Simple Synthesis and Characterization of Shell-Thickness-Controlled Ni/Ni3C Core-Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
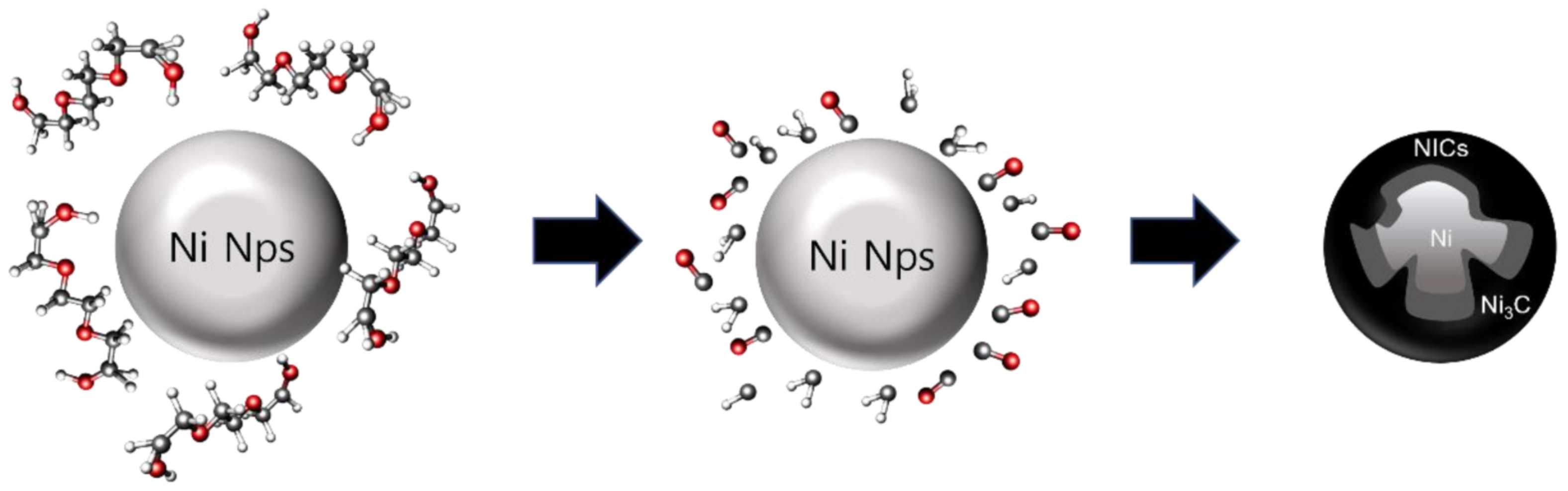
2.1. Synthesis of Ni Nps
2.2. Carburization to Obtain Ni/Ni3C Core-Shell Nanoparticles (NICs)
2.3. Analysis of Samples
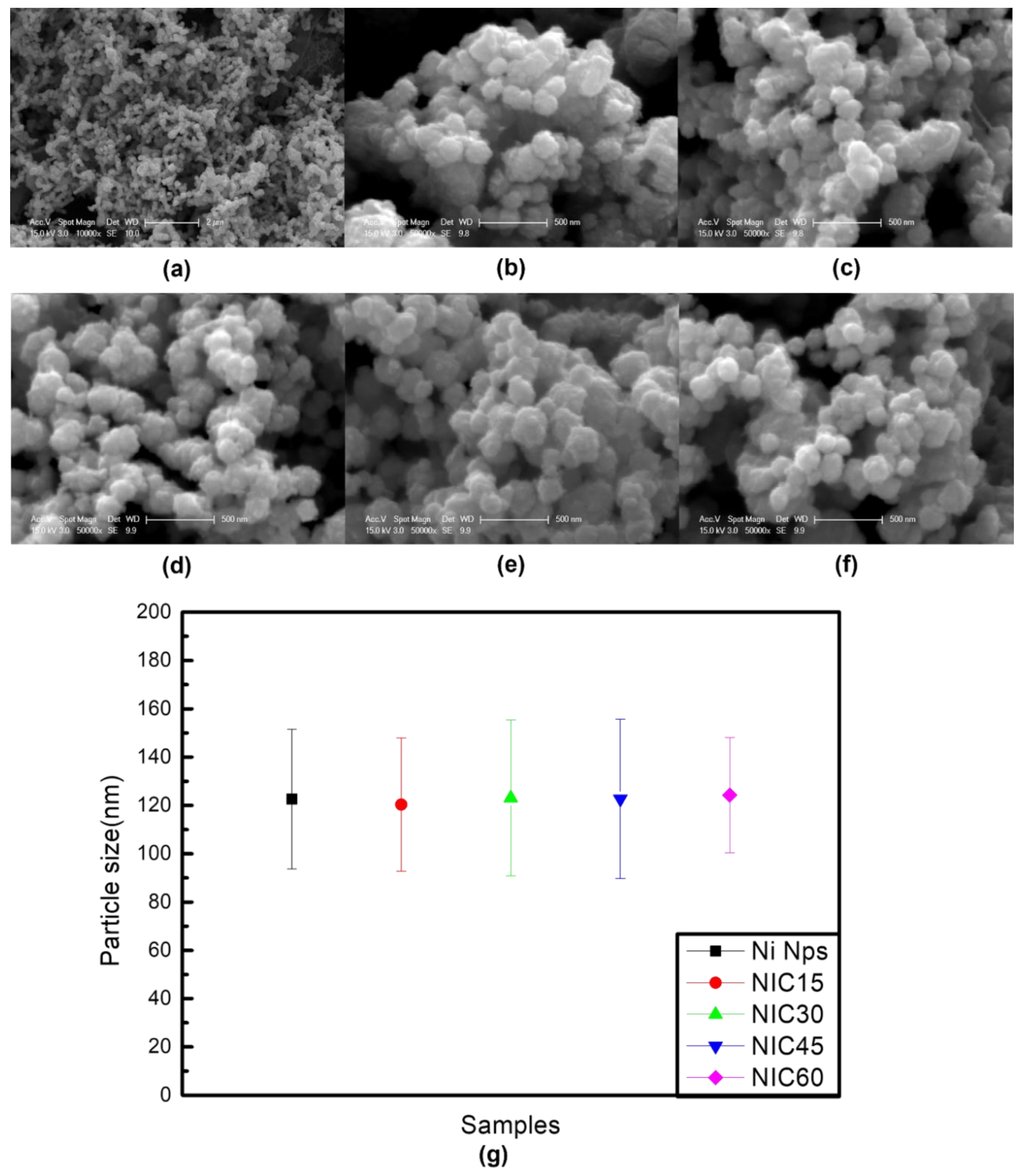
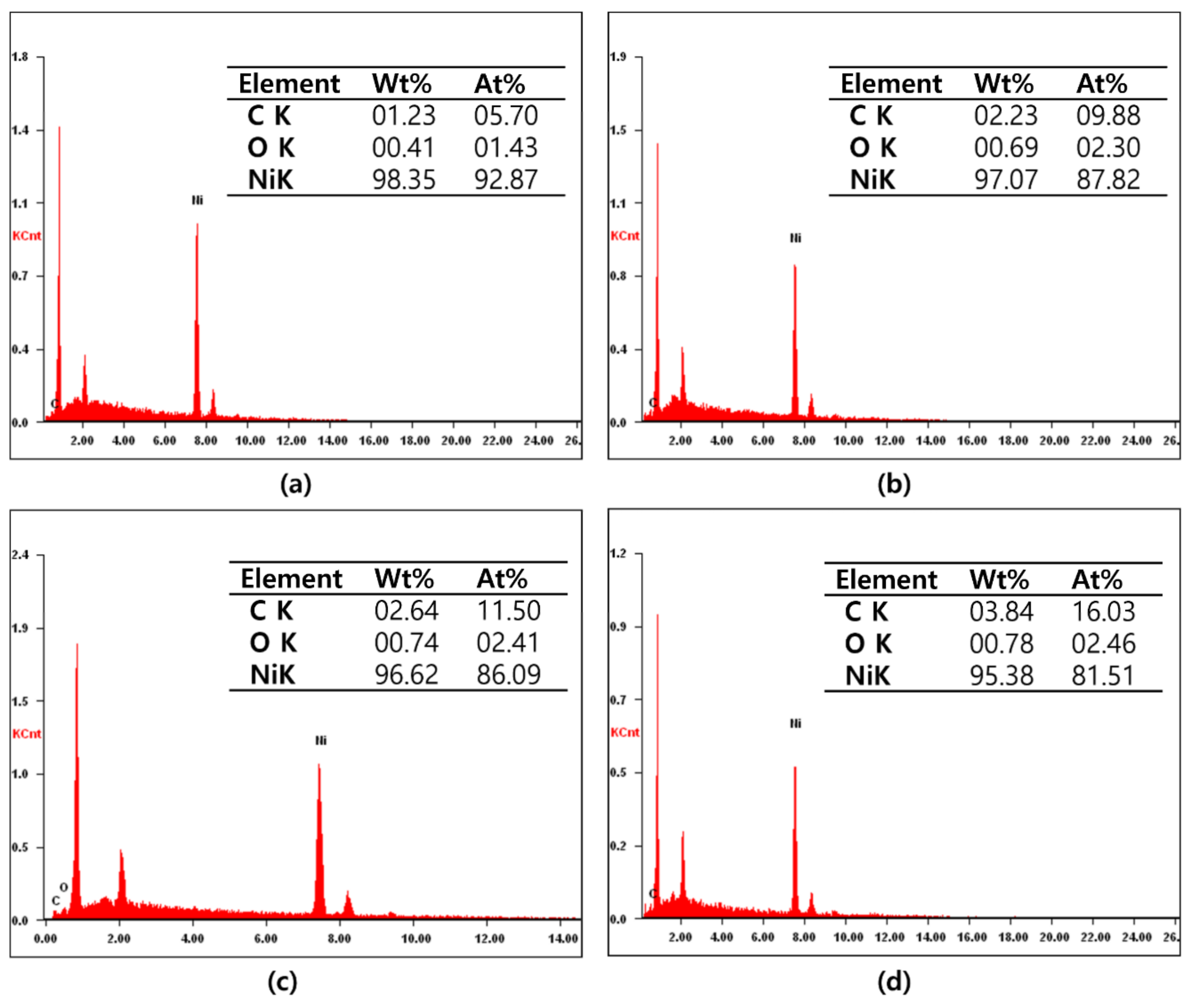
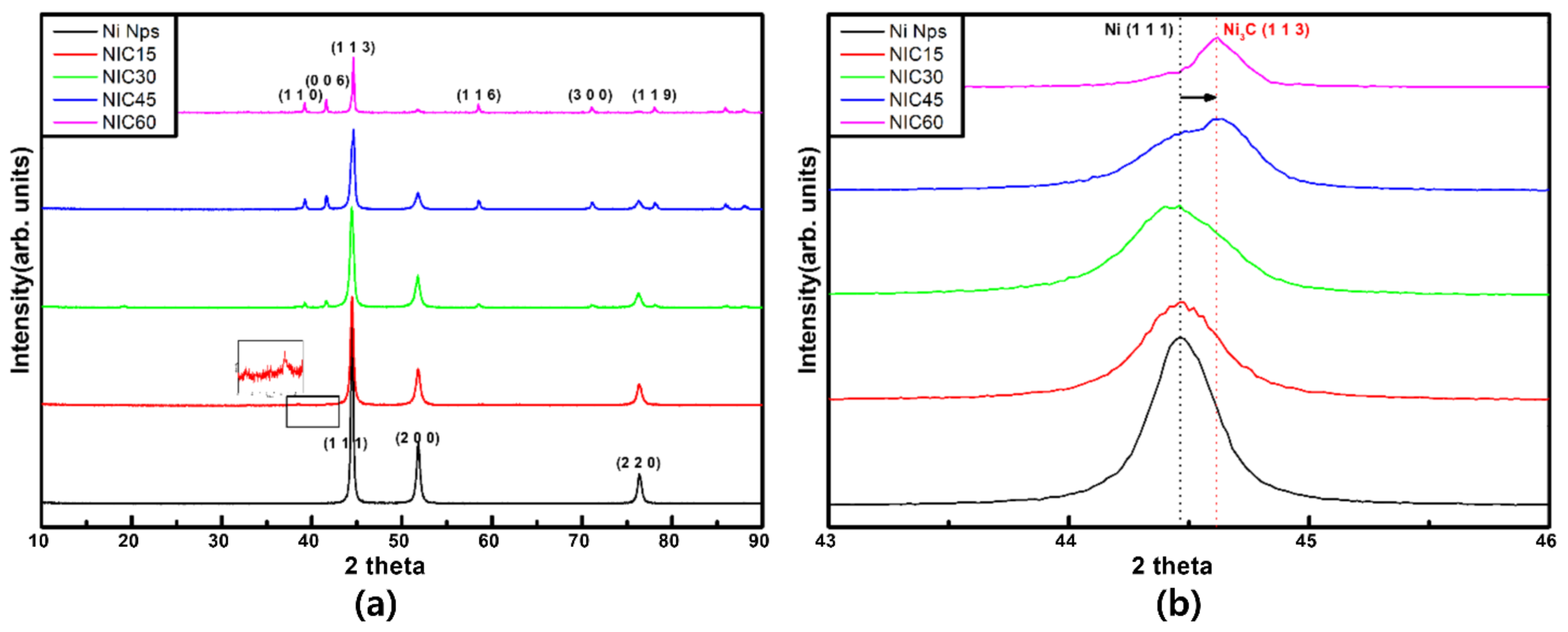
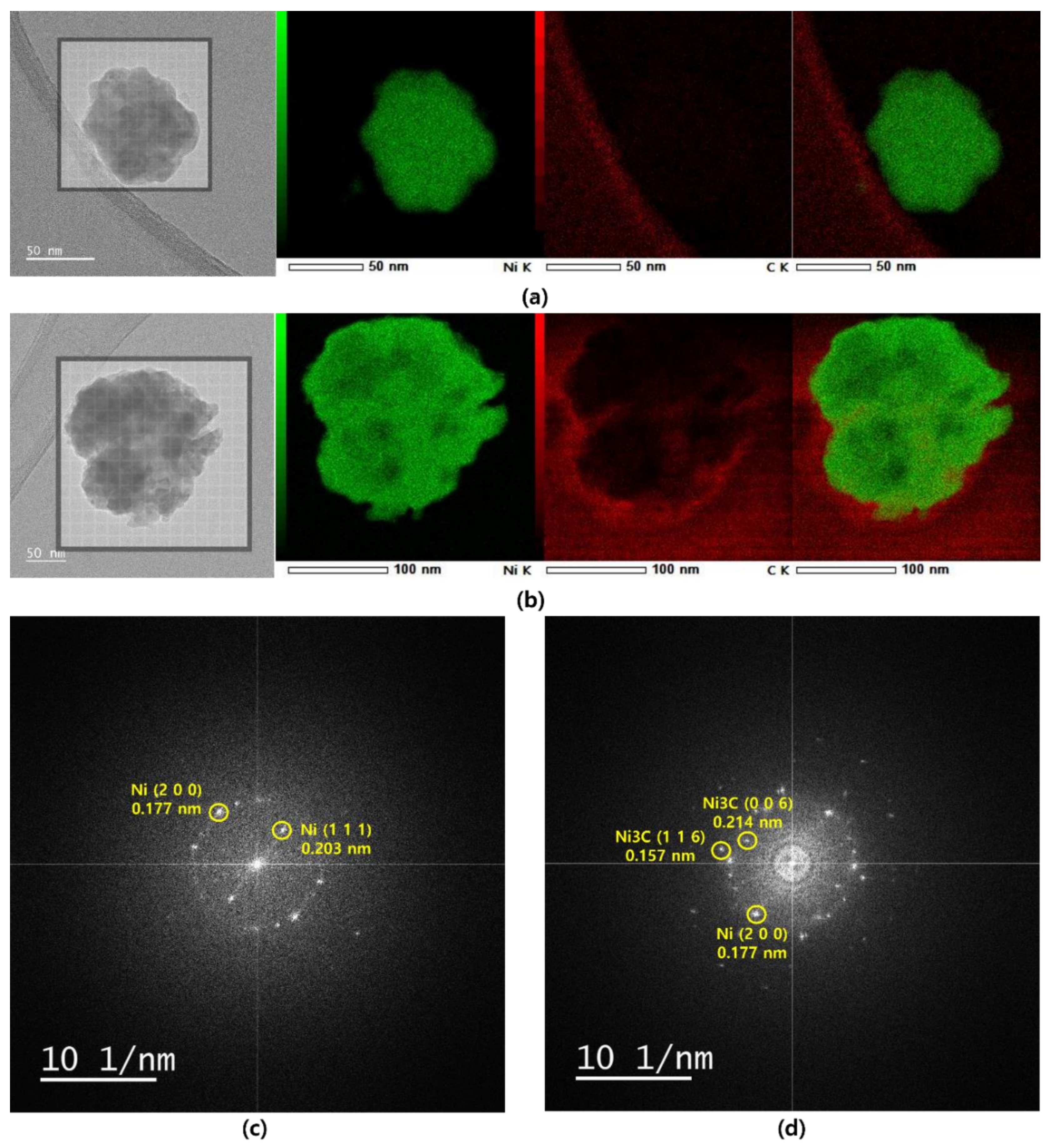
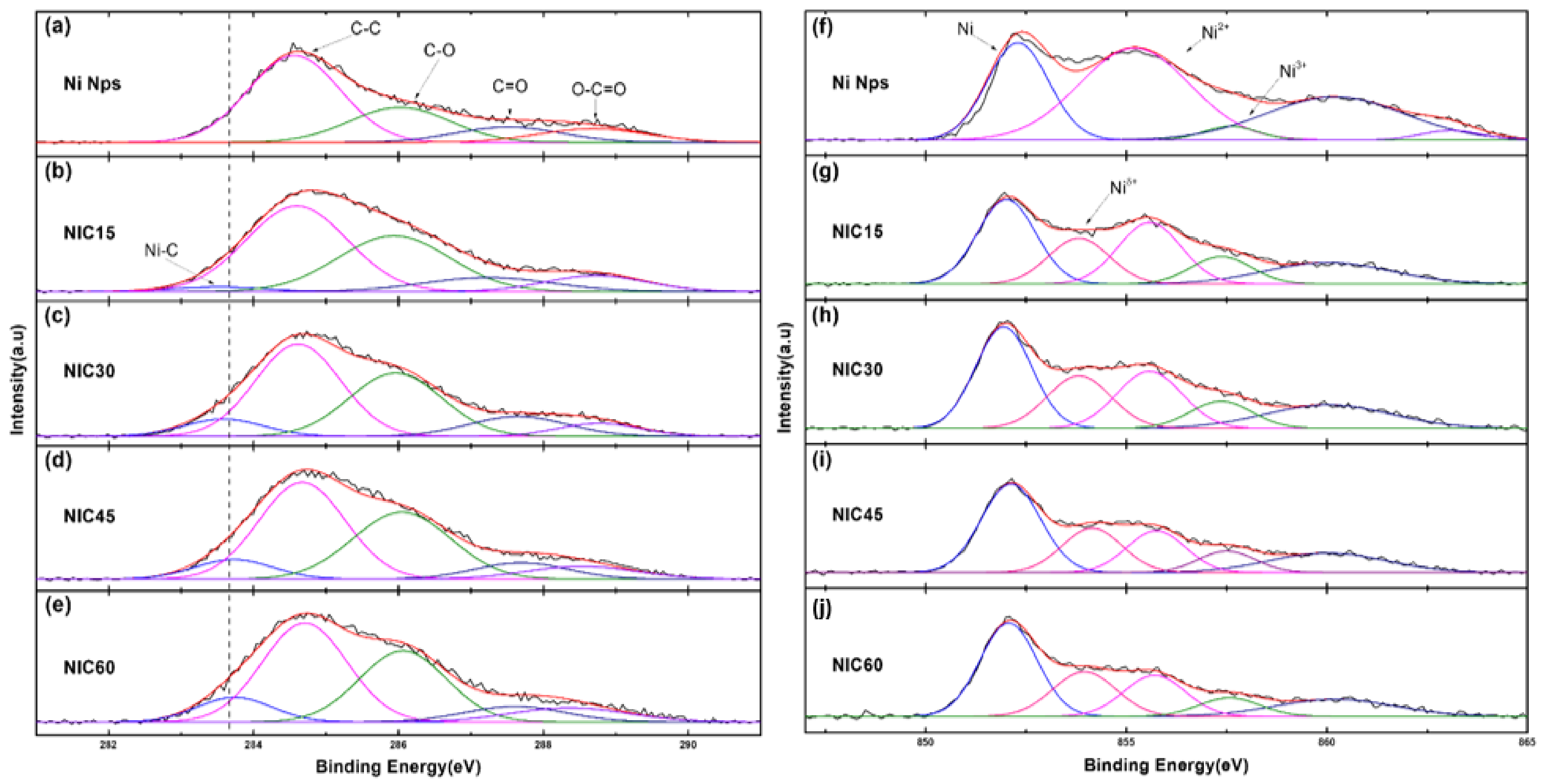
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenz, J.; Edelstein, S. Magnetic Sensors and Their Applications. IEEE Sens. J. 2006, 6, 631–649. [Google Scholar] [CrossRef]
- Khan, M.A.; Sun, J.; Li, B.; Przybysz, A.; Kosel, J. Magnetic Sensors-A Review and Recent Technologies. Eng. Res. Express 2021, 3, 022005. [Google Scholar] [CrossRef]
- Fan, H.; Xie, H.; Mao, J.; Liu, J.M.; Diao, X.; Wei, Q.; Feng, Q. Modelling of Using Hall Magnetic Sensor for Environmental Monitor of Micrometer-Sized Magnetite Particles. In Proceedings of the 15th Annual IEEE International Systems Conference (SysCon), Vancouver, BC, Canada, 15 April–15 May 2021; pp. 4–7. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A Review of Electrodeposited Ni-Co Alloy and Composite Coatings: Microstructure, Properties and Applications. Surf. Coatings Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Tolani, S.C.; Golhar, A.R.; Rewatkar, K.G. A Review of Morphological, Structural Behaviour and Technological Applications of Ferrites. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2104, p. 030032. [Google Scholar] [CrossRef]
- Radhika, M.G.; Gopalakrishna, B.; Chaitra, K.; Bhatta, L.K.G.; Venkatesh, K.; Sudha Kamath, M.K.; Kathyayini, N. Electrochemical Studies on Ni, Co & Ni/Co-MOFs for High-Performance Hybrid Supercapacitors. Mater. Res. Express 2020, 7, 054003. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Zulfiqar, M.; Zeng, S.; Ni, J. Electronic and Magnetic Properties of Fe-, Co-, and Ni-Decorated BC 3: A First-Principles Study. Phys. Lett. A 2018, 382, 1395–1400. [Google Scholar] [CrossRef]
- Kneller, E.F.; Hawig, R. The Exchange-Spring Magnet: A New Material Principle for Permanent Magnets. IEEE Trans. Magn. 1991, 27, 3588–3600. [Google Scholar] [CrossRef]
- Callen, E.; Liu, Y.J.; Cullen, J.R. Initial Magnetization, Remanence, and Coercivity of the Random Anisotropy Amorphous Ferromagnet. Phys. Rev. B 1977, 16, 263–270. [Google Scholar] [CrossRef]
