Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies
Abstract
:1. Introduction and Scope
1.1. Types and Forms of Cellulose
1.1.1. Cellulose Fibers and Fibrils (CF)
1.1.2. Regenerated Cellulose (RC)
1.1.3. Microcrystalline Cellulose (MCC)
1.1.4. Microfibrillated Cellulose (MFC)
1.1.5. Bacterial Nanocellulose (BNC)
1.1.6. Cellulose Nanocrystals (CNCs)
1.2. Cellulose Gels as Supports or Templates for Nanostructured Materials
1.2.1. Cellulose Hydrogels
1.2.2. Cellulose Aerogels
2. Cellulose Supported Metallic Nanostructures
2.1. Cellulose Supported Noble Metallic Nanostructures
2.2. Cellulose Supported Non-Precious Metallic Nanostructures
3. Cellulose Supported Metal Oxide Nanostructures
3.1. Cellulose Supported TiO2
3.2. Cellulose Supported Fe3O4 and Other Iron Oxides
3.3. Cellulose Supported ZnO
3.4. Cellulose Supported Miscellaneous Inorganic Oxides
4. Cellulose Templated Pure Metal Oxide Nanostructures
4.1. Cellulose Templated Porous TiO2
4.2. Cellulose Templated Porous Iron Oxides
4.3. Miscellaneous Cellulose Templated Porous Metallic Oxides
4.4. Inorganic Nanostructures Supported by Carbon Fibers Formed from Cellulose
5. Cellulose Supported/Templated Nanostructures of Metal Sulfides, Hydroxyapatites, and Other Inorganic Compounds
5.1. Cellulose Supported/Templated Metal Sulfides
5.2. Cellulose Supported/Templated Hydroxyapatites
5.3. Miscellaneous Cellulose Supported/Templated Inorganic Compounds
6. Cellulose Supported or Templated Hybrid Inorganic Nanostructures
6.1. Cellulose Supported Metallic Hybrid Nanostructures
6.2. Cellulose Supported Metal Oxide Hybrid Nanostructures
7. Discussion, Conclusions, and Perspectives
7.1. Discussion
7.2. Conclusions
7.3. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Madrigal, M.M.; Edo, M.G.; Alemán, C. Powering the future: Application of cellulose-based materials for supercapacitors. Green Chem. 2016, 18, 5930–5956. [Google Scholar] [CrossRef]
- Wu, Y.D.; He, J.M.; Huang, J.D.; Wang, F.W.; Tang, F. Oxidation of Regenerated Cellulose with Nitrogen Dioxide/Carbon Tetrachloride. Fibers Polym. 2012, 13, 576–581. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, S.; Huang, J. Natural Cellulose Substance Based Energy Materials. Chem. Asian J. 2021, 16, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Mendonça Cidreira, A.C.; Cappuccio de Castro, K.; Hatami, T.; Linan, L.Z.; Innocentini Mei, L.H. Cellulose nanocrystals-based materials as hemostatic agents for wound dressings: A review. Biomed. Microdevices 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Kalita, E.; Nath, B.K.; Deb, P.; Agan, F.; Islam, M.R.; Saikia, K. High quality fluorescent cellulose nanofibers from endemic rice husk: Isolation and characterization. Carbohydr. Polym. 2015, 122, 308–313. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, A.; Ibrahim, S.A.; Yang, H.; Huang, W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, X.; Gao, X.; Chen, K. Fabrication and Comparative Evaluation of Regenerated Cellulose Films using Pulp fines and Pith from Corn Stalk in DMAc/LiCl Solvent System. BioResources 2019, 14, 6421–6432. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, L.; Hao, A.; Chen, J.Y. Regenerated cellulose fibers from waste bagasse using ionic liquid. Text. Res. 2011, 81, 1949–1958. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 45, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. Cellulose: Structure and Properties. Adv. Polym. Sci. 2016, 271, 1. [Google Scholar]
- Tingaut, P.; Zimmermann, T.; Sebe, G. Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J. Mater. Chem. 2012, 22, 20105–20111. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Vo, D.V.N.; Nabgan, W. An overview on the efficiency of biohydrogen production from cellulose. Biomass Convers. Biorefin. 2020, 1–23. [Google Scholar] [CrossRef]
- Ivanova, A.; Fattakhova-Rohlfing, D.; Kayaalp, B.E.; Rathouský, J.; Bein, T. Tailoring the Morphology of Mesoporous Titania Thin Films through Biotemplating with Nanocrystalline Cellulose. J. Am. Chem. Soc. 2014, 136, 5930–5937. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, S.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Won, Y.H.; Stanciu, L.A.; Moon, R.J. Natural Biopolymers: Novel Templates for the Synthesis of Nanostructures. Langmuir 2010, 26, 8497–8502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-based Supercapacitors: Material and Performance Considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- French, A.D. Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 2017, 24, 4605–4609. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kunaver, M.; Krajnc, A.; Žagar, E. Nanocomposites of LLDPE and Surface-Modified Cellulose Nanocrystals Prepared by Melt Processing. Molecules 2018, 23, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anžlovar, A.; Krajnc, A.; Žagar, E. Silane modified cellulose nanocrystals and nanocomposites with LLDPE prepared by melt processing. Cellulose 2020, 27, 5785–5800. [Google Scholar] [CrossRef]
- Anžlovar, A.; Švab, I.; Krajnc, A.; Žagar, E. Composites of polystyrene and surface modified cellulose nanocrystals prepared by melt processing. Cellulose 2021, 28, 7813–7827. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Cellulose nanofibres for photonics and plasmonics. Curr. Opin. Green Sustain. Chem. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, X.; Gnanasekar, P.; Yan, N.; Shi, J. Review: Cascade reactions for conversion of carbohydrates using heteropolyacids as the solid catalysts. Biomass Convers. Biorefin. 2022, 12, 2313–2331. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Kalita, R.D.; Nath, Y.; Ochubiojo, M.E.; Buragohain, A.K. Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L.) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids Surf. B 2013, 108, 85–89. [Google Scholar] [CrossRef]
- Chakraborty, I.; Rongpipi, S.; Govindaraju, I.; Rakesh, B.; Mal, S.S.; Gomez, E.W.; Gomez, E.D.; Kalita, R.D.; Nath, Y.; Mazumder, N. An insight into microscopy and analytical techniques for morphological, structural, chemical, and thermal characterization of cellulose. Microsc. Res. Tech. 2022, 85, 1990–2015. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Kommul, V.P.; Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and characterization of cellulose single fibers from native African napier grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, W.; Wang, X.; Yu, J. Changes of structure and property of alkali soluble hydroxyethyl celluloses (HECs) and their regenerated films with the molar substitution. Carbohydr. Polym. 2014, 114, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.D.; Bansal, P.S.; Gorade, V.G. Extraction of Microcrystalline Cellulose from Cotton Sliver and Its Comparison with Commercial Microcrystalline Cellulose. J. Polym. Environ. 2018, 26, 355–364. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef]
- Chandra, C.S.J.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar]
- Chaker, A.; Mutje, P.; Rei Vilar, M.; Boufi, S. Agriculture crop residues as a source for the production of nanofibrillated cellulose with low energy demand. Cellulose 2014, 21, 4247–4259. [Google Scholar] [CrossRef]
- Nasir, M.; Aziz, M.A.; Zubair, M.; Manzar, M.S.; Ashraf, N.; Mu’azu, N.D.; Al-Harthi, M.A. Recent review on synthesis, evaluation, and SWOT analysis of nanostructured cellulose in construction applications. J. Build. Engin. 2022, 46, 103747. [Google Scholar] [CrossRef]
- Tajima, K.; Imai, T.; Yui, T.; Yao, M. Cellulose-synthesizing machinery in bacteria. Cellulose 2022, 29, 2755–2777. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstroem, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Kos, T.; Anžlovar, A.; Kunaver, M.; Huskić, M.; Žagar, E. Fast preparation of Nanocrystalline cellulose by microwave-assisted hydrolysis. Cellulose 2014, 21, 2579–2585. [Google Scholar] [CrossRef]
- Kunaver, M.; Anžlovar, A.; Žagar, E. The fast and effective isolation of nanocellulose from selected cellulosic feedstocks. Carbohydr. Polym. 2016, 148, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Tailoring Chemical, Physical, and Morphological Properties of Sugarcane Bagasse Cellulose Nanocrystals via Phosphorylation Method. J. Nat. Fibers 2021, 18, 1448–1460. [Google Scholar] [CrossRef]
- Su, H.; Wang, B.; Sun, Z.; Wang, S.; Feng, X.; Mao, Z.; Sui, X. High-tensile regenerated cellulose films enabled by unexpected enhancement of cellulose dissolution in cryogenic aqueous phosphoric acid. Carbohydr. Polym. 2022, 277, 118878. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose hydrolysis using ionic liquids and inorganic acids under dilute conditions: Morphological comparison of nanocellulose. RSC Adv. 2020, 10, 39413. [Google Scholar] [CrossRef]
- Huang, J.; Hou, S.; Chen, R. Ionic Liquid-assisted Fabrication of Nanocellulose from Cotton Linter by High Pressure Homogenization. BioResources 2019, 14, 7805–7820. [Google Scholar]
- Babicka, M.; Wozniak, M.; Szentner, K.; Bartkowiak, M.; Peplinska, B.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Nanocellulose Production Using Ionic Liquids with Enzymatic Pretreatment. Materials 2021, 14, 3264. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurauc, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.F.; Jong, S.J.; Yeo, Y.J. Optimization of Cellulose-Based Hydrogel Synthesis Using Response Surface Methodology. Biointerface Res. Appl. Chem. 2022, 12, 7136–7146. [Google Scholar]
- Xu, K.; Wang, Y.; Zhang, B.; Zhang, C.; Liu, T. Stretchable and self-healing polyvinyl alcohol/cellulose nanofiber nanocomposite hydrogels for strain sensors with high sensitivity and linearity. Compos. Commun. 2021, 24, 100677. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Xia Sun, X.; Xu, Z.; Yang, Y.; Song, Y.; Jiang, F. An environmentally tolerant, highly stable, cellulose nanofiber-reinforced, conductive hydrogel multifunctional sensor. Carbohydr. Polym. 2022, 284, 119199. [Google Scholar] [CrossRef]
- Cheng, Y.; Zang, J.; Zhao, X.; Wang, H.; Hu, Y. Nanocellulose-enhanced organohydrogel with high-strength, conductivity, and anti-freezing properties for wearable strain sensors. Carbohydr. Polym. 2022, 277, 118872. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Li, X.; Lou, J.; Ding, Q.; Liu, Z.; Jiang, Y.; Han, W. Bacterial cellulose-based hydrogel thermocells for low-grade heat harvesting. Chem. Engin. J. 2022, 433, 134550. [Google Scholar] [CrossRef]
- Nabipour, H.; Shi, H.; Wang, X.; Hu, X.; Song, L.; Hu, Y. Flame Retardant Cellulose-Based Hybrid Hydrogels for Firefighting and Fire Prevention. Fire Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, F.; Yang, X.; Jian, C.; Zhang, L.; Yu, A.; Lu, A. Injectable self-healing cellulose hydrogel based on host-guest interactions and acylhydrazone bonds for sustained cancer therapy. Acta Biomater. 2022, 141, 102–113. [Google Scholar] [CrossRef]
