Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
4. Thin Film Fabrication and Device Modeling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef]
- Park, N.G. Research direction toward scalable, stable, and high efficiency perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903106. [Google Scholar] [CrossRef]
- He, M.; Pang, X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z. Monodisperse dual-functional upconversion nanoparticles enabled near-infrared organolead halide perovskite solar cells. Angew. Chem. 2016, 128, 4352–4356. [Google Scholar] [CrossRef]
- Yin, X.; Yao, Z.; Luo, Q.; Dai, X.; Zhou, Y.; Zhang, Y.; Zhou, Y.; Luo, S.; Li, J.; Wang, N.; et al. High efficiency inverted planar perovskite solar cells with solution-processed NiOx hole contact. ACS Appl. Mater. Interfaces 2017, 9, 2439–2448. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Van Reenen, S.; Sutton, R.J.; Fan, J.; Haghighirad, A.A.; Johnston, M.B.; Wang, L.; Snaith, H.J. Enhanced UV-light stability of planar heterojunction perovskite solar cells with caesium bromide interface modification. Energy Environ. Sci. 2016, 9, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Annohene, G.; Tepper, G. Moisture Stability of Perovskite Solar Cells Processed in Supercritical Carbon Dioxide. Molecules 2021, 26, 7570. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Kato, Y.; Ono, L.K.; Lee, M.V.; Wang, S.; Raga, S.R.; Qi, Y. Silver iodide formation in methyl ammonium lead iodide perovskitesolar cells with silver top electrodes. Adv. Mater. Interfaces 2015, 2, 1500195. [Google Scholar] [CrossRef]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the long-term stability of perovskite solar cells with a porous Al2O3 buffer layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Lv, S.; Liu, X.; Li, X.; Wang, S.; Wei, H.; Li, D.; Xiao, Y.; Meng, Q. Energy level tuning of TPB-based hole-transporting materials for highly efficient perovskite solar cells. Chem. Commun. 2014, 50, 15239–15242. [Google Scholar] [CrossRef]
