Anomalous Metallic Phase in Molybdenum Disulphide Induced via Gate-Driven Organic Ion Intercalation
Abstract
:1. Introduction
2. Materials and Methods
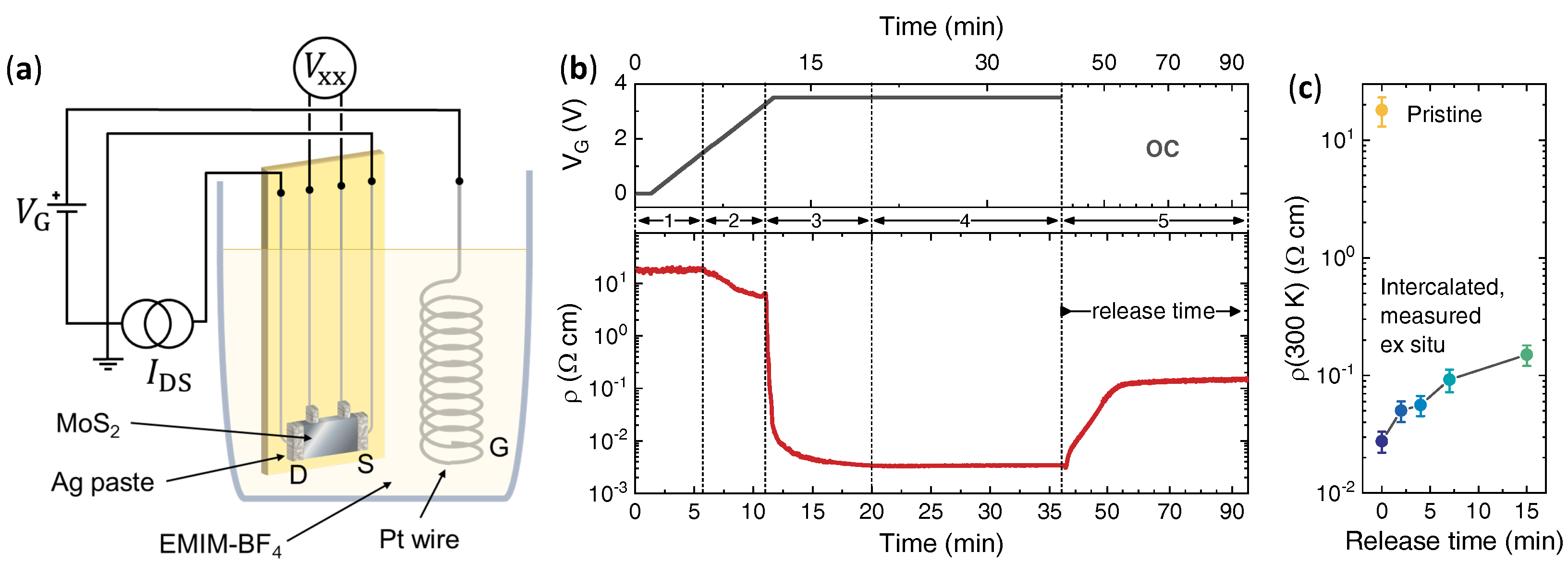
2.1. Electric-Field-Driven Ion Intercalation
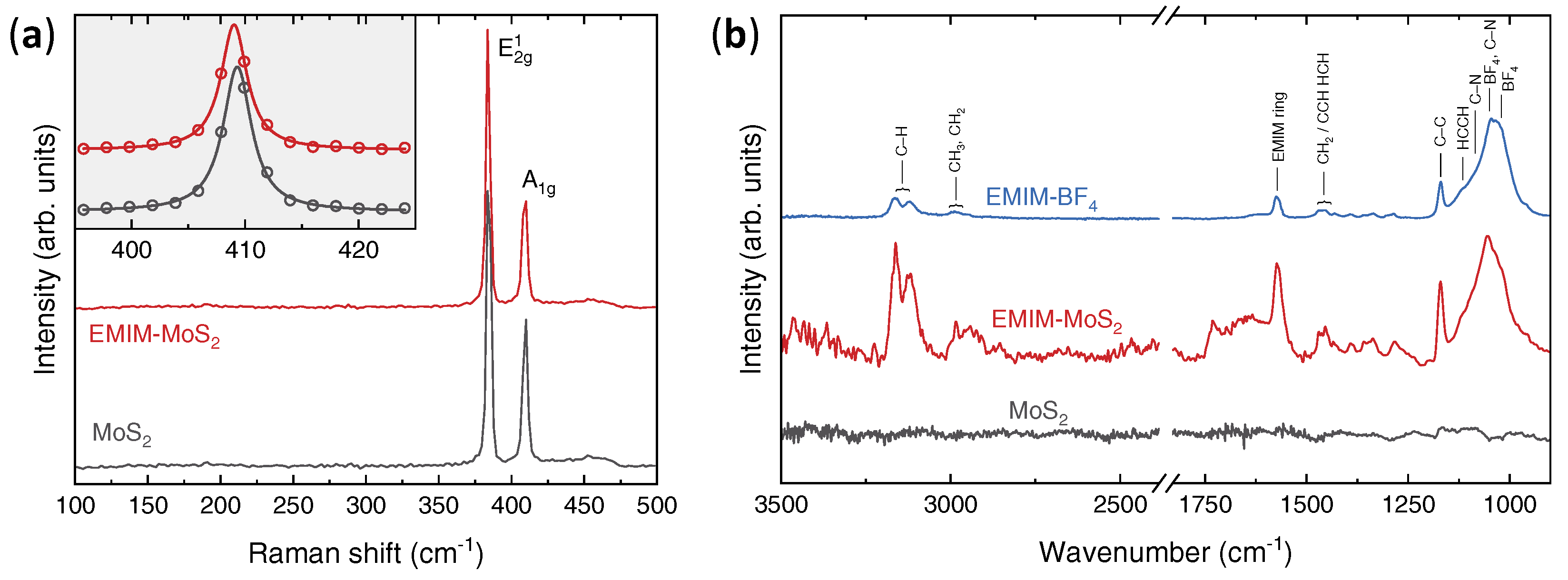
2.2. Vibrational Spectroscopies
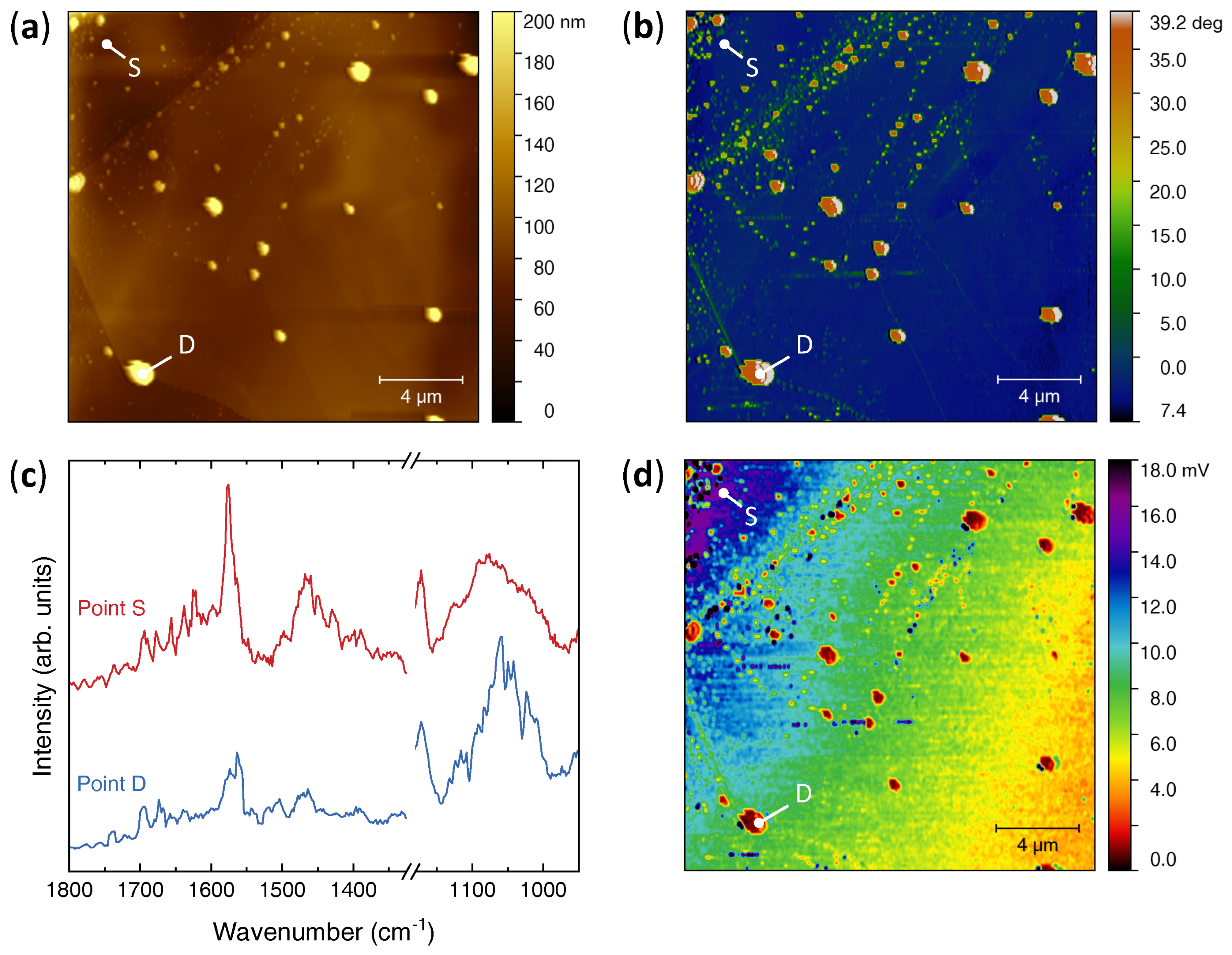
2.3. Nano-Infrared Microscopy
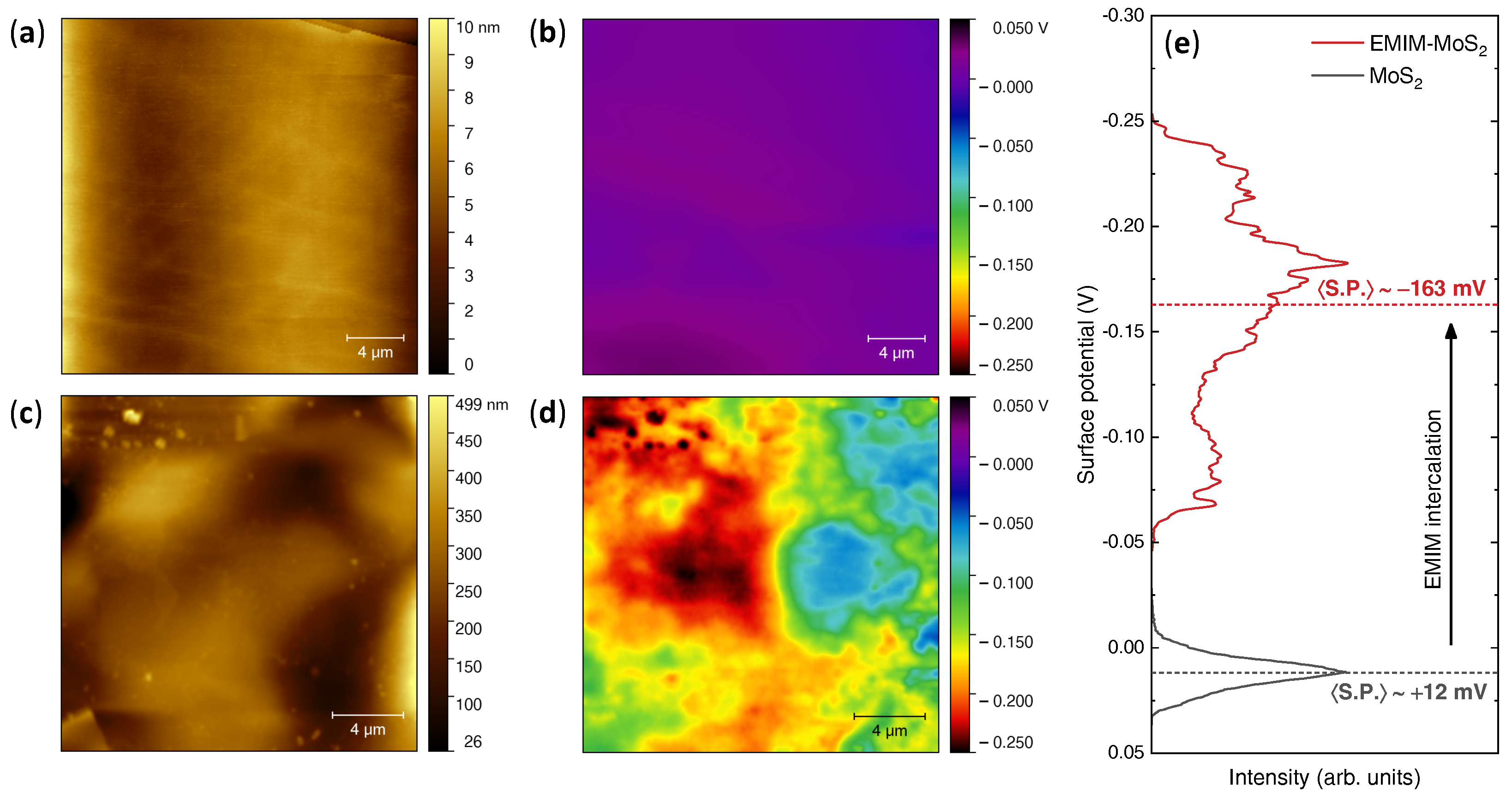
2.4. Kelvin-Probe Force Microscopy
2.5. Resistivity Measurements
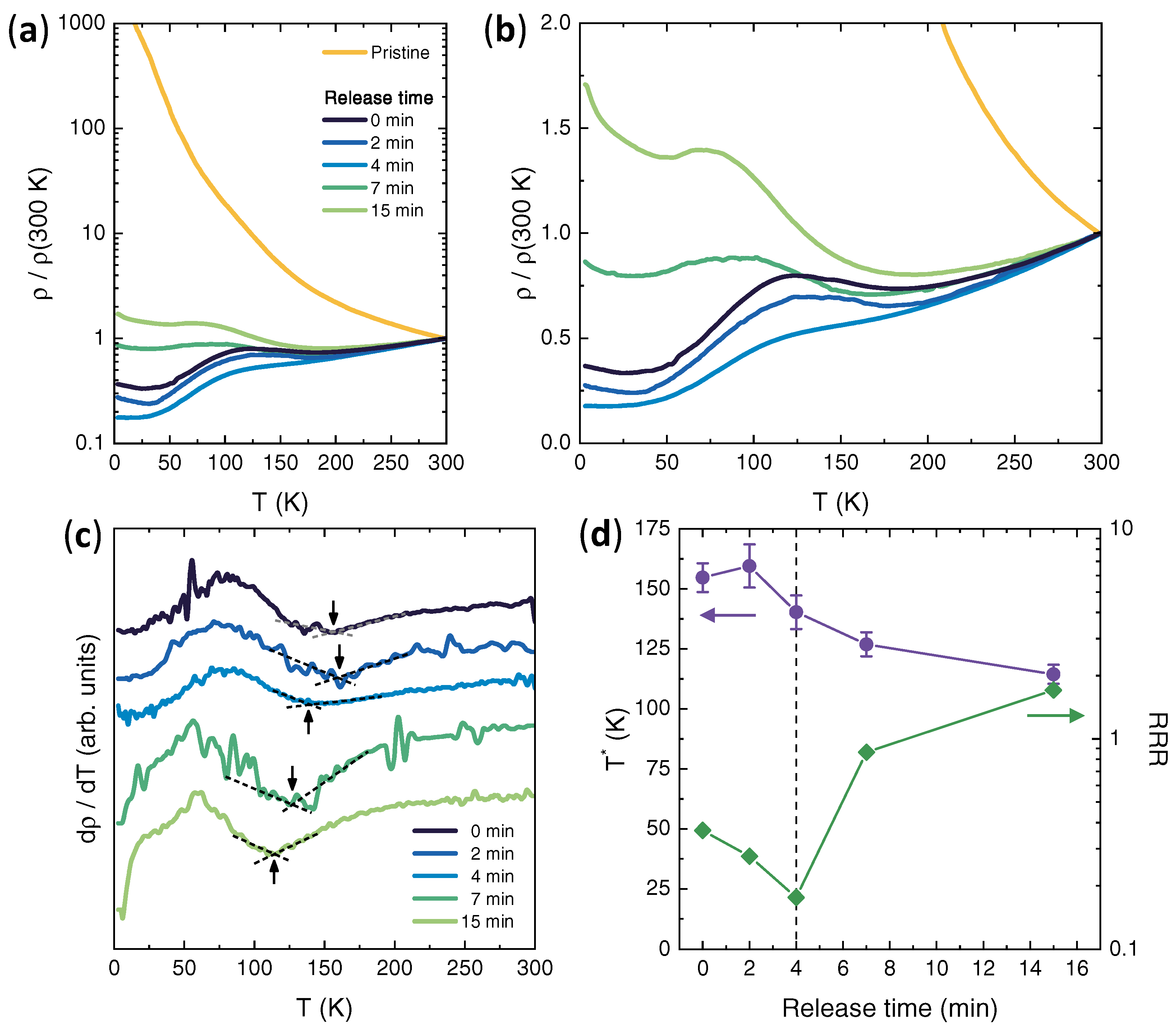
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, R.A. Pristine and intercalated transition metal dichalcogenide superconductors. Physica C 2015, 514, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Schaibley, J.R.; Yu, H.; Clark, G.; Rivera, P.; Ross, J.S.; Seyler, K.L.; Yao, W.; Xu, X. Valleytronics in 2D materials. Nat. Rev. Mater. 2016, 1, 16055. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photon. 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, A.Y. Phonon-mediated superconductivity in electron-doped single-layer MoS2: A first-principles prediction. Phys. Rev. B 2013, 87, 241408(R). [Google Scholar] [CrossRef] [Green Version]
- Piatti, E.; De Fazio, D.; Daghero, D.; Tamalampudi, S.R.; Yoon, D.; Ferrari, A.C.; Gonnelli, R.S. Multi-valley superconductivity in ion-gated MoS2 layers. Nano Lett. 2018, 18, 4821–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piatti, E.; Romanin, D.; Gonnelli, R.S. Mapping multi-valley Lifshitz transitions induced by field-effect doping in strained MoS2 nanolayers. J. Phys. Condens. Matter 2019, 31, 114002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohier, T.; Ponomarev, E.; Gibertini, M.; Berger, H.; Marzari, N.; Ubrig, N.; Morpurgo, A.F. Enhanced electron-phonon interaction in multivalley materials. Phys. Rev. X 2019, 9, 031019. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Goiricelaya, P.; Lafuente-Bartolome, J.; Gurtubay, I.G.; Eiguren, A. Long-living carriers in a strong electron–phonon interacting two-dimensional doped semiconductor. Commun. Phys. 2019, 2, 81. [Google Scholar] [CrossRef] [Green Version]
- Romanin, D.; Brumme, T.; Daghero, D.; Gonnelli, R.S.; Piatti, E. Strong band-filling-dependence of the scattering lifetime in gated MoS2 nanolayers induced by the opening of intervalley scattering channels. J. Appl. Phys. 2021, 128, 063907. [Google Scholar] [CrossRef]
- Garcia-Goiricelaya, P.; Lafuente-Bartolome, J.; Gurtubay, I.G.; Eiguren, A. Emergence of large nonadiabatic effects induced by the electron-phonon interaction on the complex vibrational quasiparticle spectrum of doped monolayer MoS2. Phys. Rev. B 2020, 101, 054304. [Google Scholar] [CrossRef] [Green Version]