- Piloto, A.M.L.; Ribeiro, D.S.M.; Rodrigues, S.S.M.; Santos, J.M.L.; Sampaio, P.; Ferreira Sales, M.G. Cellulose-based hydrogel on quantum dots with molecularly imprinted polymers for the detection of CA19-9 protein cancer biomarker. Microchim. Acta 2022, 189, 134. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper–poly(amidoxime) composite hydrogel for highly efficient uranium(VI) capture from seawater. Chem. Commun. 2020, 56, 3935–3938. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Vu, P.V.; Doan, T.D.; Tu, G.C.; Do, N.H.N.; Le, K.A.; Le, P.K. A novel application of cellulose aerogel composites from pineapple leaf fibers and cotton waste: Removal of dyes and oil in wastewater. J. Porous Mater. 2022, 1–11. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Chia, C.H.; Chen, R.S.; Ellis, A.V.; Kaco, H. Highly porous regenerated cellulose hydrogel and aerogel prepared from hydrothermal synthesized cellulose carbamate. PLoS ONE 2017, 12, e0173743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Li, X.; Mei, C.; Zheng, J.; Shiju, E.; Duan, G.; Liu, K.; Jiang, S. Liquid Transport and Real-Time Dye Purification via Lotus Petiole-Inspired Long-Range-Ordered Anisotropic Cellulose Nanofibril Aerogels. ACS Nano 2021, 15, 20666–20677. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Talari, M.; Salman Tabrizi, N.; Babaeipour, V.; Halek, F. Adsorptive removal of organic pollutants from water by carbon fiber aerogel derived from bacterial cellulose. J. Sol-Gel Sci. Technol. 2022, 101, 345–355. [Google Scholar] [CrossRef]
- Mo, L.; Tan, Y.; Shen, Y.; Zhang, S. Highly compressible nanocellulose aerogels with a cellular structure for high-performance adsorption of Cu(II). Chemosphere 2022, 291, 132887. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Cao, H.; Jia, L.; Liu, P. Cellulose nanofibers aerogels functionalized with AgO: Preparation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2022, 194, 58–65. [Google Scholar] [CrossRef]
- Wan, C.; Li, J. Cellulose aerogels functionalized with polypyrrole and silver nanoparticles: In-situ synthesis, characterization and antibacterial activity. Carbohydr. Polym. 2016, 146, 362–367. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar- Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Mei, C.; Li, Y.; Gaigai Duan, G.; Agarwal, S.; Greiner, G.; Ma, C.; Jiang, S. Wood-Inspired Anisotropic Cellulose Nanofibril Composite Sponges for Multifunctional Applications. ACS Appl. Mater. Interfaces 2020, 12, 35513–35522. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Huang, X.; Jiang, S.; Wang, G. Anisotropy-functionalized cellulose-based phase change materials with reinforced solar-thermal energy conversion and storage capacity. Chem. Engin. J. 2021, 415, 129086. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Van Rie, J.; Thielemans, W. Cellulose–gold nanoparticle hybrid materials. Nanoscale 2017, 9, 8525–8554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber Mater. 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Ashraf, S.; ur-Rehman, S.; Sher, F.; Khalid, Z.M.; Mehmood, M.; Hussain, I. Synthesis of cellulose–metal nanoparticle composites: Development and comparison of different protocols. Cellulose 2014, 21, 395–405. [Google Scholar] [CrossRef]
- Hamoud, T.; Ibrahim, H.M.; Kafafy, H.H.; Mashaly, H.M.; Mohamed, N.H.; Aly, N.M. Preparation of cellulose-based wipes treated with antimicrobial and antiviral silver nanoparticles as novel effective high-performance coronavirus fighter. Int. J. Biol. Macromol. 2021, 181, 990–1102. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B.; Sui, X. Cellulose Sponge Supported Palladium Nanoparticles as Recyclable Cross-Coupling Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef]
- Li, S.M.; Jia, N.; Zhub, J.F.; Ma, M.G.; Xua, F.; Wanga, B.; Sun, R.C. Rapid microwave-assisted preparation and characterization of cellulose–silver nanocomposites. Carbohydr. Polym. 2011, 83, 422–429. [Google Scholar] [CrossRef]
- Farag, S.; Ibrahim, H.M.; Asker, M.S.; Amr, A.; El-Shafaee, A. Impregnation of silver nanoparticles into bacterial cellulose: Green synthesis and cytotoxicity. Int. J. ChemTech Res. 2015, 8, 651–661. [Google Scholar]
- Lu, Z.; Jasinski, J.B.; Handa, S.; Hammond, G.B. Recyclable cellulose-palladium nanoparticles for clean cross-coupling chemistry. Org. Biomol. Chem. 2018, 16, 2748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated Synthesis of Gold Nanoparticle–Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Yan, W.; Chen, C.; Wang, L.; Zhang, D.; Li, A.J.; Yao, Z.; Shi, L.Y. Facile and green synthesis of cellulose nanocrystal-supported gold nanoparticles with superior catalytic activity. Carbohydr. Polym. 2016, 140, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, N.S.; Betts, J.W.; Cheng, F.; Francesconi, M.G.; Kelly, S.M.; Kornherr, A.; Priora, T.J.; Wadhawan, J.D. Synthesis and antibacterial effects of cobalt–cellulose magnetic nanocomposites. RSC Adv. 2017, 7, 20020. [Google Scholar] [CrossRef] [Green Version]
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Preparation, characterization and catalytic activity of biomaterial-supported copper nanoparticles. Res. Chem. Intermed. 2017, 43, 801–815. [Google Scholar] [CrossRef]
- Dhar, P.; Kumar, A.; Katiyar, V. Fabrication of cellulose nanocrystal supported stable Fe(0) nanoparticles: A sustainable catalyst for dye reduction, organic conversion and chemo-magnetic propulsion. Cellulose 2015, 22, 3755–3771. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, H.; Bebeko, T.; Akita, T.; Haruta, M. Aerobic oxidation of glucose over gold nanoparticles deposited on cellulose. Appl. Catal. A Gen. 2010, 377, 42–46. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, N.; Zhang, X.; Xu, J. Cellulose/silver nanoparticles composite microspheres: Eco-friendly synthesis and catalytic application. Cellulose 2012, 19, 1239–1249. [Google Scholar] [CrossRef]
- Koga, H.; Tokunaga, E.; Hidaka, M.; Umemura, Y.; Saito, T.; Isogai, A.; Kitaoka, T. Topochemical synthesis and catalysis of metal nanoparticles exposed on crystalline cellulose nanofibers. Chem. Commun. 2010, 46, 8567–8569. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Kim, C.; Nogi, M.; Suganuma, K. Electrically conductive lines on cellulose nanopaper for flexible electrical devices. Nanoscale 2013, 5, 9289–9295. [Google Scholar] [CrossRef] [PubMed]
- Azetsu, A.; Koga, H.; Isogai, A.; Kitaoka, T. Synthesis and Catalytic Features of Hybrid Metal Nanoparticles Supported on Cellulose Nanofibers. Catalysts 2011, 1, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Oracion, J.P.L.; De La Rosa, L.B.; Budlayan, M.L.M.; Rodriguez, M.J.D.; Manigo, J.P.; Patricio, J.N.; Arco, S.D.; Austria, E.S.; Alguno, A.C.; Deocaris, C.C.; et al. Simple one-pot in situ synthesis of gold and silver nanoparticles on bacterial cellulose membrane using polyethyleneimine. J. Appl. Sci. Engin. 2021, 24, 351–357. [Google Scholar]
- Johnson, L.; Thielemans, W.; Walsh, D.A. Synthesis of carbon-supported Pt nanoparticle electrocatalysts using nanocrystalline cellulose as reducing agent. Green Chem. 2011, 13, 1686–1693. [Google Scholar] [CrossRef]
- Faria-Tischer, P.C.S.; Costa, C.A.R.; Tozetti, I.; Dall’Antonia, L.H.; Vidotti, M. Structure and effects of gold nanoparticles in bacterial cellulose-polyaniline conductive membranes. RSC Adv. 2016, 6, 9571–9580. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Y.; Zhang, L.; Wu, W. Nanocellulose-based Surface-enhanced Raman spectroscopy sensor for highly sensitive detection of TNT. Carbohydr. Polym. 2020, 248, 116766. [Google Scholar] [CrossRef]
- Majoinen, J.; Hassinen, J.; Haataja, J.S.; Rekola, H.T.; Kontturi, E.; Kostiainen, M.A.; Ras, R.H.A.; Törmä, P.; Ikkala, O. Chiral Plasmonics Using Twisting along Cellulose Nanocrystals as a Template for Gold Nanoparticles. Adv. Mater. 2016, 28, 5262–5267. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Meng, Q.; Liu, R.; Fu, S.; Lucia, L.A. Physical Study of the Primary and Secondary Photothermal Events in Gold/Cellulose Nanocrystals (AuNP/CNC) Nanocomposites Embedded in PVA Matrices. ACS Sustain. Chem. Eng. 2017, 5, 1601–1609. [Google Scholar] [CrossRef]
- Wu, X.D.; Lu, C.H.; Zhou, Z.H.; Yuan, G.P.; Xiong, R.; Zhang, X.X. Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance. Environ. Sci. Nano 2014, 1, 71–79. [Google Scholar] [CrossRef]
- Schlesinger, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic cellulose-gold nanoparticle composites from mesoporous photonic cellulose. Chem. Commun. 2015, 51, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.L.; Gray, D.G.; Li, C.J. A(3)-Coupling catalyzed by robust Au nanoparticles covalently bonded to HS-functionalized cellulose nanocrystalline films. Beilstein J. Org. Chem. 2013, 9, 1388–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Cao, W.; Quinlan, P.J.; Berry, R.M.; Tam, K.C. Sustainable Catalysts from Gold-Loaded Polyamidoamine Dendrimer-Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Lam, E.; Hrapovic, S.; Majid, E.; Chong, J.H.; Luong, J.H.T. Catalysis using gold nanoparticles decorated on nanocrystalline cellulose. Nanoscale 2012, 4, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Eisa, W.H.; Abdelgawad, A.M.; Rojas, O.J. Solid-State Synthesis of Metal Nanoparticles Supported on Cellulose Nanocrystals and Their Catalytic Activity. ACS Sustain. Chem. Engin. 2018, 6, 3974–3983. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, M.; Lin, H.; Huang, M. Facile synthesis of cellulose nanofiber nanocomposite as a SERS substrate for detection of thiram in juice. Carbohydr. Polym. 2018, 189, 79–86. [Google Scholar] [CrossRef]
- Semenikhin, N.S.; Kadasala, N.R.; Moon, R.J.; Perry, J.W.; Sandhage, K.H. Individually Dispersed Gold Nanoshell-Bearing Cellulose Nanocrystals with Tailorable Plasmon Resonance. Langmuir 2018, 34, 4427–4436. [Google Scholar] [CrossRef]
- Dueñas-Mas, M.J.; Soriano, M.L.; Ruiz-Palomero, C.; Valcárcel, M. Modified nanocellulose as promising material for the extraction of gold nanoparticles. Microchem. J. 2018, 138, 379–383. [Google Scholar] [CrossRef]
- Islam, M.T.; Sultana, K.A.; Noveron, J.C. Borohydride-free catalytic reduction of organic pollutants by platinum nanoparticles supported on cellulose fibers. J. Mol. Liq. 2019, 296, 111988. [Google Scholar] [CrossRef]
- Yu, H.; Oh, S.; Han, Y.; Lee, S.; Jeong, H.S.; Hong, H.J. Modified cellulose nanofibril aerogel: Tunable catalyst support for treatment of 4-Nitrophenol from wastewater. Chemosphere 2021, 285, 131448. [Google Scholar] [CrossRef]
- Benaissi, K.; Johnson, L.; Walsh, D.A.; Thielemans, W. Synthesis of platinum nanoparticles using cellulosic reducing agents. Green Chem. 2010, 12, 220–222. [Google Scholar] [CrossRef]
- Lin, X.B.; Wu, M.; Wu, D.Y.; Kuga, S.; Endo, T.; Huang, Y. Platinum nanoparticles using wood nanomaterials: Eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem. 2011, 13, 283–287. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, H.; Yang, J.; Tang, J.; Sun, D.; Tang, W. Bacteria Cellulose Nanofibers Supported Palladium(0) Nanocomposite and Its Catalysis Evaluation in Heck Reaction. Ind. Eng. Chem. Res. 2012, 51, 5743–5748. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Liu, Q.; Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 2018, 20, 1085–1094. [Google Scholar] [CrossRef]