- Kumar, C.V.; Sfyri, G.; Raptis, D.; Stathatos, E.; Lianos, P. Perovskite solar cell with low cost Cu-phthalocyanine as hole transporting material. RSC Adv. 2015, 5, 3786–3791. [Google Scholar] [CrossRef]
- Jung, J.W.; Chueh, C.C.; Jen, A.K. High-performance semitransparent perovskite solar cells with 10% power conversion efficiency and 25% average visible transmittance based on transparent CuSCN as the hole-transporting material. Adv. Energy Mater. 2015, 5, 1500486. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhao, D.; Grice, C.R.; Cimaroli, A.J.; Fang, G.; Yan, Y. Efficient fully-vacuum-processed perovskite solar cells using copper phthalocyanine as hole selective layers. J. Mater. Chem. A 2015, 3, 23888–23894. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.L.; Xu, J.J.; Lei, H.W.; Chen, C.; Shan, H.Q.; Liu, X.Y.; Xu, Z.X.; Fang, G.J. A facile molecularly engineered copper (II) phthalocyanine as hole transport material for planar perovskite solar cells with enhanced performance and stability. Nano Energy 2017, 31, 322–330. [Google Scholar] [CrossRef]
- Sepalage, G.A.; Meyer, S.; Pascoe, A.; Scully, A.D.; Huang, F.; Bach, U.; Cheng, Y.B.; Spiccia, L. Copper (I) iodide as hole-conductor in planar perovskite solar cells: Probing the origin of J–V hysteresis. Adv. Funct. Mater. 2015, 25, 5650–5661. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Karunakaran, S.K.; Liu, C.; Zhang, C.; Guo, F.; Wu, S.; Mai, Y. Inorganic hole transport layers in inverted perovskite solar cells: A review. Nano Sel. 2021, 2, 1081–1116. