Removal of Chromium(VI) by Nanoscale Zero-Valent Iron Supported on Melamine Carbon Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
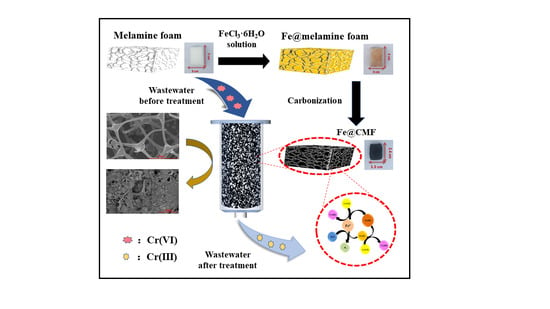
2.2. Fe@MF Composite Preparation
2.3. Analysis
2.4. Batch Experiments
2.5. Column Trial
3. Results and Discussion
3.1. Optimization of Fe@MF Synthesis
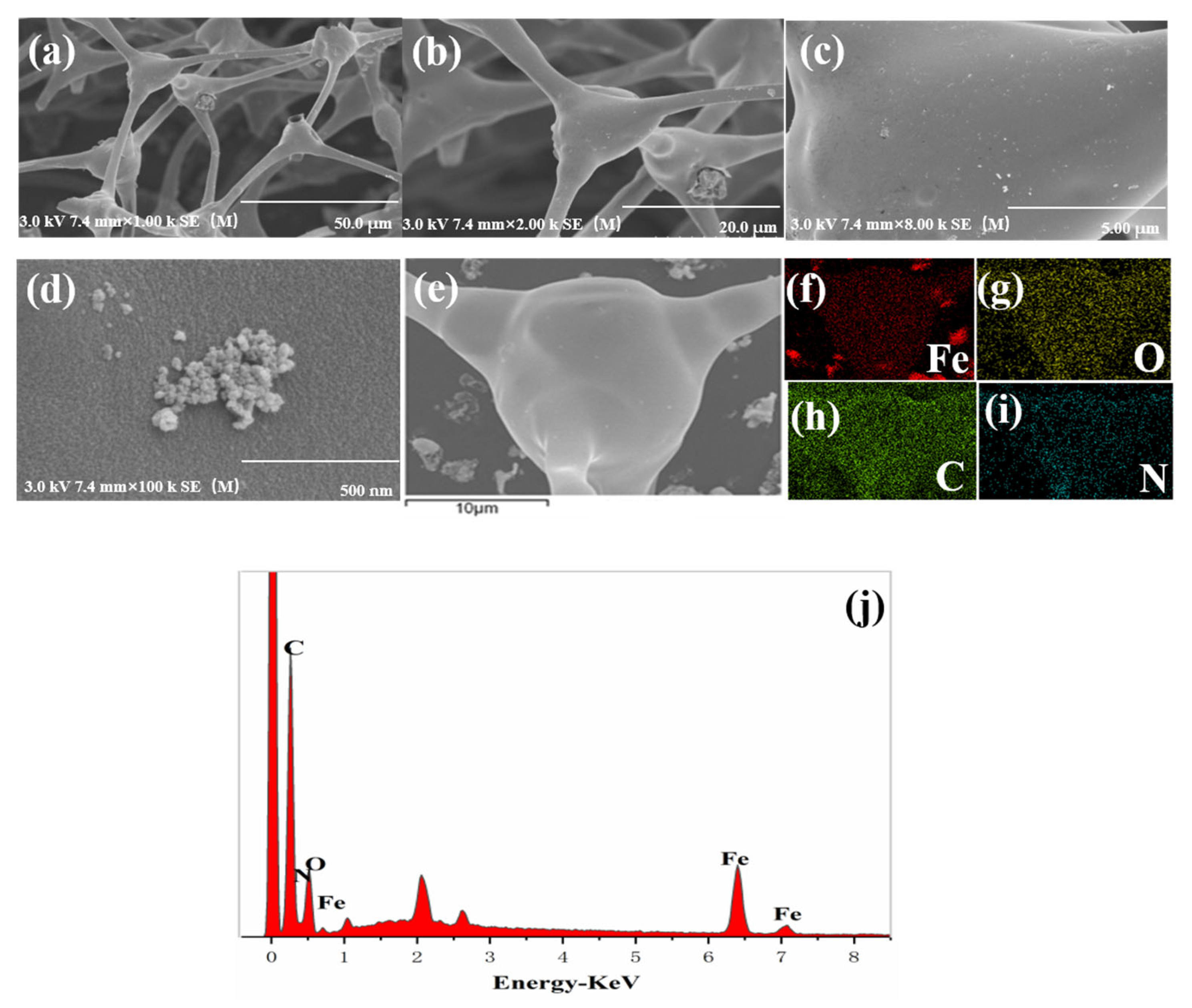
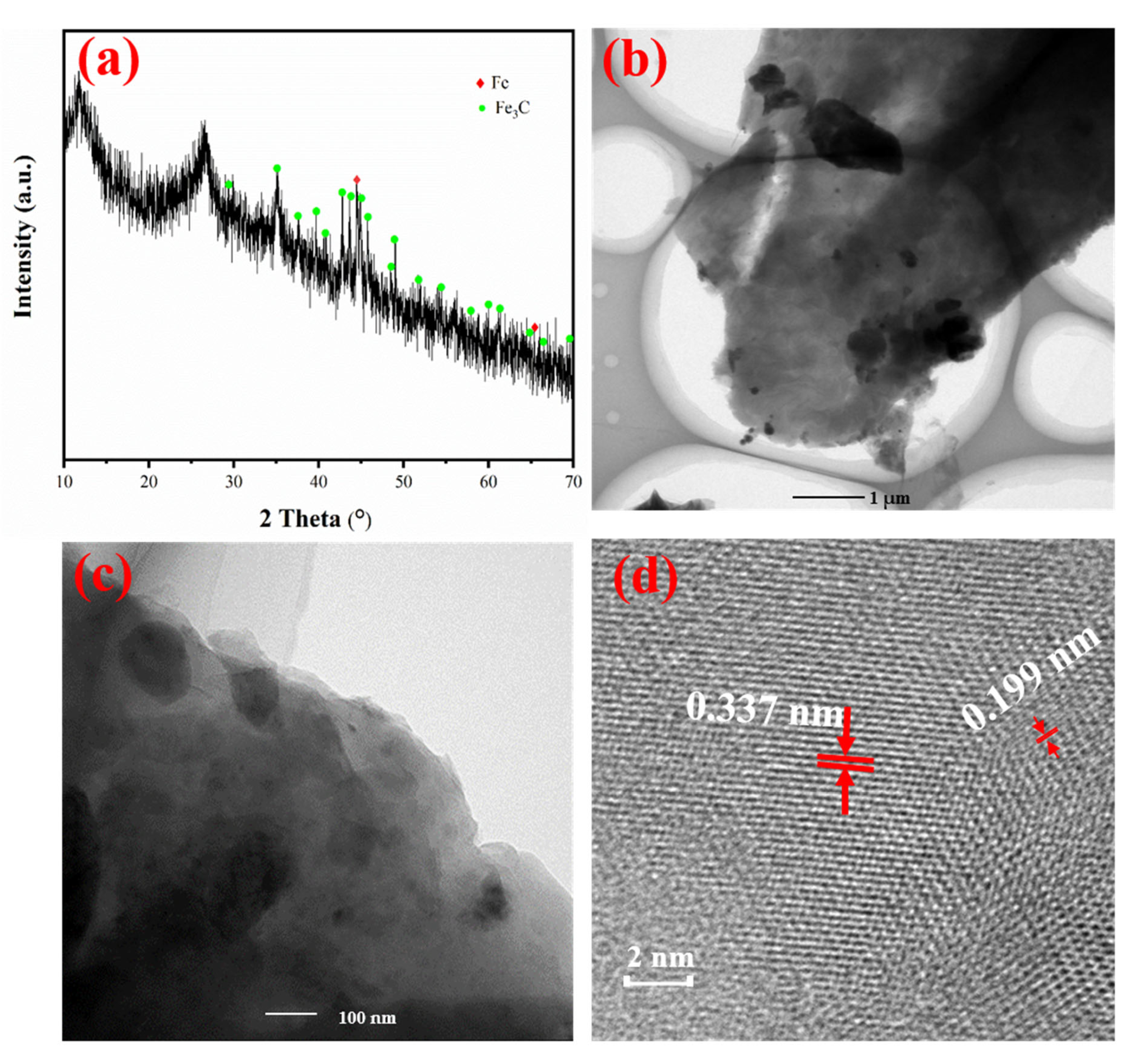
3.2. Characterization of Fe@MF-12.5-800
3.3. Batch Experiment of Cr(VI) Removal
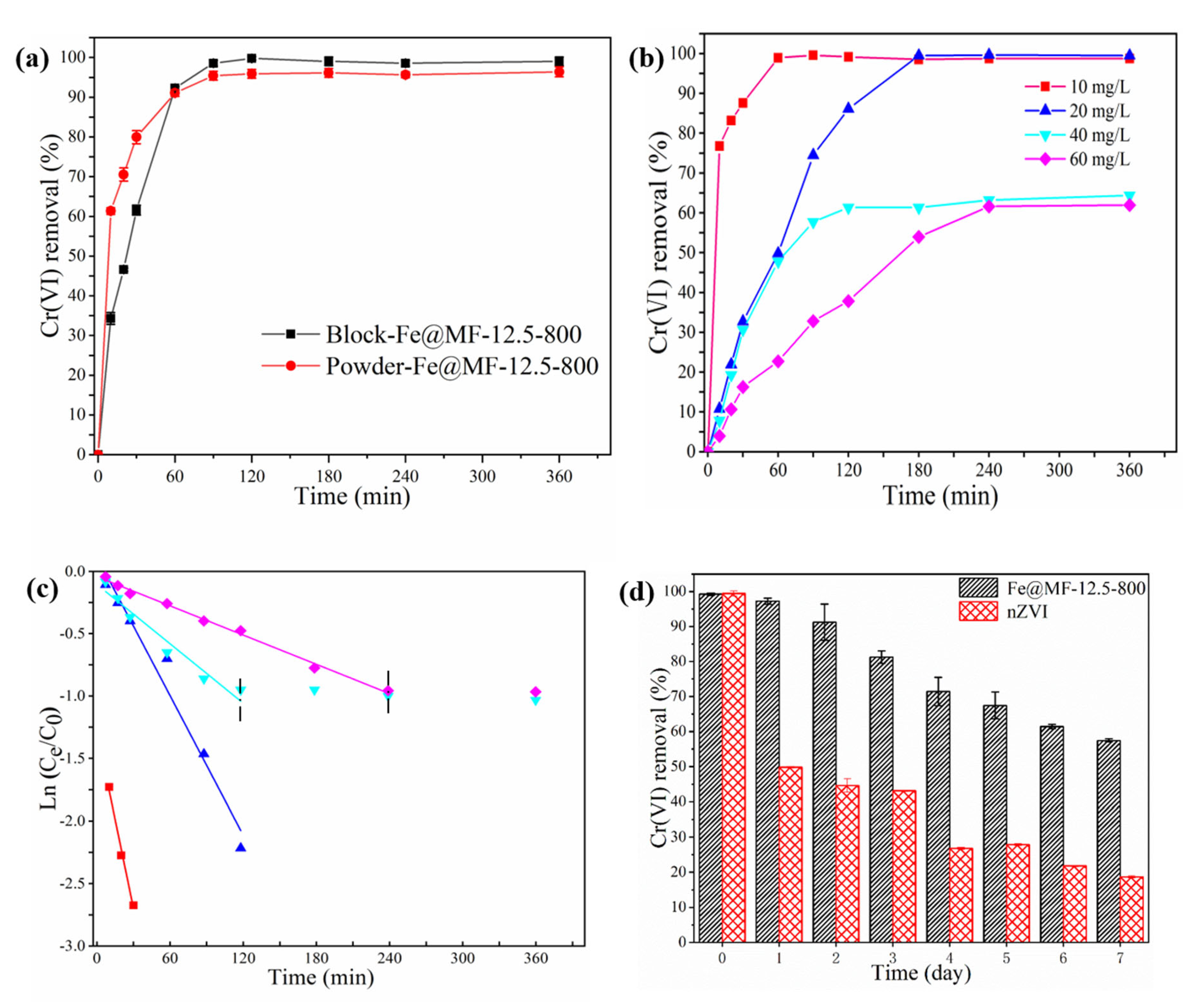
3.3.1. Effect of 3D Porous Structure
3.3.2. Effect of Initial Cr(VI) Concentration
3.3.3. Effect of the Aging Time
3.4. Mechanisms of Cr(VI) Removal by Fe@MF-12.5-800
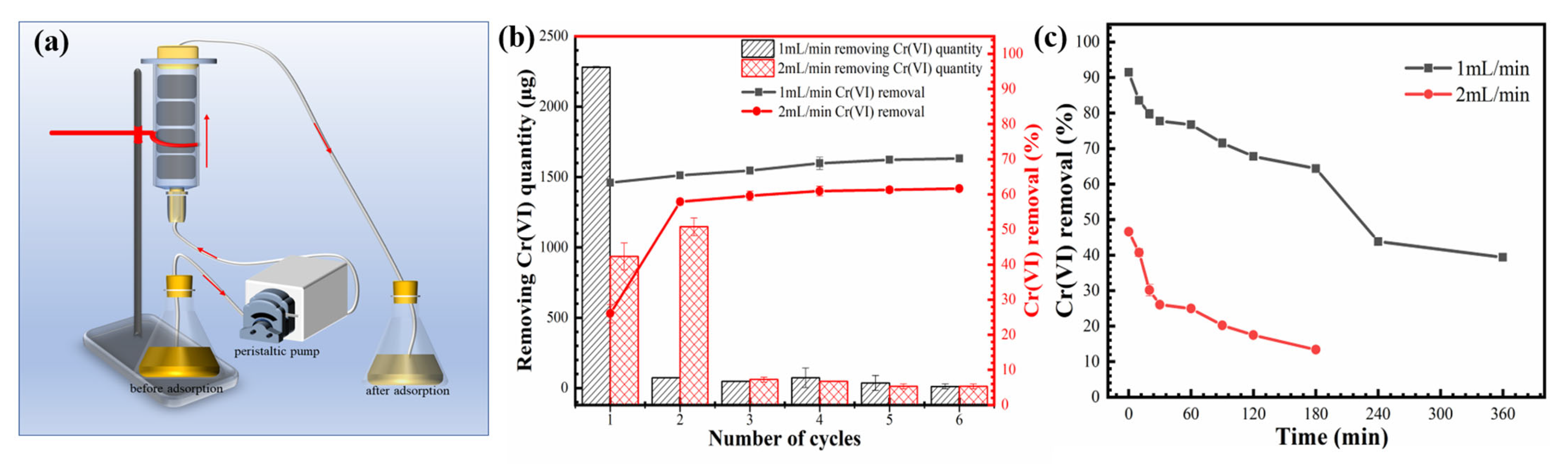
3.5. Continuous Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, W.; Xu, C.; Meng, Y.; Bai, W.; Yang, W.; Lin, A. Polyethylene glycol-stabilized nano zero-valent iron supported by biochar for highly efficient removal of Cr(VI). Ecotoxicol. Environ. Saf. 2020, 188, 109902. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, C.R.; Song, Y.E.; Heo, J.; Choi, S.M.; Lim, D.-H.; Cho, J.; Park, C.; Jang, M.; Kim, J.R. Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem. Eng. J. 2017, 328, 703–707. [Google Scholar] [CrossRef]
- Ravikumar, K.V.; Kumar, D.; Rajeshwari, A.; Madhu, G.M.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. A comparative study with biologically and chemically synthesized nZVI: Applications in Cr (VI) removal and ecotoxicity assessment using indigenous microorganisms from chromium-contaminated site. Environ. Sci. Pollut. Res. Int. 2016, 23, 2613–2627. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Jayaweera, V.; Wijesekara, H.; Kottegoda, I.R.M.; Rosa, S.R.D.; Vithanage, M. Insights into Starch Coated Nanozero Valent Iron-Graphene Composite for Cr(VI) Removal from Aqueous Medium. J. Nanomater. 2016, 2016, 2813289. [Google Scholar] [CrossRef] [Green Version]