- Nagakura, S. Study of Metallic Carbides by Electron Diffraction Part I. Formation and Decomposition of Nickel Carbide. J. Phys. Soc. Jpn. 1957, 12, 482–494. [Google Scholar] [CrossRef]
- Yue, L.; Sabiryanov, R.; Kirkpatrick, E.M.; Leslie-Pelecky, D.L. Magnetic Properties of Disordered Ni3C. Phys. Rev. B 2000, 62, 8969–8975. [Google Scholar] [CrossRef] [Green Version]
- Tokumitsu, K. Synthesis of Metastable Fe3C, Co3C and Ni3C by Mechanical Alloying Method. Mater. Sci. Forum 1996, 235–238, 127–132. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.F.; Liu, B.X.; Fang, Z.Z. Correlation of 3d Transition Metal Carbide Phase Formation with 3d Electrons by Carbon Ion Implantation. Acta Metall. Mater. 1992, 40, 1417–1420. [Google Scholar] [CrossRef]
- Sinharoy, S.; Smith, M.A.; Levenson, L.L. Thermal Decomposition of Nickel Carbide Thin Films. Surf. Sci. 1978, 72, 710–718. [Google Scholar] [CrossRef]
- Zhu, G.X.; Wei, X.W.; Jiang, S. A Facile Route to Carbon-Coated Nickel-Based Metal Nanoparticles. J. Mater. Chem. 2007, 17, 2301–2306. [Google Scholar] [CrossRef]