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Chen, C.; Wu, Y.; Bi, W.; Song, Z.; Song, H. Rare earth ions doped NiOx hole transport layer for efficient and stable inverted perovskite solar cells. J. Power Sources 2019, 444, 227267. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Tress, W. Interfaces and interfacial layers in inorganic perovskite solar cells. Angew. Chem. Int. Ed. 2021, 60, 26440–26453. [Google Scholar] [CrossRef]
- Capasso, A.; Matteocci, F.; Najafi, L.; Prato, M.; Buha, J.; Cinà, L.; Pellegrini, V.; Carlo, A.D.; Bonaccorso, F. Few-layer MoS2 flakes as active buffer layer for stable perovskite solar cells. Adv. Energy Mater. 2016, 6, 1600920. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Tremolet de Villers, B.J.; Schulz, P.; Reese, M.O.; Ferrere, S.; Zhu, K.; Lin, L.Y.; Berry, J.J.; Luther, J.M. Influence of electrode interfaces on the stability of perovskite solar cells: Reduced degradation using MoOx/Al for hole collection. ACS Energy Lett. 2016, 1, 38–45. [Google Scholar] [CrossRef]
- Koo, B.; Jung, H.; Park, M.; Kim, J.Y.; Son, H.J.; Cho, J.; Ko, M.J. Pyrite-Based Bi-Functional Layer for Long-Term Stability and High-Performance of Organo-Lead Halide Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 5400–5407. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Gratzel, M. Not all that glitters is gold: Metal-migration-induced degradation in perovskite solar cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; et al. Efficient inorganic solid solar cells composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907. [Google Scholar] [CrossRef]
- Zheng, X.; Lei, H.; Yang, G.; Ke, W.; Chen, Z.; Chen, C.; Ma, J.; Guo, Q.; Yao, F.; Zhang, Q.; et al. Enhancing efficiency and stability of perovskite solar cells via a high mobility p-type PbS buffer layer. Nano Energy 2017, 38, 1–11. [Google Scholar] [CrossRef]
