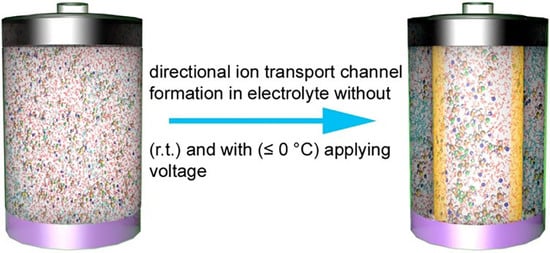
Electrolytes with Micelle-Assisted Formation of Directional Ion Transport Channels for Aqueous Rechargeable Batteries with Impressive Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of NTP
2.3. Preparation of LN-SDS-n Electrolytes
2.4. Characterization
2.5. Electrochemical Test
2.6. Computational Method
3. Results and Discussion
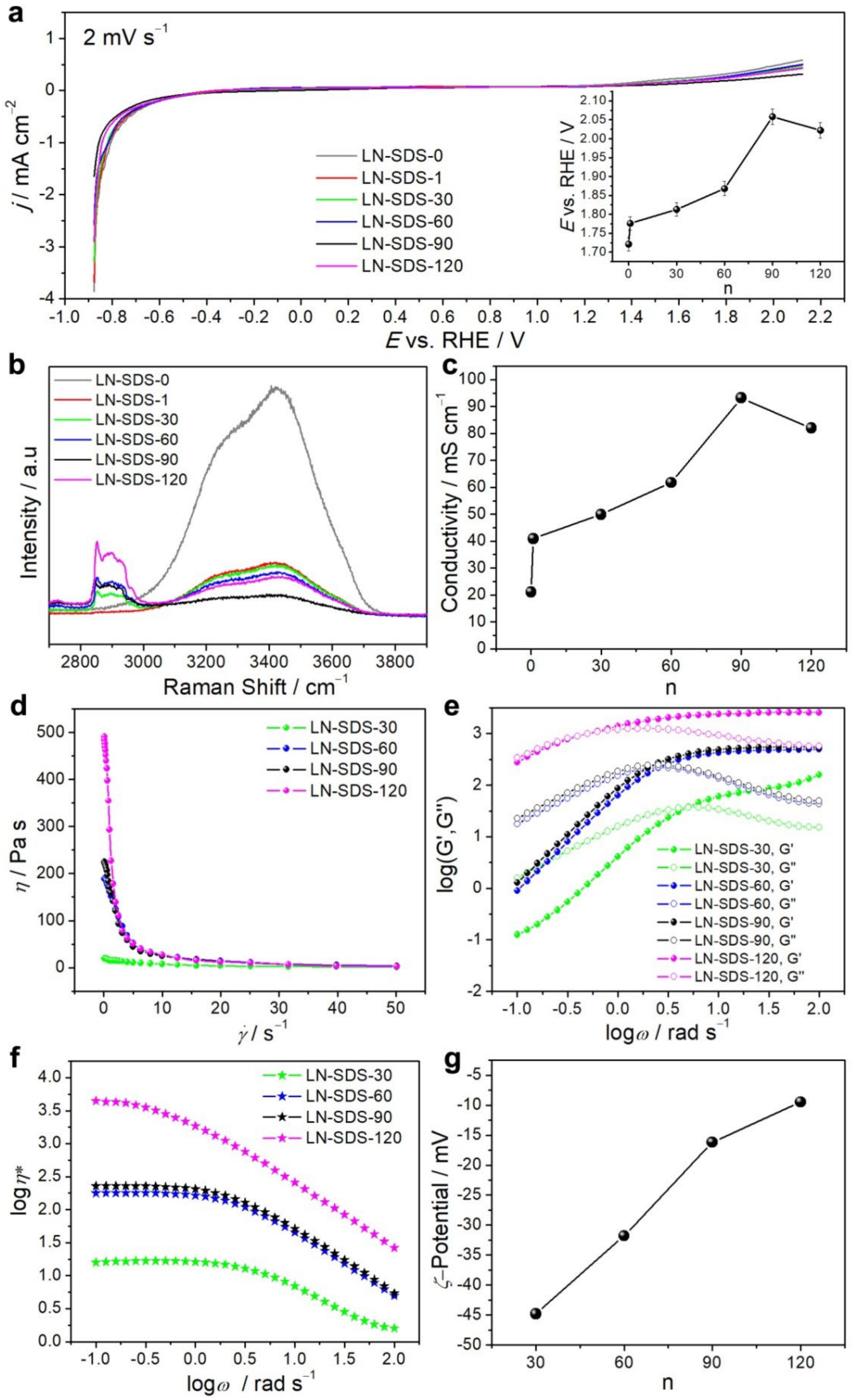
3.1. LN-SDS-n Electrolytes
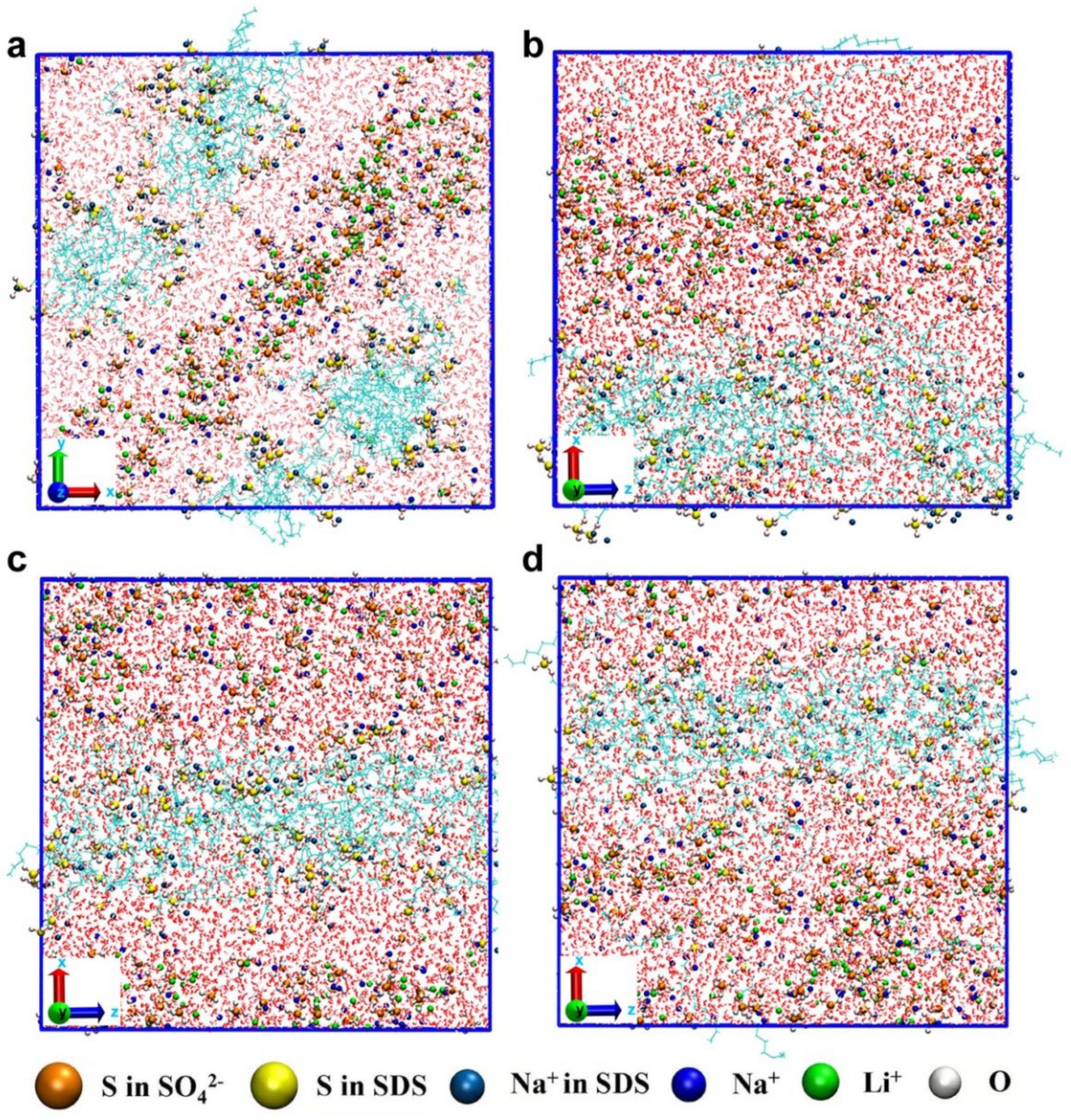
3.2. Conductive Mechanism of LN-SDS-n
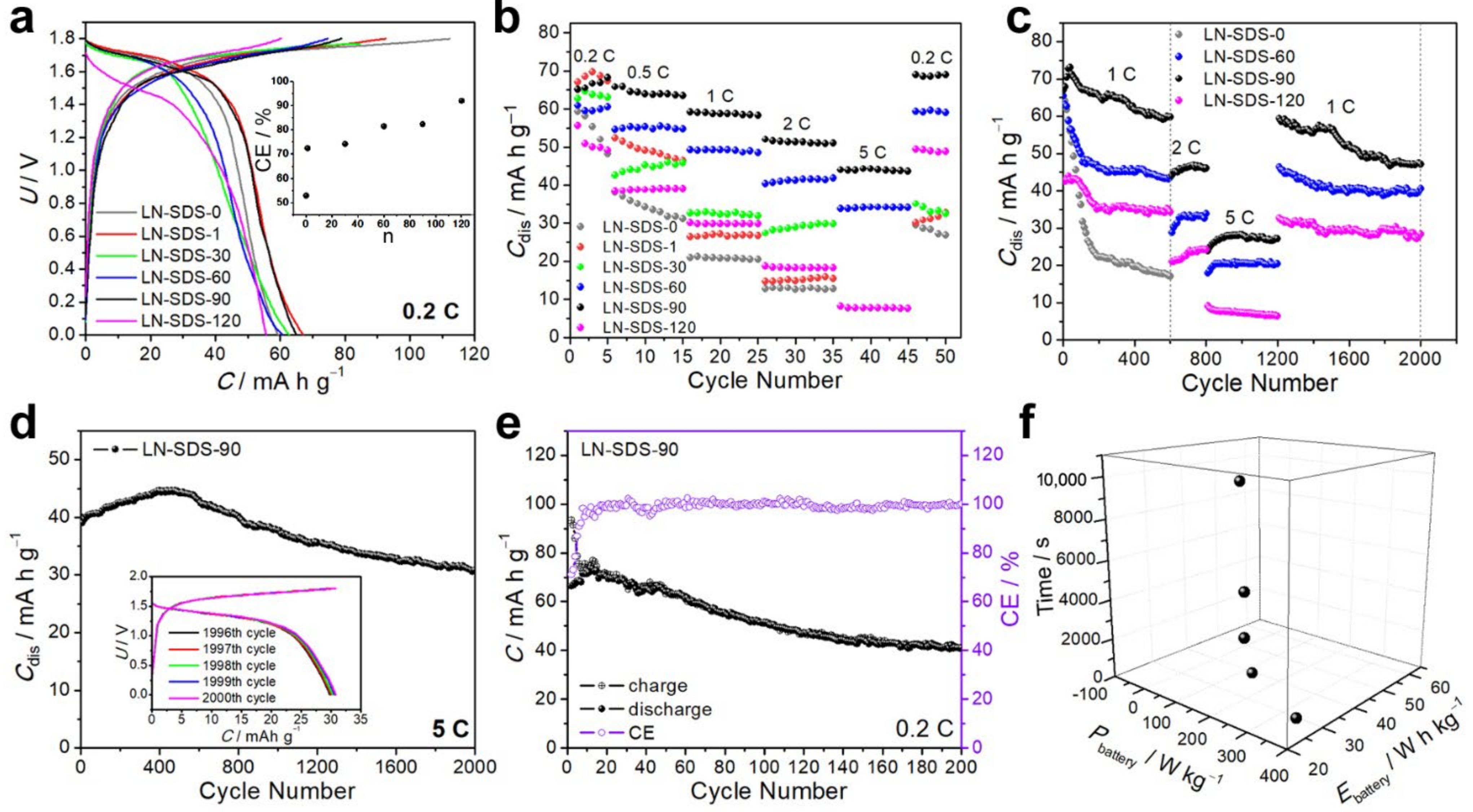
3.3. NTP‖LMO Operating at Room Temperature
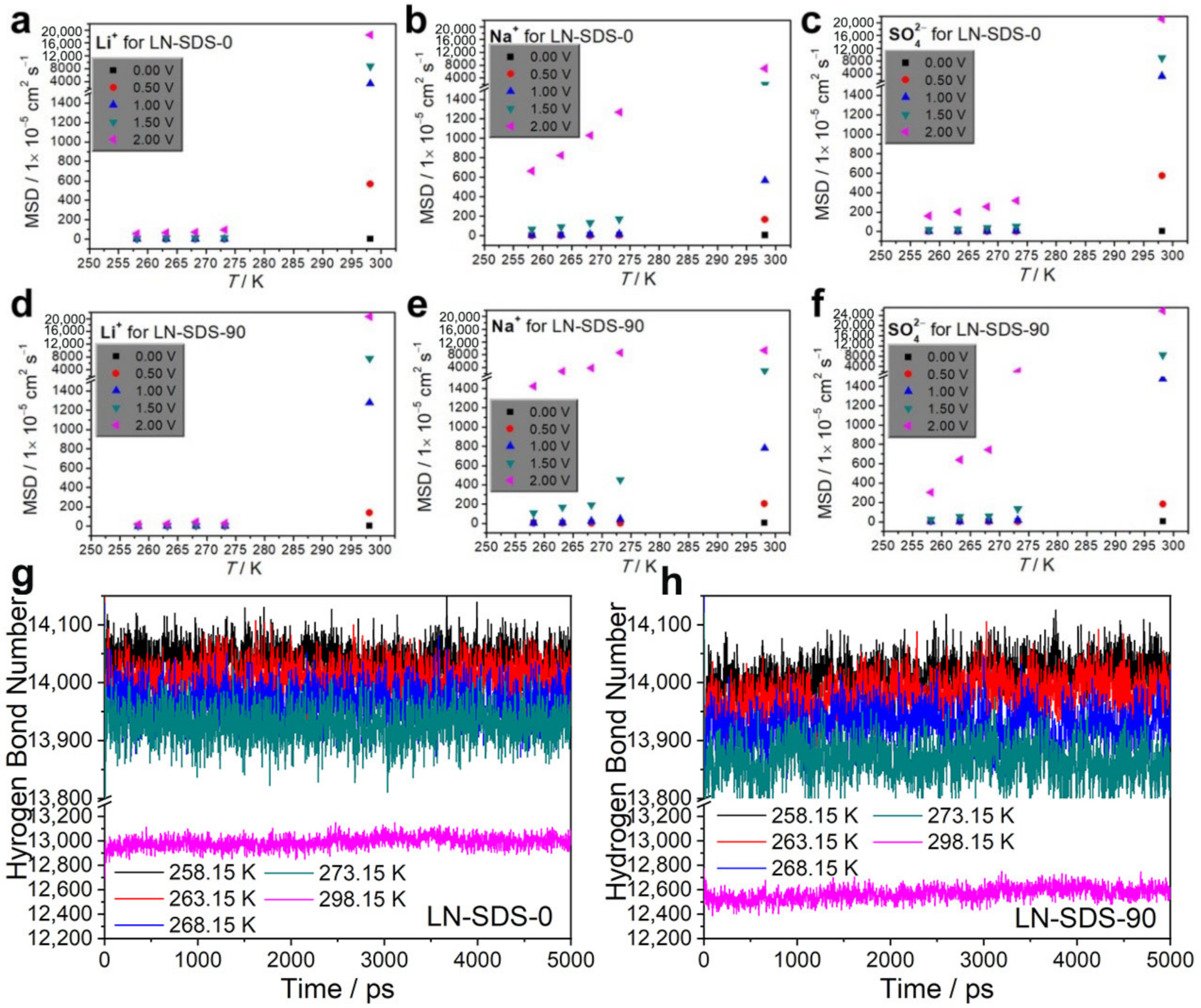
3.4. NTP‖LMO Operating at Zero and Subzero Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X.; Huang, Z.; Zhi, C. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Cui, W.J.; He, P.; Xia, Y.Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, D.; Wang, S.; Cai, X.; Qian, K.; Kang, F.; Li, B. Understanding the cathode electrolyte interface formation in aqueous electrolyte by scanning electrochemical microscopy. J. Mater. Chem. A 2019, 7, 12993–12996. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.; Suo, L.; Fan, X.; Gao, T.; Yang, C.; Han, F.; Qi, Y.; Xu, K.; Wang, C. Stabilizing high voltage LiCoO2 cathode in aqueous electrolyte with interphase-forming additive. Energy Environ. Sci. 2016, 9, 3666–3673. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Yang, X.; Li, J.; Huang, Z.-H.; Li, B.; Kang, F. A dual-carbon-anchoring strategy to fabricate flexible LiMn2O4 cathode for advanced lithium-ion batteries with high areal capacity. Nano Energy 2020, 67, 104256. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Q.; Zhu, X.; Gu, L.; Yue, J.; Xia, Q.; Xing, T.; Chen, T.; Yao, Y.; Xia, H. 3D LiCoO2 nanosheets assembled nanorod arrays via confined dissolution-recrystallization for advanced aqueous lithium-ion batteries. Nano Energy 2019, 56, 463–472. [Google Scholar] [CrossRef]
- Xie, J.; Liang, Z.; Lu, Y.-C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 2020, 19, 1006–1011. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Greenblatt, M.; Dismukes, G.C. Water Oxidation by λ-MnO2: Catalysis by the Cubical Mn4O4 Subcluster Obtained by Delithiation of Spinel LiMn2O4. J. Am. Chem. Soc. 2010, 132, 11467–11469. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Ji, X.; Pollard, T.P.; Lü, X.; Sun, C.