- Habibul, N.; Hu, Y.; Wang, Y.K.; Chen, W.; Yu, H.Q.; Sheng, G.P. Bioelectrochemical Chromium(VI) Removal in Plant-Microbial Fuel Cells. Environ. Sci. Technol. 2016, 50, 3882–3889. [Google Scholar] [CrossRef]
- Gao, G.; Nie, L.; Yang, S.; Jin, P.; Chen, R.; Ding, D.; Wang, X.C.; Wang, W.; Wu, K.; Zhang, Q. Well-defined strategy for development of adsorbent using metal organic frameworks (MOF) template for high performance removal of hexavalent chromium. Appl. Surf. Sci. 2018, 457, 1208–1217. [Google Scholar] [CrossRef]
- Mamais, D.; Noutsopoulos, C.; Kavallari, I.; Nyktari, E.; Kaldis, A.; Panousi, E.; Nikitopoulos, G.; Antoniou, K.; Nasioka, M. Biological groundwater treatment for chromium removal at low hexavalent chromium concentrations. Chemosphere 2016, 152, 238–244. [Google Scholar] [CrossRef]
- Zou, W.; Li, C.; Hu, J.; Hou, X. Selective determination of Cr() and non-chromatographic speciation analysis of inorganic chromium by chemical vapor generation-inductively coupled plasma mass spectrometry. Talanta 2020, 218, 121128. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, Z.; Zhang, Z.; Wang, X.; Yang, Z. Active biochar support nano zero-valent iron for efficient removal of U (VI) from sewage water. J. Alloy. Compd. 2021, 852, 156993. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Baltazar, S.E.; Garcia, A.; Munoz-Lira, D.; Sepulveda, P.; Rubio, M.A.; Altbir, D. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2016, 301, 371–380. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Qu, G.; Zeng, D.; Chu, R.; Wang, T.; Liang, D.; Qiang, H. Magnetic Fe3O4 assembled on nZVI supported on activated carbon fiber for Cr(VI) and Cu(II) removal from aqueous solution through a permeable reactive column. Environ. Sci. Pollut. Res. Int. 2019, 26, 5176–5188. [Google Scholar] [CrossRef]
