Novel MOF-Based Photocatalyst AgBr/AgCl@ZIF-8 with Enhanced Photocatalytic Degradation and Antibacterial Properties
Abstract
:1. Introduction
2. Experimental Section
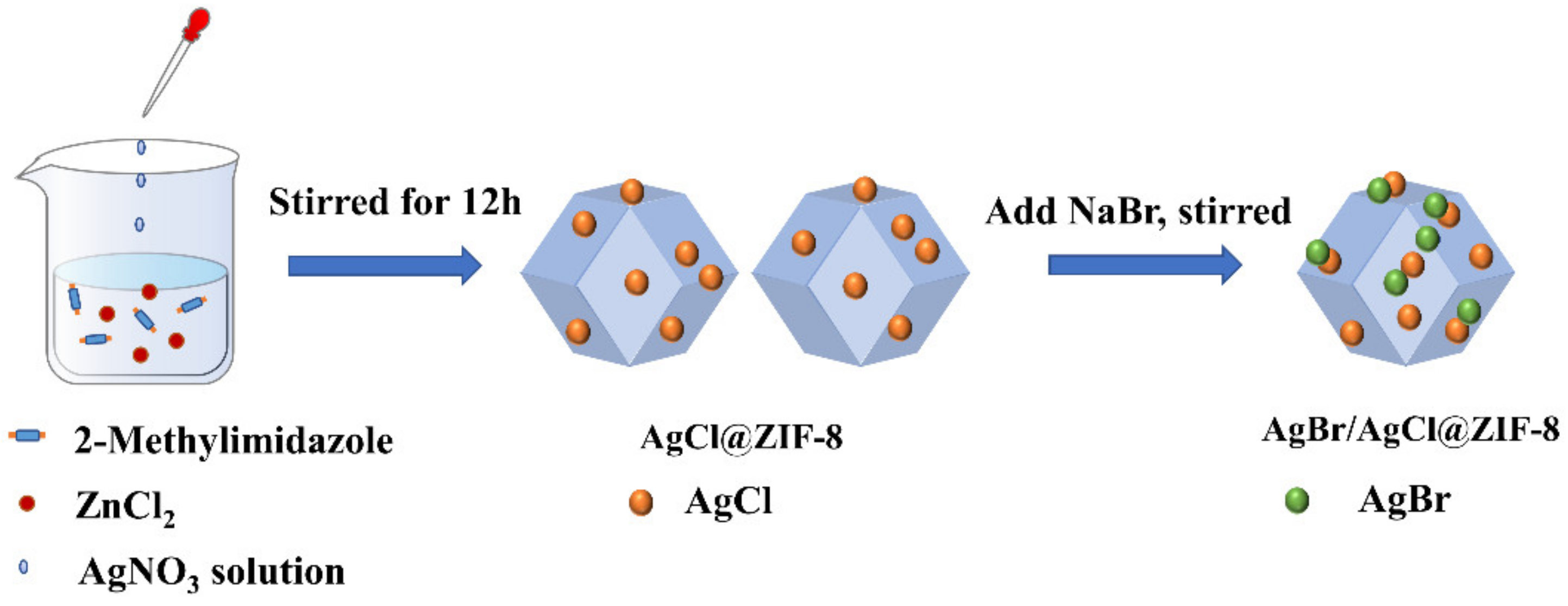
2.1. Preparation of AgBr/AgCl@ZIF-8
2.2. Characterization
2.3. Photocatalytic Performance
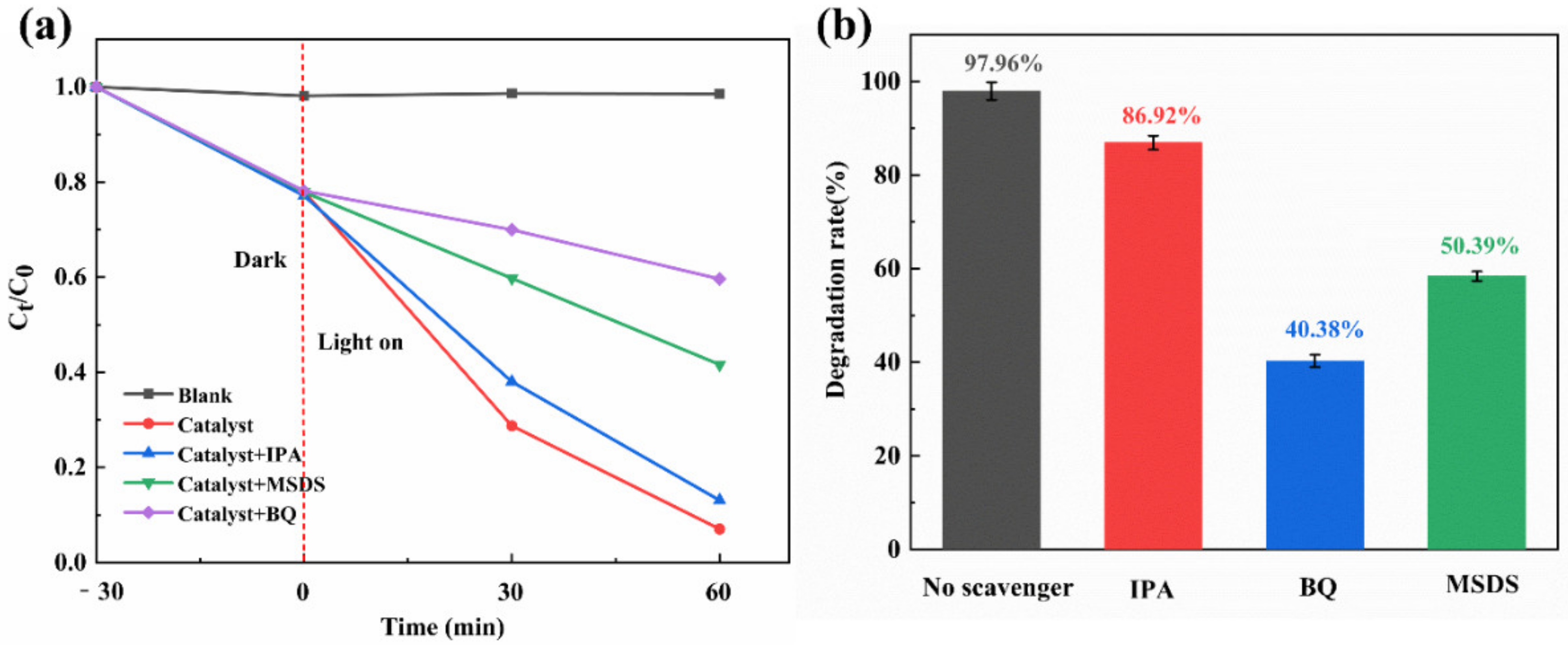
2.4. Free Radical Scavenging Experiment
3. Results and Discussion
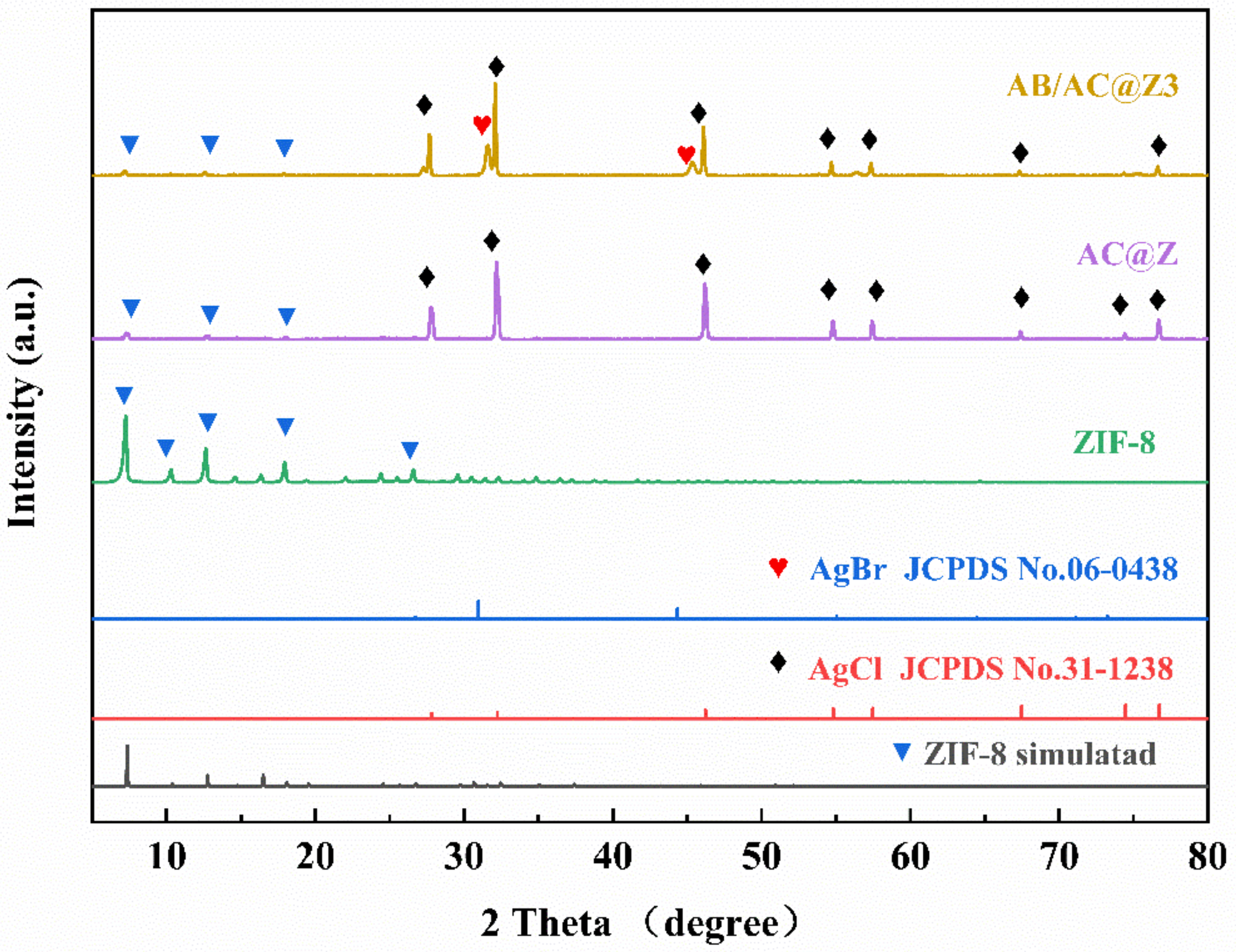
3.1. The Structure of Photocatalysts
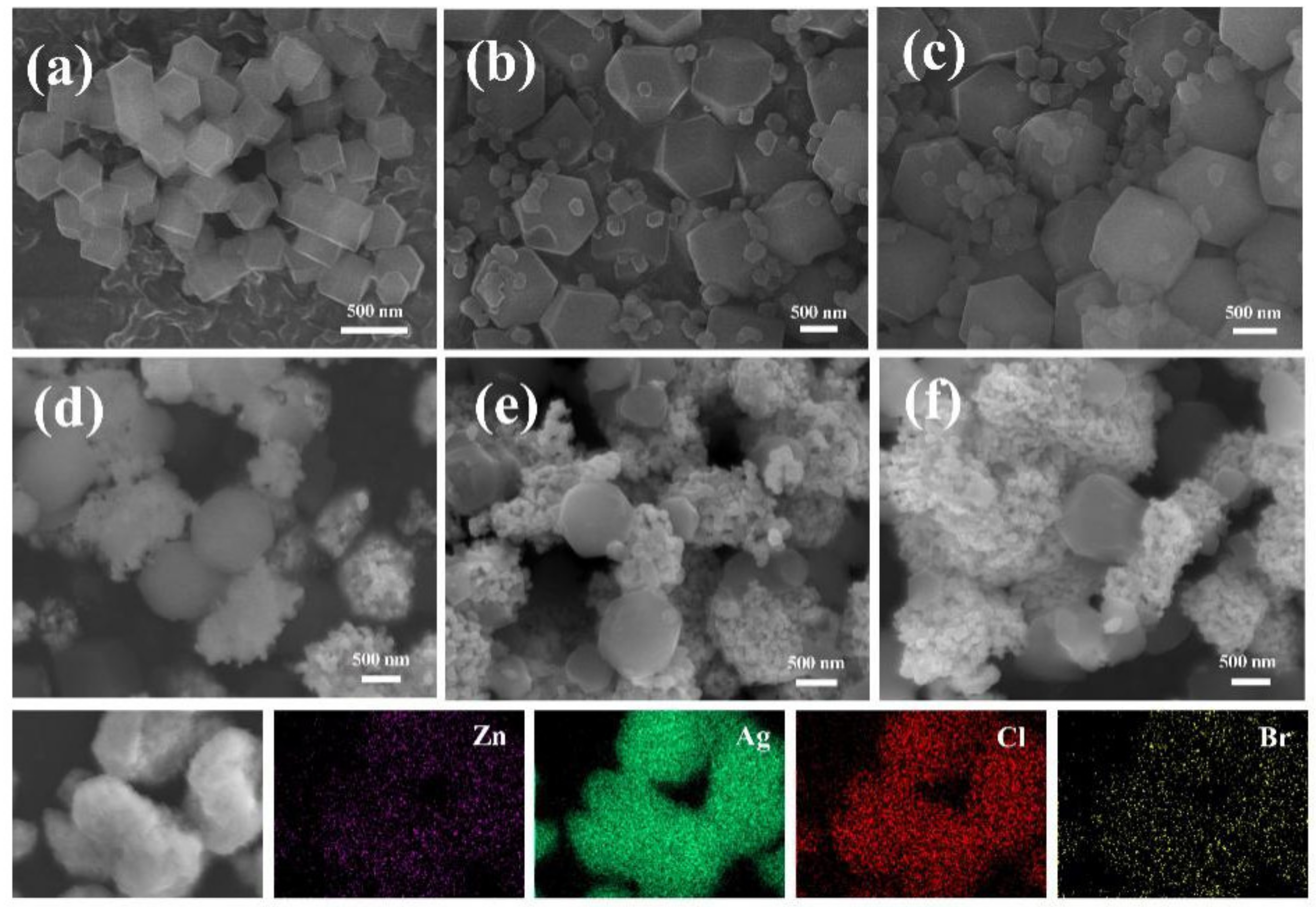
3.2. The Morphologies of the Photocatalysts
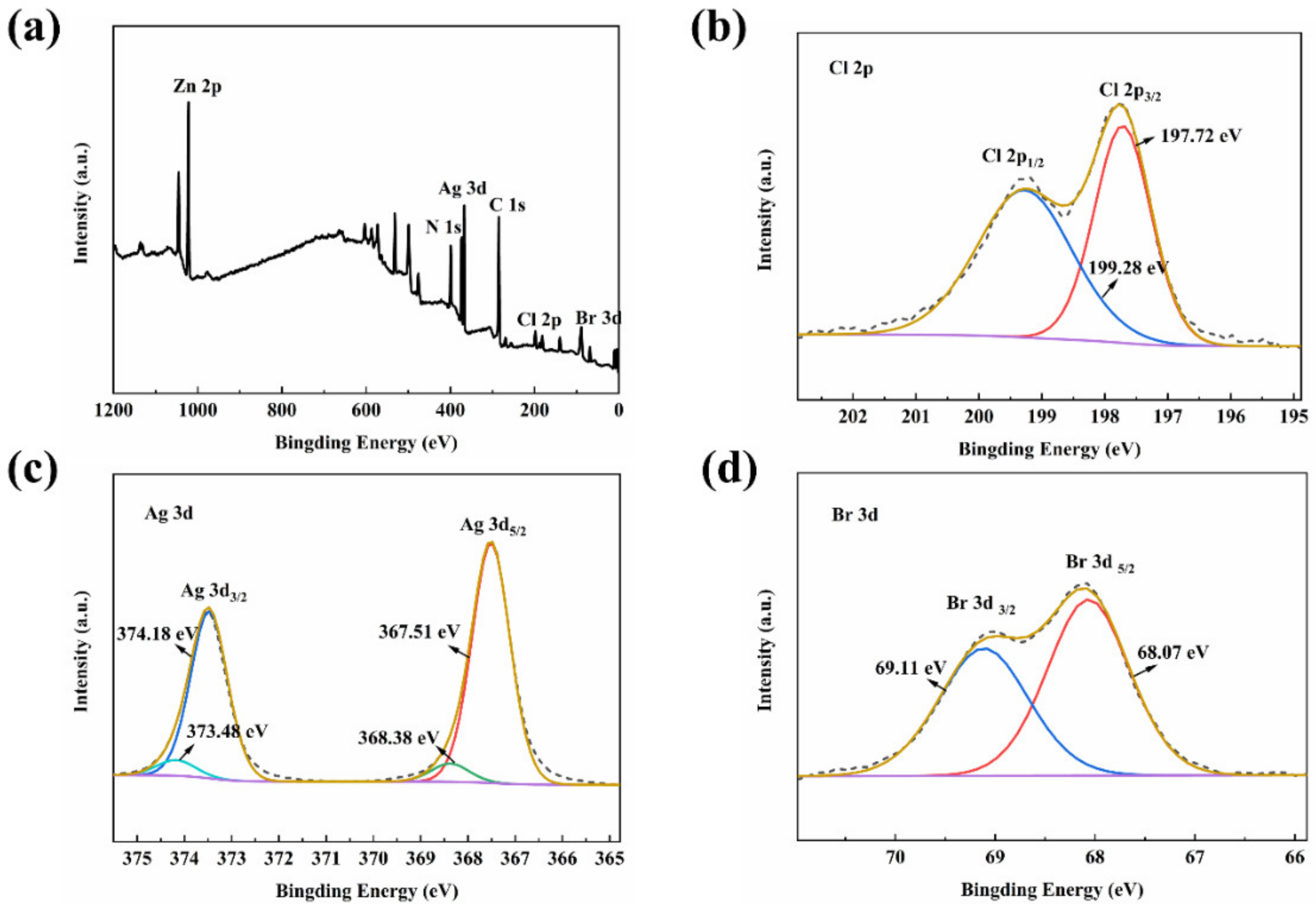
3.3. The Surface Elemental Compositions
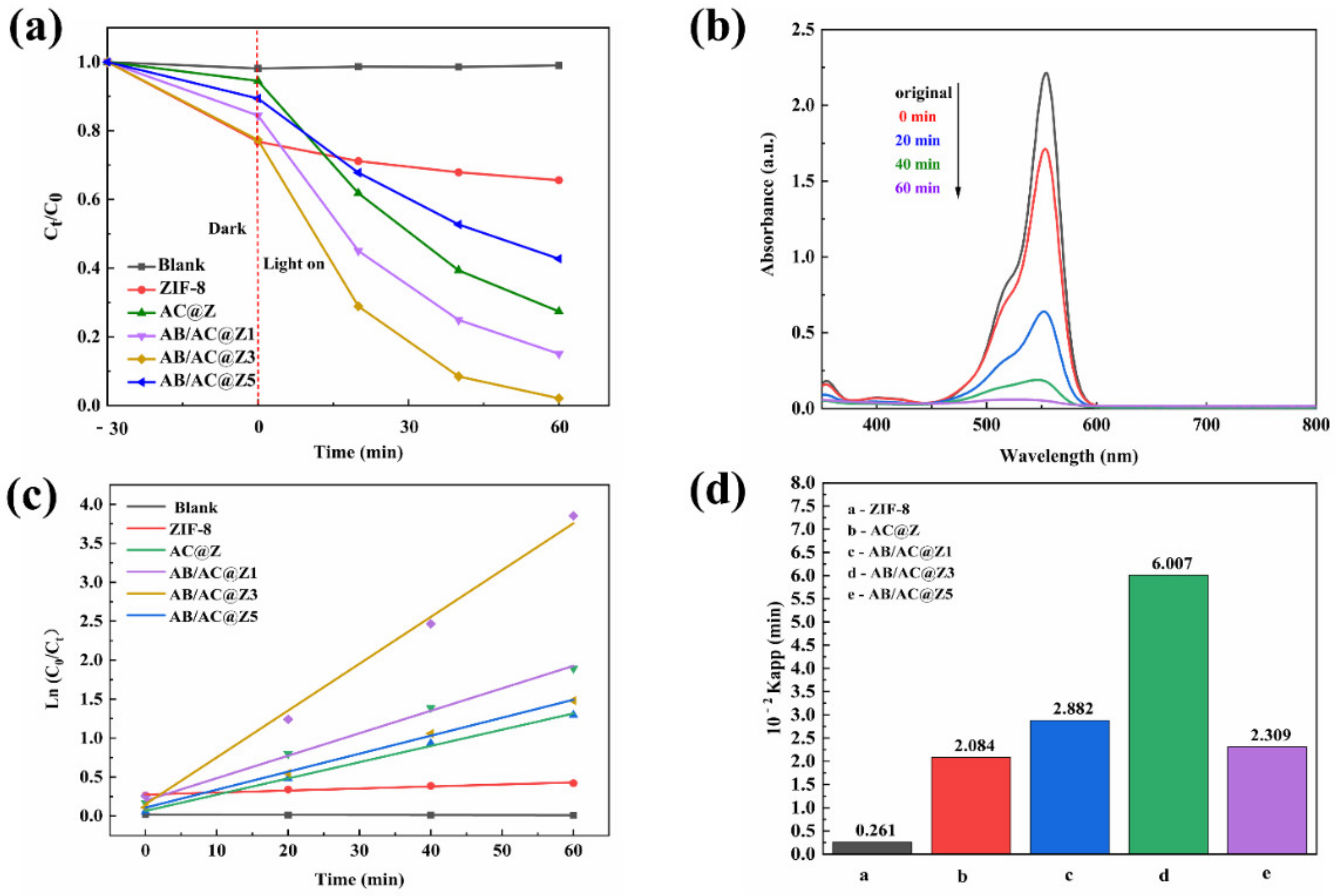
3.4. The Photocatalytic Activity
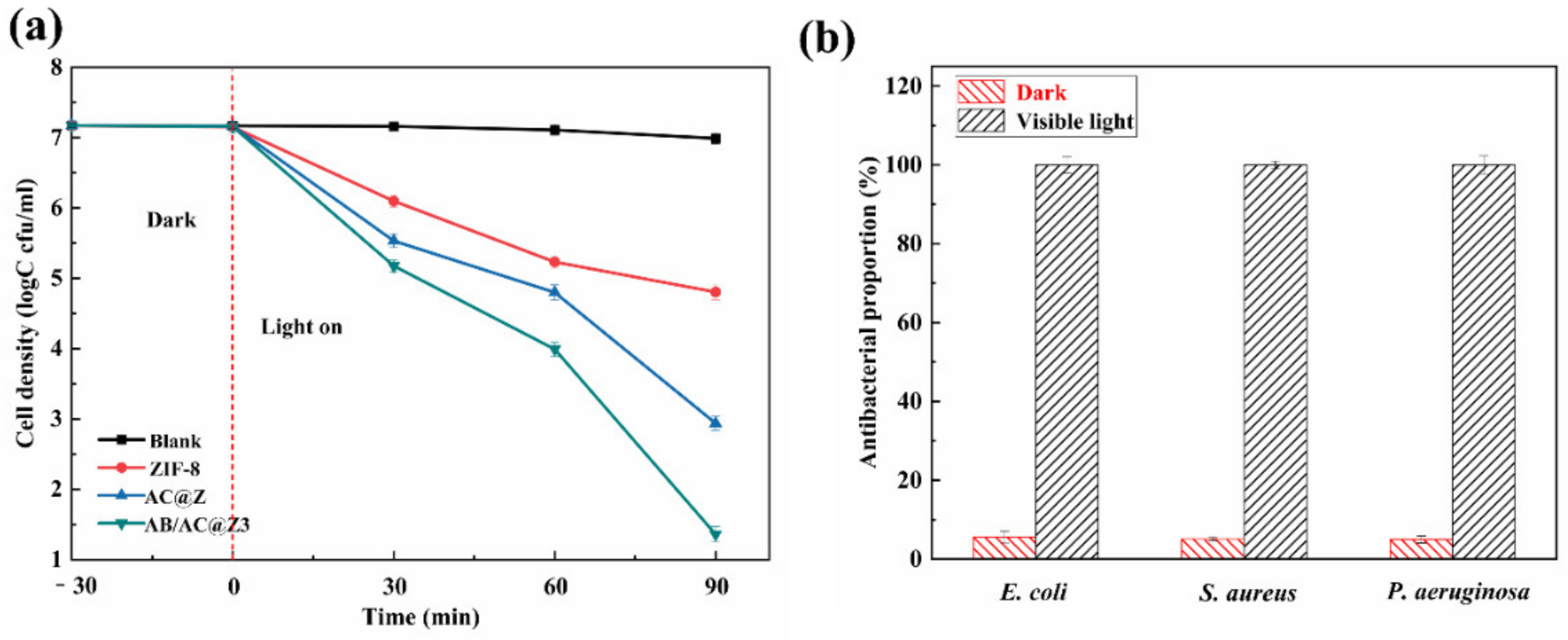
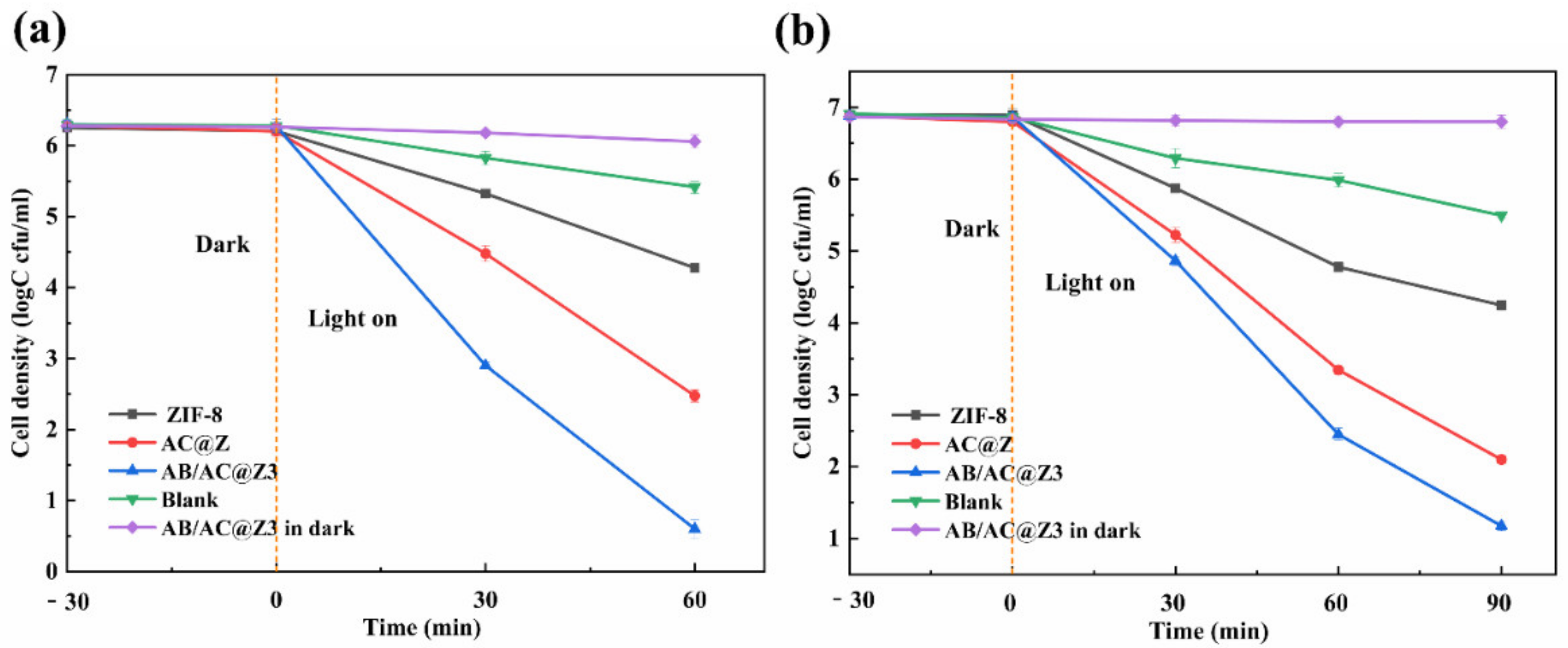
3.5. Photocatalytic Antibacterial Efficiency
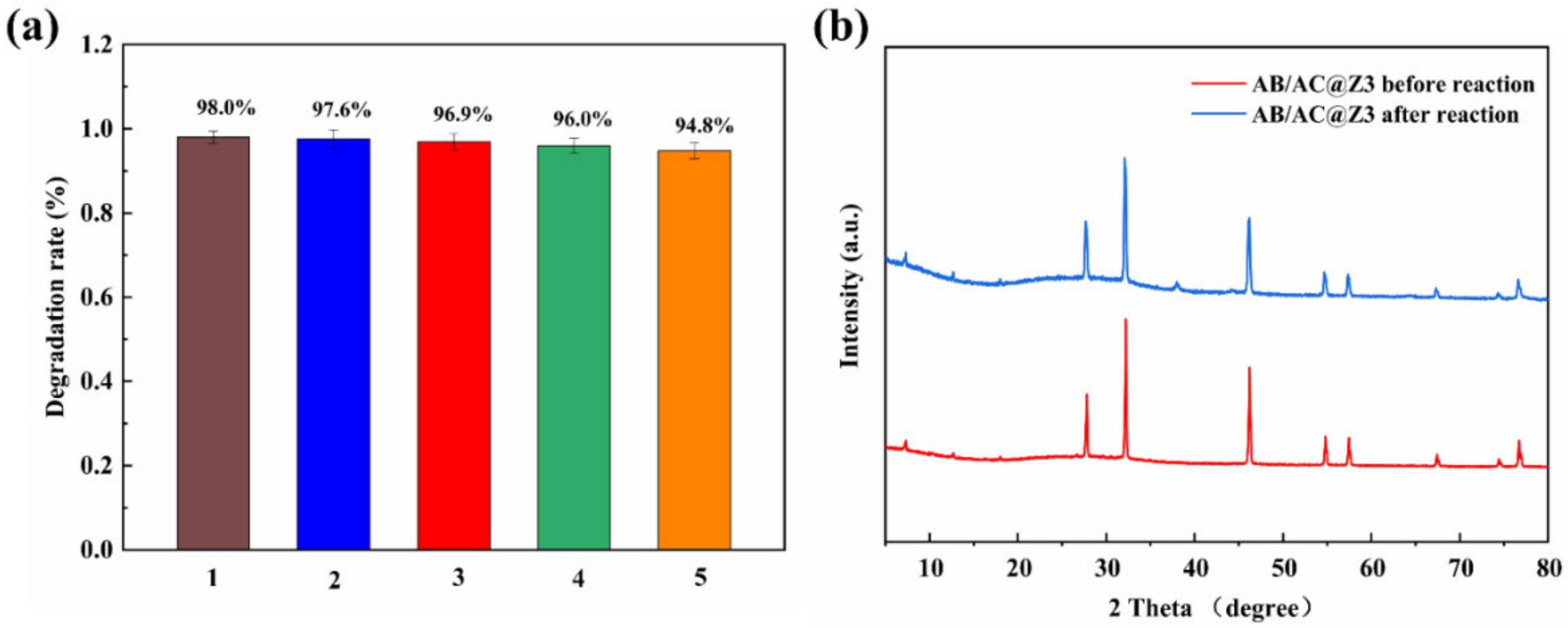
3.6. Stability and Reusability
3.7. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Abdi, J. Synthesis of Ag-doped ZIF-8 photocatalyst with excellent performance for dye degradation and antibacterial activity. Colloids Surf. A 2020, 604, 125330. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yu, J.Q.; Zhang, Y.; Cui, Z.X.; Sun, Y.; Hou, B.R. Fabrication of InVO4/AgVO3 heterojunctions with enhanced photocatalytic antifouling efficiency under visible-light. Appl. Catal. B 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.Y.; Lan, X.; Liu, Y.; Yang, W.W.; Zhang, J.; Yu, Y.S. Building P-doped MoS2/g-C3N4 layered heterojunction with a dual-internal electric field for efficient photocatalytic sterilization. Chem. Eng. J. 2022, 429, 132588. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Liu, J.H.; Wu, D.; Zhu, N.; Wu, Y.N.; Li, G.L. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Fan, G.D.; Luo, J.; Guo, L.; Lin, R.J.; Zheng, X.M.; Snyder, S.A. Doping Ag/AgCl in zeolitic imidazolate framework-8 (ZIF-8) to enhance the performance of photodegradation of methylene blue. Chemosphere 2018, 209, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.B.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Urchin-Inspired TiO2@MIL-101 Double-Shell Hollow Particles: Adsorption and Highly Efficient Photocatalytic Degradation of Hydrogen Sulfide. Chem. Mater. 2017, 29, 5612–5616. [Google Scholar] [CrossRef]
- Guo, J.; Wan, Y.; Zhu, Y.F.; Zhao, M.T.; Tang, Z.Y. Advanced photocatalysts based on metal nanoparticle/metal-organic framework composites. Nano Res. 2021, 14, 2037–2052. [Google Scholar] [CrossRef]
- Wang, C.L.; Liu, N.Z.; Liu, X.J.; Tian, Y.; Zhai, X.F.; Chen, X.W.; Hou, B.R. Fluoro-Substituted Covalent Organic Framework Particles Anchored on TiO2 Nanotube Arrays for Photoelectrochemical Determination of Dopamine. ACS Appl. Nano Mater. 2021, 4, 8801–8812. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; Keeffe, M.O.