-J.; Hou, S.; Liu, Q.; Liu, C.; Qing, T.; et al. Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. Nature 2019, 569, 245–250. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Dong, X.; Huang, J.; Wang, Y.; Xia, Y. An Environmentally Friendly and Flexible Aqueous Zinc Battery Using an Organic Cathode. Angew. Chem. Int. Ed. 2018, 57, 11737–11741. [Google Scholar] [CrossRef] [PubMed]
- Tron, A.; Jo, Y.N.; Oh, S.H.; Park, Y.D.; Mun, J. Surface Modification of the LiFePO4 Cathode for the Aqueous Rechargeable Lithium Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 12391–12399. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, L.; Liu, J.; Yang, Z.; Yu, X.; Gu, L.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L.; et al. A Novel High Capacity Positive Electrode Material with Tunnel-Type Structure for Aqueous Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501005. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.; Zhong, Y.; Li, Y.; Sun, C.; Han, X.; Deng, Y.; Zhao, N.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, M.; Xiong, Y.; Hou, Z.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. Aqueous Rechargeable Li+/Na+ Hybrid Ion Battery with High Energy Density and Long Cycle Life. Small 2020, 16, 2003585. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Guo, Z.; Wang, Y.; Wang, C.; Che, Y.; Xia, Y. Aqueous Lithium-Ion Batteries Using O2 Self-Elimination Polyimides Electrodes. J. Electrochem. Soc. 2015, 162, A1972–A1977. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angew. Chem. Int. Ed. 2016, 55, 7136–7141. [Google Scholar] [CrossRef]
- Gordon, D.; Wu, M.Y.; Ramanujapuram, A.; Benson, J.; Lee, J.T.; Magasinski, A.; Nitta, N.; Huang, C.; Yushin, G. Enhancing Cycle Stability of Lithium Iron Phosphate in Aqueous Electrolytes by Increasing Electrolyte Molarity. Adv. Energy Mater. 2016, 6, 1501805. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Li, X.; Zhu, Y.; Liang, J.; Qian, Y. Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery. J. Mater. Chem. A 2017, 5, 730–738. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, B.; Han, J.; Passerini, S. Aqueous/Nonaqueous Hybrid Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1769–1770. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Reber, D.; Battaglia, C. A High-Voltage Aqueous Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2017, 2, 2005–2006. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z. Concentrated Mixed Cation Acetate “Water-in-Salt” Solutions as Green and Low Cost High Voltage Electrolytes for Aqueous Batteries. Energy Environ. Sci. 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