- Pimenta Martins, L.G.; Carvalho, B.R.; Occhialini, C.A.; Neme, N.P.; Park, J.H.; Song, Q.; Venezuela, P.; Mazzoni, M.S.C.; Matos, M.J.S.; Kong, J.; et al. Electronic Band Tuning and Multivalley Raman Scattering in Monolayer Transition Metal Dichalcogenides at High Pressures. ACS Nano 2022, 16, 8064–8075. [Google Scholar] [CrossRef]
- Fujimoto, T.; Awaga, K. Electric-double-layer field-effect transistors with ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 8983. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Shimotani, H.; Yuan, H.; Ye, J.; Kawasaki, M.; Iwasa, Y. Field-Induced Superconductivity in Electric Double Layer Transistors. J. Phys. Soc. Jpn. 2014, 83, 032001. [Google Scholar] [CrossRef]
- Liu, P.; Lei, B.; Chen, X.; Wang, L.; Wang, X. Superior carrier tuning in ultrathin superconducting materials by electric-field gating. Nat. Rev. Phys. 2022, 4, 336–352. [Google Scholar] [CrossRef]
- Ye, J.T.; Zhang, Y.J.; Akashi, R.; Bahramy, M.S.; Arita, R.; Iwasa, Y. Superconducting dome in a gate-tuned band insulator. Science 2012, 338, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Piatti, E. Ionic gating in metallic superconductors: A brief review. Nano Ex. 2021, 2, 024003. [Google Scholar] [CrossRef]
- Ye, J.T.; Inoue, S.; Kobayashi, K.; Kasahara, Y.; Yuan, H.T.; Shimotani, H.; Iwasa, Y. Liquid-gated interface superconductivity on an atomically flat film. Nat. Mater. 2010, 9, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Costanzo, D.; Berger, H.; Morpurgo, A.F. Electrostatically induced superconductivity at the surface of WS2. Nano Lett. 2015, 15, 1197–1202. [Google Scholar] [CrossRef] [Green Version]
- Piatti, E.; Daghero, D.; Ummarino, G.A.; Laviano, F.; Nair, J.R.; Cristiano, R.; Casaburi, A.; Portesi, C.; Sola, A.; Gonnelli, R.S. Control of bulk superconductivity in a BCS superconductor by surface charge doping via electrochemical gating. Phys. Rev. B 2017, 95, 140501(R). [Google Scholar] [CrossRef] [Green Version]
- Xi, X.; Berger, H.; Forró, L.; Shan, J.; Mak, K.F. Gate tuning of electronic phase transitions in two-dimensional NbSe2. Phys. Rev. Lett. 2016, 117, 106801. [Google Scholar] [CrossRef] [Green Version]
- Piatti, E.; Romanin, D.; Daghero, D.; Gonnelli, R.S. Two-dimensional hole transport in ion-gated diamond surfaces: A brief review. Low Temp. Phys. 2019, 45, 1143–1155. [Google Scholar] [CrossRef]
- Li, L.J.; O’Farrell, E.C.T.; Loh, K.P.; Eda, G.; Özyilmaz, B.; Castro Neto, A.H. Controlling many-body states by the electric-field effect in a two-dimensional material. Nature 2016, 529, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Cui, J.H.; Xiang, Z.J.; Shang, C.; Wang, N.Z.; Ye, G.J.; Luo, X.G.; Wu, T.; Sun, Z.; Chen, X.H. Evolution of high-temperature superconductivity from a low-T c phase tuned by carrier concentration in FeSe thin flakes. Phys. Rev. Lett. 2016, 116, 077002. [Google Scholar] [CrossRef] [Green Version]