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 6149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.F.; Forghani, F.; Kong, X.Q.; Liu, D.H.; Ye, X.Q.; Chen, S.Q.; Ding, T. Antibacterial applications of metal-organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.B.; Wen, Z.D.; Wu, Z.L.; Luo, X.T. Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. Front. 2018, 5, 687–693. [Google Scholar] [CrossRef]
- Wan, J.; Du, X.; Liu, E.Z.; Hu, Y.; Fan, J.; Hu, X.Y. Z-scheme visible-light-driven Ag3PO4 nanoparticle@MoS2 quantum dot/few-layered MoS2 nanosheet heterostructures with high efficiency and stability for photocatalytic selective oxidation. J. Catal. 2017, 345, 281–294. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.L.; Gan, C.X.; Zhang, J.Y.; Liu, Y.F.; Zhou, M.; Zhang, C.; Fang, Y.Z. Heterostructural Ag3PO4/UiO-66 composite for highly efficient visible-light photocatalysts with long-term stability. J. Photochem. Photobiol. A 2019, 376, 305–315. [Google Scholar] [CrossRef]
- Yuan, X.; Qu, S.L.; Huang, X.Y.; Xue, X.G.; Yuan, C.L.; Wang, S.W.; Wei, L.; Cai, P. Design of core-shelled g-C3N4@ZIF-8 photocatalyst with enhanced tetracycline adsorption for boosting photocatalytic degradation. Chem. Eng. J. 2021, 416, 129148. [Google Scholar] [CrossRef]
- Zeng, M.; Chai, Z.G.; Deng, X.; Li, Q.; Feng, S.Q.; Wang, J.; Xu, D.S. Core-shell CdS@ZIF-8 structures for improved selectivity in photocatalytic H2 generation from formic acid. Nano Res. 2016, 9, 2729–2734. [Google Scholar] [CrossRef]