- Saran, R.; Curry, R.J. Lead sulphide nanocrystal photodetector technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Liu, M.; Voznyy, O.; Sabatini, R.; De Arquer, F.P.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic–inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nat. Mater. 2017, 16, 258–263. [Google Scholar] [CrossRef]
- Li, S.S.; Chang, C.H.; Wang, Y.C.; Lin, C.W.; Wang, D.Y.; Lin, J.C.; Chen, C.C.; Sheu, H.S.; Chia, H.C.; Wu, W.R.; et al. Intermixing-seeded growth for high-performance planar heterojunction perovskite solar cells assisted by precursor-capped nanoparticles. Energy Environ. Sci. 2016, 9, 1282–1289. [Google Scholar] [CrossRef]
- Bendjedid, S.; Bazine, I.; Tadjine, A.; Djelloul, R.; Boukhari, A.; Bensouici, C. Analysis of Phytochemical Constituents by using LC-MS, Antifungal and Allelopathic Activities of Leaves Extracts of Aloe vera. Jordan J. Biol. Sci. 2022, 15, 1. [Google Scholar]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Future J. Pharm. Sci. 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tizard, I.R. Analytical Methodology: The Gel-Analysis of Aloe Pulp and Its Derivatives; CRC Press: Boca Raton, FL, USA, 2004; pp. 111–126. [Google Scholar]
- Mahdi, A.A.; Mahmoud, N.E.; Abdelhameed, R.M. The Effect of Incorporated Aloe vera Extracts onto Zeolitic Imidazolate Framework on Physiological, Biochemical, and Molecular Behavior of Quinoa (Chenopodium quinoa Willd.) Genotype Under Saline Conditions. J. Soil Sci. Plant Nutr. 2021, 22, 306–323. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huo, N.; Li, F.; Gu, M.; Ling, X.; Ma, W. Room-temperature direct synthesis of semi-conductive PbS nanocrystal inks for optoelectronic applications. Nat. Commun. 