- Ren, J.; Woo, Y.C.; Yao, M.; Lim, S.; Tijing, L.D.; Shon, H.K. Nanoscale zero-valent iron (nZVI) immobilization onto graphene oxide (GO)-incorporated electrospun polyvinylidene fluoride (PVDF) nanofiber membrane for groundwater remediation via gravity-driven membrane filtration. Sci. Total Environ. 2019, 688, 787–796. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Wei, E.; Gong, T.; Xian, Q. Facile synthesis of graphene nano zero-valent iron composites and their efficient removal of trichloronitromethane from drinking water. Chemosphere 2016, 146, 32–39. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Marshall, D.L.; Hou, K.; Ayoko, G.A.; Millar, G.J.; Xi, Y. Simultaneous adsorption and degradation of 2,4-dichlorophenol on sepiolite-supported bimetallic Fe/Ni nanoparticles. J. Environ. Chem. Eng. 2019, 7, 102955. [Google Scholar] [CrossRef]
- Petala, E.; Dimos, K.; Douvalis, A.; Bakas, T.; Tucek, J.; Zboril, R.; Karakassides, M.A. Nanoscale zero-valent iron supported on mesoporous silica: Characterization and reactivity for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2013, 261, 295–306. [Google Scholar] [CrossRef]
- Fu, F.; Ma, J.; Xie, L.; Tang, B.; Han, W.; Lin, S. Chromium removal using resin supported nanoscale zero-valent iron. J. Environ. Manag. 2013, 128, 822–827. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef]
- Zhou, N.; Gong, K.; Hu, Q.; Cheng, X.; Zhou, J.; Dong, M.; Wang, N.; Ding, T.; Qiu, B.; Guo, Z. Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr(VI) reduction. Chemosphere 2020, 242, 125235. [Google Scholar] [CrossRef]