- Chen, M.; Long, Z.; Dong, R.H.; Wang, L.; Zhang, J.J.; Li, S.X.; Zhao, X.H.; Hou, X.D.; Shao, H.W.; Jiang, X.Y. Titanium Incorporation into Zr-Porphyrinic Metal-Organic Frameworks with Enhanced Antibacterial Activity against Multidrug-Resistant Pathogens. Small 2020, 16, 1906240. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lai, C.; Qin, L.; Fu, Y.K.; He, J.F.; Huang, D.L.; Li, B.S.; Zhang, M.M.; Liu, S.Y.; Li, L.; et al. ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn-O-Fe structure for TC degradation. Chem. Eng. J. 2020, 392, 124851. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Cao, J.; Ye, J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef]
- Ye, L.Q.; Liu, J.Y.; Gong, C.Q.; Tian, L.H.; Peng, T.Y.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z-Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Gamage McEvoy, J.; Cui, W.Q.; Zhang, Z.S. Synthesis and characterization of Ag/AgCl-activated carbon composites for enhanced visible light photocatalysis. Appl. Catal. B 2014, 144, 702–712. [Google Scholar] [CrossRef]
- Jing, Y.; Lei, Q.; Xia, C.; Guan, Y.; Yang, Y.; He, J.; Yang, Y.; Zhang, Y.; Yan, M. Synthesis of Ag and AgCl co-doped ZIF-8 hybrid photocatalysts with enhanced photocatalytic activity through a synergistic effect. RSC Adv. 2020, 1, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; Xu, H.H.; Lv, X.Z.; Zeng, Y.X.; Duan, J.Z.; Hou, B.R. The effective photocatalysis and antibacterial properties of AgBr/AgVO3 composites