- Liu, T.; Tang, L.; Luo, H.; Cheng, S.; Liu, M. A promising water-in-salt electrolyte for aqueous based electrochemical energy storage cells with a wide potential window: Highly concentrated HCOOK. Chem. Commun. 2019, 55, 12817–12820. [Google Scholar] [CrossRef]
- He, X.; Yan, B.; Zhang, X.; Liu, Z.; Bresser, D.; Wang, J.; Wang, R.; Cao, X.; Su, Y.; Jia, H.; et al. Fluorine-free water-in-ionomer electrolytes for sustainable lithium-ion batteries. Nat. Commun. 2018, 9, 5320. [Google Scholar] [CrossRef]
- Jaumaux, P.; Yang, X.; Zhang, B.; Safaei, J.; Tang, X.; Zhou, D.; Wang, C.; Wang, G. Localized Water-In-Salt Electrolyte for Aqueous Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 19965–19973. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, J.; Ko, S.; Watanabe, E.; Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 2019, 4, 269–280. [Google Scholar] [CrossRef]
- Khan, Z.; Ail, U.; Ajjan, F.N.; Phopase, J.; Kim, N.; Kumar, D.; Khan, Z.U.; Nilsson, J.; Inganäs, O.; Berggren, M.; et al. Towards printable water-in-polymer salt electrolytes for high power organic batteries. J. Power Sources 2022, 524, 231103. [Google Scholar] [CrossRef]
- Khan, Z.; Ail, U.; Nadia Ajjan, F.; Phopase, J.; Ullah Khan, Z.; Kim, N.; Nilsson, J.; Inganäs, O.; Berggren, M.; Crispin, X. Water-in-Polymer Salt Electrolyte for Slow Self-Discharge in Organic Batteries. Adv. Energy Sustain. Res. 2021, 3, 2100165. [Google Scholar] [CrossRef]
- Cekic-Laskovic, I.; Aspern, N.v.; Imholt, L.; Kaymaksiz, S.; Oldiges, K.; Rad, B.R.; Winter, M. Synergistic Effect of Blended Components in Nonaqueous Electrolytes for Lithium Ion Batteries. Top Curr. Chem. 2017, 375, 37. [Google Scholar] [CrossRef] [PubMed]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Chen, M.; Lu, X.; Liu, X.; Hou, Q.; Zhu, Y.; Zhou, H. Specific Counterion Effects on the Atomistic Structure and Capillary-Waves Fluctuation of the Water/Vapor Interface Covered by Sodium Dodecyl Sulfate. J. Phys. Chem. C 2014, 118, 19205–19213. [Google Scholar] [CrossRef]