- Cirtiu, C.M.; Dunlop-Brière, A.F.; Moores, A. Cellulose nanocrystallites as an efficient support for nanoparticles of palladium: Application for catalytic hydrogenation and Heck coupling under mild conditions. Green Chem. 2011, 13, 288–291. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhang, W.; Yuan, G.; Xiong, R.; Zhang, X. A novel reagentless approach for synthesizing cellulose nanocrystal-supported palladium nanoparticles with enhanced catalytic performance. J. Mater. Chem. A 2013, 1, 8645–8652. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Li, J.; Yu, J.; Xu, X.; Yang, X. Novel Pd-Cu/bacterial cellulose nanofibers: Preparation and excellent performance in catalytic denitrification. Appl. Surf. Sci. 2010, 256, 2241–2244. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.H.; Lee, J.S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Fu, F.; Yang, B.; Zhang, J.; Zhang, Y.; Touhid, S.S.B.; Liu, L.; Dong, Y.; Liu, X.; et al. Synthesis of bimetallic silver-gold nanoparticle composites using a cellulose dope: Tunable nanostructure and its biological activity. Carbohydr. Polym. 2020, 248, 116777. [Google Scholar] [CrossRef]
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose 2017, 24, 2331–2345. [Google Scholar] [CrossRef]
- Hu, H.; Wu, X.; Wang, H.; Wang, H.; Zhou, J. Photo-reduction of Ag nanoparticles by using cellulose-based micelles as soft templates: Catalytic and antimicrobial activities. Carbohydr. Polym. 2019, 213, 419–427. [Google Scholar] [CrossRef]
- Ramaraju, B.; Imae, T.; Destaye, A.G. Ag nanoparticle-immobilized cellulose nanofibril films for environmental conservation. Appl. Catal. A Gen. 2015, 492, 184–189. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, Y.; Shi, L.; Cao, S.; Feng, X.; Miao, M.; Fang, J. Solution-processed assembly of ultrathin transparent conductive cellulose nanopaper embedding AgNWs. Nanoscale 2015, 7, 13694–13701. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Yang, G.; Xie, J.; Hong, F.; Cao, Z.; Yang, X. Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: Effect of fermentation carbon sources of bacterial cellulose. Carbohydr. Polym. 2012, 87, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Jiang, F.; Hsieh, Y.L.; Nitin, N. Cellulose nanofibrils improve dispersibility and stability of silver nanoparticles and induce production of bacterial extracellular polysaccharides. J. Mater. Chem. B 2014, 2, 6226–6235. [Google Scholar] [CrossRef] [Green Version]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef] [Green Version]
- Parnsubsakul, A.; Ngoensawat, U.; Wutikhun, T.; Sukmanee, T.; Sapcharoenkun, C.; Pienpinijtham, P.; Ekgasit, S. Silver nanoparticle/bacterial nanocellulose paper composites for paste-and-read SERS detection of pesticides on fruit surfaces. Carbohydr. Polym. 2020, 235, 115956. [Google Scholar] [CrossRef]
- Fu, L.H.; Liu, B.; Meng, L.Y.; Ma, M.G. Comparative study of cellulose/Ag nanocomposites using four cellulose types. Mater. Lett. 2016, 171, 277–280. [Google Scholar] [CrossRef]
- Yang, G.; Xie, J.; Deng, Y.; Bian, Y.; Hong, F. Hydrothermal synthesis of bacterial cellulose/AgNPs composite: A “green” route for antibacterial application. Carbohydr. Polym. 2012, 87, 2482–2487. [Google Scholar] [CrossRef]
- Maria, L.; Santos, A.L.; Oliveira, P.C.; Valle, A.S.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J. Preparation and Antibacterial Activity of Silver Nanoparticles Impregnated in Bacterial Cellulose. Polimeros 2010, 20, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Barud, H.S.; Regiani, T.; Marques, R.F.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 721631. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lin, H.; Xu, T.; Lai, K.; Han, X.; Lin, M. Cellulose nanofibers coated with silver nanoparticles as a flexible nanocomposite for measurement of flusilazole residues in Oolong tea by surface-enhanced Raman spectroscopy. Food Chem. 2020, 315, 126276. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, C.; Xie, L.; Yuan, X.; Wei, X.; Huang, Q.; Chen, Y.; Lu, Y. Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim. Nanomaterials 2019, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Thielemans, W.; Walsh, D.A. Nanocomposite oxygen reduction electrocatalysts formed using bioderived reducing agents. J. Mater. Chem. 2010, 20, 1737–1743. [Google Scholar] [CrossRef]
- Wu, C.N.; Fuh, S.C.; Lin, S.P.; Lin, Y.Y.; Chen, H.Y.; Liu, J.M.; Cheng, K.C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Song, Z.; Shang, S. Preparation of silver nanoparticles on cellulose nanocrystals and the application in electrochemical detection of DNA hybridization. Cellulose 2011, 18, 67–74. [Google Scholar] [CrossRef]
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose Nanocrystal/Silver Nanoparticle Composites as Bifunctional Nanofillers within Waterborne Polyurethane. ACS Appl. Mater. Interfaces 2012, 4, 2413–2419. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Zhang, W.; Zhou, Z.; Zhang, X. Facile synthesis of tunable silver nanostructures for antibacterial application using cellulose nanocrystals. Carbohydr. Polym. 2013, 95, 214–219. [Google Scholar] [CrossRef]
- Drogat, N.; Granet, R.; Sol, V.; Memmi, A.; Saad, N.; Klein Koerkamp, C.; Bressollier, P.; Krausz, P. Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J. Nanopart. Res. 2011, 13, 1557–1562. [Google Scholar] [CrossRef]
- Lokanathan, A.R.; Uddin, K.M.A.; Rojas, O.J.; Laine, J. Cellulose Nanocrystal-Mediated Synthesis of Silver Nanoparticles: Role of Sulfate Groups in Nucleation Phenomena. Biomacromolecules 2014, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, Z.; Berry, R.M.; Tam, K.C. Mussel-Inspired Green Metallization of Silver Nanoparticles on Cellulose Nanocrystals and Their Enhanced Catalytic Reduction of 4-Nitrophenol in the Presence of beta-Cyclodextrin. Ind. Eng. Chem. Res. 2015, 54, 3299–3308. [Google Scholar] [CrossRef]
- Shi, Z.; Tang, J.; Chen, L.; Yan, C.; Tanvir, S.; Anderson, W.A.; Berry, R.M.; Tam, K.C. Enhanced colloidal stability and antibacterial performance of silver nanoparticles/cellulose nanocrystal hybrids. J. Mater. Chem. B 2015, 3, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, F.; Denneulin, A.; Neuman, C.; Bras, J. Charge density modification of carboxylated cellulose nanocrystals for stable silver nanoparticles suspension preparation. J. Nanopart. Res. 2015, 17, 244. [Google Scholar] [CrossRef]
- Kaushik, M.; Li, A.Y.; Hudson, R.; Masnadi, M.; Li, C.J.; Moores, A. Reversing aggregation: Direct synthesis of nanocatalysts from bulk metal. Cellulose nanocrystals as active support to access efficient hydrogenation silver nanocatalysts. Green Chem. 2016, 18, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Bendi, R.; Imae, T. Renewable catalyst with Cu nanoparticles embedded into cellulose nano-fiber film. RSC Adv. 2013, 3, 16279–16282. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green Synthesis of Copper Nanoparticles Using Cotton. Polymers 2021, 13, 1906. [Google Scholar] [CrossRef]
- Toncheva, A.; Khelifa, F.; Paint, Y.; Voue, M.; Lambert, P.; Dubois, P.; Raquez, J.M. Fast IR-Actuated Shape-Memory Polymers Using in Situ Silver Nanoparticle-Grafted Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2018, 10, 29933–29942. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Development of cellulose nanowhisker- polyacrylamide copolymer as a highly functional precursor in the synthesis of nanometal particles for conductive textiles. Cellulose 2014, 21, 3055–3071. [Google Scholar] [CrossRef]
- Goswami, M.; Das, A.M. Synthesis of cellulose impregnated copper nanoparticles as an efficient heterogeneous catalyst for CeN coupling reactions under mild conditions. Carbohydr. Polym. 2018, 195, 189–198. [Google Scholar] [CrossRef]
- Dutta, A.; Chetia, M.; Ali, A.A.; Bordoloi, A.; Gehlot, P.S.; Kumar, A.; Sarma, D. Copper Nanoparticles Immobilized on Nanocellulose: A Novel and Efficient Heterogeneous Catalyst for Controlled and Selective Oxidation of Sulfides and Alcohols. Catal. Lett. 2019, 149, 141–150. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Z.; Peng, Z.; Zeng, G.; Chen, G.; Zhang, C.; Cheng, M.; Wan, J.; Wang, X.; Qin, X. Cadmium immobilization in river sediment using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating. J. Environ. Manag. 2018, 210, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Cao, L.; Yang, C. Promoting dynamic adsorption of Pb2+ in a single pass flow using fibrous nano-TiO2/cellulose membranes. Chem. Eng. J. 2016, 283, 1145–1153. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Li, L.; Yang, C. In situ growing directional spindle TiO2 nanocrystals on cellulose fibers for enhanced Pb2+ adsorption from water. J. Hazard. Mater. 2015, 289, 140–148. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 Immobilized in Cellulose Matrix for Photocatalytic Degradation of Phenol under Weak UV Light Irradiation. J. Phys. Chem. C 2010, 114, 7806–7811. [Google Scholar] [CrossRef]
- Kettunen, M.; Silvennoinen, R.J.; Houbenov, N.; Nykänen, A.; Ruokolainen, J.; Sainio, J.; Pore, V.; Kemell, M.; Ankerfors, M.; Lindström, T.; et al. Photoswitchable Superabsorbency Based on Nanocellulose Aerogels. Adv. Funct. Mater. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Abouzayed, F.I. Preparation and characterization of nano-magnetic cellulose with fast kinetic properties towards the adsorption of some metal ions. Chem. Eng. J. 2012, 191, 22–30. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Nata, I.F.; Sureshkumar, M.; Lee, C.K. One-pot preparation of amine-rich magnetite/bacterial cellulose nanocomposite and its application for arsenate removal. RSC Adv. 2011, 1, 625–631. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Z.; Liu, Y.; Cheng, M.; Qian, P.; Wang, Q.; Zhu, M. Superparamagnetic iron oxide coated on the surface of cellulose nanospheres for the rapid removal of textile dye under mild condition. Appl. Surf. Sci. 2015, 357, 2103–2111. [Google Scholar] [CrossRef]
- John, A.; Ko, H.U.; Kim, D.G.; Kim, J. Preparation of cellulose-ZnO hybrid films by a wet chemical method and their characterization. Cellulose 2011, 18, 675–680. [Google Scholar] [CrossRef]
- Yu, H.Y.; Chen, G.Y.; Wang, Y.B.; Yao, J.M. A facile one-pot route for preparing cellulose nanocrystal/zinc oxide nanohybrids with high antibacterial and photocatalytic activity. Cellulose 2015, 22, 261–273. [Google Scholar] [CrossRef]
- Zheng, W.L.; Hu, W.L.; Chen, S.Y.; Zheng, Y.; Zhou, B.H.; Wang, H.P. High Photocatalytic Properties of Zinc Oxide Nanoparticles with Amidoximated Bacterial Cellulose Nanofibers as Templates. Chin. J. Polym. Sci. 2014, 32, 169–176. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, B.; Hu, W.; Zhang, W.; Yin, N.; Wang, H. Polyol mediated synthesis of ZnO nanoparticles templated by bacterial cellulose. Carbohydr. Polym. 2013, 92, 1953–1959. [Google Scholar] [CrossRef]