under visible-light. RSC Adv. 2019, 9, 3719–37118. [Google Scholar] [CrossRef] [Green Version]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. JACS 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Dong, R.; Tian, B.; Zeng, C.; Li, T.; Wang, T.; Zhang, J. Ecofriendly Synthesis and Photocatalytic Activity of Uniform Cubic Ag@AgCl Plasmonic Photocatalyst. J. Phys. Chem. C 2013, 117, 213–220. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. A visible light driven AgBr/g-C3N4 photocatalyst composite in methyl orange photodegradation: Focus on photoluminescence, mole ratio, synthesis method of g-C3N4 and scavengers. Compos. Part B 2020, 183, 107712. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Yeung, K.W.K.; Wu, S. AgBr Nanoparticles in Situ Growth on 2D MoS2 Nanosheets for Rapid Bacteria-Killing and Photodisinfection. ACS Appl. Mater. Interfaces 2019, 11, 34364–34375. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.B.; Lou, Z.Z.; Zhang, X.Y.; Qin, X.Y.; Dai, Y.; Zheng, Z.K.; Wang, X.N. Synthesis of Highly Efficient Ag@AgCl Plasmonic Photocatalysts with Various Structures. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, C.; Zhuang, J.; Zhong, Y.; Wang, D.; Zhou, H. AgBr/MgBi2O6 heterostructured composites with highly efficient visible-light-driven photocatalytic activity. J. Phys. Chem. Solids 2018, 117, 94–100. [Google Scholar] [CrossRef]