- Huang, M.; Zhen, S.; Ren, X.; Ju, X. High-voltage hydrous electrolytes for electrochemical energy storage. J. Power Sources 2020, 465, 228265. [Google Scholar] [CrossRef]
- Zakarina, R.; Kurmanbayeva, I.; Bakenov, Z. Suppression of zinc dendrite formation on anode of Zn/LiFePO4 aqueous rechargeable batteries using electrodeposition. Mater. Today Proc. 2020, 25, 93–96. [Google Scholar] [CrossRef]
- Sun, K.E.K.; Hoang, T.K.A.; Doan, T.N.L.; Yu, Y.; Zhu, X.; Tian, Y.; Chen, P. Suppression of Dendrite Formation and Corrosion on Zinc Anode of Secondary Aqueous Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9681–9687. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Mateja, A.; Ożóg, A.; Miśkowiec, P. Influence of metal ions on the aggregation of anionic surfactants. Studies on the interactions between environmental pollutants in aqueous solutions. J. Mol. Liq. 2017, 240, 514–521. [Google Scholar] [CrossRef]
- Lu, J.R.; Marrocco, A.; Su, T.J.; Thomas, R.K.; Penfold, J. Adsorption of Dodecyl Sulfate Surfactants with Monovalent Metal Counterions at the Air-Water Interface Studied by Neutron Reflection and Surface Tension. J. Colloid Interface Sci. 1993, 158, 303–316. [Google Scholar] [CrossRef]
- Sharker, K.K.; Nazrul Islam, M.; Das, S. Interactions of Some Hofmeister Cations with Sodium Dodecyl Sulfate in Aqueous Solution. J. Surfactants Deterg. 2019, 22, 249–258. [Google Scholar] [CrossRef]
- Prajapati, R.R.; Bhagwat, S.S. Effect of Foam Boosters on Krafft Temperature. J. Chem. Eng. Data 2012, 57, 869–874. [Google Scholar] [CrossRef]
- Xiang, W.; Preisig, N.; Ketola, A.; Tardy, B.L.; Bai, L.; Ketoja, J.A.; Stubenrauch, C.; Rojas, O.J. How Cellulose Nanofibrils Affect Bulk, Surface, and Foam Properties of Anionic Surfactant Solutions. Biomacromolecules 2019, 20, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Q.; Zhou, X.; Lee, S.; Xia, Y.; Liu, Z. New-concept Batteries Based on Aqueous Li+/Na+ Mixed-ion Electrolytes. Sci. Rep. 2013, 3, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhang, L.; Zhou, X.; Liu, Z. Aqueous Batteries Based on Mixed Monovalence Metal Ions: A New Battery Family. ChemSusChem 2014, 7, 2295–2302. [Google Scholar] [CrossRef]
- Guo, X.; Luan, Z.; Lu, Y.; Fu, L.; Gai, L. NaTi2(PO4)3/C‖carbon package asymmetric flexible supercapacitors with the positive material recycled from spent Zn–Mn dry batteries. J. Alloys Compd. 2019, 782, 576–585. [Google Scholar] [CrossRef]