- Katepetch, C.; Rujiravanit, R.; Tamura, H. Formation of nanocrystalline ZnO particles into bacterial cellulose pellicle by ultrasonic-assisted in situ synthesis. Cellulose 2013, 20, 1275–1292. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Yang, R.T.; Yu, H.Y.; Song, M.L.; Zhou, Y.W.; Yao, J.M. Flower-like zinc oxide nanorod clusters grown on spherical cellulose nanocrystals via simple chemical precipitation method. Cellulose 2016, 23, 1871–1884. [Google Scholar] [CrossRef]
- Rull-Barrull, J.; d’Halluin, M.; Le Grognec, E.; Felpin, F.X. Harnessing the Dual Properties of Thiol-Grafted Cellulose Paper for Click Reactions: A Powerful Reducing Agent and Adsorbent for Cu. Angew. Chem. Int. Ed. 2016, 55, 13549–13552. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, Q.; Tao, D.; Yu, T.; Liu, X. Highly flexible magnetic composite aerogels prepared by using cellulose nanofibril networks as templates. Carbohydr. Polym. 2012, 89, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.T.; Azizi Samir, M.A.S.; Salazar-Alvarez, G.; Belova, L.; Stroem, V.; Berglund, L.A.; Ikkala, O.; Nogue’s, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Rendon, M.; Reece, L.M.; Pastrana, F.; Arias, S.L.; Shetty, A.R.; Pavón, J.J.; Allain, J.P. Bacterial Nanocellulose Magnetically Functionalized for Neuro-Endovascular Treatment. Macromol. Biosci. 2017, 17, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Nypelo, T.; Rodriguez-Abreu, C.; Rivas, J.; Dickey, M.D.; Rojas, O.J. Magneto-responsive hybrid materials based on cellulose nanocrystals. Cellulose 2014, 21, 2557–2566. [Google Scholar] [CrossRef]
- Dong, P.; Cheng, X.; Huang, Z.; Chen, Y.; Zhang, Y.; Nie, X.; Zhang, X. In-situ and phase controllable synthesis of nanocrystalline TiO2 on flexible cellulose fabrics via a simple hydrothermal method. Mater. Res. Bull. 2018, 97, 89–95. [Google Scholar] [CrossRef]
- Chauhan, I.; Mohanty, P. In situ decoration of TiO2 nanoparticles on the surface of cellulose fibers and study of their photocatalytic and antibacterial activities. Cellulose 2015, 22, 507–519. [Google Scholar] [CrossRef]
- Noman, M.T.; Wienera, J.; Saskova, J.; Ashrafa, M.A.; Vikova, M.; Jamshaid, H.; Kejzlar, P. In-situ development of highly photocatalytic multifunctional nanocomposites by ultrasonic acoustic method. Ultrason. Sonochem. 2018, 40, 41–56. [Google Scholar] [CrossRef]
- Chauhan, I.; Mohanty, P. Immobilization of titania nanoparticles on the surface of cellulose fibres by a facile single step hydrothermal method and study of their photocatalytic and antibacterial activities. RSC Adv. 2014, 4, 57885–57890. [Google Scholar] [CrossRef]
- Zong, E.; Wang, C.; Yang, J.; Zhu, H.; Jiang, S.; Liu, X.; Song, P. Preparation of TiO2/cellulose nanocomposites as antibacterial bio-adsorbents for effective phosphate removal from aqueous medium. Int. J. Biol. Macromol. 2021, 182, 434–444. [Google Scholar] [CrossRef]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M.; Tashakkorian, H.; Amouei, A. TiO2-grafted cellulose via click reaction: An efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2018, 25, 639–660. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; dos Santos, M.S.; Brandao, L.M.S.; de Resende, I.T.F.; Leo, I.M.; Morillo, E.S.; Yerga, R.M.N.; Fierro, J.L.G.; Egues, S.M.S.; Figueiredo, R.T. The effect of cellulose loading on the photoactivity of cellulose-TiO2 hybrids for hydrogen production under simulated sunlight. Int. J. Hydrogen Energy 2017, 42, 28747–28754. [Google Scholar] [CrossRef]
- Schütz, C.; Sort, J.; Bacsik, Z.; Oliynyk, V.; Pellicer, E.; Fall, A.; Wågberg, L.; Berglund, L.; Bergström, L.; Salazar-Alvarez, G. Hard and Transparent Films Formed by Nanocellulose-TiO2 Nanoparticle Hybrids. PLoS ONE 2012, 7, e45828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missoum, K.; Sadocco, P.; Causio, J.; Belgacem, M.N.; Bras, J. Antibacterial activity and biodegradability assessment of chemically grafted nanofibrillated cellulose. J. Mater. Sci. Eng. C 2014, 45, 477–483. [Google Scholar] [CrossRef]
- Wesarg, F.; Schlott, F.; Grabow, J.; Kurland, H.D.; Heßler, N.; Kralisch, D.; Müller, F.A. In Situ Synthesis of Photocatalytically Active Hybrids Consisting of Bacterial Nanocellulose and Anatase Nanoparticles. Langmuir 2012, 28, 13518–13525. [Google Scholar] [CrossRef]
- Gao, R.; Chen, X.; Chen, C.; Shi, R.; Ouyang, F.; Yang, J.; Sun, D.; Liu, J. Synthesis of BC@mTiO2 hybrid nanofibers for highly efficient enrichment and detection of phosphopeptides. Cellulose 2016, 23, 2475–2485. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Bravo, Y.; Sanchez-Herencia, A.J.; Rodrıguez, A.; Dewalque, J.; Manceriu, L.; Henrist, C.; Ferrari, B. Heteroacoagulation of lignocellulose fibers-based biotemplates and functionalized TiO2 nanoparticles to tailor film microstructures. Cellulose 2020, 27, 7543–7559. [Google Scholar] [CrossRef]
- Janpetch, N.; Vanichvattanadecha, C.; Rujiravanit, R. Photocatalytic disinfection of water by bacterial cellulose/N-F co-doped TiO2 under fluorescent light. Cellulose 2015, 22, 3321–3335. [Google Scholar] [CrossRef]
- Brandes, R.; Trindade, E.C.A.; Vanin, D.F.; Vargas, V.M.M.; Carminatti, C.A.; Al-Qureshi, H.A.; Recouvreux, D.O.S. Spherical Bacterial Cellulose/TiO2 Nanocomposite with Potential Application in Contaminants Removal from Wastewater by Photocatalysis. Fibers Polym. 2018, 19, 1861–1868. [Google Scholar] [CrossRef]
- Nabeela, K.; Thorat, M.N.; Backer, S.N.; Ramachandran, A.M.; Thomas, R.T.; Preethikumar, G.; Mohamed, A.P.; Asok, A.; Dastager, S.G.; Pillai, S. Hydrophilic 3D Interconnected Network of Bacterial Nanocellulose/Black Titania Photothermal Foams as an Efficient Interfacial Solar Evaporator. ACS Appl. Bio Mater. 2021, 4, 4373–4383. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, N.; Capron, I. Safer-by-design hybrid nanostructures: An alternative to conventional titanium dioxide UV filters in skin care products. RSC Adv. 2017, 7, 20430–20439. [Google Scholar] [CrossRef] [Green Version]
- Evdokimova, O.L.; Svensson, F.G.; Agafonov, A.V.; Håkansson, S.; Seisenbaeva, G.A.; Kessler, V.G. Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania–Synthesis and Antibacterial Properties. Nanomaterials 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Li, L.; Xie, R.; Zhao, Z.; Mao, Z. Preparation of magnetic cotton fabric by surface micro-dissolution treatment. Cellulose 2017, 24, 1099–1106. [Google Scholar] [CrossRef]
- Rotaru, R.; Savin, M.; Tudorachi, N.; Peptu, C.; Samoila, P.; Sacarescu, L.; Harabagiu, V. Ferromagnetic iron oxide–cellulose nanocomposites prepared by ultrasonication. Polym. Chem. 2018, 9, 860–868. [Google Scholar] [CrossRef]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.Y.; Ali, R.R.; Siew, K.K.; Chan, H.Y.; Wong, M.M.T.; Lim, W.L.; Kuča, K. 5-Fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef]
- Spiridonova, V.V.; Panova, I.G.; Makarova, L.A.; Afanasova, M.I.; Zezina, S.B.; Sybachina, A.V.; Yaroslavov, A.A. The one-step synthesis of polymer-based magnetic γ-Fe2O3/carboxymethyl cellulose nanocomposites. Carbohydr. Polym. 2017, 177, 269–274. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, L. In situ synthesis of plate-like Fe2O3 nanoparticles in porous cellulose films with obvious magnetic anisotropy. Cellulose 2011, 18, 663–673. [Google Scholar] [CrossRef]
- Ni, W.; Zheng, Z.; Liu, H.; Wang, P.; Wang, L.; Wang, H.; Sun, X.; Yang, Q.; Tang, H.; Zhao, G. Synthesis of the carboxymethyl cellulose magnetic nanoparticles for efficient immobilization of prenyltransferase NovQ. Carbohydr. Polym. 2020, 235, 115955. [Google Scholar] [CrossRef]
- Rashid, M.; Gafur, M.A.; Sharafat, M.K.; Minami, H.; Miah, M.A.J.; Ahmad, H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr. Polym. 2017, 170, 72–79. [Google Scholar] [CrossRef]
- Biliuta, G.; Sacarescu, L.; Socoliu, V.; Iacob, M.; Gheorghe, L.; Negru, D.; Coseri, S. Carboxylated Polysaccharides Decorated with Ultrasmall Magnetic Nanoparticles with Antibacterial and MRI Properties. Macromol. Chem. Phys. 2017, 218, 1700062. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Feng, W.; Levkin, P.A.; Roig, A. Origami magnetic cellulose: Controlled magnetic fraction and patterning of flexible bacterial cellulose. J. Mater. Chem. C 2014, 2, 6312–6318. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef]
- An, X.; Cheng, D.; Dai, L.; Wang, B.; Ocampo, H.J.; Nasrallah, J.; Jia, X.; Zou, J.; Long, Y.; Ni, Y. Synthesis of nano-fibrillated cellulose/magnetite/titanium dioxide(NFC@Fe3O4@TNP) nanocomposites and their application in thephotocatalytic hydrogen generation. Appl. Catal. B 2017, 206, 53–64. [Google Scholar] [CrossRef]
- Guo, J.; Filpponen, I.; Johansson, L.S.; Mohammadi, P.; Latikka, M.; Linder, M.B.; Ras, R.H.A.; Rojas, O.J. Complexes of Magnetic Nanoparticles with Cellulose Nanocrystals as Regenerable, Highly Efficient, and Selective Platform for Protein Separation. Biomacromolecules 2017, 18, 898–905. [Google Scholar] [CrossRef]
- Karimian, A.; Yousefi, B.; Sadeghi, F.; Feizi, F.; Najafzadehvarzi, H.; Parsian, H. Synthesis of biocompatible nanocrystalline cellulose against folate receptors as a novel carrier for targeted delivery of doxorubicin. Chem.-Biol. Interact. 2022, 351, 109731. [Google Scholar] [CrossRef]
- Liu, K.; Nasrallah, J.; Chen, L.; Huang, L.; Ni, Y. Preparation of CNC-dispersed Fe3O4 nanoparticles and their application in conductive paper. Carbohydr. Polym. 2015, 126, 175–178. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, Y.; Cai, P. Cellulose-based antimicrobial films incorporated with ZnO nanopillars on surface as biodegradable and antimicrobial packaging. Food Chem. 2022, 368, 130784. [Google Scholar] [CrossRef]
- Wang, Y.H.; Song, P.; Li, X.; Ru, C.; Ferrari, G.; Balasubramanian, P.; Amabili, M.; Sun, Y.; Liu, X. A Paper-Based Piezoelectric Accelerometer. Micromachines 2018, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.A.; Nada, A.A.; Hassabo, A.G.; Eid, B.M.; Noor El-Deen, A.M.; Abou-Zeid, N.Y. Effect of different capping agents on physicochemical and antimicrobial properties of ZnO nanoparticles. Chem. Pap. 2017, 71, 1365–1375. [Google Scholar] [CrossRef]
- Ibupoto, Z.; Khun, K.; Eriksson, M.; Al Salhi, M.; Atif, M.; Ansari, A.; Willander, M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials 2013, 6, 3584–3597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Zhang, Y.; Xu, M.; Shi, S.Q. The three-dimensional heterostructure synthesis of ZnO/cellulosic fibers and its application for rubber composites. Compos. Sci. Technol. 2019, 177, 10–17. [Google Scholar] [CrossRef]