- Piatti, E.; Galanti, F.; Pippione, G.; Pasquarelli, A.; Gonnelli, R.S. Towards the insulator-to-metal transition at the surface of ion-gated nanocrystalline diamond films. Eur. Phys. J. Spec. Top. 2019, 228, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, T.; Ding, M.; Dong, B.; Li, Y.; Chen, M.; Li, X.; Huang, J.; Wang, H.; Zhao, X.; et al. Electric-field control of magnetism in a few-layered van der Waals ferromagnetic semiconductor. Nat. Nanotechnol. 2018, 13, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Piatti, E.; Pasquarelli, A.; Gonnelli, R.S. Orientation-dependent electric transport and band filling in hole co-doped epitaxial diamond films. Appl. Surf. Sci. 2020, 528, 146795. [Google Scholar] [CrossRef]
- Shi, W.; Ye, J.; Zhang, Y.; Suzuki, R.; Yoshida, M.; Miyazaki, J.; Inoue, N.; Saito, Y.; Iwasa, Y. Superconductivity series in transition metal dichalcogenides by ionic gating. Sci. Rep. 2015, 5, 12534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Yang, F.; Lu, X.F.; Yan, Y.J.; Cho, Y.H.; Ma, L.; Niu, X.; Kim, S.; Son, Y.W.; Feng, D.; et al. Gate-tunable phase transitions in thin flakes of 1T-TaS2. Nat. Nanotechnol. 2015, 10, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiogai, J.; Ito, Y.; Mitsuhashi, T.; Nojima, T.; Tsukazaki, A. Electric-field-induced superconductivity in electrochemically etched ultrathin FeSe films on SrTiO3 and MgO. Nat. Phys. 2016, 12, 42–46. [Google Scholar] [CrossRef]
- Piatti, E.; Chen, Q.; Ye, J. Strong dopant dependence of electric transport in ion-gated MoS2. Appl. Phys. Lett. 2017, 111, 013106. [Google Scholar] [CrossRef]
- Piatti, E.; Chen, Q.; Tortello, M.; Ye, J.; Gonnelli, R.S. Possible charge-density-wave signatures in the anomalous resistivity of Li-intercalated multilayer MoS2. Appl. Surf. Sci. 2018, 461, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Ying, T.P.; Wang, M.X.; Wu, X.X.; Zhao, Z.Y.; Zhang, Z.Z.; Song, B.Q.; Li, Y.C.; Lei, B.; Li, Q.; Yu, Y.; et al. Discrete superconducting phases in FeSe-derived superconductors. Phys. Rev. Lett. 2018, 121, 207003. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Aetukuri, N.; Graf, T.; Schladt, T.D.; Samant, M.G.; Parkin, S.S.P. Suppression of metal-insulator transition in VO2 by electric field–induced oxygen vacancy formation. Science 2013, 339, 1402–1405. [Google Scholar] [CrossRef]