2019, 10, 5136. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.G.; Bartels, K.; Bedard, P.L. Lead chloride solubility and density data in binary aqueous solutions. Hydrometallurgy 1987, 17, 335–356. [Google Scholar] [CrossRef]
- Scaife, C.W.; Hall, C.D. Recycling lead (II) halides from solubility experiments. J. Chem. Educ. 1990, 67, 605. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, X.; Gao, W.; Wang, C.; Feng, H.; Zhao, X.; Chen, L. Synthesis of PbS nanoflowers by biomolecule-assisted method in the presence of supercritical carbon dioxide. Solid State Sci. 2009, 11, 976–981. [Google Scholar] [CrossRef]
- Jiao, Y.; Gao, X.; Lu, J.; Chen, Y.; Zhou, J.; Li, X. A novel method for PbS quantum dot synthesis. Mater. Lett. 2012, 72, 116–118. [Google Scholar] [CrossRef]
- Mondal, A.; Mukherjee, N. Cubic PbS thin films on TCO glass substrate by galvanic technique. Mater. Lett. 2006, 60, 2672–2674. [Google Scholar] [CrossRef]
- Mubiayi, K.P.; Revaprasadu, N.; Garje, S.S.; Moloto, M.J. Designing the morphology of PbS nanoparticles through a single source precursor method. J. Saudi Chem. Soc. 2017, 21, 593–598. [Google Scholar] [CrossRef]
- Islam, M.A.; Wahab, Y.A.; Khandaker, M.U.; Alsubaie, A.; Almalki, A.S.; Bradley, D.A.; Amin, N. High Mobility Reactive Sputtered CuxO Thin Film for Highly Efficient and Stable Perovskite Solar Cells. Crystals 2021, 11, 389. [Google Scholar] [CrossRef]
- Islam, M.A.; Misran, H.; Akhtaruzzaman, M.; Amin, N. Influence of oxygen on structural and optoelectronic properties of CdS thin film deposited by magnetron sputtering technique. Chin. J. Phys. 2020, 67, 170–179. [Google Scholar] [CrossRef]