- Sun, X.C.; Dong, X.L. Magnetic Properties and Microstructure of Carbon Encapsulated Ni Nanoparticles and Pure Ni Nanoparticles Coated with NiO Layer. Mater. Res. Bull. 2002, 37, 991–1004. [Google Scholar] [CrossRef]
- Sun, X.; Gutierrez, A.; Yacaman, M.J.; Dong, X.; Jin, S. Investigations on Magnetic Properties and Structure for Carbon Encapsulated Nanoparticles of Fe, Co, Ni. Mater. Sci. Eng. A 2000, 286, 157–160. [Google Scholar] [CrossRef]
- Nishijo, J.; Okabe, C.; Oishi, O.; Nishi, N. Synthesis, Structures and Magnetic Properties of Carbon-Encapsulated Nanoparticles via Thermal Decomposition of Metal Acetylide. Carbon N. Y. 2006, 44, 2943–2949. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, K.; He, L.; Wang, R.; Guo, L.; Chen, C.; Han, X.; Zhang, Z. Ni/Ni3C Core-Shell Nanochains and Its Magnetic Properties: One-Step Synthesis at Low Temperature. Nano Lett. 2008, 8, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, S.; Struis, R.; Schmid-Engel, H.; Bock, J.; Probst, A.C.; Freitag-Weber, O.; Zizak, I.; Chernikov, R.; Schultes, G. Piezoresistive Ni:A-C:H Thin Films Containing Hcp-Ni or Ni3C Investigated by XRD, EXAFS, and Wavelet Analysis. Diam. Relat. Mater. 