- Li, M.; Han, W.; Jiang, X.; Jeong, J.; Samant, M.G.; Parkin, S.S.P. Suppression of ionic liquid gate-induced metallization of SrTiO3 (001) by oxygen. Nano Lett. 2013, 13, 4675–4678. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, S.; Yin, X.; Asmara, T.C.; Yang, P.; Han, K.; Cao, Y.; Zhou, W.; Wan, D.; Tang, C.S.; et al. The Mechanism of Electrolyte Gating on High-Tc Cuprates: The Role of Oxygen Migration and Electrostatics. ACS Nano 2017, 11, 9950–9956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Li, Q.; Gong, B.C.; Wei, X.; Hu, W.; Ni, Z.; He, G.; Qin, M.; Kusmartseva, A.; Kusmartsev, F.V.; et al. Two superconductor-insulator phase transitions in the spinel oxide Li1±xTi2O4−δ induced by ionic liquid gating. Phys. Rev. B 2021, 103, L140501. [Google Scholar] [CrossRef]
- Petach, T.A.; Lee, M.; Davis, R.C.; Mehta, A.; Goldhaber-Gordon, D. Mechanism for the large conductance modulation in electrolyte-gated thin gold films. Phys. Rev. B 2014, 90, 081108. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, S.; Fu, H.; Gao, M.; Ren, Z.; Chen, G. Dominant role of processing temperature in electric field induced superconductivity in layered ZrNBr. New J. Phys. 2019, 21, 023002. [Google Scholar] [CrossRef]
- Zakhidov, D.; Rehn, D.A.; Reed, E.J.; Salleo, A. Reversible electrochemical phase change in monolayer to bulk-like MoTe2 by ionic liquid gating. ACS Nano 2020, 14, 2894–2903. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Kis, A. Mobility engineering and a metal-insulator transition in monolayer MoS2. Nat. Mater. 2013, 12, 815–820. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, S.; Lu, H.; Khamoshi, A.; Liu, G.B.; Han, T.; Wu, Y.; Lin, J.; Long, G.; He, Y.; et al. Even–odd layer-dependent magnetotransport of high-mobility Q-valley electrons in transition metal disulfides. Nat. Commun. 2016, 7, 12955. [Google Scholar] [CrossRef] [Green Version]
- Piatti, E.; Arbab, A.; Galanti, F.; Carey, T.; Anzi, L.; Spurling, D.; Roy, A.; Zhussupbekova, A.; Patel, K.A.; Kim, J.M.; et al. Charge transport mechanisms in inkjet-printed thin-film transistors based on two-dimensional materials. Nat. Electron. 2021, 4, 893–905. [Google Scholar] [CrossRef]
- Biscaras, J.; Chen, Z.; Paradisi, A.; Shukla, A. Onset of two-dimensional superconductivity in space charge doped few-layer molybdenum disulfide. Nat. Commun. 2015, 6, 8826. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, D.; Zhang, H.; Reddy, B.A.; Berger, H.; Morpurgo, A.F. Tunnelling spectroscopy of gate-induced superconductivity in MoS2. Nat. Nanotechnol. 