- Maharaz, M.N.; Halimah, M.K.; Paiman, S.; Saiden, N.M.; Alibe, I.M. Influence of solvents and irradiation time on structural and optical properties of cubic PbS nanoparticles. Int. J. Electrochem. Sci. 2018, 13, 9317–9332. [Google Scholar] [CrossRef]
- Himadri, D.; Pranayee, D.; Kumar, S.K. Synthesis of PbS nanoparticles and its potential as a biosensor based on memristic properties. J. Nanosci. Technol. 2018, 4, 500–502. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, P.; Jia, D. Solvothermal synthesis, growth mechanism, and photoluminescence property of sub-micrometer PbS anisotropic structures. Nanoscale Res. Lett. 2012, 7, 668. [Google Scholar] [CrossRef] [Green Version]
- Kaewmuang, P.; Thongtem, T.; Thongtem, S.; Kittiwachana, S.; Kaowphong, S. Influence of calcination temperature on particle size and photocatalytic activity of nanosized NiO powder. Russ. J. Phys. Chem. A 2018, 92, 1777–1781. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Patel, J.D.; Mighri, F.; Ajji, A. Room temperature synthesis of aminocaproic acid-capped lead sulphide nanoparticles. Mater. Sci. Appl. 2012, 3, 17264. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, U.; Akshay Gowda, K.M.; Elisha, M.G.; Nitish, N.; Raj Mohan, B. Biologically synthesized PbS nanoparticles for the detection of arsenic in water. Int. Biodeterior. Biodegrad. 2017, 119, 78–86. [Google Scholar]
- Vankhade, D.; Chaudhuri, T.K. Effect of thickness on structural and optical properties of spin-coated nanocrystalline PbS thin films. Opt. Mater. 2019, 98, 109491. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Ramakrishnan, V.M.; Palanisamy, S.E.; Sundaram, S.; Velauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2020, 13, 2526–2557. [Google Scholar] [CrossRef]