2013, 34, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, Z.L.; Weeber, K.M.; Misra, R.; Schiffer, P.; Schaak, R.E. Bridging Hcp-Ni and Ni3C via a Ni3C 1-x Solid Solution: Tunable Composition and Magnetism in Colloidal Nickel Carbide Nanoparticles. Chem. Mater. 2011, 23, 2475–2480. [Google Scholar] [CrossRef]
- Xing, M.; Mohapatra, J.; Zeng, F.; Ping Liu, J. Magnetic Properties of Nickel Carbide Nanoparticles with Enhanced Coercivity. AIP Adv. 2018, 8, 10–14. [Google Scholar] [CrossRef]
- Kovács, G.J.; Bertóti, I.; Radnóczi, G. X-ray Photoelectron Spectroscopic Study of Magnetron Sputtered Carbon-Nickel Composite Films. Thin Solid Films 2008, 516, 7942–7946. [Google Scholar] [CrossRef]
- Poppv, C.; Kulisch, W.; Boycheva, S.; Yamamoto, K.; Ceccone, G.; Koga, Y. Structural Investigation of Nanocrystalline Diamond/Amorphous Carbon Composite Films. Diam. Relat. Mater. 2004, 13, 2071–2075. [Google Scholar] [CrossRef]
- Díaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the and Components in the C1s Photoemission Spectra of Amorphous Carbon Films. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 8064–8069. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U.; Magnuson, M. Crystallization Characteristics and Chemical Bonding Properties of Nickel Carbide Thin Film Nanocomposites. J. Phys. Condens. Matter 