2018, 13, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Tsai, I.L.; Chapman, J.; Khestanova, E.; Waters, J.; Grigorieva, I.V. Superconductivity in potassium-doped metallic polymorphs of MoS2. Nano Lett. 2016, 16, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Subhan, M.K.; Suleman, A.; Moore, G.; Phu, P.; Hoesch, M.; Kurebayashi, H.; Howard, C.A.; Schofield, S.R. Charge density waves in electron-doped molybdenum disulfide. Nano Lett. 2021, 21, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Seifert, G. Density-functional study of LixMoS2 intercalates (0 ≤ x ≤ 1). Comput. Theor. Chem. 2012, 999, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.L.; Johannes, M.D.; Singh, A.K.; Hennig, R.G. Doping-controlled phase transitions in single-layer MoS2. Phys. Rev. B 2017, 96, 165305. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, Z.; Li, J. Critical electronic structures controlling phase transitions induced by lithium ion intercalation in molybdenum disulphide. Chin. Sci. Bull. 2013, 58, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Rösner, M.; Haas, S.; Wehling, T.O. Phase diagram of electron-doped dichalcogenides. Phys. Rev. B 2014, 90, 245105. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Sun, D.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the dynamics of the metallic-to-semiconducting structural phase transformation in MoS2 crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase restructuring in transition metal dichalcogenides for highly stable energy storage. ACS Nano 2016, 10, 9208–9215. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wang, J.; Chao, D.; Chen, Z.; Liu, Z.; Kuo, J.L.; Yan, J.; Shen, Z.X. Phase evolution of lithium intercalation dynamics in 2H-MoS2. Nanoscale 2017, 9, 7533–7540. [Google Scholar] [CrossRef]
- Boeri, L.; Hennig, R.G.; Hirschfeld, P.J.; Profeta, G.; Sanna, A.; Zurek, E.; Pickett, W.E.; Amsler, M.; Dias, R.; Eremets, M.; et al. The 2021 room-temperature superconductivity roadmap. J. Phys. Condens. Matter 2021, 34, 183002. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, Z.; Zhang, J.S.; Ma, W.L.; Ma, M.W.; Ma, Z.; Wang, C.; Yan, J.Q.; Sun, J.P.; Cheng, J.G.; et al. Ionic-Liquid-Gating Induced Protonation and Superconductivity in FeSe, FeSe0.93S0.07, ZrNCl, 1T-TaS2 and Bi2Se3. Chin. Phys. Lett. 2019, 36, 077401. [Google Scholar] [CrossRef] [Green Version]
- Rafique, M.; Feng, Z.; Lin, Z.; Wei, X.; Liao, M.; Zhang, D.; Jin, K.; Xue, Q.K. Ionic liquid gating induced protonation of electron-doped cuprate superconductors. Nano Lett. 2019, 19, 7775–7780. [Google Scholar] [CrossRef]
- Meng, Y.; Xing, X.; Yi, X.; Li, B.; Zhou, N.; Li, M.; Zhang, Y.; Wei, W.; Feng, J.; Terashima, K.; et al. Protonation-induced discrete superconducting phases in bulk FeSe single crystals. Phys. Rev. B 2022, 105, 134506. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Xie, W.; Chen, G.; Zhu, X.; Wen, H.H. Superconductivity at 44.4 K achieved by intercalating EMIM+ into FeSe. Chin. Phys. B 2021, 30, 107402. [Google Scholar] [CrossRef]