- Nitta, Y.; Yoshimura, M.; Nishinari, K. The effect of thermal history on the elasticity of K-type gellan gels. Carbohydr. Polym. 2014, 113, 189–193. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.-M. Towards High Power High Energy Aqueous Sodium-Ion Batteries: The NaTi2(PO4)3/Na0.44MnO2 System. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Li, Z.; Ravnsbæk, D.B.; Xiang, K.; Chiang, Y.-M. Na3Ti2(PO4)3 as a sodium-bearing anode for rechargeable aqueous sodium-ion batteries. Electrochem. Commun. 2014, 44, 12–15. [Google Scholar] [CrossRef]
- Hou, Z.; Li, X.; Liang, J.; Zhu, Y.; Qian, Y. An aqueous rechargeable sodium ion battery based on a NaMnO2–NaTi2(PO4)3 hybrid system for stationary energy storage. J. Mater. Chem. A 2015, 3, 1400–1404. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Spoel, D.v.d.; Drunen, R.v. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Modeling 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.V.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Springer: Dordecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Abascal, J.L.F.; Sanz, E.; García Fernández, R.; Vega, C. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: AnN⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, H. Self-Aggregation of the SDS Surfactant at a Solid-Liquid Interface. J. Phys. Chem. B 2007, 111, 4054–4059. [Google Scholar] [CrossRef]
- Mukherjee, P.; Das, A.; Sen, P. Solvation dynamics in SDS micelle revisited with femtosecond time resolution to reveal the probe and concentration dependence. Chem. Phys. 2018, 513, 141–148. [Google Scholar] [CrossRef]
- Dolenko, T.A.; Burikov, S.A.; Dolenko, S.A.; Efitorov, A.O.; Mirgorod, Y.A. Raman spectroscopy of micellization-induced liquid–liquid fluctuations in sodium dodecyl sulfate aqueous solutions. J. Mol. Liq. 2015, 204, 44–49. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P. Micelle–monomer equilibria in solutions of ionic surfactants and in ionic–nonionic mixtures: A generalized phase separation model. Adv. Colloid Interface Sci. 2014, 206, 17–45. [Google Scholar] [CrossRef]
