 35.26 | 0.22 |
| 2,3,5-Trimethoxy toluene | C10H14O3 | 37.68 | 0.41 |
| 9-Octadecenoic acid, methyl ester | C19H36O2 | 50.05 | 0.03 |
| Oleic acid | C18H34O2 | 51.84 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Armeanu, A. Cr/13X Zeolite and Zn/13X Zeolite Nanocatalysts Used in Pyrolysis of Pretreated Residual Biomass to Produce Bio-Oil with Improved Quality. Nanomaterials 2022, 12, 1960. https://doi.org/10.3390/nano12121960
David E, Armeanu A. Cr/13X Zeolite and Zn/13X Zeolite Nanocatalysts Used in Pyrolysis of Pretreated Residual Biomass to Produce Bio-Oil with Improved Quality. Nanomaterials. 2022; 12(12):1960. https://doi.org/10.3390/nano12121960
Chicago/Turabian StyleDavid, Elena, and Adrian Armeanu. 2022. "Cr/13X Zeolite and Zn/13X Zeolite Nanocatalysts Used in Pyrolysis of Pretreated Residual Biomass to Produce Bio-Oil with Improved Quality" Nanomaterials 12, no. 12: 1960. https://doi.org/10.3390/nano12121960
APA StyleDavid, E., & Armeanu, A. (2022). Cr/13X Zeolite and Zn/13X Zeolite Nanocatalysts Used in Pyrolysis of Pretreated Residual Biomass to Produce Bio-Oil with Improved Quality. Nanomaterials, 12(12), 1960. https://doi.org/10.3390/nano12121960