- Shi, M.; Wang, N.; Lei, B.; Ying, J.; Zhu, C.; Sun, Z.; Cui, J.; Meng, F.; Shang, C.; Ma, L.; et al. FeSe-based superconductors with a superconducting transition temperature of 50 K. New J. Phys. 2018, 20, 123007. [Google Scholar] [CrossRef]
- Xu, H.S.; Wu, S.; Zheng, H.; Yin, R.; Li, Y.; Wang, X.; Tang, K. Research Progress of FeSe-based Superconductors Containing Ammonia/Organic Molecules Intercalation. Top. Curr. Chem. 2022, 380, 11. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, T.; Wang, Z.; Ren, W.; Nan, H.; Ni, Z.; Chen, Q.; Yuan, S.; Miao, F.; Song, F.; et al. Hopping transport through defect-induced localized states in molybdenum disulphide. Nat. Commun. 2013, 4, 2642. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single-and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, B.; Bera, A.; Muthu, D.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 2012, 85, 161403. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Utama, M.I.B.; Wang, X.; Xu, W.; Zhao, W.; Owen, M.H.S.; Xiong, Q. Gate-Tunable resonant Raman spectroscopy of bilayer MoS2. Small 2017, 13, 1701039. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Berner, N.C.; Backes, C.; Duesberg, G.S.; McDonald, A.R. Functionalization of Two-Dimensional MoS2: On the Reaction Between MoS2 and Organic Thiols. Angew. Chem. 2016, 128, 5897–5902. [Google Scholar] [CrossRef]
- Höfft, O.; Bahr, S.; Kempter, V. Investigations with infrared spectroscopy on films of the ionic liquid [EMIM]Tf2N. Langmuir 2008, 24, 11562–11566. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, M.; Haddad, B.; Paolone, A.; Drai, M.; Villemin, D.; Rahmouni, M.; Bresson, S.; Abbas, O. Synthesis, thermal stability, vibrational spectra and conformational studies of novel dicationic meta-xylyl linked bis-1-methylimidazolium ionic liquids. J. Mol. Struct. 2019, 1186, 68–79. [Google Scholar] [CrossRef]
- Heimer, N.E.; Del Sesto, R.E.; Meng, Z.; Wilkes, J.S.; Carper, W.R. Vibrational spectra of imidazolium tetrafluoroborate ionic liquids. J. Mol. Liq. 2006, 124, 84–95. [Google Scholar] [CrossRef]
- Prater, C.; Kjoller, K.; Shetty, R. Nanoscale infrared spectroscopy. Mater. Today 2010, 13, 56–60. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Almadori, Y.; Bendiab, N.; Grevin, B. Multimodal Kelvin probe force microscopy investigations of a photovoltaic WSe2/MoS2 type-II interface. ACS Appl. Mater. Interfaces 2018, 10, 1363–1373. [Google Scholar] [CrossRef]
- Ding, S.; Xiao, X.; Liu, S.; Wu, J.; Huang, Z.; Qi, X.; Li, J. Detection of interfacial charge transfer in MoS2/PbI2 heterostructures via Kelvin probe force microscope. Appl. Phys. A 2019, 125, 287. [Google Scholar] [CrossRef]