- Evageliou, V. Shear and extensional rheology of selected polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 1853–1861. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Wang, J.; Gai, L.; Ji, X.; Jiang, H.; Liu, L. Antifreezing Zwitterionic Hydrogel Electrolyte with High Conductivity of 12.6 mS cm at −40 °C through Hydrated Lithium Ion Hopping Migration. Adv. Funct. Mater. 2021, 31, 2009438. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, X.; Ok, Y.S.; Elliott, J.A.W.; Chang, S.X.; Chung, H.-J. Flexible and Self-Healing Aqueous Supercapacitors for Low Temperature Applications: Polyampholyte Gel Electrolytes with Biochar Electrodes. Sci. Rep. 2017, 7, 1685. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qi, F.; Zeng, G.; Shi, L.; Li, X.; Gu, Y.; Shi, Y. Repeating recovery and reuse of SDS micelles from MEUF retentate containing Cd2+ by acidification UF. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 361–368. [Google Scholar] [CrossRef]
- Geanta, R.M.; Olga Ruiz, M.; Escudero, I. Micellar-enhanced ultrafiltration for the recovery of lactic acid and citric acid from beet molasses with sodium dodecyl sulphate. J. Membr. Sci. 2013, 430, 11–23. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Li, T. Novel polymer electrolytes based on cationic polyurethane with different alkyl chain length. J. Power Sources 2014, 249, 397–404. [Google Scholar] [CrossRef]
- García, A.; Torres-González, L.C.; Padmasree, K.P.; Benavides-Garcia, M.G.; Sánchez, E.M. Conductivity and viscosity properties of associated ionic liquids phosphonium orthoborates. J. Mol. Liq. 2013, 178, 57–62. [Google Scholar] [CrossRef]
- Tiyapiboonchaiya, C.; Pringle, J.M.; Sun, J.; Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 2003, 3, 29–32. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Yin, Q.; Wu, J.; Chen, P.; Zhang, G.; Liu, G.; Wu, C.; Xie, Y. A zwitterionic gel electrolyte for efficient solid-state supercapacitors. Nat. Commun. 2016, 7, 11782. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, J.; Gai, L.; Ma, X.; Zhou, J. NaTi2(PO4)3/C‖LiMn2O4 rechargeable battery operating with Li+/Na+-mixed aqueous electrolyte exhibits superior electrochemical performance. Electrochim. Acta 2017, 255, 220–229. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, G.; Wang, X.; Zhang, S.; Deng, C. Cross-linked Na2VTi(PO4)3@C hierarchical nanofibers as high-performance bi-functional electrodes for symmetric aqueous rechargeable sodium batteries. J. Mater. Chem. A 2017, 5, 18725–18736. [Google Scholar] [CrossRef]