- Xiao, H.; Shan, Y.; Zhang, W.; Huang, L.; Chen, L.; Ni, Y.; Boury, B.; Wu, H. C-nanocoated ZnO by TEMPO-oxidized cellulose templating for improved photocatalytic performance. Carbohydr. Polym. 2020, 235, 115958. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.V.; Gonçalves, A.S.; Zaguete, M.A.; Mazon, T.; Nogueira, A.F. ZnO nanostructures directly grown on paper and bacterial cellulose substrates without any surface modification layer. Chem. Commun. 2013, 49, 8096–8098. [Google Scholar] [CrossRef]
- Janpetch, N.; Saito, N.; Rujiravanit, R. Fabrication of bacterial cellulose-ZnO composite via solution plasma process for antibacterial applications. Carbohydr. Polym. 2016, 148, 335–344. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Xuan, R.; Wang, Y.; Zou, C.; Zhang, Z.; Wan, Y.; Xu, Y. Flexible and monolithic zinc oxide bionanocomposite foams by a bacterial cellulose mediated approach for antibacterial applications. Dalton Trans. 2014, 43, 6762–6768. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Lizundia, E.; Urruchi, A.; Vilas, J.L.; León, L.M. Increased functional properties and thermal stability of flexible cellulose nanocrystal/ZnO films. Carbohydr. Polym. 2016, 136, 250–258. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.; Mahdavi, M.; Abdolmohammadi, S. Preparation, Characterization, and Antimicrobial Activities of ZnO Nanoparticles/Cellulose Nanocrystal Nanocomposites. BioResources 2013, 8, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Soltani, R.D.C.; Mashayekhi, M.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Safari, M. Sonocatalytic degradation of tetracycline antibiotic using zinc oxide nanostructures loaded on nano-cellulose from waste straw as Nanosonocatalyst. Ultrason. Sonochem. 2019, 55, 117–124. [Google Scholar] [CrossRef]
- Tavker, N.; Gaur, U.; Sharma, M. Cellulose supported bismuth vanadate nanocomposite for effective removal of organic pollutant. J. Environ. Chem. Eng. 2020, 8, 104027. [Google Scholar] [CrossRef]
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon—cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Tao, J.; Xiong, J.; Wang, X.; Zhang, D.; Lin, H.; Chen, Y. In situ synthesis of MnO2-loaded biocomposite based on microcrystalline cellulose for Pb2+ removal from wastewater. Cellulose 2017, 24, 2591–2604. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Gao, R.; Xiao, S.; Zhang, M.; Yin, Y.; Wang, S.; Li, J.; Yang, D. Coherent-Interface-Assembled Ag2O Anchored Nanofibrillated Cellulose Porous Aerogels for Radioactive Iodine Capture. ACS Appl. Mater. Interf. 2014, 24, 29179–29185. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.V.; Barud, H.S.; Alencar, M.A.S.; Nalin, M.; Toma, S.H.; Araki, K.; Benedetti, A.V.; Maciel, I.O.; Fragneaud, B.; Legnani, C.; et al. Self-Supported Smart Bacterial Nanocellulose–Phosphotungstic Acid Nanocomposites for Photochromic Applications. Front. Mater. 2021, 8, 668835. [Google Scholar] [CrossRef]
- Yin, J.; Gao, F.; Wu, Y.; Wang, J.; Lu, Q. Synthesis of Mn3O4 octahedrons and other manganese-based nanostructures through a simple and green route. CrystEngComm 2010, 12, 3401–3403. [Google Scholar] [CrossRef]
- Dai, B.; Chao, C.; Lu, X.; Xia, Q.; Han, J.; Mao, F.; Yang, J.; Sun, D. Preparation of ZnO/CdS/BC Photocatalyst Hybrid Fiber and Research of Its Photocatalytic Properties. J. Nanotechnol. 2015, 2015, 614170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liao, Q.; Zhang, Z.; Liang, Q.; Zhao, Y.; Zheng, X.; Zhang, Y. Novel Piezoelectric Paper-Based Flexible Nanogenerators Composed of BaTiO3 Nanoparticles and Bacterial Cellulose. Adv. Sci. 2016, 3, 1500257. [Google Scholar] [CrossRef]
- Choi, H.Y.; Jeong, Y.G. Microstructures and piezoelectric performance of eco-friendly composite films based on nanocellulose and barium titanate nanoparticle. Compos. Part B 2019, 168, 58–65. [Google Scholar] [CrossRef]
- Yang, J.; Tang, W.; Liu, X.; Chao, C.; Liu, J.; Sun, D. Bacterial cellulose-assisted hydrothermal synthesis and catalytic performance of La2CuO4 nanofiber for methanol steam reforming. Int. J. Hydrogen Energy 2013, 38, 10813–10818. [Google Scholar] [CrossRef]
- Menchaca-Nal, S.; Londono-Calderon, C.L.; Cerrutti, P.; Foresti, M.L.; Pampillo, L.; Bilovol, V.; Candal, R.; Martinez-Garcia, R. Facile synthesis of cobalt ferrite nanotubes using bacterial nanocellulose as template. Carbohydr. Polym. 2016, 137, 726–731. [Google Scholar] [CrossRef]
- Gutierrez, J.; Fernandes, S.C.M.; Mondragon, I.; Tercjak, A. Conductive Photoswitchable Vanadium Oxide Nanopaper based on Bacterial Cellulose. ChemSusChem 2012, 5, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Mehryar, L.; Ghadertaj, A. Characterization of CuO-bacterial cellulose nanohybrids fabricated by in-situ and ex-situ impregnation methods. Carbohydr. Polym. 2019, 222, 114995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Lu, C.H.; Wu, X.D.; Zhang, X.X. Cellulose nanocrystals as a novel support for CuO nanoparticles catalysts: Facile synthesis and their application to 4-nitrophenol reduction. RSC Adv. 2013, 3, 26066–26073. [Google Scholar] [CrossRef]
- Lou, Z.; Cao, Z.; Xu, J.; Zhou, X.; Zhu, J.; Liu, X.; Baig, S.A.; Zhou, J.; Xu, X. Enhanced removal of As(III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids. Chem. Eng. J. 2017, 322, 710–721. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Jiang, S.; Bai, H.; Zhang, S. Use of CeO2 Nanoparticles to Enhance UV-Shielding of Transparent Regenerated Cellulose Films. Polymers 2019, 11, 458. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, J.T.; Hiekkataipale, P.; Malm, J.; Karpinen, M.; Ikkala, O.; Ras, R.H.A. Inorganic Hollow Nanotube Aerogels by Atomic Layer Deposition onto Native Nanocellulose Templates. ACS Nano 2011, 5, 1967–1974. [Google Scholar] [CrossRef]
- Shi, F.; Yu, T.; Hu, S.C.; Liu, J.X.; Yu, L.; Liu, S.H. Synthesis of highly porous SiO2-(WO3)xTiO2 composite aerogels using bacterial cellulose as template with solvothermal assisted crystallization. Chem. Eng. J. 2016, 292, 105–112. [Google Scholar] [CrossRef]
- Qiu, J.; Li, M.; Ding, M.; Yao, J. Cellulose tailored semiconductors for advanced photocatalysis. Renew. Sustain. Energy Rev. 2022, 154, 111820. [Google Scholar] [CrossRef]
- Hiltunen, A.; Or, T.; Lahtonen, K.; Ali-Löytty, H.; Tkachenko, N.; Valden, M.; Sarlin, E.; Cranston, E.D.; Moran-Mirabal, J.M.; Vapaavuori, J. Ultrathin-Walled 3D Inorganic Nanostructured Networks Templated from Cross-Linked Cellulose Nanocrystal Aerogels. Adv. Mater. Interfaces 2021, 8, 2001181. [Google Scholar] [CrossRef]
- Sun, J.; Wenqiang Liu, W.; Wang, W.; Hu, Y.; Yang, X.; Chen, H.; Peng, Y.; Xu, M. CO2 Sorption Enhancement of Extruded-Spheronized CaO-Based Pellets by Sacrificial Biomass Templating Technique. Energy Fuels 2016, 30, 9605–9612. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Giese, M.; Blusch, L.K.; Khan, M.K.; MacLachlan, M.J. Functional Materials from Cellulose-Derived Liquid-Crystal Templates. Angew. Chem. Int. Ed. 2015, 54, 2888–2910. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Lee, S.Y.; Gwon, J.G.; Cho, H.J.; Wu, Q.; Kim, Y.H.; Lee, W.H. Photocatalytic performance of highly transparent and mesoporous molybdenum-doped titania films fabricated by templating cellulose nanocrystals. Ceram. Int. 2018, 44, 16647–16653. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Stahl, A.; Hamad, W.Y.; MacLachlan, M.J. Hard Templating of Nanocrystalline Titanium Dioxide with Chiral Nematic Ordering. Angew. Chem. Int. Ed. 2012, 51, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, J.; Liu, Z.; Sang, T.; Song, B.; Zhu, H.; Wong, C. Facile preparation and microwave absorption properties of porous hollow BaFe12O19/CoFe2O4 composite microrods. J. Alloys Compd. 2015, 648, 1072–1075. [Google Scholar] [CrossRef]
- Ivanova, A.; Fominykh, K.; Fattakhova-Rohlfing, D.; Zeller, P.; Döblinger, M.; Bein, T. Nanocellulose-Assisted Formation of Porous Hematite Nanostructures. Inorg. Chem. 2015, 54, 1129–1135. [Google Scholar] [CrossRef]
- Guo, H.; Mao, R.; Yang, X.; Wang, S.; Chen, J. Hollow nanotubular SnO2 with improved lithium storage. J. Power Sources 2012, 219, 280–284. [Google Scholar] [CrossRef]
- Teng, Y.; Song, L.X.; Wang, L.B.; Xia, J. Face-Raised Octahedral Co3O4 Nanocrystals and Their Catalytic Activity in the Selective Oxidation of Alcohols. J. Phys. Chem. C 2014, 118, 4767–4773. [Google Scholar] [CrossRef]
- Ma, M.G.; Qing, S.J.; Li, S.M.; Zhu, J.F.; Fu, L.H.; Sun, R.C. Microwave synthesis of cellulose/CuO nanocomposites in ionic liquid and its thermal transformation to CuO. Carbohydr. Polym. 2013, 91, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, S.; Zhou, B.; Wang, H. Facile synthesis of ZnO nanoparticles based on bacterial cellulose. Mater. Sci. Eng. B 2010, 170, 88–92. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Wang, J.; Long, D.; Qiao, W.; Ling, L. Ultrahigh intercalation pseudocapacitance of mesoporous orthorhombic niobium pentoxide from a novel cellulose nanocrystal template. Mater. Chem. Phys. 2015, 149–150, 495–504. [Google Scholar] [CrossRef]
- Chu, G.; Feng, J.; Wang, Y.; Zhang, X.; Xu, Y.; Zhang, H. Chiral nematic mesoporous films of ZrO2:Eu3+: New luminescent materials. Dalton Trans. 2014, 43, 15321–15327. [Google Scholar] [CrossRef]
- Wang, F.; Cheong, J.Y.; Lee, J.; Ahn, J.; Duan, G.; Chen, H.; Zhang, Q.; Kim, I.D.; Jiang, S. Pyrolysis of Enzymolysis-Treated Wood: Hierarchically Assembled Porous Carbon Electrode for Advanced Energy Storage Devices. Adv. Funct. Mater. 2021, 31, 2101077. [Google Scholar] [CrossRef]
- Li, L.; Xiao, R.; Tao, X.; Wu, Y.; Jiang, L.; Zhang, Z.; Qing, Y. Free-standing electrodes via coupling nanostructured Ni–NiO with hierarchical wood carbon for high-performance supercapacitors and Ni–Zn batteries. J. Power Sources 2021, 491, 229618. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Zhang, X.; Guo, C.; Chen, C.; Shuangfei Wang, S.; Min, D. High performance supercapacitors assembled with hierarchical porous carbonized wood electrode prepared through self-activation. Ind. Crops Prod. 2022, 181, 114802. [Google Scholar] [CrossRef]
- Subramaniam, T.; Krishnan, S.G.; Ansari, M.N.M.; Hamid, N.A.; Khalid, M. Recent progress on supercapacitive performance of agrowaste fibers: A review. Crit. Rev. Solid State Mater. Sci. 2022, 1–43. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, B.; Fu, F.; Zhang, H.; Zhang, L.; Zhou, J. Fabrication of Hollow Materials by Fast Pyrolysis of Cellulose Composite Fibers with Heterogeneous Structures. Angew. Chem. Int. Ed. 2016, 55, 13504–13508. [Google Scholar] [CrossRef]
- Long, C.; Qi, D.; Wei, T.; Yan, J.; Jiang, L.; Fan, Z. Nitrogen-Doped Carbon Networks for High Energy Density Supercapacitors Derived from Polyaniline Coated Bacterial Cellulose. Adv. Funct. Mater. 2014, 24, 3953–3961. [Google Scholar] [CrossRef]