2014, 26, 415501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisoni, J.G.; Goux, L.; Hoffmann, T.; Diaz-Droguett, D.E.; Jurczak, M. Influence of the Microstructure on the Oxidation of Ni Thin Films. Corros. Sci. 2012, 59, 282–289. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray Photoelectron Spectroscopic Chemical State Quantification of Mixed Nickel Metal, Oxide and Hydroxide Systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Yoon, J.S.; Park, M.B.; Kim, Y.; Hwang, D.W.; Chae, H.-J. Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts 2019, 9, 933. [Google Scholar] [CrossRef] [Green Version]
- González-castaño, M.; Le Saché, E.; Berry, C.; Pastor-pérez, L.; Arellano-garcía, H.; Wang, Q.; Reina, T.R. Article Nickel Phosphide Catalysts as Efficient Systems for Co2 Upgrading via Dry Reforming of Methane. Catalysts 2021, 11, 446. [Google Scholar] [CrossRef]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, 2001642. [Google Scholar] [CrossRef]
- Kang, J.; Han, R.; Wang, J.; Yang, L.; Fan, G.; Li, F. In Situ Synthesis of Nickel Carbide-Promoted Nickel/Carbon Nanofibers Nanocomposite Catalysts for Catalytic Applications. Chem. Eng. J. 2015, 275, 36–44. [Google Scholar] [CrossRef]