- Wu, X.; Cao, Y.; Ai, X.; Qian, J.; Yang, H. A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3–Na2NiFe(CN)6 intercalation chemistry. Electrochem. Commun. 2013, 31, 145–148. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Hybrid Aqueous Energy Storage Cells Using Activated Carbon and Lithium-Intercalated Compounds. J. Electrochem. Soc. 2006, 153, A450. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Ma-trix-flushing method for quantitative multicomponent analysis. J. Appl. Cryst 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guo, Z.; Wang, Y.; Wang, C.; Xia, Y. Electrochemical profile of LiTi2(PO4)3 and NaTi2 (PO4)3 in lithium, sodium or mixed ion aqueous solutions. J. Electrochem. Soc. 2016, 163, A904–A910. [Google Scholar] [CrossRef]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680. [Google Scholar] [CrossRef] [Green Version]
- Park, S.I.; Gocheva, I.; Okada, S.; Yamaki, J.-i. Electrochemical properties of NaTi2(PO4)3 anode for re-chargeable aqueous sodium-ion batteries. J. Electrochem. Soc. 2011, 158, A1067. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Whitacre, J.F. Capacity fade of NaTi2 (PO4) 3 in aqueous electrolyte solutions: Relating pH increases to long term stability. Electrochim. Acta 2017, 235, 730–739. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Shanbhag, S.; Mohamed, A.; Polonsky, A.; Carlisle, K.; Gulakowski, J.; Wu, W.; Smith, C.; Cooney, L.; Blackwood, D.; et al. A Polyionic, Large–Format Energy Storage Device Using an Aqueous Electrolyte and Thick–Format Composite NaTi2(PO4)3/Activated Carbon Negative Electrodes. Energy Technol. 2015, 3, 20–31. [Google Scholar] [CrossRef] [Green Version]


| Electrolyte | Conductivity at Different Temperatures (mS cm−1) | Ref. | |||
|---|---|---|---|---|---|
| 25 °C/r.t. | 10 °C | 0 °C | −15 °C | ||
| 21 m LiTFSI (WIS) | 10, 10.9 a | 6.6 a | 2.4 a | 1.0 a | [17] |
| Li(TFSI)0.7(BETI)0.3·2H2O (WIBS) | 3 at 30 °C | – | – | – | [18] |
| 7 m NaOTf in H2O + 8 m NaOTf in PC | 25 at 20 °C | – | – | – | [22] |
| 15–35 m NaFSI (WIS) | 8–90 at 20 °C | – | – | – | [23] |
| 27 m KOAc | 31.4 | – | – | – | [25] |
| 32 m KOAc–8 m LiOAc | 5.3 | – | – | – | [25] |
| 10–30 m KOAc | 32–90 | – | – | – | [26] |
| 10–40 m HCOOK | 46–130 | – | – | – | [26] |
| 50 wt% LiPAA gel | 6.5 at 20 °C | – | – | – | [27] |
| 25 m LiNO3 in water (WIS) | 73.8 | 0.016 | – | – | [28] |
| 25 m LiNO3 in H2O:PD | 22.8 | 14.4 | – | – | [28] |
| low WIS gel | 16.2 | – | – | – | [28] |
| 2 m LiTFSI–xPEG–(1–x)H2O | 0.8–3.4 (x = 71–94%) | – | – | – | [8] |