- Xu, H.; Jin, M.; Geng, J.; Zhang, S.; Zhang, H. Bacterial cellulose-regulated synthesis of metallic Ni catalysts for high-efficiency electrosynthesis of hydrogen peroxide. Sci. China Mater. 2021, 65, 721–731. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. A new platform for facile synthesis of hybrid TiO2 nanostructures by various functionalizations of cellulose to be used in highly-efficient photocatalysis. Mater. Lett. 2020, 274, 128016. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Synthesis of TiO2 nanoparticles coated on cellulose nanofibers with different morphologies: Effect of the template and sol-gel parameters. Mater. Sci. Semicond. Proc. 2020, 109, 104927. [Google Scholar] [CrossRef]
- Ghani, M. Nanocrystalline cellulose as a biotemplate for preparation of porous titania thin film as a sorbent for thin film microextraction of ketorolac, meloxicam, diclofenac and mefenamic acid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1142, 122039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.R.H.; Wan, J.M.; Hu, Z.W.; Jiang, W.B. Preparation and Photocatalytic Activity of TiO2-Wrapped Cotton Nanofiber Composite Catalysts. BioResources 2017, 12, 6062–6081. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, J.; Shi, X.; Yu, Z.; Song, Z.; Dong, L.; Jiang, G.; Han, S. Synthesis of Mesoporous TiO2 Induced by Nano-Cellulose and Its Photocatalytic Properties. BioResources 2016, 11, 3084–3093. [Google Scholar] [CrossRef] [Green Version]
- Tajik, E.; Naeimi, A.; Amiri, A. Fabrication of iron oxide nanoparticles, and green catalytic application of an immobilized novel iron Schiff on wood cellulose. Cellulose 2018, 25, 915–923. [Google Scholar] [CrossRef]
- Mikhaylov, V.I.; Krivoshapkina, E.F.; Belyy, V.A.; Krivoshapkin, P.V. Synthesis and characterization of sponge-like α-Fe microtubes. Chem. Eng. Sci. 2017, 163, 27–30. [Google Scholar] [CrossRef]
- Mikhailov, V.I.; Krivoshapkina, E.F.; Ryabkov, Y.I.; Krivoshapkin, P.V. Influence of the Electrokinetic Properties of Cellulose on the Morphology of Iron(III) Oxide upon Template Synthesis. Glass Phys. Chem. 2016, 42, 582–589. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Zhang, L. Cellulose scaffold: A green template for the controlling synthesis of magnetic inorganic nanoparticles. Powder Technol. 2012, 217, 502–509. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Z.; Xiong, G.; Luo, H. A general strategy of decorating 3D carbon nanofiber aerogels derived from bacterial cellulose with nano-Fe3O4 for high performance flexible and binder-free lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 15386–15393. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Z.; Xiong, G.; Guo, R.; Liu, Z.; Luo, H. Anchoring Fe3O4 nanoparticles on three-dimensional carbon nanofibers toward flexible high performance anodes for lithium-ion batteries. J. Power Sources 2015, 294, 414–419. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 nanoshells coated on carbonized bacterial cellulose nanofibers as a flexible anode for high-performance lithium ion batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Yuan, Y.; Fu, A.; Wang, Y.; Guo, P.; Wu, G.; Li, H.; Zhao, X.S. Spray drying assisted assembly of ZnO nanocrystals using cellulose as sacrificial template and studies on their photoluminescent and photocatalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Zhang, W.; Wei, Y.; Chen, L. Carbon/ZnO nanorods composites templated by TEMPO oxidized cellulose and photocatalytic activity for dye degradation. Cellulose 2018, 25, 1809–1819. [Google Scholar] [CrossRef]
- Liu, S.; Yao, K.; Wang, B.; Ma, M.G. Microwave assisted hydrothermal synthesis of cellulose/ZnO composites and its thermal transformation to ZnO/carbon composites. Iran. Polym. J. 2017, 26, 681–691. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Jia, Y.; Yang, Y.; Cai, P.; Huang, J.; Li, J. Co-assembly of photosystem II in nanotubular indium–tin oxide multilayer films templated by cellulose substance for photocurrent generation. J. Mater. Chem. A 2017, 5, 19826. [Google Scholar] [CrossRef]
- Liu, S.; Hu, H.; Zhou, J.; Zhang, L. Cellulose scaffolds modulated synthesis of Co3O4 nanocrystals: Preparation, characterization and properties. Cellulose 2011, 18, 1273–1283. [Google Scholar] [CrossRef]
- Fu, L.H.; Li, S.M.; Bian, J.; Ma, M.G.; Long, X.L.; Zhang, X.M.; Liu, S.J. Compare study cellulose/Mn3O4 composites using four types of alkalis by sonochemistry method. Carbohydr. Polym. 2015, 115, 373–378. [Google Scholar] [CrossRef]
- Ounnunkad, K.; Phanichphant, S. Cellulose-precursor synthesis of nanocrystalline Co0.5Cu0.5Fe2O4 spinel ferrites. Mater. Res. Bull. 2012, 47, 473–477. [Google Scholar] [CrossRef]
- Henry, A.; Hesemann, P.; Alauzun, J.G.; Boury, B. Reductive mineralization of cellulose with vanadium, iron and tungsten chlorides and access to MxOy metal oxides and MxOy/C metal oxide/carbon composites. Carbohydr. Polym. 2017, 174, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, W.; Cao, X.; Zhou, Z.; Yang, R. Bacterial-cellulose-derived carbon nanofiber supported CoFe2O4 as efficient electrocatalyst for oxygen reduction and evolution reactions. Int. J. Hydrogen Energy 2016, 41, 5351–5360. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Guan, Q.F.; Yu, S.H. Bacterial-Cellulose-Derived Carbon Nanofiber@MnO2 and Nitrogen-Doped Carbon Nanofiber Electrode Materials: An Asymmetric Supercapacitor with High Energy and Power Density. Adv. Mater. 2013, 25, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Haseen, U.; Umar, K.; Ansari, M.S.; Nasir, M.; Ibrahim, M. Bioinspired 2D carbon sheets decorated with MnFe2O4 nanoparticles for preconcentration of inorganic arsenic, and its determination by ICP-OES. Microchim. Acta 2019, 186, 649. [Google Scholar] [CrossRef]
- Yang, E.H.; Moon, D.J. Synthesis of LaNiO3 perovskite using an EDTA cellulose method and comparison with the conventional Pechini method: Application to steam CO2 reforming of methane. RSC Adv. 2016, 6, 112885–112898. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Zhang, Y.; Huang, J. Hierarchical SnO2/Carbon Nanofibrous Composite Derived from Cellulose Substance as Anode Material for Lithium-Ion Batteries. Chem. Eur. J. 2015, 21, 16195–16202. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Luo, B.; Yang, J.; Wang, X.; Song, Q.; Chen, S.; Zhi, L. Pyrolyzed Bacterial Cellulose: A Versatile Support for Lithium Ion Battery Anode Materials. Small 2013, 9, 2399–2404. [Google Scholar] [CrossRef]
- Cazaña, F.; Galetti, A.; Meyer, C.; Sebastián, V.; Centeno, M.A.; Romeo, E.; Monzón, A. Synthesis of Pd-Al/biomorphic carbon catalysts using cellulose as carbon precursor. Catal. Today 2018, 301, 226–238. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, S.; Xie, C.; Yu, H.; Liu, Y.; Yu, S.; Huang, L. Ni-doped mesoporous carbon obtained from hydrothermal carbonization of cellulose and their catalytic hydrogenation activity study. J. Mater. Sci. 2018, 53, 7900–7910. [Google Scholar] [CrossRef]
- Foo, Y.T.; Chan, J.E.M.; Ngoh, G.C.; Abdullah, A.Z.; Horrid, B.A.; Salamatinia, B. Synthesis and characterization of NiO and Ni nanoparticles using nanocrystalline cellulose (NCC) as a template. Ceram. Int. 2017, 43, 16331–16339. [Google Scholar] [CrossRef]
- Tong, S.; Zheng, M.; Lu, Y.; Lin, Z.; Zhang, X.; He, P.; Zhou, H. Binder-free carbonized bacterial cellulose-supported ruthenium nanoparticles for Li–O2 batteries. Chem. Commun. 2015, 51, 7302–7304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Schutz, C.; Salazar-Alvarez, G.; Titirici, M.M. Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv. 2014, 4, 17549–17554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Tang, Y.; Yang, Y.; Zhang, X.; Lee, C.S. In-situ assembly of three-dimensional MoS2 nanoleaves/carbon nanofiber composites derived from bacterial cellulose as flexible and binder-free anodes for enhanced lithium-ion batteries. Electrochim. Acta 2016, 211, 404–410. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.E.; Huang, Y.; Zhang, Y.; Liu, T. Nitrogen-Doped Carbon Nanofiber/Molybdenum Disulfide Nanocomposites Derived from Bacterial Cellulose for High-Efficiency Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interf. 2016, 8, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, W.; Shen, W. Honeycomb-like composite structure for advanced solid state asymmetric supercapacitors. Chem. Eng. J. 2017, 326, 518–527. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ke, D.; Zeng, J.; Zhou, J.; Peng, T.; Zhang, L. Construction of inorganic nanoparticles by micro-nanoporous structure of cellulose matrix. Cellulose 2011, 18, 945–956. [Google Scholar] [CrossRef]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Yong, Q.; Chen, L.; Xing, Y.; Hamel, J.; Zhu, H. Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462. [Google Scholar] [CrossRef]
- Padalkar, S.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Moon, R.J.; Stanciu, L.A. Self-assembly and alignment of semiconductor nanoparticles on cellulose nanocrystals. J. Mater.Sci. 2011, 46, 5672–5679. [Google Scholar] [CrossRef] [Green Version]
- Hokkanen, S.; Repo, E.; Johansson Westholm, L.; Lou, S.; Sainio, T.; Sillanpää, M. Adsorption of Ni2+, Cd2+, PO43+ and NO3− from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite. Chem. Eng. J. 2014, 252, 64–74. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shi, H.H.; Zheng, L.; Zhang, H.; Cha, Y.Y.; Ruan, H.X.; Zhang, Y.; Zhang, X.C. A new cancellous bone material of silk fibroin/cellulose dual network composite aerogel reinforced by nano-hydroxyapatite filler. Int. J. Biol. Mol. 2021, 182, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Garcia, R.P.; Munoz, J.; Pérez de Larraya, U.; Garmendia, N.; Yao, Q.; Boccaccini, A.R. Cellulose Nanocrystals–Bioactive Glass Hybrid Coating as Bone Substitutes by Electrophoretic Co-deposition: In Situ Control of Mineralization of Bioactive Glass and Enhancement of Osteoblastic Performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef]
- Kulpinski, P.; Namyslak, M.; Grzyb, T.; Lis, S. Luminescent cellulose fibers activated by Eu3+-doped nanoparticles. Cellulose 2012, 19, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Hobisch, M.A.; Müller, D.; Fischer, W.J.; Zankel, A.; Eckhart, R.; Bauer, W.; Zabler, S.; Spirk, S. Cobalt Ferrite Nanoparticles for Three-Dimensional Visualization of Micro- and Nanostructured Cellulose in Paper. ACS Appl. Nano Mater. 2019, 2, 3864–3869. [Google Scholar] [CrossRef]
- Butwong, N.; Kunthadong, P.; Soisungnoen, P.; Chotichayapong, C.; Srijaranai, S.; Luong, J.H.T. Silver-doped CdS quantum dots incorporated into chitosan-coated cellulose as a colorimetric paper test stripe for mercury. Microchim. Acta 2018, 185, 126. [Google Scholar] [CrossRef]
- Gomathi, P.T.; Sahatiya, P.; Badhulika, S. Large-Area, Flexible Broadband Photodetector Based on ZnS–MoS2 Hybrid on Paper Substrate. Adv. Funct. Mater. 2017, 27, 1701611. [Google Scholar] [CrossRef]
- Phiwchaia, I.; Thongtem, T.; Thongtem, S.; Pilapong, C. Deferoxamine-conjugated AgInS2 nanoparticles as new nanodrug for synergistic therapy for hepatocellular carcinoma. Int. J. Pharm. 2017, 524, 30–40. [Google Scholar] [CrossRef]