- Cui, S.N.; Ye, Z.Q.; Qian, C.; Liu, J.; Jin, J.; Liang, Q.; Liu, C.H.; Xu, S.; Li, Z.Y. Construction of ternary Ag/AgBr@UIO-66(NH2) heterojunctions with enhanced photocatalytic performance for the degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2018, 29, 15138–15146. [Google Scholar] [CrossRef]
- Chang, N.; Chen, Y.R.; Xie, F.; Liu, Y.P.; Wang, H.T. Facile construction of Z-scheme AgCl/Ag-doped-ZIF-8 heterojunction with narrow band gaps for efficient visible-light photocatalysis. Colloids Surf. A 2021, 616, 126351. [Google Scholar] [CrossRef]
- Wang, X.; Rong, X.; Zhang, Y.; Luo, F.; Qiu, B.; Wang, J.; Lin, Z.Y. Homogeneous photoelectrochemical aptasensors for tetracycline based on sulfur-doped g-C3N4/n-GaN heterostructures formed through self-assembly. Anal. Chem. 2022, 94, 3735–3742. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, Y.; Ju, P.; Long, Y.; Zhang, D. Facile fabrication of AgI/BiVO4 composites with enhanced visible photocatalytic degradation and antibacterial ability. J. Alloys Compd. 2017, 721, 622–627. [Google Scholar] [CrossRef]
- Xue, S.; Wei, Z.; Hou, X.; Xie, W.; Li, S.; Shang, X.; He, D. Enhanced visible-light photocatalytic activities and mechanism insight of BiVO4/Bi2WO6 composites with virus-like structures. Appl. Surf. Sci. 2015, 355, 1107–1115. [Google Scholar] [CrossRef]
- Xiang, Z.B.; Wang, Y.; Zhang, D.; Ju, P. BiOI/BiVO4 p-n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Ind. Eng. Chem. 2016, 40, 83–92. [Google Scholar] [CrossRef]