| polyacrylate derivatives with K+ (WIPSE) | 45–87 | [30] | |||
| potassium polyacrylate (WIPSE) | 40–120 | [31] | |||
| organic carbonate-based Li+ electrolytes | 4.5–10.7 | – | – | – | [32] |
| LN-SDS-90 | 93.2 | 35.8 | 26.1 | 15.2 | this work |
| LN-SDS-120 | 82.0 | 33.7 | 24.9 | 14.0 | this work |
| Electrolytes | σ at Different Temperatures (K)/mS cm−1 | Ea/eV | ||||||
|---|---|---|---|---|---|---|---|---|
| 298 | 283 | 278 | 273 | 268 | 263 | 258 | ||
| LN-SDS -0 | 21.1 | 15.6 | 13.6 | 11.6 | 9.5 | 8 | 1.6 | 0.587 |
| LN-SDS-30 | 50.1 | 37.2 | 29 | 22.3 | 18.7 | 15.4 | 12.6 | 0.252 |
| LN-SDS-60 | 65.7 | 50.6 | 43.2 | 28.1 | 23.3 | 19.1 | 15.5 | 0.242 |
| LN-SDS-90 | 87.2 | 60.7 | 33.2 | 29.5 | 25 | 20.8 | 16.9 | 0.211 |
| LN-SDS-120 | 82.8 | 45.5 | 28.5 | 24.9 | 21.1 | 17.4 | 14 | 0.221 |
| 21 m LITFSI * | 10.9 | 6.6 | 2.9 | 2.3 | 1.7 | 1.3 | 1 | 0.334 |
| Temperature (K) | HBNs of Water in Electrolytes | |
|---|---|---|
| LN-SDS-0 | LN-SDS-90 | |
| 258.15 | 14,050 ± 50 | 14,020 ± 50 |
| 263.15 | 14,020 ± 50 | 13,990 ± 50 |
| 268.15 | 13,960 ± 50 | 13,920 ± 60 |
| 273.15 | 13,930 ± 50 | 13,850 ± 50 |
| 298.15 | 12,980 ± 100 | 12,570 ± 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, F.; Lu, X.; Jiang, H.; Hu, W.; Liu, L.; Gai, L. Electrolytes with Micelle-Assisted Formation of Directional Ion Transport Channels for Aqueous Rechargeable Batteries with Impressive Performance. Nanomaterials 2022, 12, 1920. https://doi.org/10.3390/nano12111920
Lu Y, Zhang F, Lu X, Jiang H, Hu W, Liu L, Gai L. Electrolytes with Micelle-Assisted Formation of Directional Ion Transport Channels for Aqueous Rechargeable Batteries with Impressive Performance. Nanomaterials. 2022; 12(11):1920. https://doi.org/10.3390/nano12111920
Chicago/Turabian StyleLu, Yanmin, Fengxiang Zhang, Xifeng Lu, Haihui Jiang, Wei Hu, Libin Liu, and Ligang Gai. 2022. "Electrolytes with Micelle-Assisted Formation of Directional Ion Transport Channels for Aqueous Rechargeable Batteries with Impressive Performance" Nanomaterials 12, no. 11: 1920. https://doi.org/10.3390/nano12111920
APA StyleLu, Y., Zhang, F., Lu, X., Jiang, H., Hu, W., Liu, L., & Gai, L. (2022). Electrolytes with Micelle-Assisted Formation of Directional Ion Transport Channels for Aqueous Rechargeable Batteries with Impressive Performance. Nanomaterials, 12(11), 1920. https://doi.org/10.3390/nano12111920