- Jing, S.; Gong, X.; Ji, S.; Jia, L.; Pollet, B.G.; Yan, S.; Liang, H. Self-Standing Heterostructured NiCx-NiFe-NC/Biochar as a Highly Efficient Cathode for Lithium–Oxygen Batteries. Beilstein J. Nanotechnol. 2020, 11, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Drake, N.L.; Smith, T.B. The Decomposition of Ethylene Glycol in the Presence of Catalysts. I. Vanadium Pentoxide as Catalyst. J. Am. Chem. Soc. 1930, 52, 4558–4566. [Google Scholar] [CrossRef]
- Xiong, J.; Ding, Y.; Wang, T.; Yan, L.; Chen, W.; Zhu, H.; Lu, Y. The Formation of Co2C Species in Activated Carbon Supported Cobalt-Based Catalysts and Its Impact on Fischer-Tropsch Reaction. Catal. Lett. 2005, 102, 265–269. [Google Scholar] [CrossRef]
- Poudyal, N.; Chaubey, G.S.; Rong, C.B.; Cui, J.; Liu, J.P. Synthesis of Monodisperse FeCo Nanoparticles by Reductive Salt-Matrix Annealing. Nanotechnology 2013, 24, 345605. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.I.; Martínez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3 Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and Magnetic Properties of Uniform Magnetite Nanoparticles Prepared by High Temperature Decomposition of Organic Precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar] [CrossRef]