- Huang, X.; Hu, N.; Wang, X.; Zhang, Y.S.; Sun, R. Copper Sulfide Nanoparticle/Cellulose Composite Paper: Room-Temperature Green Fabrication for NIR Laser-Inducible Ablation of Pathogenic Microorganisms. ACS Sustain. Chem. Eng. 2017, 5, 2648–2655. [Google Scholar] [CrossRef]
- Peng, S.; Fan, L.; Wei, C.; Liu, X.; Zhang, H.; Xu, W.; Xu, J. Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes. Carbohydr. Polym. 2017, 157, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.; Fan, J.; Sun, D.; Tang, W.; Yang, X. Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011, 189, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tavker, N.; Sharma, M. Designing of waste fruit peels extracted cellulose supported molybdenum sulfide nanostructures for photocatalytic degradation of RhB dye and industrial effluent. J. Environ. Manag. 2020, 255, 109906. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, S.; Zhao, S.; Zheng, Y.; Wang, H. Zinc Sulfide Nanoparticles Template by Bacterial Cellulose and Their Optical Properties. J. Appl. Polym. Sci. 2014, 131, 40874. [Google Scholar] [CrossRef]
- Wang, P.; Geng, Z.; Gao, J.; Xuan, R.; Liu, P.; Wang, Y.; Huang, K.; Wan, Y.; Xu, Y. ZnxCd1-xS /bacterial cellulose bionanocomposite foams with hierarchical architecture and enhanced visible-light photocatalytic hydrogen production activity. J. Mater. Chem. A 2015, 3, 1709–1716. [Google Scholar] [CrossRef]
- Guo, X.; Cao, G.L.; Ding, F.; Li, X.; Zhen, S.; Xue, Y.F.; Yan, Y.M.; Liu, T.; Sun, K.N. A bulky and flexible electrocatalyst for efficient hydrogen evolution based on the growth of MoS2 nanoparticles on carbon nanofiber foam. J. Mater. Chem. A 2015, 3, 5041–5046. [Google Scholar] [CrossRef]
- Li, Z.; Ottmann, A.; Thauer, E.; Neef, C.; Sai, H.; Sun, Q.; Cendrowski, K.; Meyer, H.P.; Vaynzof, Y.; Mijowska, E.; et al. A facile synthesis method and electrochemical studies of a hierarchical structured MoS2/C nanocomposite. RSC Adv. 2016, 6, 76084–76092. [Google Scholar] [CrossRef] [Green Version]
- Luna-Martinez, J.F.; Hernandez-Uresti, D.B.; Reyes-Melo, M.E.; Guerrero-Salazar, C.A.; Gonzalez-Gonzalez, V.A.; Sepulveda-Guzman, S. Synthesis and optical characterization of ZnS-sodium carboxymethyl cellulose nanocomposite films. Carbohydr. Polym. 2011, 84, 566–570. [Google Scholar] [CrossRef]
- Abdullah, H.; Hsu, C.N.; Shuwanto, H.; Gultom, N.S.; Kebede, W.L.; Wua, C.M.; Lai, C.C.; Murakami, R.I.; Hirota, M.; Nakagaito, A.N.; et al. Immobilization of cross-linked In-doped Mo(O,S)2 on cellulose nanofiber for effective organic-compound degradation under visible light illumination. Progr. Nat. Sci. Mater. Int. 2021, 31, 404–413. [Google Scholar] [CrossRef]
- Shao, D.; Ren, X.; Wen, J.; Hu, S.; Xiong, J.; Jiang, T.; Wang, X.; Wang, X. Immobilization of uranium by biomaterial stabilized FeS nanoparticles: Effects of stabilizer and enrichment mechanism. J. Hazard. Mater. 2016, 302, 1–9. [Google Scholar] [CrossRef]
- Yu, W.; Lin, W.; Shao, X.; Hu, Z.; Li, R.; Yuan, D. High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J. Power Sources 2014, 272, 137–143. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Sundberg, J.; Götherström, C.; Gatenholm, P. Biosynthesis and in vitro evaluation of macroporous mineralized bacterial nanocellulose scaffolds for bone tissue engineering. Bio-Med. Mater. Eng. 2015, 25, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Tabaght, F.E.; Azzaoui, K.; Elidrissi, A.; Hamed, O.; Mejdoubi, E.; Jodeh, S.; Akartasse, N.; Lakrat, M.; Lamhamdi, A. New nanostructure based on hydroxyapatite modified cellulose for bone substitute, synthesis, and characterization. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 437–448. [Google Scholar] [CrossRef]
- Salehia, G.; Behnamghader, A.; Hesaraki, S.; Mozafari, M. Synergistic effects of carbohydrate polymers on the performance of hybrid injectable bone pastes. Eur. Polym. J. 2019, 119, 523–530. [Google Scholar] [CrossRef]
- Ohland, A.L.; Martins Salim, V.M.; Piacsek Borges, C. Nanocomposite membranes for osmotic processes: Incorporation of functionalized hydroxyapatite in porous substrate and in selective layer. Desalination 2019, 463, 23–31. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Herdocia-Lluberes, C.S.; Laboy-López, S.; Morales, S.; Gonzalez-Robles, T.J.; González-Feliciano, J.A.; Nicolau, E. Evaluation of Synthesized Nanohydroxyapatite-Nanocellulose Composites as Biocompatible Scaffolds for Applications in Bone Tissue Engineering. J. Nanomater. 2015, 2015, 310935. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ni, Y.; Pudukudy, M.; Zhang, H.; Wang, H.; Jia, Q.; Shan, S. Synthesis of nanocrystalline cellulose/hydroxyapatite nanocomposites for the efficient removal of chlortetracycline hydrochloride in aqueous medium. Mater. Chem. Phys. 2022, 275, 125135. [Google Scholar] [CrossRef]
- Ma, M.G.; Donga, Y.Y.; Fua, L.H.; Li, S.M.; Sun, R.C. Cellulose/CaCO3 nanocomposites: Microwave ionic liquid synthesis, characterization, and biological activity. Carbohydr. Polym. 2013, 92, 1669–1676. [Google Scholar] [CrossRef]
- Van Opdenbosch, D.; Maischa, P.; Fritz-Popovski, G.; Paris, O.; Zollfrank, C. Transparent cellulose sheets as synthesis matrices for inorganic functional particles. Carbohydr. Polym. 2012, 87, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, H.; Guan, Y.; Zhu, M.; Liu, S.; Yao, J. A novel chemical reduction method to fabricate tunable selenium nanosphere and nanobelt based on cellulose nanocrystals. Mater. Lett. 2018, 230, 44–47. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Q.; Deng, J.; Okosi, N.; Xia, J.; Su, M. Nanocellulose templated growth of ultra-small bismuth nanoparticles for enhanced radiation therapy. Nanoscale 2018, 10, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, R.; Liu, L.; Wang, F.; Niu, H.; Huang, Y. Facile synthesis of Ni(OH)2/graphene/bacterial cellulose paper for large areal mass, mechanically tough and flexible supercapacitor electrodes. J. Power Sources 2016, 335, 76–83. [Google Scholar] [CrossRef]
- Vosmanská, V.; Kolářová, K.; Pišlová, M.; Švorčík, V. Reaction parameters of in situ silver chloride precipitation on cellulose fibres. Mater. Sci. Eng. C 2019, 95, 134–142. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.Q.; Si, C.L. Simple and green fabrication of AgCl/Ag-cellulose paper with antibacterial and photocatalytic activity. Carbohydr. Polym. 2017, 174, 450–455. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.N.; Niu, G.D.; Li, X.L.; Li, E.Y.; Bai, Y.; Liu, Y.; Yuan, G.H. Flexible Ti-Doped FeOOH Quantum Dots/Graphene/Bacterial Cellulose Anode for High-Energy Asymmetric Supercapacitors. Part. Part. Syst. Charact. 2017, 34, 1700213. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Oun, A.A.; Shankar, S.; Rhim, J.W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Zhang, Y.; Lan, F.; Yana, M.; Yu, J. Nanomaterials-modified cellulose paper as a platform for biosensing applications. Nanoscale 2017, 9, 4366–4382. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, C.; Zheng, X.; Liu, H.; Yu, J. Paper-based electrochemiluminescence origami device for protein detection using assembled cascade DNA-carbon dots nanotags based on rolling circle amplification. Biosens. Bioelectron. 2015, 68, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Incel, A.; Akın, O.; Cagır, A.; Yıldız, Ü.H.; Demir, M.M. Smart phone assisted detection and quantification of cyanide in drinking water by paper based sensing platform. Sens. Actuators B 2017, 252, 886–893. [Google Scholar] [CrossRef]
- Qi, H.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Chiral Nematic Assemblies of Silver Nanoparticles in Mesoporous Silica Thin Films. J. Am. Chem. Soc. 2011, 133, 3728–3731. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, G.; Mourdikoudis, S.; Yate, L.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Nickel Nanoparticle-Doped Paper as a Bioactive Scaffold for Targeted and Robust Immobilization of Functional Proteins. ACS Nano 2014, 8, 6221–6231. [Google Scholar] [CrossRef] [PubMed]
- Bazant, P.; Kuritka, I.; Munster, L.; Kalina, L. Microwave solvothermal decoration of the cellulose surface by nanostructured hybrid Ag/ZnO particles: A joint XPS, XRD and SEM study. Cellulose 2015, 22, 1275–1293. [Google Scholar] [CrossRef]
- Khatri, V.; Halász, K.; Trandafilović, L.V.; Dimitrijević-Branković, S.; Mohanty, P.; Djoković, V.; Csóka, L. ZnO-modified cellulose fiber sheets for antibody immobilization. Carbohydr. Polym. 2014, 109, 139–147. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhang, Y.; Huang, J. Bioinspired Hierarchical Nanotubular Titania Immobilized with Platinum Nanoparticles for Photocatalytic Hydrogen Production. Chem. Eur. J. 2015, 21, 7345–7349. [Google Scholar] [CrossRef]
- Nagaraju, G.; Ko, Y.H.; Yu, J.S. Facile synthesis of ZnO/CuO nanostructures on cellulose paper and their p–n junction properties. Mater. Lett. 2014, 116, 64–67. [Google Scholar] [CrossRef]
- Lv, P.; Xu, W.; Li, D.; Feng, Q.; Yao, Y.; Pang, Z.; Lucia, L.A.; Wei, Q. Metal-based bacterial cellulose of sandwich nanomaterials for anti-oxidation electromagnetic interference shielding. Mater. Des. 2016, 112, 374–382. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Hu, W.; Zhou, B.; Yang, Z.; Yin, N.; Wang, H. Facile fabrication of flexible magnetic nanohybrid membrane with amphiphobic surface based on bacterial cellulose. Carbohydr. Polym. 2011, 86, 1760–1767. [Google Scholar] [CrossRef]
- Yang, X.; Shi, K.; Zhitomirsky, I.; Cranston, E.D. Cellulose Nanocrystal Aerogels as Universal 3D Lightweight Substrates for Supercapacitor Materials. Adv. Mater. 2015, 27, 6104–6109. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Berry, R.M.; Tam, K.C. Synthesis of β-Cyclodextrin-Modified Cellulose Nanocrystals (CNCs)@Fe3O4@SiO2 Superparamagnetic Nanorods. ACS Sustain. Chem. Eng. 2014, 2, 951–958. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Lam, E.; Hrapovic, S.; Luong, J.H.T. Preparation of Well-Dispersed Gold/Magnetite Nanoparticles Embedded on Cellulose Nanocrystals for Efficient Immobilization of Papain Enzyme. ACS Appl. Mater. Interfaces 2013, 5, 4978–4985. [Google Scholar] [CrossRef] [Green Version]
- Gesesse, G.D.; Li, C.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863. [Google Scholar] [CrossRef]
- Busbee, B.D.; Obare, S.O.; Murphy, C.J. An Improved Synthesis of High-Aspect-Ration Gold Nanorods. Adv. Mater. 2013, 15, 414–416. [Google Scholar] [CrossRef]
- Ge, S.; Liu, W.; Ge, L.; Yan, M.; Yan, J.; Huang, J.; Yu, J. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for Microcystin-LR immunoassay. Biosens. Bioelectron. 2013, 49, 111–117. [Google Scholar] [CrossRef]
- Sun, G.; Liu, H.; Zhang, Y.; Yu, J.; Yan, M.; Song, X.; He, W. Gold nanorods-paper electrode based enzyme-free electrochemical immunoassay for prostate specific antigen using porous zinc oxide spheres-silver nanoparticles nanocomposites as labels. New J. Chem. 2015, 39, 6062–6067. [Google Scholar] [CrossRef]