- Chen, Q.H.; Lu, J.M.; Liang, L.; Zheliuk, O.; Ali, A.; Sheng, P.; Ye, J.T. Inducing and Manipulating Heteroelectronic States in a Single MoS2 Thin Flake. Phys. Rev. Lett. 2017, 119, 147002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, P.; Lee, M.; Petach, T.A.; Stanwyck, S.W.; Williams, J.R.; Watanabe, K.; Taniguchi, T.; Goldhaber-Gordon, D. A high-mobility electronic system at an electrolyte-gated oxide surface. Nat. Commun. 2015, 6, 6437. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Iwasa, Y. Ambipolar insulator-to-metal transition in black phosphorus by ionic-liquid gating. ACS Nano 2015, 9, 3192–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piatti, E.; Galasso, S.; Tortello, M.; Nair, J.; Gerbaldi, C.; Bruna, M.; Borini, S.; Daghero, D.; Gonnelli, R.S. Carrier mobility and scattering lifetime in electric double-layer gated few-layer graphene. Appl. Surf. Sci. 2017, 395, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Gonnelli, R.S.; Piatti, E.; Sola, A.; Tortello, M.; Dolcini, F.; Galasso, S.; Nair, J.R.; Gerbaldi, C.; Cappelluti, E.; Bruna, M.; et al. Weak localization in electric-double-layer gated few-layer graphene. 2D Mater. 2017, 4, 035006. [Google Scholar] [CrossRef]
- Piatti, E.; Hatano, T.; Daghero, D.; Galanti, F.; Gerbaldi, C.; Guastella, S.; Portesi, C.; Nakamura, I.; Fujimoto, R.; Iida, K.; et al. Ambipolar suppression of superconductivity by ionic gating in optimally doped BaFe2(As,P)2 ultrathin films. Phys. Rev. Mater. 2019, 3, 044801. [Google Scholar] [CrossRef] [Green Version]
- Ovchinnikov, D.; Gargiulo, F.; Allain, A.; Pasquier, D.J.; Dumcenco, D.; Ho, C.H.; Yazyev, O.V.; Kis, A. Disorder engineering and conductivity dome in ReS2 with electrolyte gating. Nat. Commun. 2016, 7, 12391. [Google Scholar] [CrossRef]
- Lu, J.; Zheliuk, O.; Chen, Q.; Leermakers, I.; Hussey, N.E.; Zeitler, U.; Ye, J. Full superconducting dome of strong Ising protection in gated monolayer WS2. Proc. Natl. Acad. Sci. USA 2018, 115, 3551–3556. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Ding, D.; Li, S.; Han, X.; Niu, R.; Yin, D.; Qu, Z.; Watanabe, K.; Taniguchi, T.; Liao, Z.M.; et al. Probing the nature of an emergent insulator in ionic gated monolayer transition metal dichalcogenides. Res. Sq. 2020; Preprint. [Google Scholar] [CrossRef]
- Gor’kov, L.P.; Grüner, G. Charge Density Waves in Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Rossnagel, K. On the origin of charge-density waves in select layered transition-metal dichalcogenides. J. Phys. Condens. Matter 2011, 23, 213001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Y.; Zhang, J.; Plummer, E.; Guo, J. Classification of charge density waves based on their nature. Proc. Natl. Acad. Sci. USA 2015, 112, 2367–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morosan, E.; Zandbergen, H.W.; Dennis, B.; Bos, J.; Onose, Y.; Klimczuk, T.; Ramirez, A.; Ong, N.; Cava, R.J. Superconductivity in CuxTiSe2. Nat. Phys. 2006, 2, 544–550. [Google Scholar] [CrossRef]