- Jaymin, R.; Tapas, K.C.; Chetan, P.; Kinjal, P.; Keyur, P.; Gopal, B.; Priya, S. PbS-ZnO Solar Cell: A Numerical Simulation. J. Nano Electron. Phys. 2017, 9, 030411–030414. [Google Scholar]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Bisquert, J.; Bogdanoff, P.; Zaban, A. Mott-Schottky analysis of nanoporous semiconductor electrodes in dielectric state deposited on SnO2 (F) conducting substrates. J. Electrochem. Soc. 2003, 150, E293. [Google Scholar] [CrossRef]
- Lin, P.Y.; Wu, T.; Ahmadi, M.; Liu, L.; Haacke, S.; Guo, T.F.; Hu, B. Simultaneously enhancing dissociation and suppressing recombination in perovskite solar cells. Nano Energy 2017, 36, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Hsiao, Y.C.; Li, M.; Kang, N.G.; Mays, J.W.; Hu, B. Dynamic coupling between electrode interface and donor/acceptor interface via charge dissociation in organic solar cells at device-operating condition. J. Phys. Chem. C 2015, 119, 2727–2732. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Qiu, Y.; Zhang, M.; Liu, B. Device engineering for all-inorganic perovskite light-emitting diodes. Nanomaterials 2019, 9, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, H.; Zhang, Y.; Xu, B.; Liu, L.; Chen, G.; Im, C.; Tian, W. Influence of hole transport layers on internal absorption, charge recombination and collection in HC(NH2)2PbI3 perovskite solar cells. J. Mater. Chem. A 2018, 6, 7922–7932. [Google Scholar] [CrossRef]
- Gottesman, R.; Lopez-Varo, P.; Gouda, L.; Jimenez-Tejada, J.A.; Hu, J.; Tirosh, S.; Zaban, A.; Bisquert, J. Dynamic phenomena at perovskite/electron-selective contact interface as interpreted from photovoltage decays. Chem 2016, 1, 776–789. [Google Scholar] [CrossRef] [Green Version]

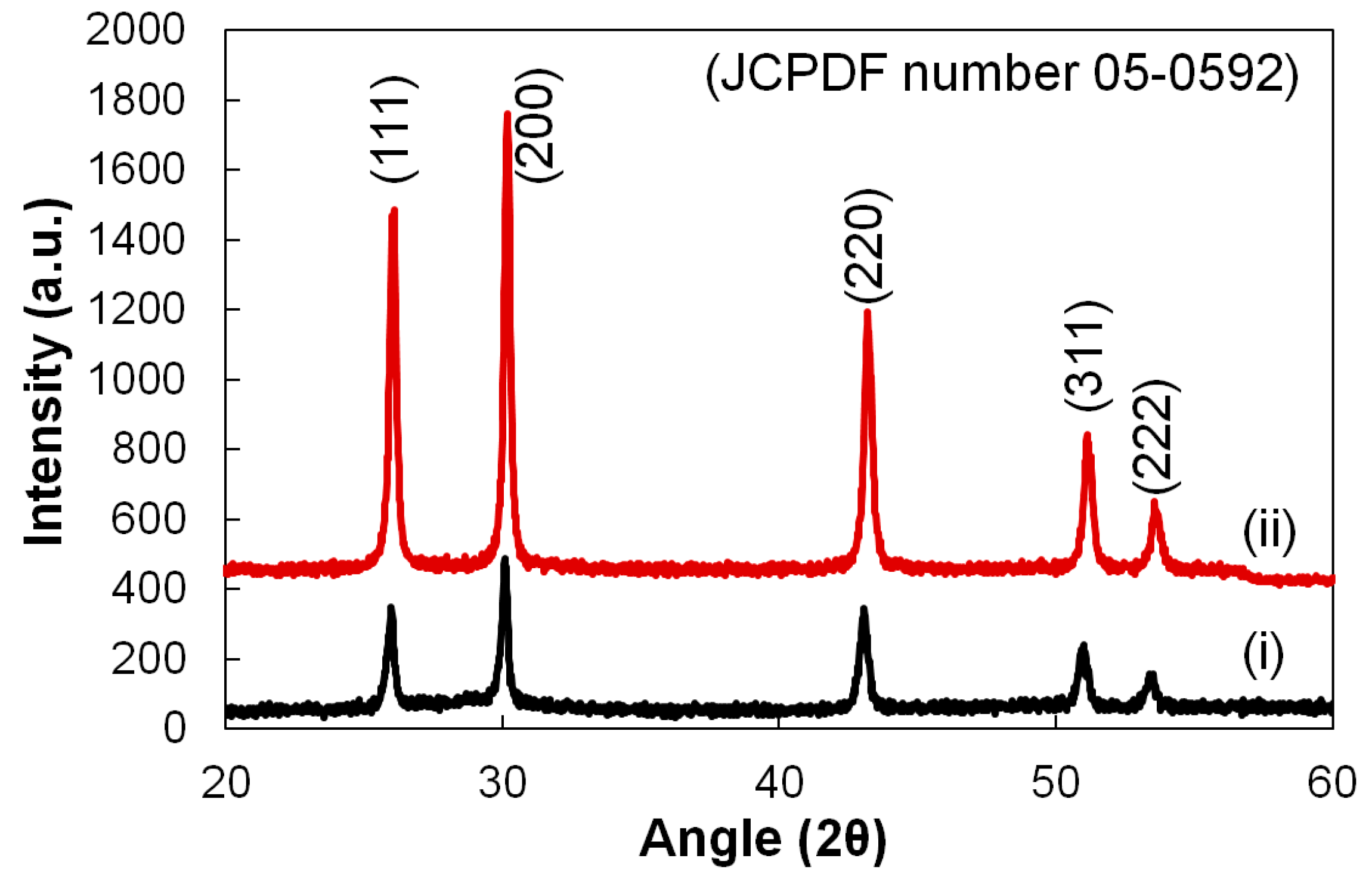

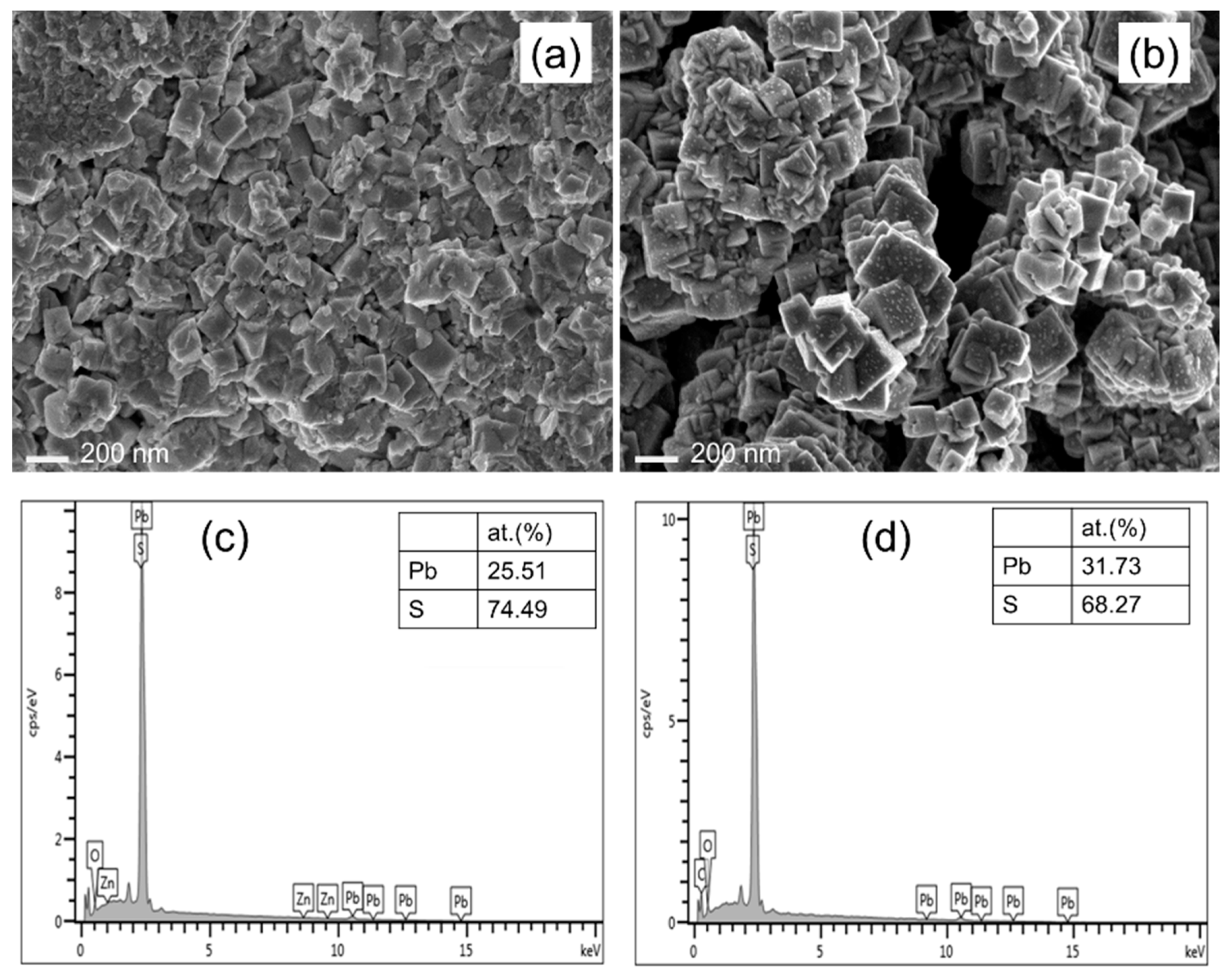
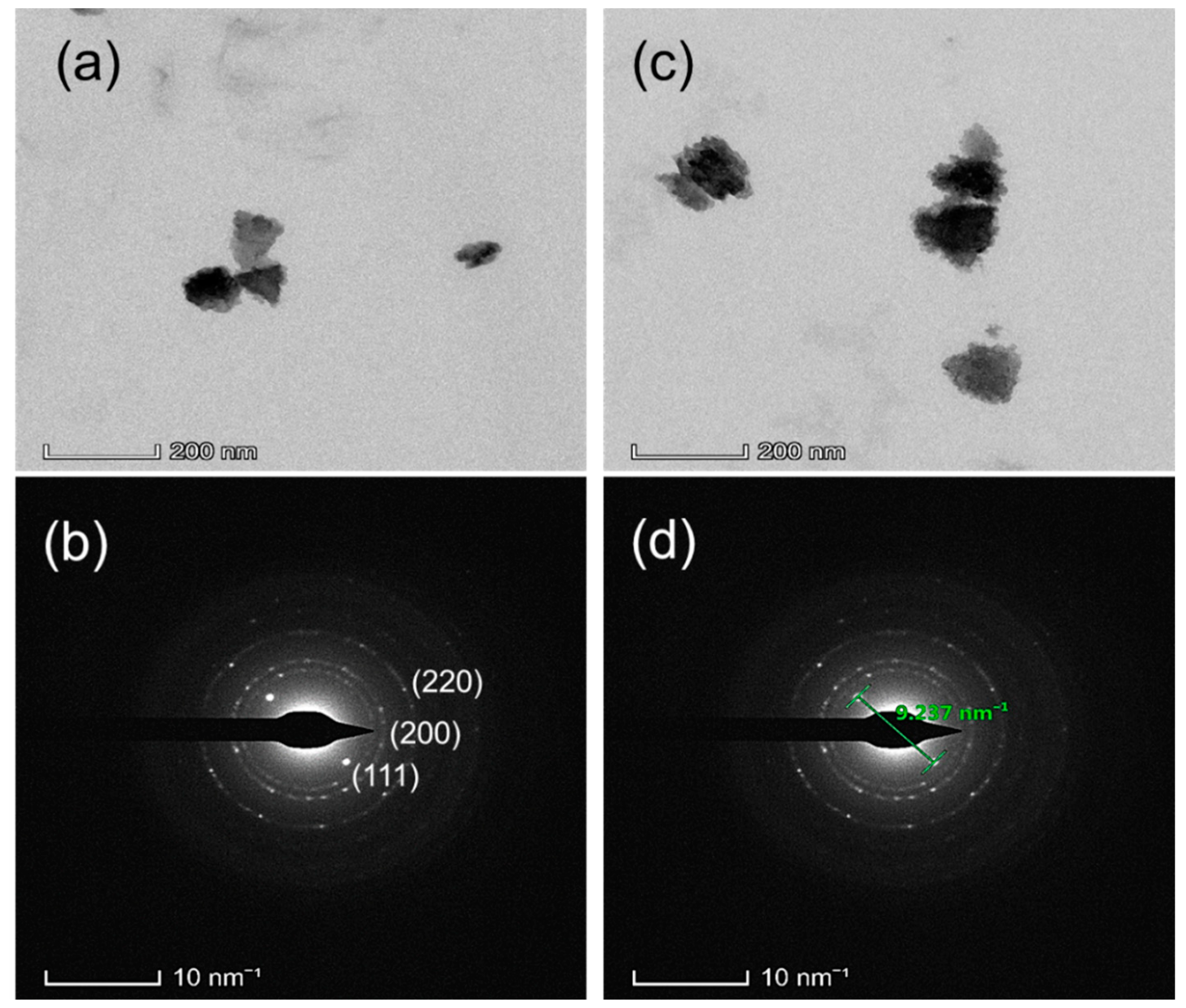

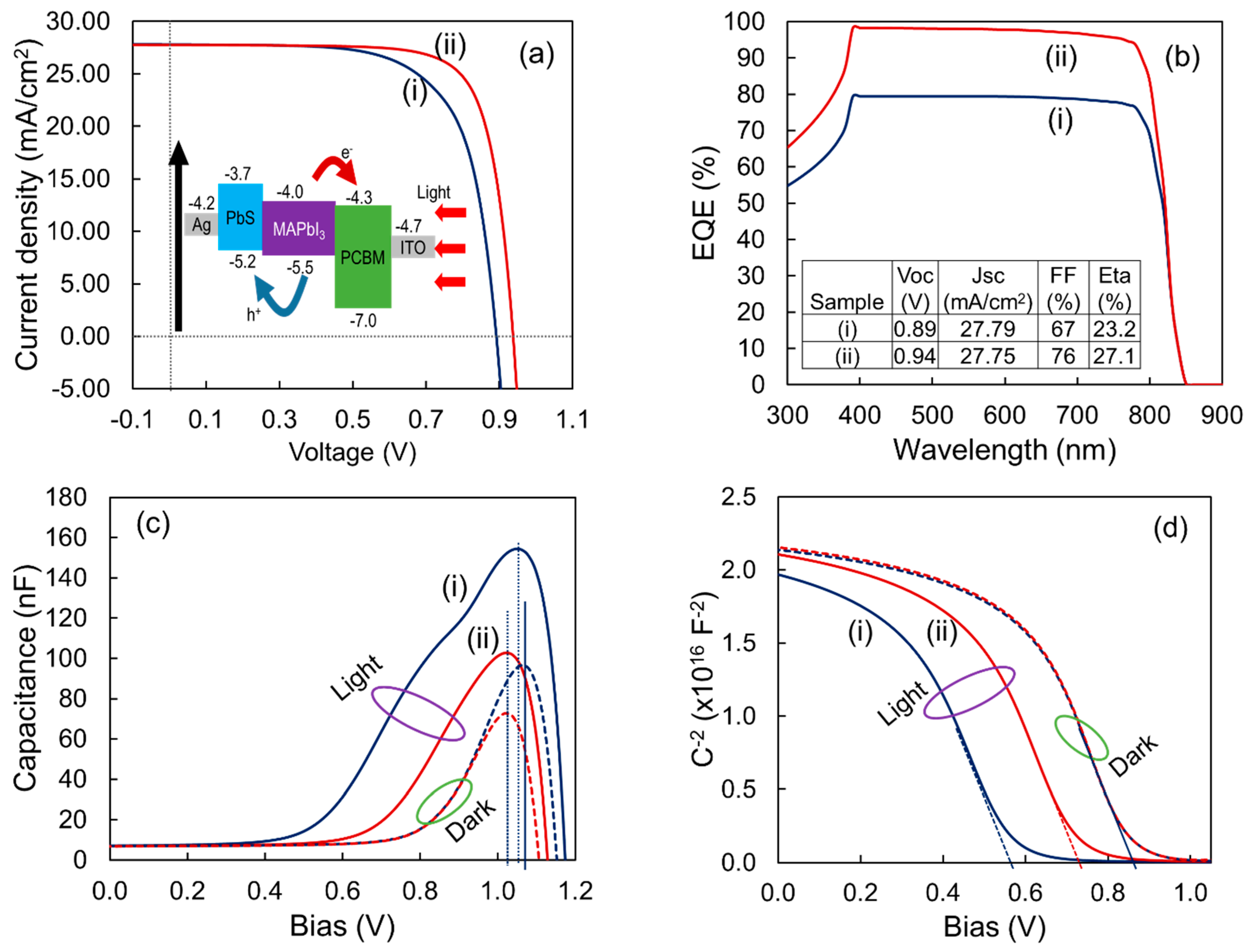