- Ren, L.; Dong, J.; Chi, Z.; Huang, H. Reduced graphene oxide-nano zero value iron (rGO-nZVI) micro-electrolysis accelerating Cr(VI) removal in aquifer. J. Environ. Sci. 2018, 73, 96–106. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Lv, B.; Chang, G.; Jiao, W.; Liu, Y. Removal of trace Cr(VI) from aqueous solution by porous activated carbon balls supported by nanoscale zero-valent iron composites. Environ. Sci. Pollut. Res. Int. 2020, 27, 7015–7024. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Li, J.; Xu, J.; Wang, X. Fabrication of Fe/Fe3C@porous carbon sheets from biomass and their application for simultaneous reduction and adsorption of uranium(VI) from solution. Inorg. Chem. Front. 2014, 1, 641–648. [Google Scholar] [CrossRef]
- Zou, H.; Hu, E.; Yang, S.; Gong, L.; He, F. Chromium(VI) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci. Total Environ. 2019, 650, 419–426. [Google Scholar] [CrossRef]
- Qian, L.; Shang, X.; Zhang, B.; Zhang, W.; Su, A.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, P.; Zhang, L.; Zhou, F.; Li, T.; Bai, R.; Sun, R.; Wong, C. Electrodeposition of Co(OH)2 Improving Carbonized Melamine Foam Performance for Compressible Supercapacitor Application. ACS Sustain. Chem. Eng. 2019, 7, 16803–16813. [Google Scholar] [CrossRef]
- Zhao, M.; Nie, J.; Li, H.; Xia, M.; Liu, M.; Zhang, Z.; Liang, X.; Qi, R.; Wang, Z.L.; Lu, X. High-frequency supercapacitors based on carbonized melamine foam as energy storage devices for triboelectric nanogenerators. Nano Energy 2019, 55, 447–453. [Google Scholar] [CrossRef]