- Chang, N.; Chen, Y.; Xie, F.; Liu, Y.; Wang, H. A promising Z-scheme heterojunction via loading Ag/AgCl into porous Co3O4 derived from ZIF-67 for visible light driven photocatalysis. Microporous Mesoporous Mater. 2020, 307, 110530. [Google Scholar] [CrossRef]
- Kong, W.; Wang, S.; Wu, D.; Chen, C.; Luo, Y.; Pei, Y.; Tian, B.; Zhang, J. Fabrication of 3D Sponge@AgBr-AgCl/Ag and Tubular Photoreactor for Continuous Wastewater Purification under Sunlight Irradiation. ACS Sustain. Chem. Eng. 2019, 7, 14051–14063. [Google Scholar] [CrossRef]
- Thakur, P.; Raizada, P.; Singh, P.; Kumar, A.; Khan, A.A.P.; Asiri, A.M. Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications. Arab. J. Chem. 2020, 13, 8271–8300. [Google Scholar] [CrossRef]
- Liu, J.X.; Li, R.; Hu, Y.Y.; Li, T.; Jia, Z.H.; Wang, Y.F.; Wang, Y.W.; Zhang, X.C.; Fan, C.M. Harnessing Ag nanofilm as an electrons transfer mediator for enhanced visible light photocatalytic performance of Ag@AgCl/Ag nanofilm/ZIF-8 photocatalyst. Appl. Catal. B 2017, 202, 64–71. [Google Scholar] [CrossRef]
- Xu, H.H.; Zhang, J.; Lv, X.Z.; Niu, T.; Zeng, Y.X.; Duan, J.Z.; Hou, B.R. The effective photocatalysis and antibacterial properties of AgBr/Ag2MoO4@ZnO composites under visible light irradiation. Biofouling 2019, 35, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Y.; An, T.; Li, G.; Yip, H.Y.; Yu, J.C.; Wong, P.K. Visible-Light-Driven Photocatalytic Inactivation of E. coli K-12 by Bismuth Vanadate Nanotubes: Bactericidal Performance and Mechanism. Environ. Sci. Technol. 2012, 46, 4599–4606. [Google Scholar] [CrossRef]