| Process Temperature | Peak Position, (200) (2θ°) | Crystallite Size, (nm) | Microstrain, ε (×10−3) | Dislocation Density, δ (×1014 cm−2) | |
|---|---|---|---|---|---|
| (i) | 300 °C | 30.08 | 24.21 | 5.52 | 17.07 |
| (ii) | 360 °C | 30.20 | 31.66 | 4.20 | 9.97 |
| Peak Position, (111) (2θo) | Crystallite Size, (nm) | Microstrain, ε (×10−3) | Dislocation Density, δ (×1014 cm−2) | Carrier Con. (cm−3) | Mobility (cm2 V−1 s−1) | Resistivity (×102) (Ω-cm) | |
|---|---|---|---|---|---|---|---|
| (i) | 30.22 | 17.89 | 7.43 | 31.22 | 4.1 × 1014 | 43 | 9.17 |
| (ii) | 30.06 | 30.48 | 4.39 | 10.76 | 10.6 × 1014 | 54 | 7.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Sarkar, D.K.; Shahinuzzaman, M.; Wahab, Y.A.; Khandaker, M.U.; Tamam, N.; Sulieman, A.; Amin, N.; Akhtaruzzaman, M. Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications. Nanomaterials 2022, 12, 1933. https://doi.org/10.3390/nano12111933
Islam MA, Sarkar DK, Shahinuzzaman M, Wahab YA, Khandaker MU, Tamam N, Sulieman A, Amin N, Akhtaruzzaman M. Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications. Nanomaterials. 2022; 12(11):1933. https://doi.org/10.3390/nano12111933
Chicago/Turabian StyleIslam, Mohammad Aminul, Dilip Kumar Sarkar, Md. Shahinuzzaman, Yasmin Abdul Wahab, Mayeen Uddin Khandaker, Nissren Tamam, Abdelmoneim Sulieman, Nowshad Amin, and Md. Akhtaruzzaman. 2022. "Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications" Nanomaterials 12, no. 11: 1933. https://doi.org/10.3390/nano12111933
APA StyleIslam, M. A., Sarkar, D. K., Shahinuzzaman, M., Wahab, Y. A., Khandaker, M. U., Tamam, N., Sulieman, A., Amin, N., & Akhtaruzzaman, M. (2022). Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications. Nanomaterials, 12(11), 1933. https://doi.org/10.3390/nano12111933