- Stolz, A.; Le Floch, S.; Reinert, L.; Ramos, S.M.M.; Tuaillon-Combes, J.; Soneda, Y.; Chaudet, P.; Baillis, D.; Blanchard, N.; Duclaux, L.; et al. Melamine-derived carbon sponges for oil-water separation. Carbon 2016, 107, 198–208. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, X.; Chu, Y.; Wang, L.; Kang, W.; Wei, D.; Li, H.; Xiong, S. Nitrogen/oxygen co-doped monolithic carbon electrodes derived from melamine foam for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 17730–17739. [Google Scholar] [CrossRef]
- Wu, K.; Guo, J.; Wang, C. An Elastic Monolithic Catalyst: A Microporous Metalloporphyrin-Containing Framework-Wrapped Melamine Foam for Process-Intensified Acyl Transfer. Angew. Chem. Int. Ed. Engl. 2016, 55, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, D.; Liang, H.; Huo, B.; Liu, C.; Yang, W.; Liu, J. Superelastic and Arbitrary-Shaped Graphene Aerogels with Sacrificial Skeleton of Melamine Foam for Varied Applications. Adv. Funct. Mater. 2018, 28, 1704674. [Google Scholar] [CrossRef]
- Rodon Fores, J.; Criado-Gonzalez, M.; Chaumont, A.; Carvalho, A.; Blanck, C.; Schmutz, M.; Serra, C.A.; Boulmedais, F.; Schaaf, P.; Jierry, L. Supported Catalytically Active Supramolecular Hydrogels for Continuous Flow Chemistry. Angew. Chem. Int. Ed. Engl. 2019, 58, 18817–18822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaouen, L.; Renault, A.; Deverge, M. Elastic and damping characterizations of acoustical porous materials: Available experimental methods and applications to a melamine foam. Appl. Acoust. 2008, 69, 1129–1140. [Google Scholar] [CrossRef]
- Milačič, R.; Štupar, J.; Kožuh, N.; Korošin, J. Critical evaluation of three analytical techniques for the determination of chromium(VI) in soil extracts. Analyst 1992, 117, 125–130. [Google Scholar] [CrossRef]