- Husain, H.; Hariyanto, B.; Sulthonul, M.; Klysubun, W.; Darminto, D.; Pratapa, S. Structure and Magnetic Properties of Silica-Coated Magnetite-Nanoparticle Composites. Mater. Res. Express 2019, 6, 086117. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.; Chul Ro, J.; Suh, S.J. Yolk–Shell Fe–Fe3O4@C Nanoparticles with Excellent Reflection Loss and Wide Bandwidth as Electromagnetic Wave Absorbers in the High-Frequency Band. Appl. Surf. Sci. 2022, 573, 151469. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Ro, J.C.; Suh, S.-J. Simple Synthesis and Characterization of Shell-Thickness-Controlled Ni/Ni3C Core-Shell Nanoparticles. Nanomaterials 2022, 12, 1954. https://doi.org/10.3390/nano12121954
Kim S-W, Ro JC, Suh S-J. Simple Synthesis and Characterization of Shell-Thickness-Controlled Ni/Ni3C Core-Shell Nanoparticles. Nanomaterials. 2022; 12(12):1954. https://doi.org/10.3390/nano12121954
Chicago/Turabian StyleKim, Sun-Woo, Jae Chul Ro, and Su-Jeong Suh. 2022. "Simple Synthesis and Characterization of Shell-Thickness-Controlled Ni/Ni3C Core-Shell Nanoparticles" Nanomaterials 12, no. 12: 1954. https://doi.org/10.3390/nano12121954