- Wang, M.J.; Nian, L.Y.; Cheng, Y.L.; Yuan, B.; Cheng, S.J.; Cao, C.J. Encapsulation of colloidal semiconductor quantum dots into metal-organic frameworks for enhanced antibacterial activity through interfacial electron transfer. Chem. Eng. J. 2021, 426, 130832. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhang, J.; Wang, Y.; Zhu, Q.; Zhang, X.; Duan, J.; Hou, B. Novel MOF-Based Photocatalyst AgBr/AgCl@ZIF-8 with Enhanced Photocatalytic Degradation and Antibacterial Properties. Nanomaterials 2022, 12, 1946. https://doi.org/10.3390/nano12111946
Liu N, Zhang J, Wang Y, Zhu Q, Zhang X, Duan J, Hou B. Novel MOF-Based Photocatalyst AgBr/AgCl@ZIF-8 with Enhanced Photocatalytic Degradation and Antibacterial Properties. Nanomaterials. 2022; 12(11):1946. https://doi.org/10.3390/nano12111946
Chicago/Turabian StyleLiu, Ning, Jie Zhang, Yanhua Wang, Qingjun Zhu, Xuan Zhang, Jizhou Duan, and Baorong Hou. 2022. "Novel MOF-Based Photocatalyst AgBr/AgCl@ZIF-8 with Enhanced Photocatalytic Degradation and Antibacterial Properties" Nanomaterials 12, no. 11: 1946. https://doi.org/10.3390/nano12111946
APA StyleLiu, N., Zhang, J., Wang, Y., Zhu, Q., Zhang, X., Duan, J., & Hou, B. (2022). Novel MOF-Based Photocatalyst AgBr/AgCl@ZIF-8 with Enhanced Photocatalytic Degradation and Antibacterial Properties. Nanomaterials, 12(11), 1946. https://doi.org/10.3390/nano12111946