- Bhoi, D.; Khim, S.; Nam, W.; Lee, B.; Kim, C.; Jeon, B.G.; Min, B.; Park, S.; Kim, K.H. Interplay of charge density wave and multiband superconductivity in 2H-PdxTaSe2. Sci. Rep. 2016, 6, 24068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Wang, Y.; Zou, P.Y.; Tang, L.; Xu, Z.; Chen, H.; Dong, C.; Shan, L.; Wen, H.H. Fabrication and superconductivity of NaxTaS2 crystals. Phys. Rev. B 2005, 72, 014534. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.Y.; Hu, J.W.; Goncharov, A.F.; Chen, X.J. Nontrivial metallic state of MoS2. Phys. Rev. B 2018, 97, 214519. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, D.; Jo, S.; Berger, H.; Morpurgo, A.F. Gate-induced superconductivity in atomically thin MoS2 crystals. Nat. Nanotechnol. 2016, 11, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liu, E.; Yuan, H.; Tang, P.; Lian, B.; Xu, G.; Zeng, J.; Chen, Z.; Wang, Y.; Zhou, W.; et al. Gated tuned superconductivity and phonon softening in monolayer and bilayer MoS2. NPJ Quant. Mater. 2017, 2, 52. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Li, S.; Susner, M.; Kwon, S.; Kim, M.; Haugan, T.; Lv, B. Spacing dependent and cation doping independent superconductivity in intercalated 1T 2D SnSe2. 2D Mater. 2019, 6, 045048. [Google Scholar] [CrossRef]
- Ma, L.; Shi, M.; Kang, B.; Peng, K.; Meng, F.; Zhu, C.; Cui, J.; Sun, Z.; Ma, D.; Wang, H.; et al. Quasi-two-dimensional superconductivity in SnSe2 via organic ion intercalation. Phys. Rev. Mater. 2020, 4, 124803. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatti, E.; Montagna Bozzone, J.; Daghero, D. Anomalous Metallic Phase in Molybdenum Disulphide Induced via Gate-Driven Organic Ion Intercalation. Nanomaterials 2022, 12, 1842. https://doi.org/10.3390/nano12111842
Piatti E, Montagna Bozzone J, Daghero D. Anomalous Metallic Phase in Molybdenum Disulphide Induced via Gate-Driven Organic Ion Intercalation. Nanomaterials. 2022; 12(11):1842. https://doi.org/10.3390/nano12111842
Chicago/Turabian StylePiatti, Erik, Jessica Montagna Bozzone, and Dario Daghero. 2022. "Anomalous Metallic Phase in Molybdenum Disulphide Induced via Gate-Driven Organic Ion Intercalation" Nanomaterials 12, no. 11: 1842. https://doi.org/10.3390/nano12111842
APA StylePiatti, E., Montagna Bozzone, J., & Daghero, D. (2022). Anomalous Metallic Phase in Molybdenum Disulphide Induced via Gate-Driven Organic Ion Intercalation. Nanomaterials, 12(11), 1842. https://doi.org/10.3390/nano12111842