- Wu, M.; Qiu, X.; Chen, C.; Chen, K.; Li, M.; Xu, H.; Wu, X.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts of sublethal exposure to diazepam on behavioral traits and brain GABA levels in juvenile zebrafish (Danio rerio). Sci. Total Environ. 2020, 740, 140392. [Google Scholar] [CrossRef]
- Chen, K.; Wu, M.; Chen, C.; Xu, H.; Wu, X.; Qiu, X. Impacts of chronic exposure to sublethal diazepam on behavioral traits of female and male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111747. [Google Scholar] [CrossRef]
- Qiu, X.; Kim, S.; Kang, I.J.; Hano, T.; Shimasaki, Y.; Oshima, Y. Combined toxicities of tributyltin and polychlorinated biphenyls on the development and hatching of Japanese medaka (Oryzias latipes) embryos via in ovo nanoinjection. Chemosphere 2019, 225, 927–934. [Google Scholar] [CrossRef]
- Qiu, X.; Iwasaki, N.; Chen, K.; Shimasaki, Y.; Oshima, Y. Tributyltin and perfluorooctane sulfonate play a synergistic role in promoting excess fat accumulation in Japanese medaka (Oryzias latipes) via in ovo exposure. Chemosphere 2019, 220, 687–695. [Google Scholar] [CrossRef]
- Calderon, B.; Smith, F.; Aracil, I.; Fullana, A. Green Synthesis of Thin Shell Carbon-Encapsulated Iron Nanoparticles via Hydrothermal Carbonization. ACS Sustain. Chem. Eng. 2018, 6, 7995–8002. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Yao, M.; Woo, Y.C.; Tijing, L.D.; Kim, J.-H.; Shon, H.K. Recyclable nanoscale zerovalent iron (nZVI)-immobilized electrospun nanofiber composites with improved mechanical strength for groundwater remediation. Compos. Part B Eng. 2019, 171, 339–346. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Su, C.; Jiang, Z.; Lu, Z. Ultrasonic-microwave assisted synthesis of stable reduced graphene oxide modified melamine foam with superhydrophobicity and high oil adsorption capacities. Chem. Eng. J. 2016, 306, 504–511. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Zhang, Y.; Lan, Y.; Cheng, K. Performance of lead ion removal by the three-dimensional carbon foam supported nanoscale zero-valent iron composite. J. Clean. Prod. 2021, 294, 125350. [Google Scholar] [CrossRef]
- Su, Q.; Su, Z.; Xie, W.; Tian, C.; Su, X.; Lin, Z. Preparation of 2D nitrogen-doped magnetic Fe3C/C by in-situ self-assembled double-template method for enhanced removal of Cr(VI). Environ. Pollut. 2020, 263, 114374. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, X.; Ai, Z.; Jia, F.; Zhang, L. Liquid Nitrogen Activation of Zero-Valent Iron and Its Enhanced Cr(VI) Removal Performance. Environ. Sci. Technol. 2019, 53, 8333–8341. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, M.; Qiu, X.; Liu, X.; Dapaah, M.F.; Niu, Q.; Cheng, L. Removal of Chromium(VI) by Nanoscale Zero-Valent Iron Supported on Melamine Carbon Foam. Nanomaterials 2022, 12, 1866. https://doi.org/10.3390/nano12111866
Li Q, Liu M, Qiu X, Liu X, Dapaah MF, Niu Q, Cheng L. Removal of Chromium(VI) by Nanoscale Zero-Valent Iron Supported on Melamine Carbon Foam. Nanomaterials. 2022; 12(11):1866. https://doi.org/10.3390/nano12111866
Chicago/Turabian StyleLi, Qiming, Meili Liu, Xuchun Qiu, Xiang Liu, Malcom Frimpong Dapaah, Qijian Niu, and Liang Cheng. 2022. "Removal of Chromium(VI) by Nanoscale Zero-Valent Iron Supported on Melamine Carbon Foam" Nanomaterials 12, no. 11: 1866. https://doi.org/10.3390/nano12111866
APA StyleLi, Q., Liu, M., Qiu, X., Liu, X., Dapaah, M. F., Niu, Q., & Cheng, L. (2022). Removal of Chromium(VI) by Nanoscale Zero-Valent Iron Supported on Melamine Carbon Foam. Nanomaterials, 12(11), 1866. https://doi.org/10.3390/nano12111866