- Yan, J.; Yan, M.; Ge, L.; Yu, J.; Ge, S.; Huang, J. A microfluidic origami electrochemiluminescence aptamer-device based on a porous Au-paper electrode and a phenyleneethynylene derivative. Chem. Commun. 2013, 49, 1383–1385. [Google Scholar] [CrossRef]
- Alba-Patino, A.; Russell, S.M.; Borges, M.; Pazos-Perez, N.; Alvarez-Puebla, R.A.; de la Rica, R. Nanoparticle-based mobile biosensors for the rapid detection of sepsis biomarkers in whole blood. Nanoscale Adv. 2020, 2, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Wang, P.; Zhu, P.; Ge, L.; Ge, S.; Yan, M.; Song, X.; Yu, J. A near-infrared light photoelectrochemical immunosensor based on a Au-paper electrode and naphthalocyanine sensitized ZnO nanorods. J. Mater. Chem. B 2014, 2, 4811–4817. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wu, K.; Zhang, Y.; Yan, M.; Yu, J. Paper-based biosensor relying on flower-like reduced graphene guided enzymatically deposition of polyaniline for Pb2+ detection. Biosens. Bioelectron. 2016, 80, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Y.; Wang, Y.; Ge, S.; Yan, M.; Yu, J.; Song, X. Paper-Based Analytical Devices Relying on Visible-Light-Enhanced Glucose/Air Biofuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 24330–24337. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kong, Q.; Zhang, Y.; Dong, C.; Ge, S.; Yu, J. A 3D electrochemical immunodevice based on a porous Pt-paper electrode and metal ion functionalized flower-like Au nanoparticles. J. Mater. Chem. B 2015, 3, 2764–2769. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, L.N.; Ge, S.G.; Liu, H.Y.; Ren, N.; Yan, M.; Yu, J.H. Paper-Based Device for Colorimetric and Photoelectrochemical Quantification of the Flux of H2O2 Releasing from MCF-7 Cancer Cells. Anal. Chem. 2016, 88, 5369–5377. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, L.; Ma, C.; Kong, Q.; Yan, M.; Ge, S.; Yu, J. Self-Powered and Sensitive DNA Detection in a Three-Dimensional Origami-Based Biofuel Cell Based on a Porous Pt-Paper Cathode. Chem. Eur. J. 2014, 20, 12453–12462. [Google Scholar] [CrossRef]
- Liu, F.; Ge, S.; Yu, J.; Yan, M.; Song, X. Electrochemical device based on a Pt nanosphere-paper working electrode for in situ and real-time determination of the flux of H2O2 releasing from SK-BR-3 cancer cells. Chem. Commun. 2014, 50, 10315–10318. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Ge, S.; Song, X.; Ge, L.; Yan, M.; Yu, J. A 3D origami multiple electrochemiluminescence immunodevice based on a porous silver-paper electrode and multi-labeled nanoporous gold-carbon spheres. Chem. Commun. 2013, 49, 7687–7689. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Ge, S.; Song, X.; Ge, L.; Yan, M.; Yu, J. Multiplex electrochemical origami immunodevice based on cuboid silver-paper electrode and metal ions tagged nanoporous silver-chitosan. Biosens. Bioelectron. 2014, 56, 167–173. [Google Scholar] [CrossRef]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-Nanoparticle-Colored Cotton Fabrics with Tunable Colors and Durable Antibacterial and Self-Healing Superhydrophobic Properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Liang, L.L.; Lan, F.F.; Li, L.; Su, M.; Ge, S.G.; Yu, J.H.; Liu, H.Y.; Yan, M. Fluorescence “turn-on” determination of H2O2 using multilayer porous SiO2/NGQDs and PdAu mimetics enzymatic/oxidative cleavage of single-stranded DNA. Biosens. Bioelectron. 2016, 82, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Lan, F.; Li, L.; Ge, S.; Yu, J.; Ren, N.; Liu, H.; Yan, M. Paper analytical devices for dynamic evaluation of cell surface N-glycan expression via a bimodal biosensor based on multibranched hybridization chain reaction amplification. Biosens. Bioelectron. 2016, 86, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, W.; Yan, M.; Song, X.; Yu, J. Photoelectrochemical detection of tumor markers based on a CdS quantum dot/ZnO nanorod/Au@Pt-paper electrode 3D origami immunodevice. J. Mater. Chem. B 2015, 3, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, C.; Kong, Q.; Li, W.; Zhang, Y.; Ge, S.; Yan, M.; Yu, J. A 3D origami electrochemical immunodevice based on a Au@Pd alloy nanoparticle-paper electrode for the detection of carcinoembryonic antigen. J. Mater. Chem. B 2014, 2, 6669–6674. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Subnanomolar Sensitivity of Filter Paper-Based SERS Sensor for Pesticide Detection by Hydrophobicity Change of Paper Surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Saklya, N.; Marzouka, W.; Ouada, H.B.; Majdou, H. Enhancing performances of colorimetric response of carboxymethylcellulose-stabilized silver nanoparticles: A fully eco-friendly assay for Hg2+ detection. Sens. Actuators B 2017, 253, 918–927. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. Cellulose acetate nanofibers embedded with AgNPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar] [CrossRef]
- Li, G.; Sun, Y.; Liu, H. Gold-Carboxymethyl Cellulose Nanocomposites Greenly Synthesized for Fluorescent Sensitive Detection of Hg(II). J. Cluster Sci. 2018, 29, 177–184. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Liu, F.; Su, M.; Liang, L.; Ge, S.; Yu, J. Real-time visual determination of the flux of hydrogen sulphide using a hollow-channel paper electrode. Chem. Commun. 2015, 51, 14030–14033. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhu, S.; Yan, M.; Ge, S.; Yu, J. 3D origami electrochemical device for sensitive Pb2+ testing based on DNA functionalized iron-porphyrinic metal-organic framework. Biosens. Bioelectron. 2017, 87, 108–115. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, H.Y.; Zhang, D.W.; Jiang, J.; Cui, Y.R.; Qiu, S.; Zhou, Y.L.; Zhang, X.X. Fabrication of Bienzymatic Glucose Biosensor Based on Novel Gold Nanoparticles-Bacteria Cellulose Nanofibers Nanocomposite. Electroanalysis 2010, 22, 2543–2550. [Google Scholar] [CrossRef]
- Lan, F.; Sun, G.; Liang, L.; Ge, S.; Yan, M.; Yu, J. Microfluidic paper-based analytical device for photoelectrochemical immunoassay with multiplex signal amplification using multibranched hybridization chain reaction and PdAu enzyme mimetics. Biosens. Bioelectron. 2016, 79, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Yang, H.; Zhang, Y.; Yu, J.; Ge, S.; Yan, M.; Song, X. Branched zinc oxide nanorods arrays modified paper electrode for electrochemical immunosensing by combining biocatalytic precipitation reaction and competitive immunoassay mode. Biosens. Bioelectron. 2015, 74, 823–829. [Google Scholar] [CrossRef]
- Sun, G.; Yang, H.; Ma, C.; Zhang, Y.; Yu, J.; He, W.; Song, X. Application of CuS-functionalized ZnO nanoflakes for a paper-based photoelectrochemical immunoassay using an insitu electron donor producing strategy. New J. Chem. 2015, 39, 7012–7018. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, L.; Li, M.; Yan, M.; Ge, S.; Yu, J.; Song, X.; Cao, B. Flexible paper-based ZnO nanorod light-emitting diodes induced multiplexed photoelectrochemical immunoassay. Chem. Commun. 2014, 50, 1417–1419. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Ma, C.; Li, S.; Yu, J.; Ge, S.; Yan, M. A chemiluminescence excited photoelectrochemistry aptamer-device equipped with a tin dioxide quantum dot/reduced graphene oxide nanocomposite modified porous Au-paper electrode. J. Mater. Chem. B 2014, 2, 3462–3468. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Zheng, X.; Ma, C.; Song, X.; Ge, S.; Yu, J.; Yan, M. Growth of gold-manganese oxide nanostructures on a 3D origami device for glucose-oxidase label based electrochemical immunosensor. Biosens. Bioelectron. 2014, 61, 76–82. [Google Scholar] [CrossRef]
- Ge, L.; Wang, P.; Ge, S.; Li, N.; Yu, J.; Yan, M.; Huang, J. Photoelectrochemical Lab-on-Paper Device Based on an Integrated Paper Supercapacitor and Internal Light Source. Anal. Chem. 2013, 85, 3961–3970. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Wu, H.; Yue, X.; Wang, Z.; Rooney, D.; Feng, J.; Sun, K. Facile synthesis of copper-manganese spinel anodes with high capacity and cycling performance for lithium-ion batteries. Mater. Lett. 2016, 182, 147–150. [Google Scholar] [CrossRef]
- Lin, F.; Cai, J.; Li, Y.; Yu, H.; Li, S. Constituting fully integrated colorimetric analysis system for Fe(III) on multifunctional nitrogen-doped MoO3/cellulose paper. Talanta 2018, 180, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xu, Q.; Fan, L.; Wei, C.; Bao, H.; Xu, W.; Xu, J. Flexible polypyrrole/cobalt sulfide /bacterial cellulose composite membranes for supercapacitor application. Synth. Met. 2016, 222, 285–292. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ramireddy, H.; Baidya, A.; Amala, A.K.; Sudhakar, C.; Mondal, B.; Philip, L.; Pradeep, T. Nanocellulose-Reinforced Organo-Inorganic Nanocomposite for Synergistic and Affordable Defluoridation of Water and an Evaluation of Its Sustainability Metrics. ACS Sustain. Chem. Eng. 2020, 8, 139–147. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A. Synthesis, Antibacterial and Thermal Studies of Cellulose Nanocrystal Stabilized ZnO-Ag Heterostructure Nanoparticles. Molecules 2013, 18, 6269–6280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, J. Natural cellulose derived nanofibrous Ag-nanoparticle/SnO2/carbon ternary composite as an anodic material for lithium-ion batteries. J. Phys. Chem. Solids 2019, 126, 155–163. [Google Scholar] [CrossRef]
- Kargar, P.G.; Bagherzade, G.; Eshghi, H. Novel biocompatible core/shell Fe3O4@NFC@Co(II) as a new catalyst in a multicomponent reaction: An efficient and sustainable methodology and novel reusable material for one-pot synthesis of 4H-pyran and pyranopyrazole in aqueous media. RSC Adv. 2020, 10, 37086. [Google Scholar] [CrossRef]
- Nair, S.S.; Chen, J.; Slabon, A.; Mathew, A.P. Converting cellulose nanocrystals into photocatalysts by functionalisation with titanium dioxide nanorods and gold nanocrystals. RSC Adv. 2020, 10, 37374. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.; Guo, H.; Liu, K.; Mei, C.; Li, Y.; Duan, G.; He, S.; Han, J.; Zheng, J.; et al. Anisotropic cellulose nanofibril composite sponges for electromagnetic interference shielding with low reflection loss. Carbohydr. Polym. 2022, 276, 118799. [Google Scholar] [CrossRef]
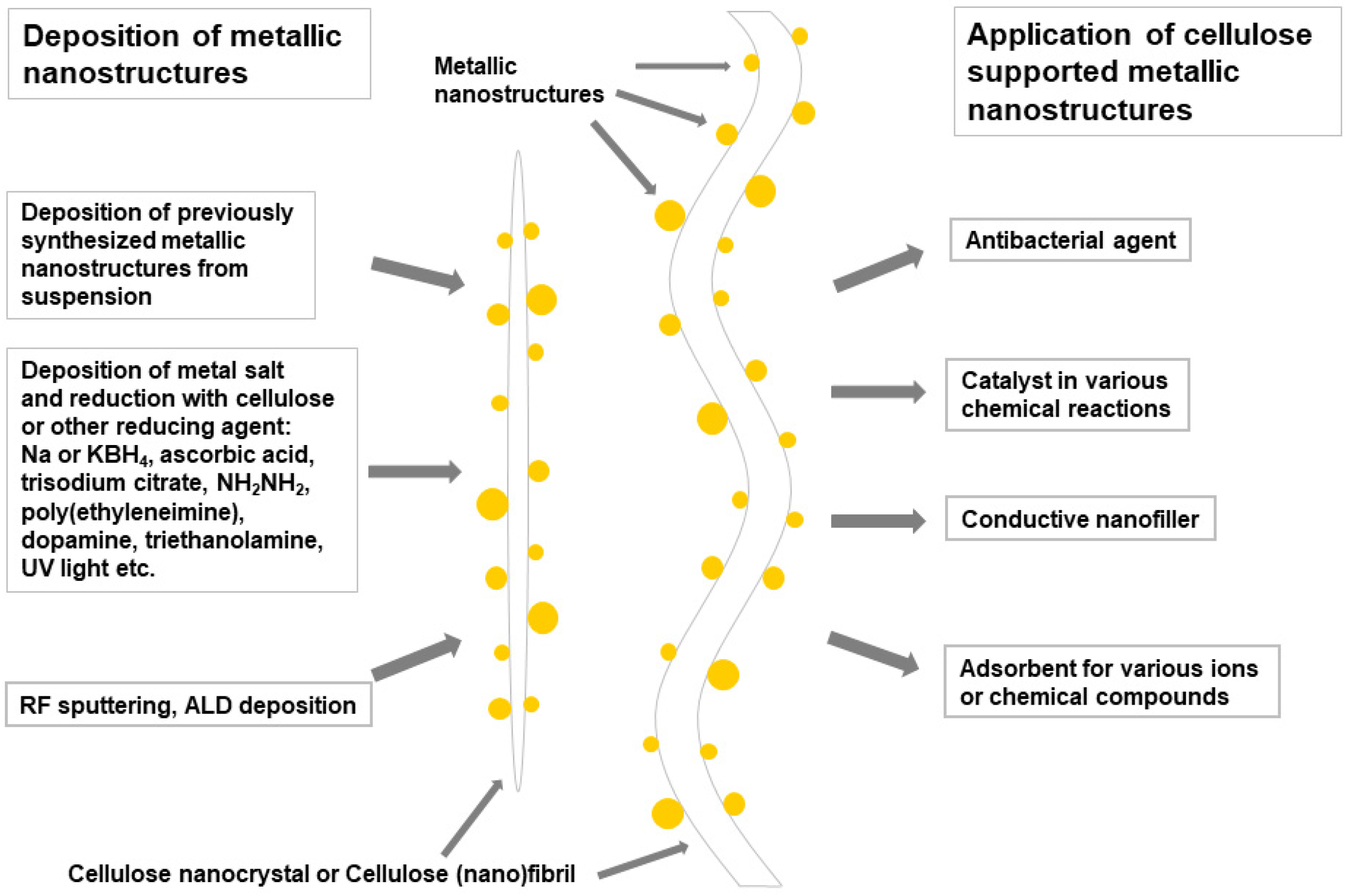
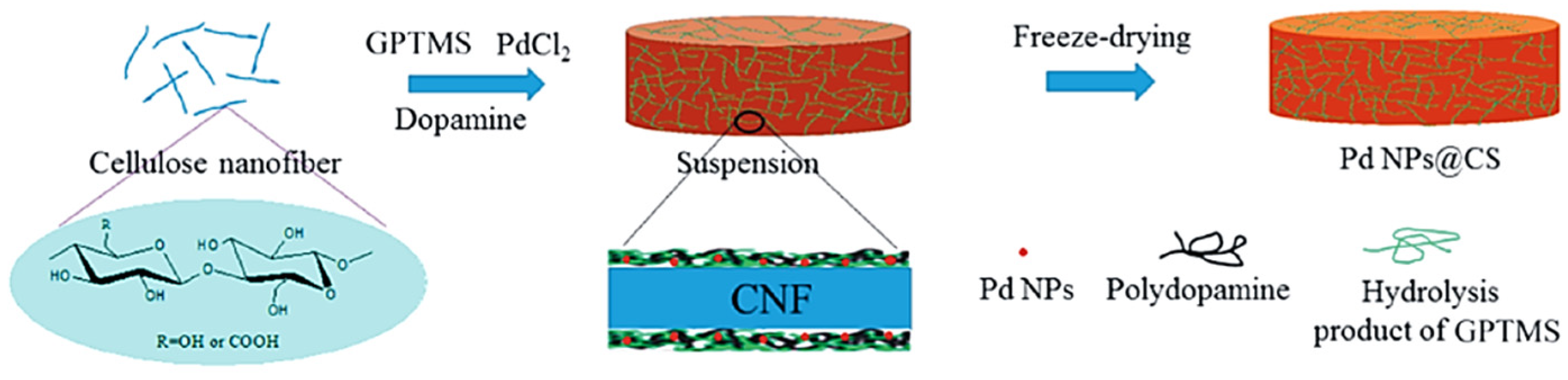
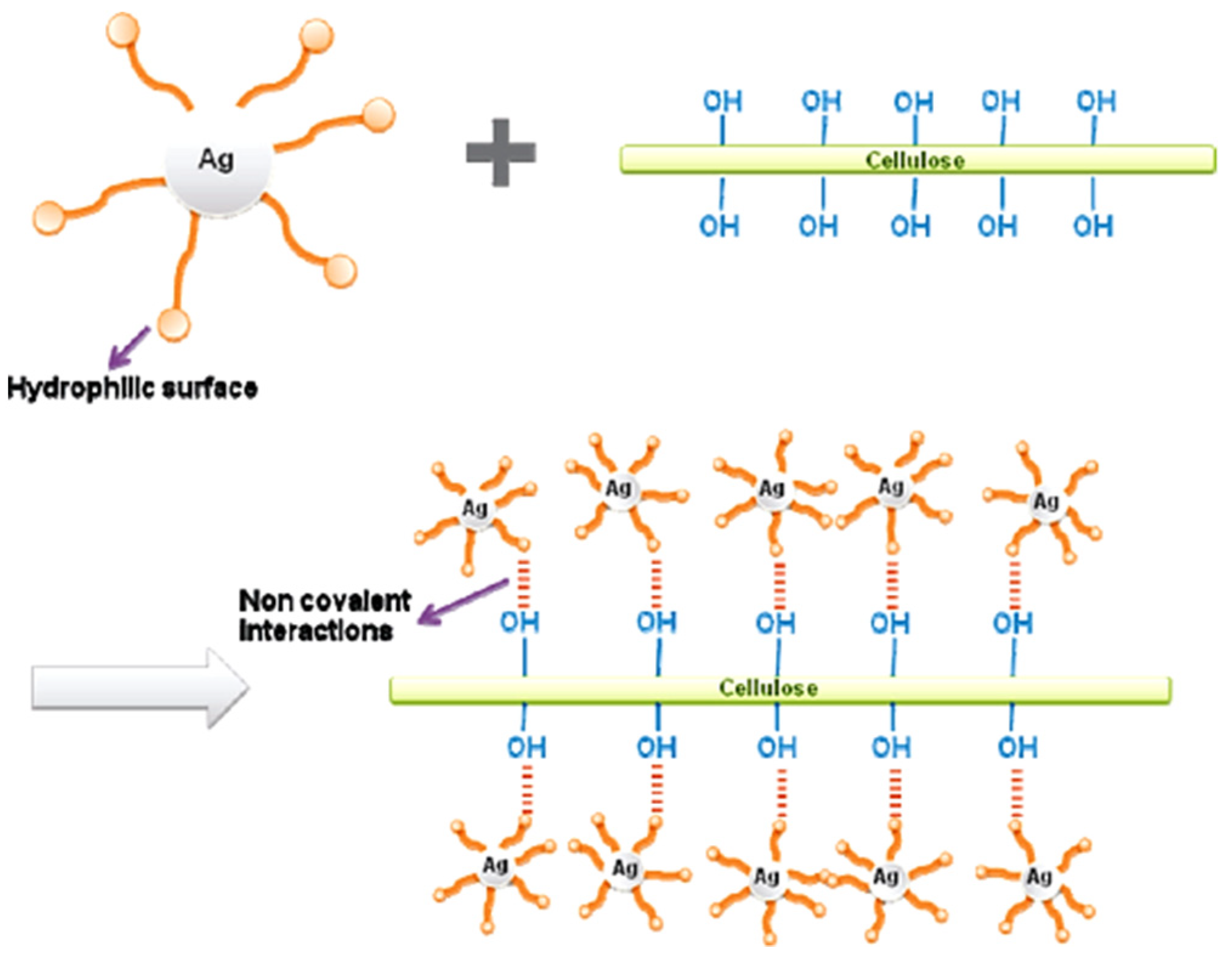
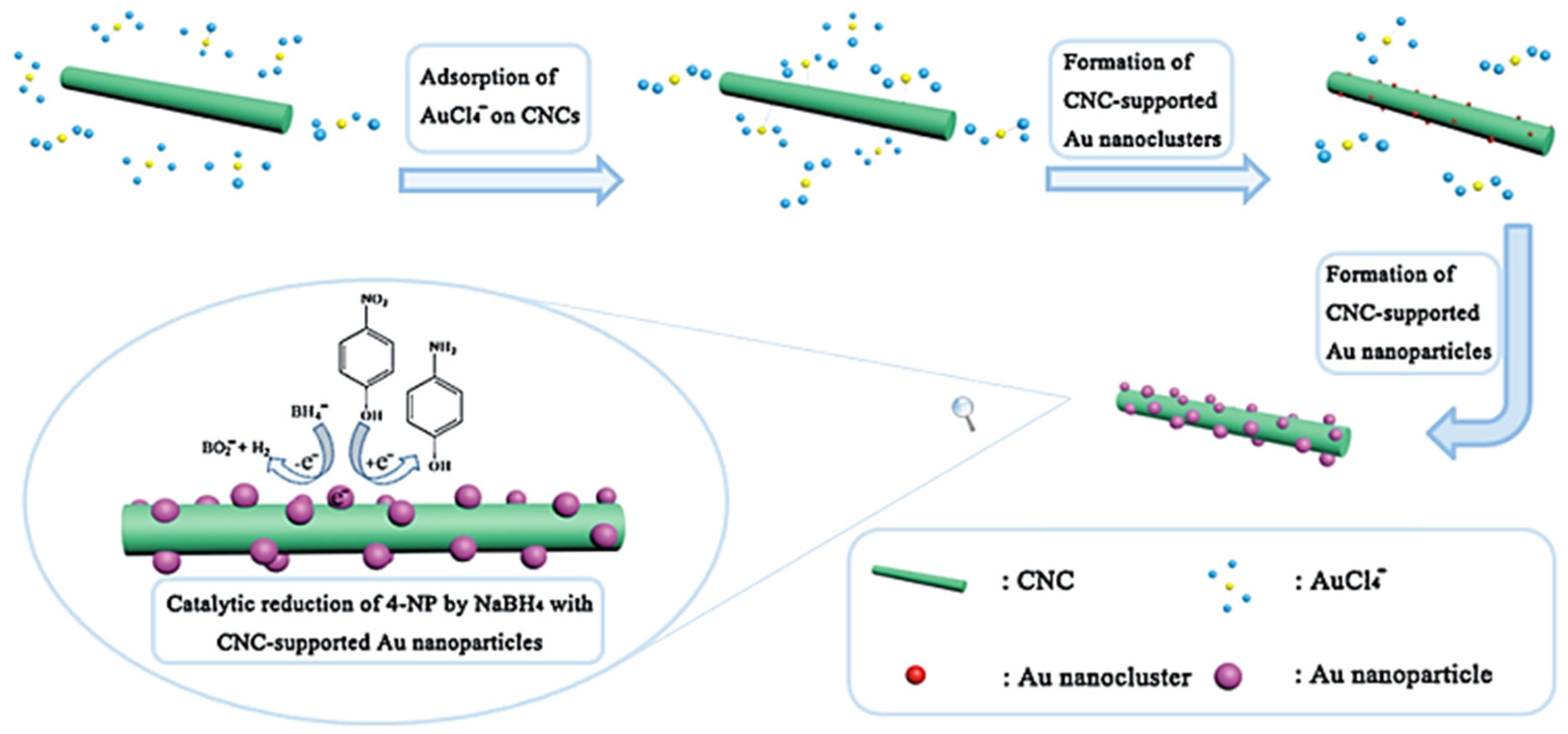
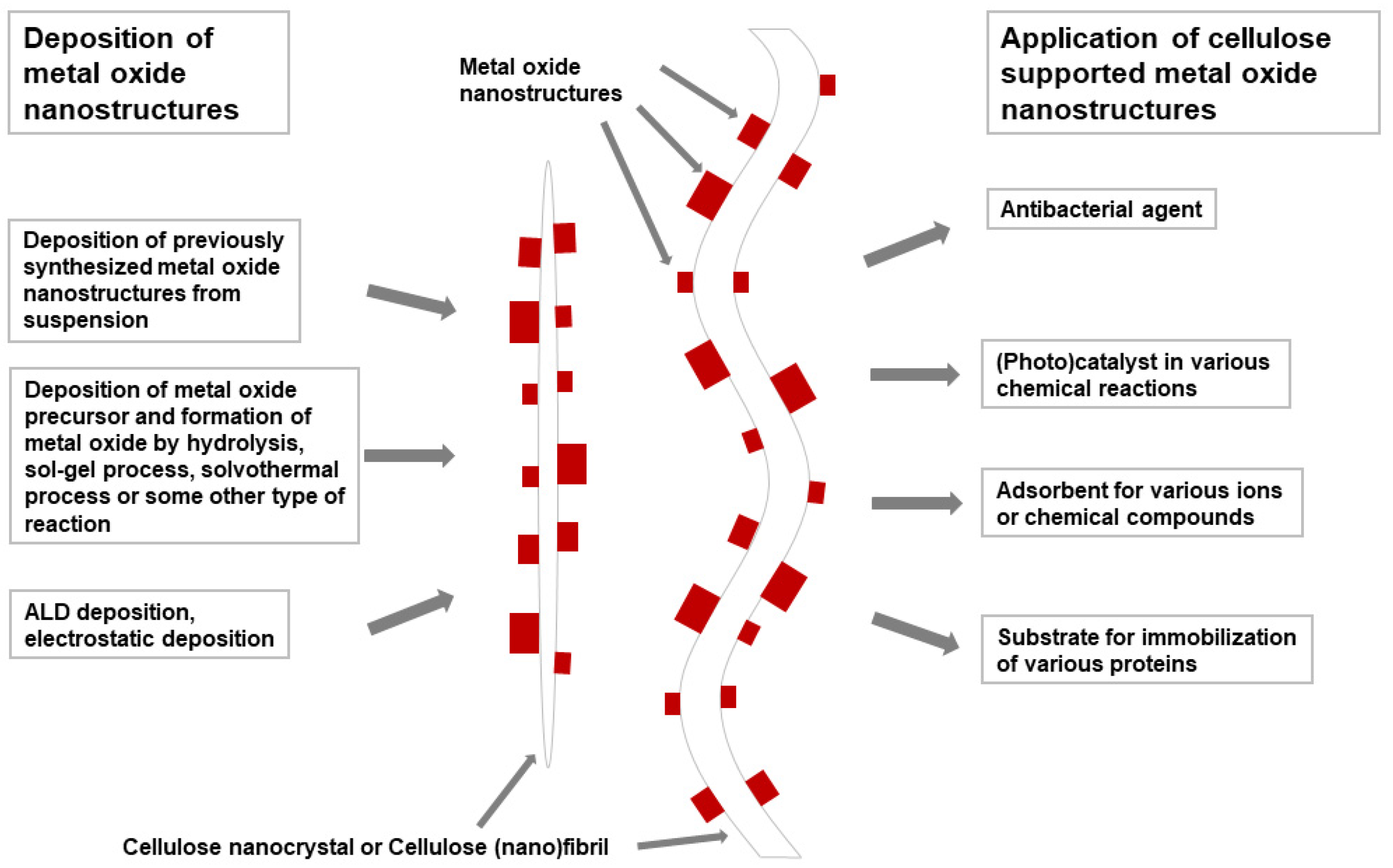
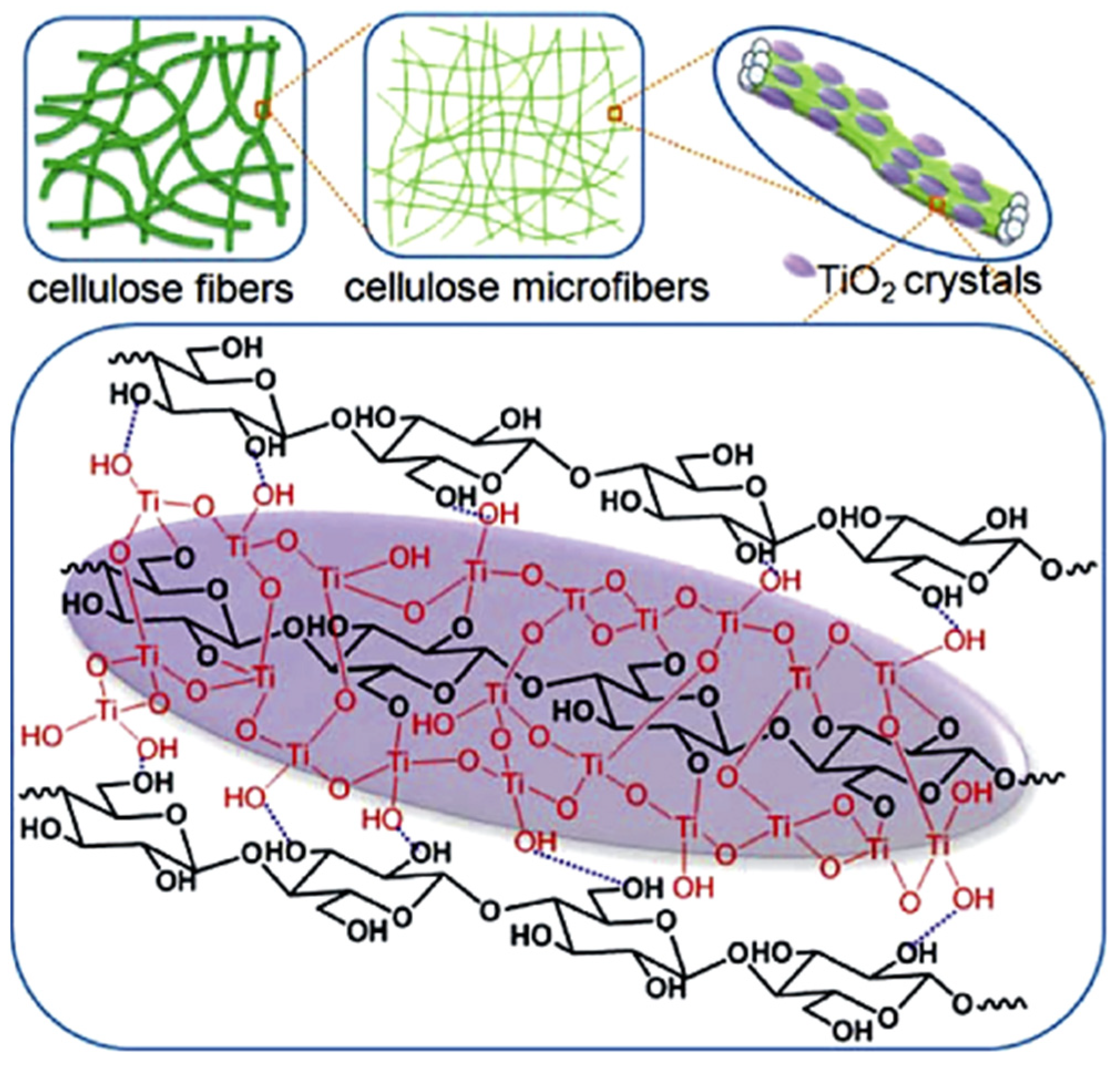
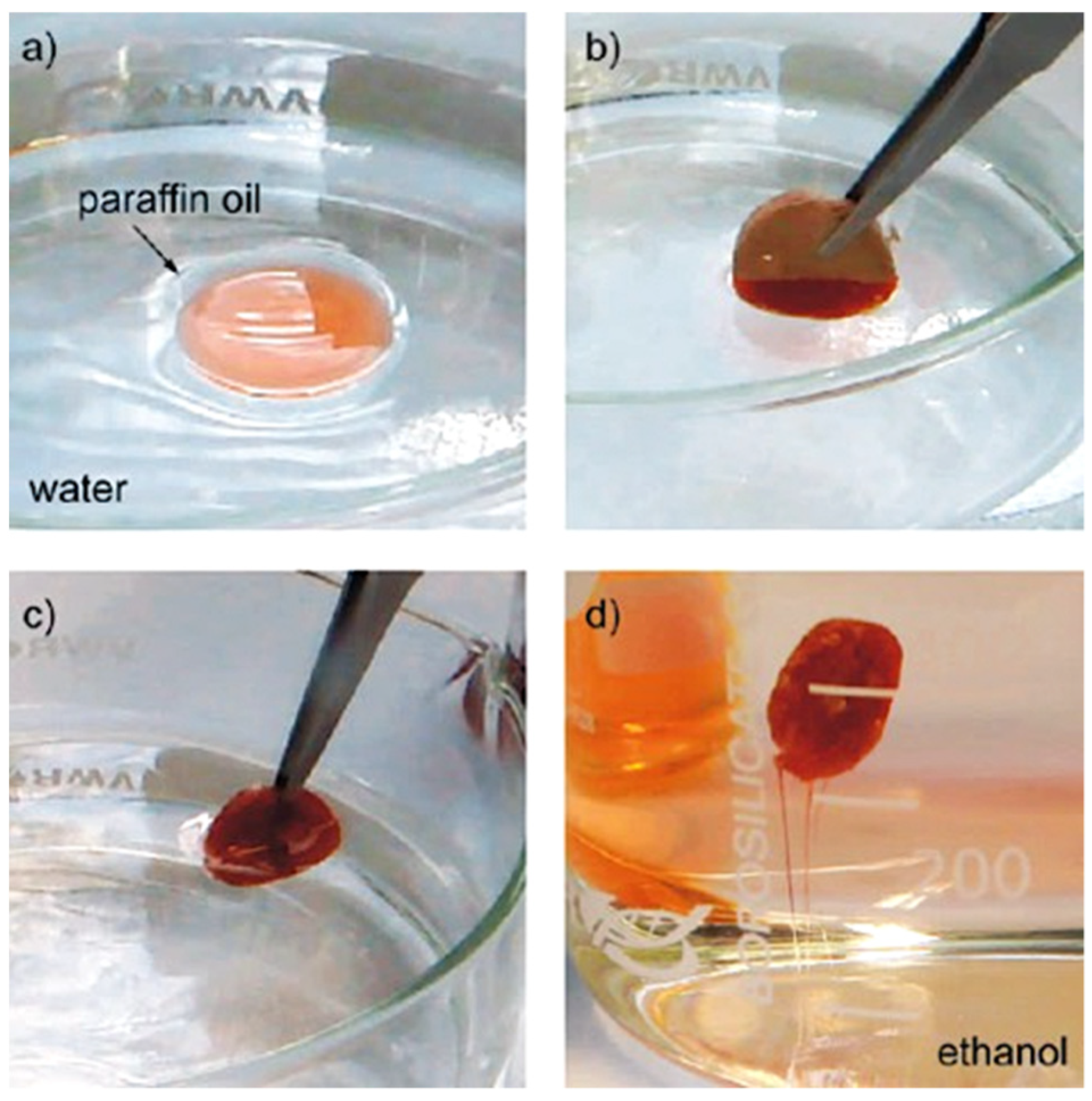
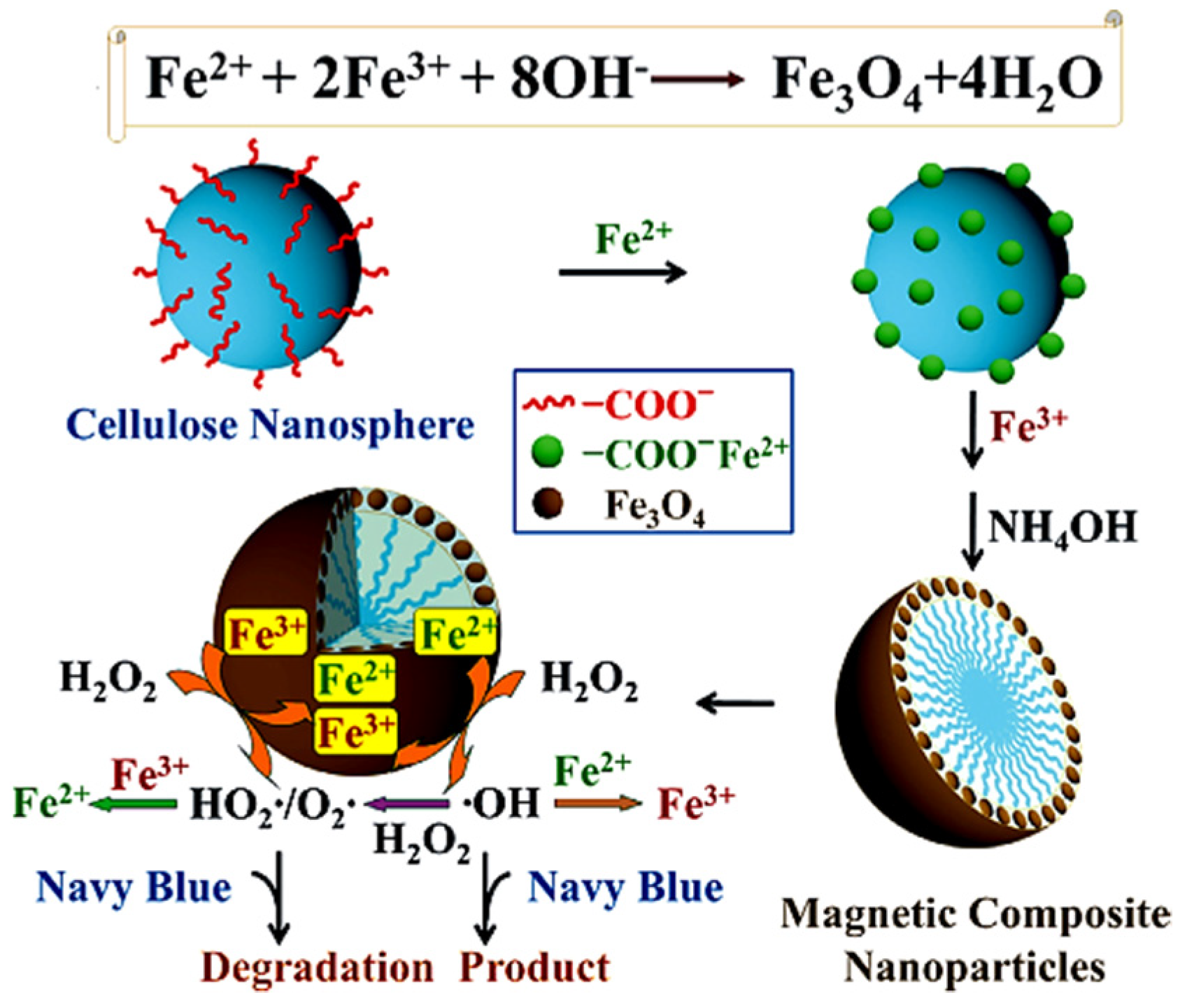
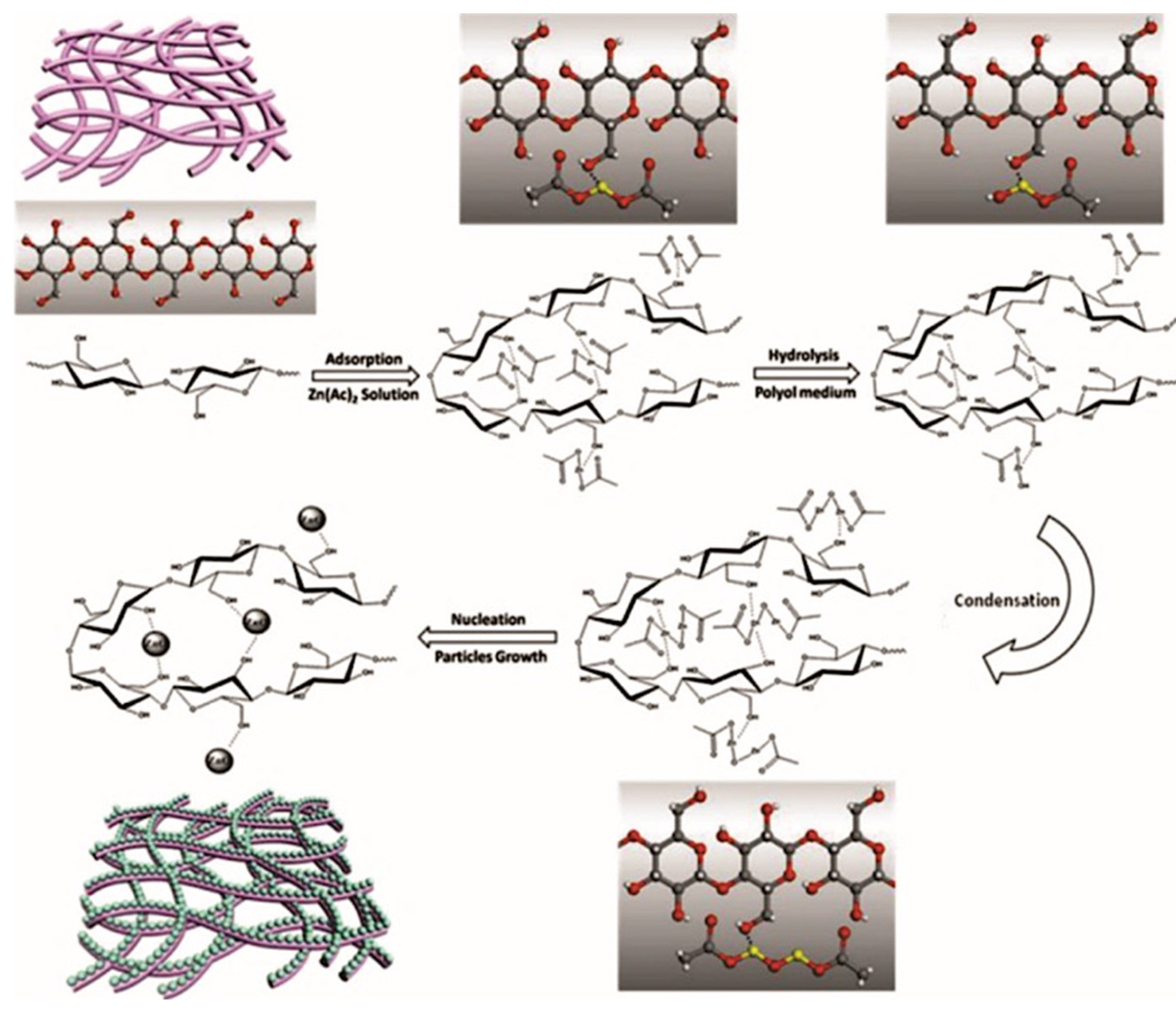
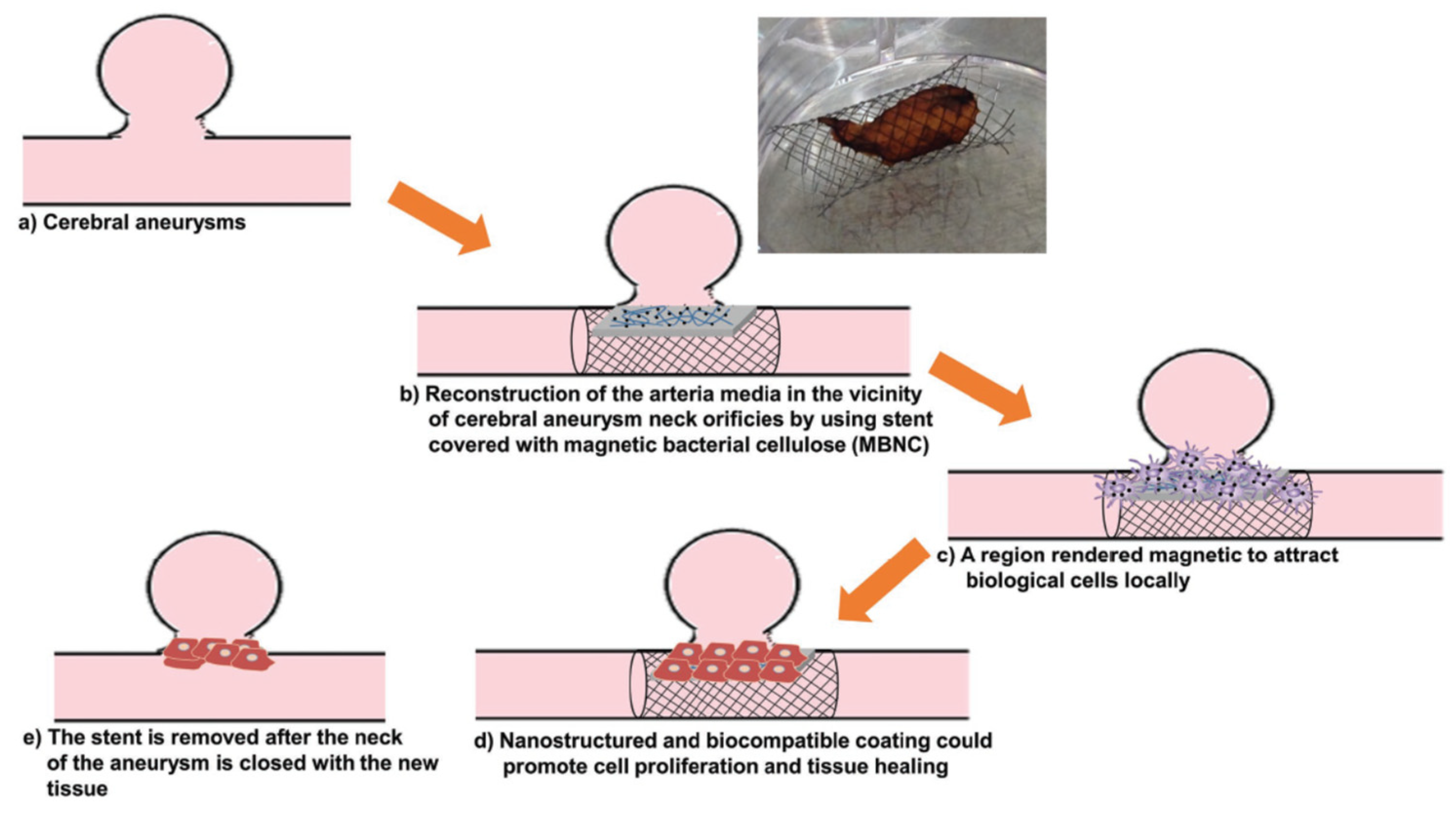
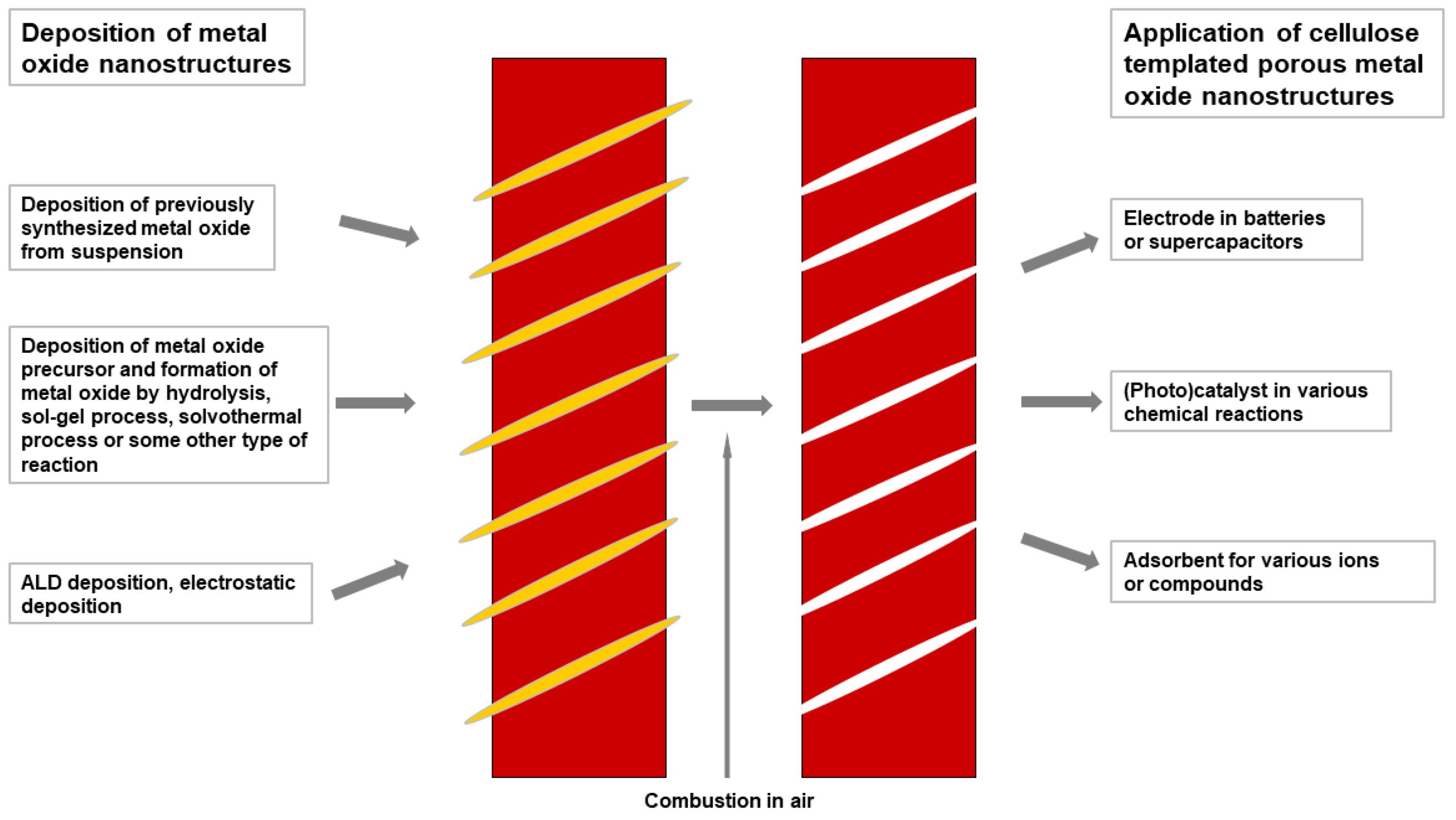
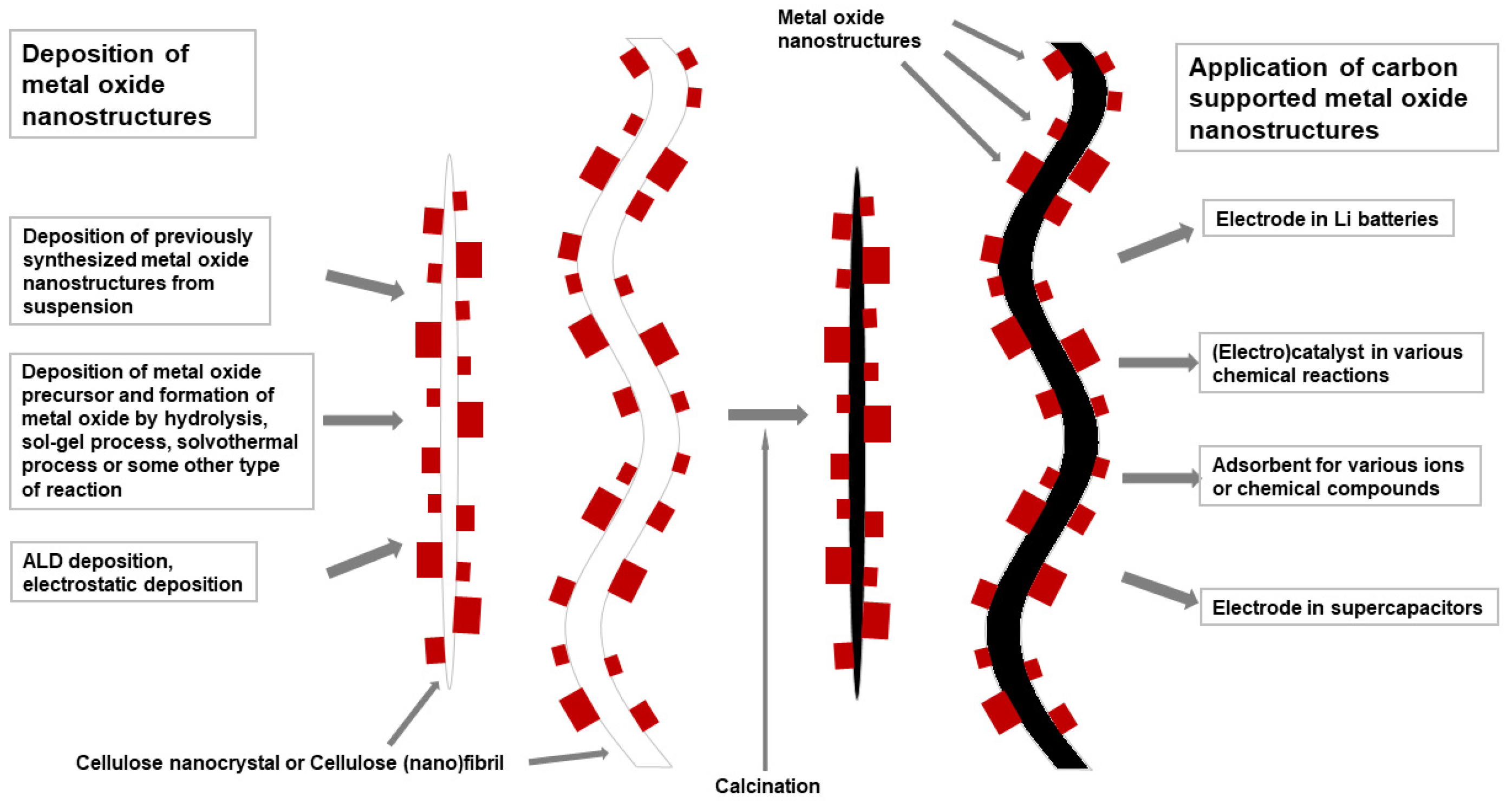
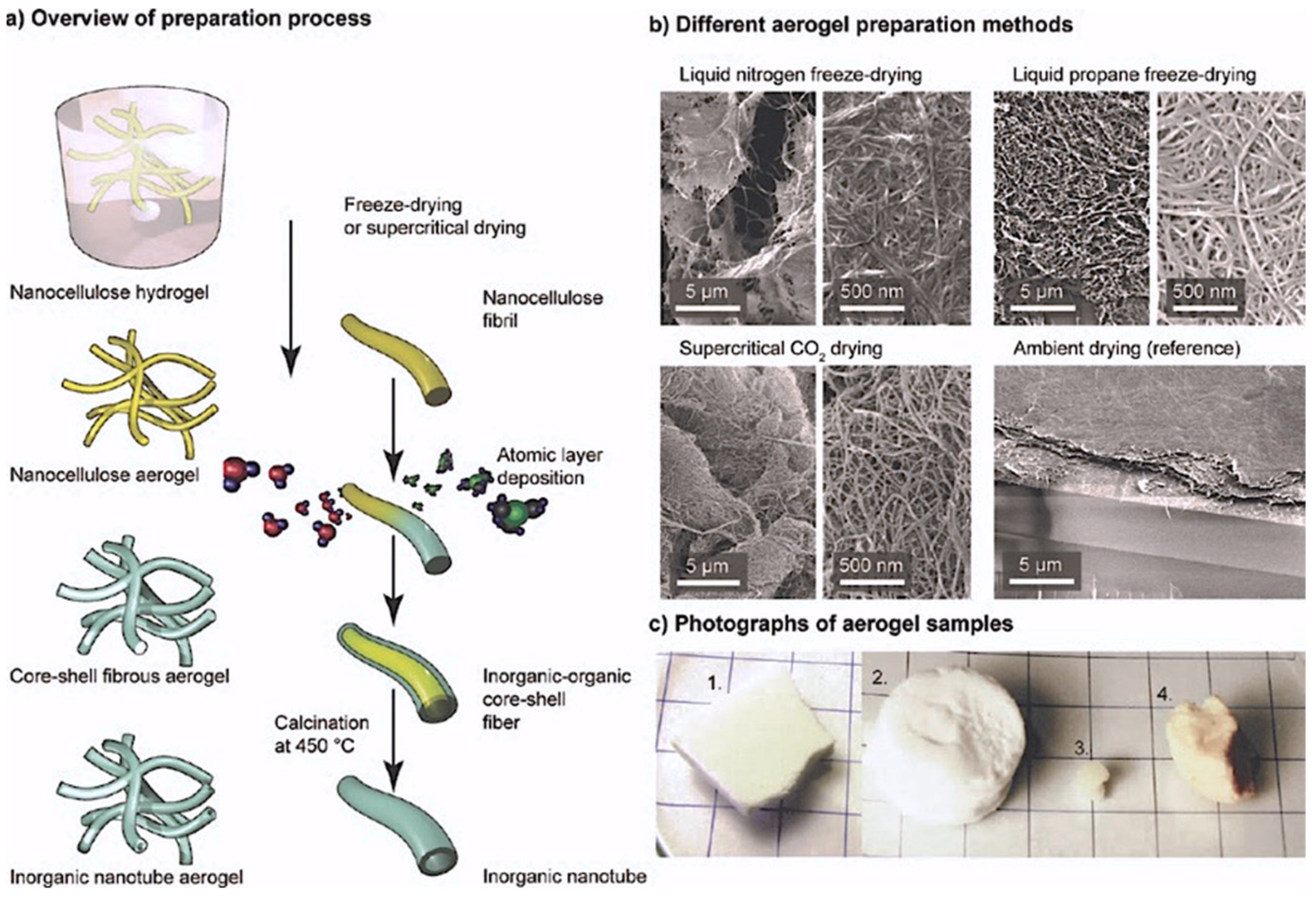

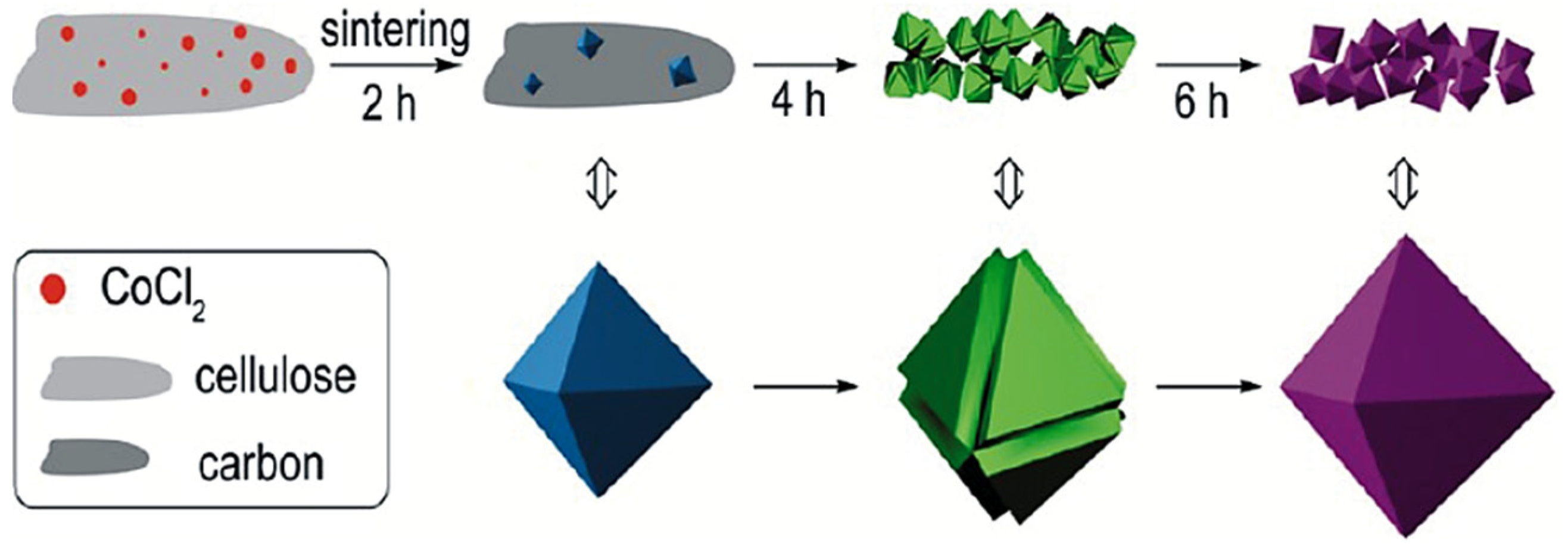
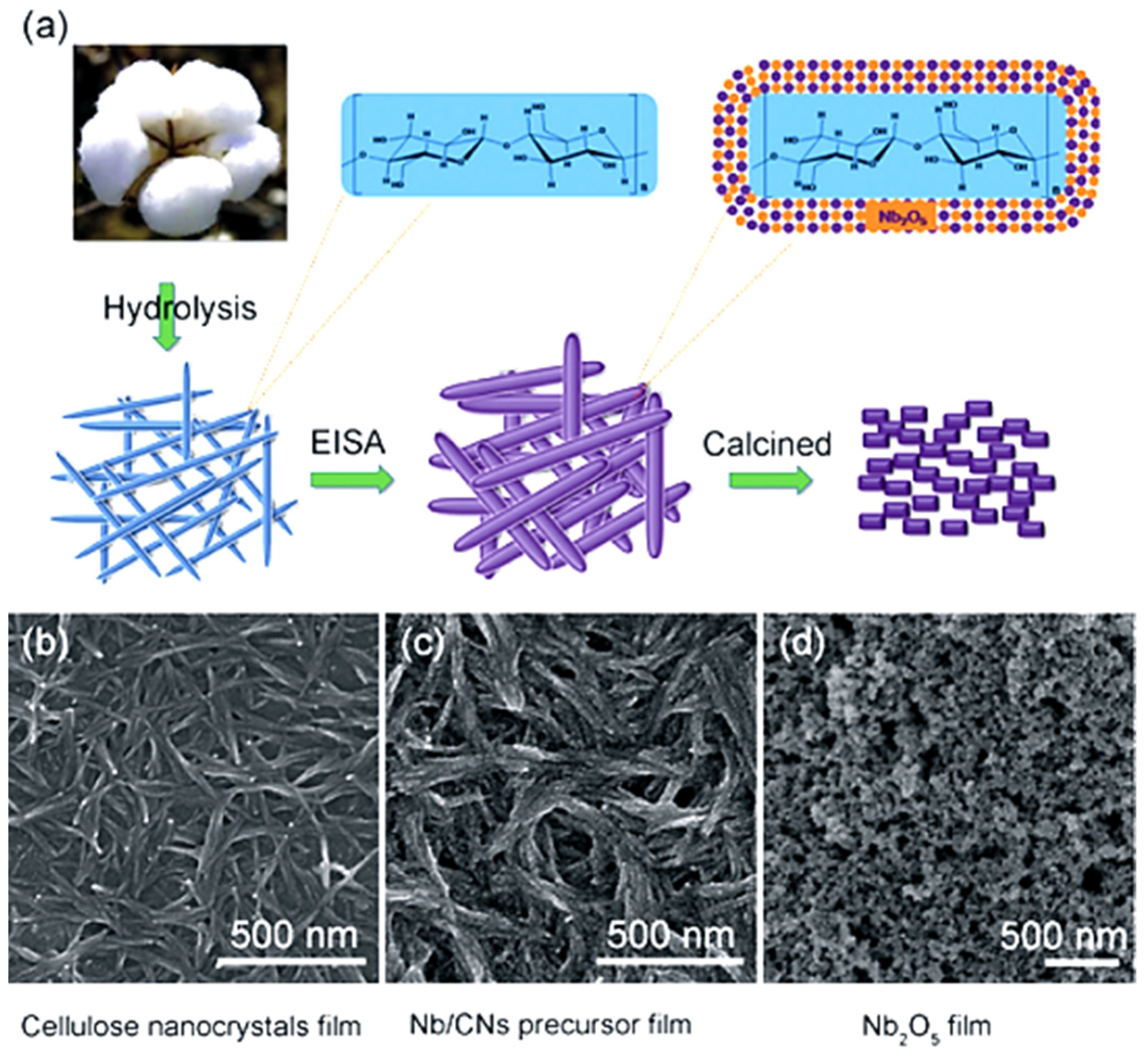
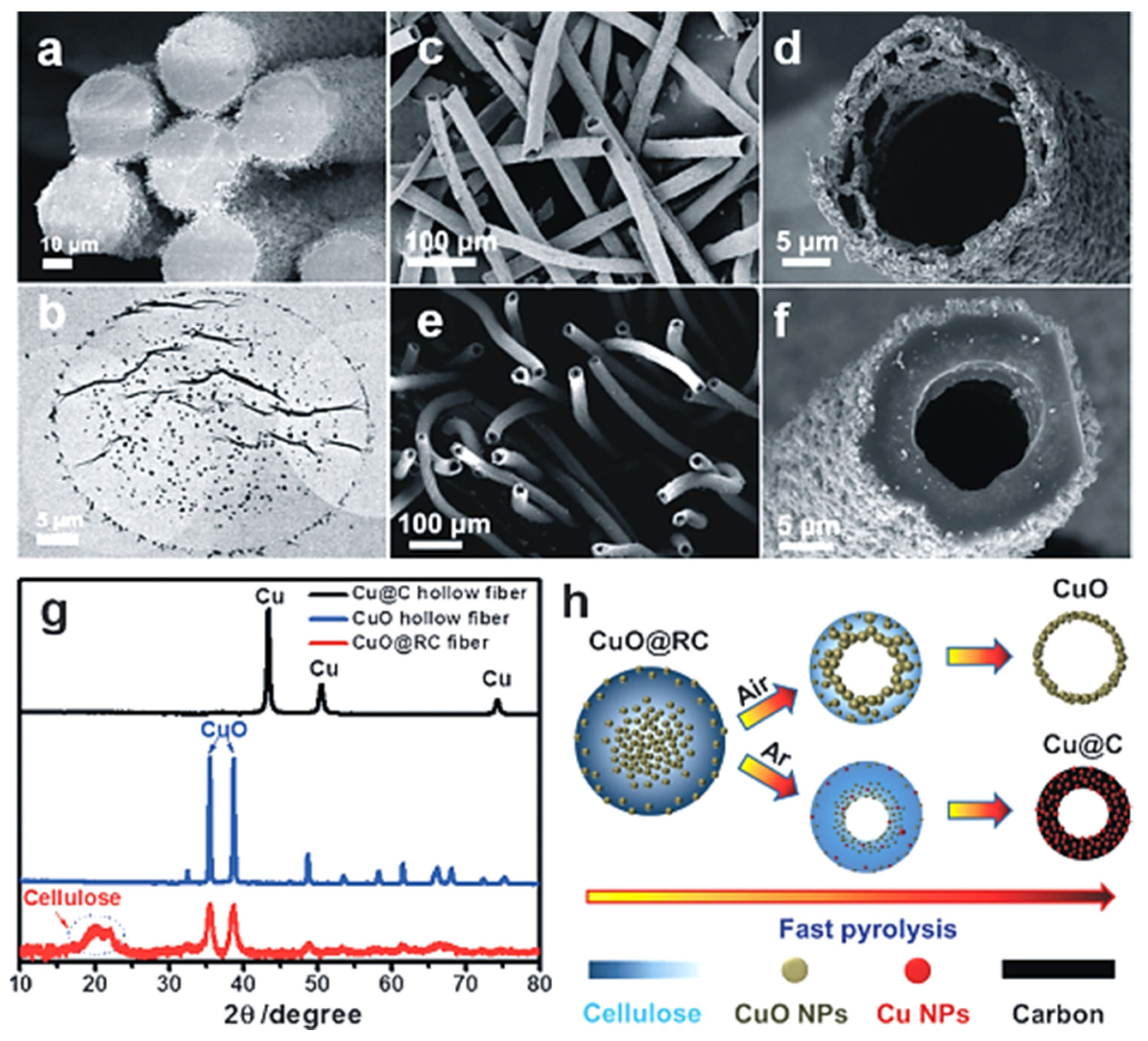
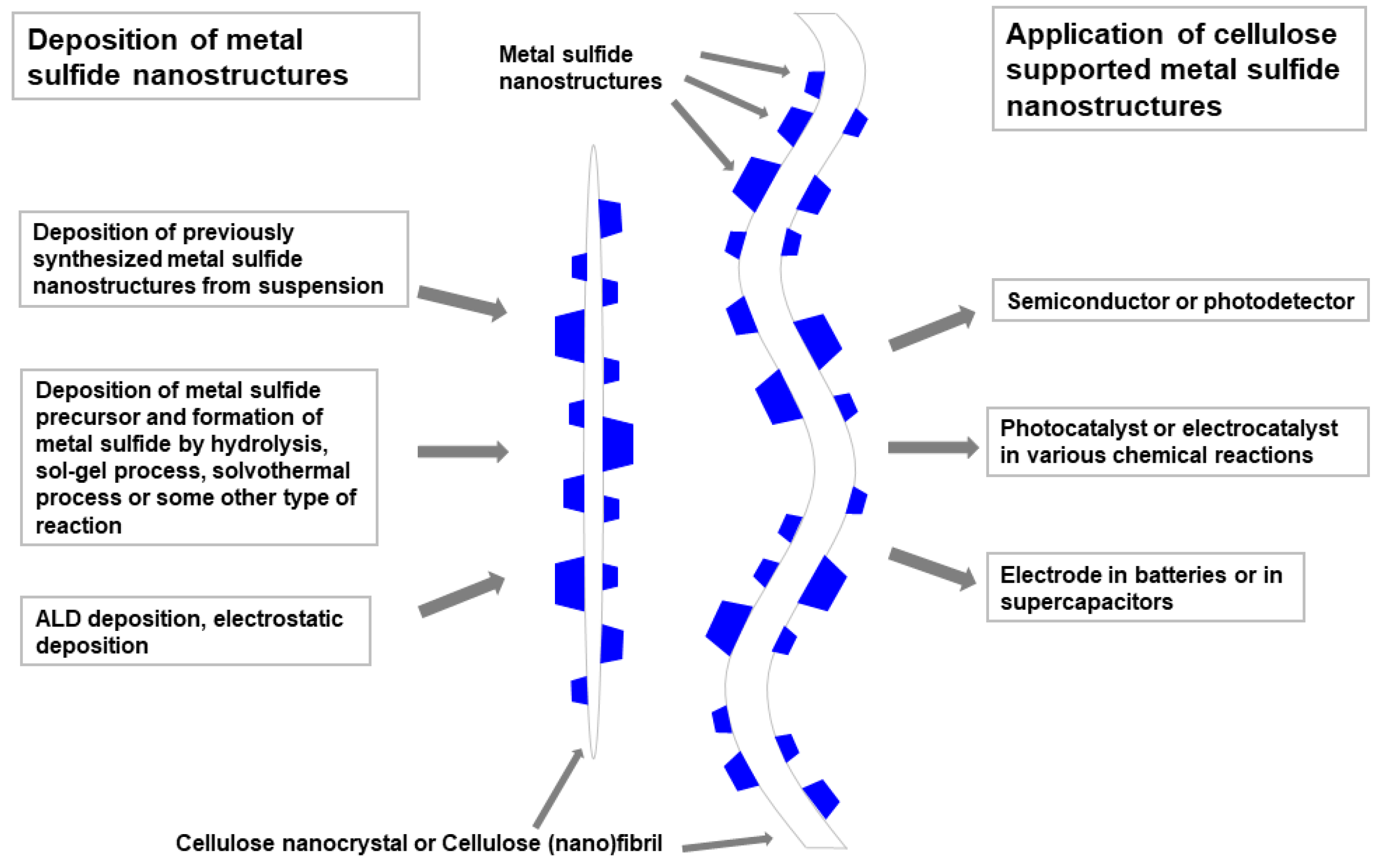
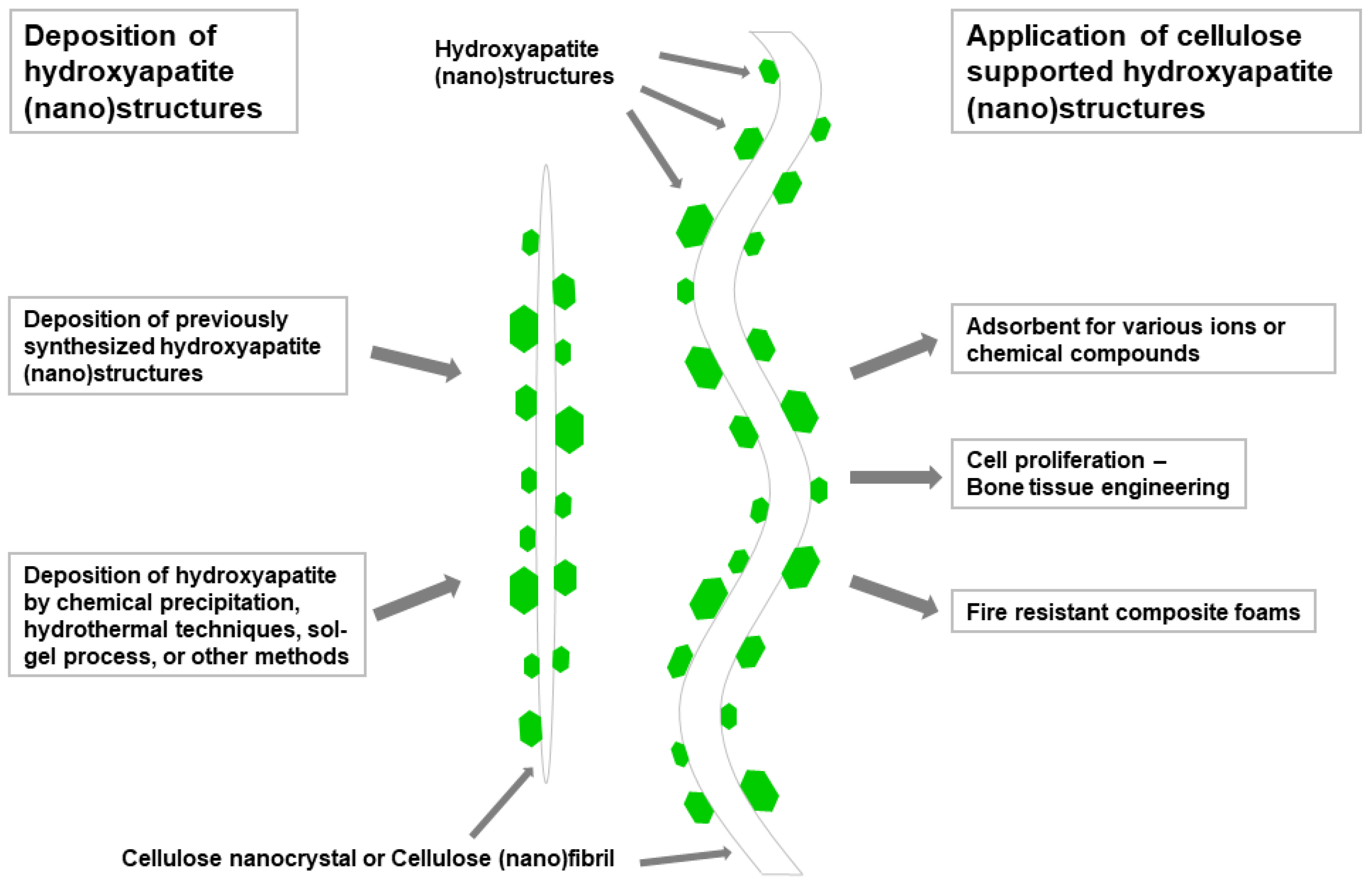
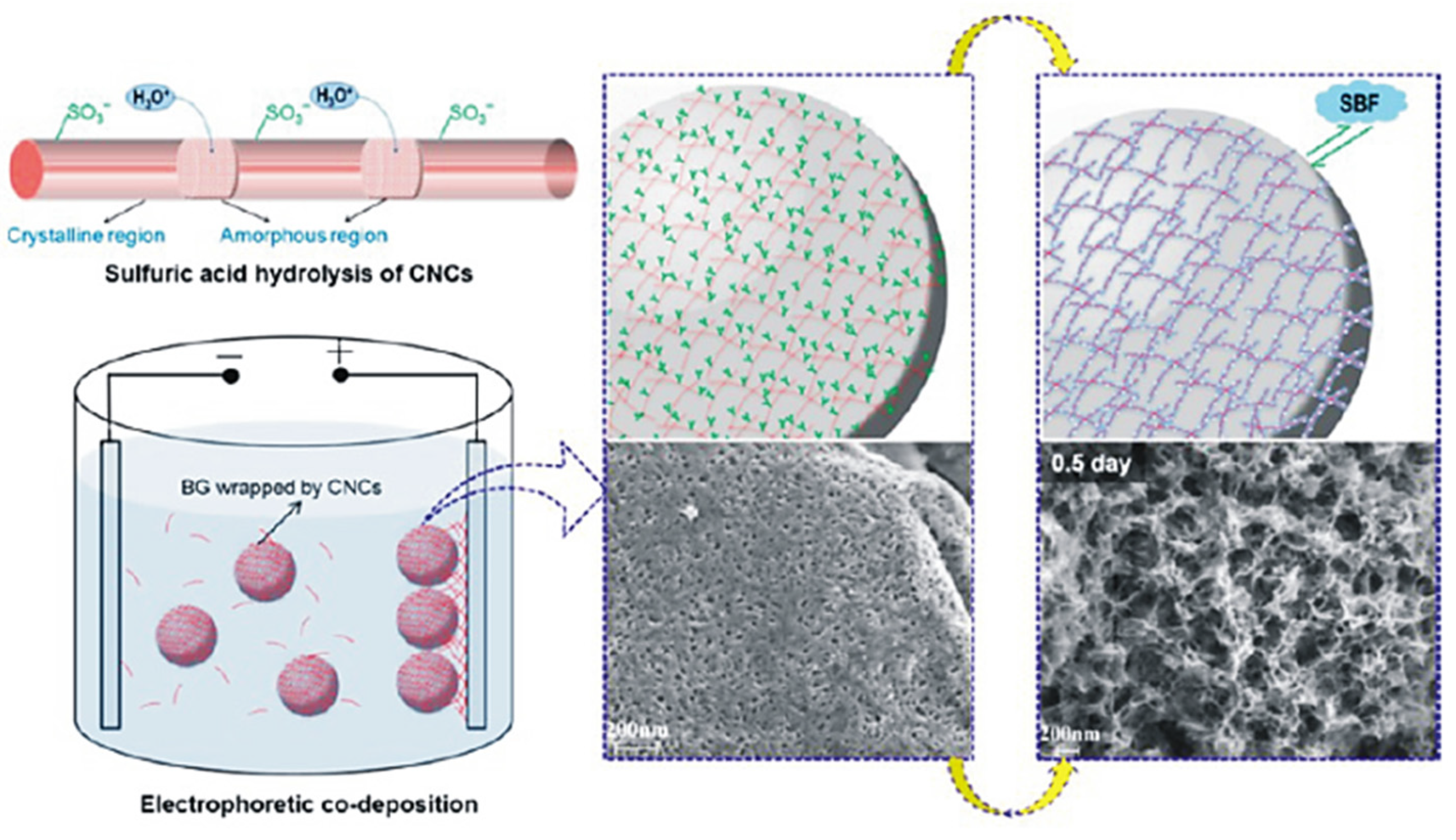
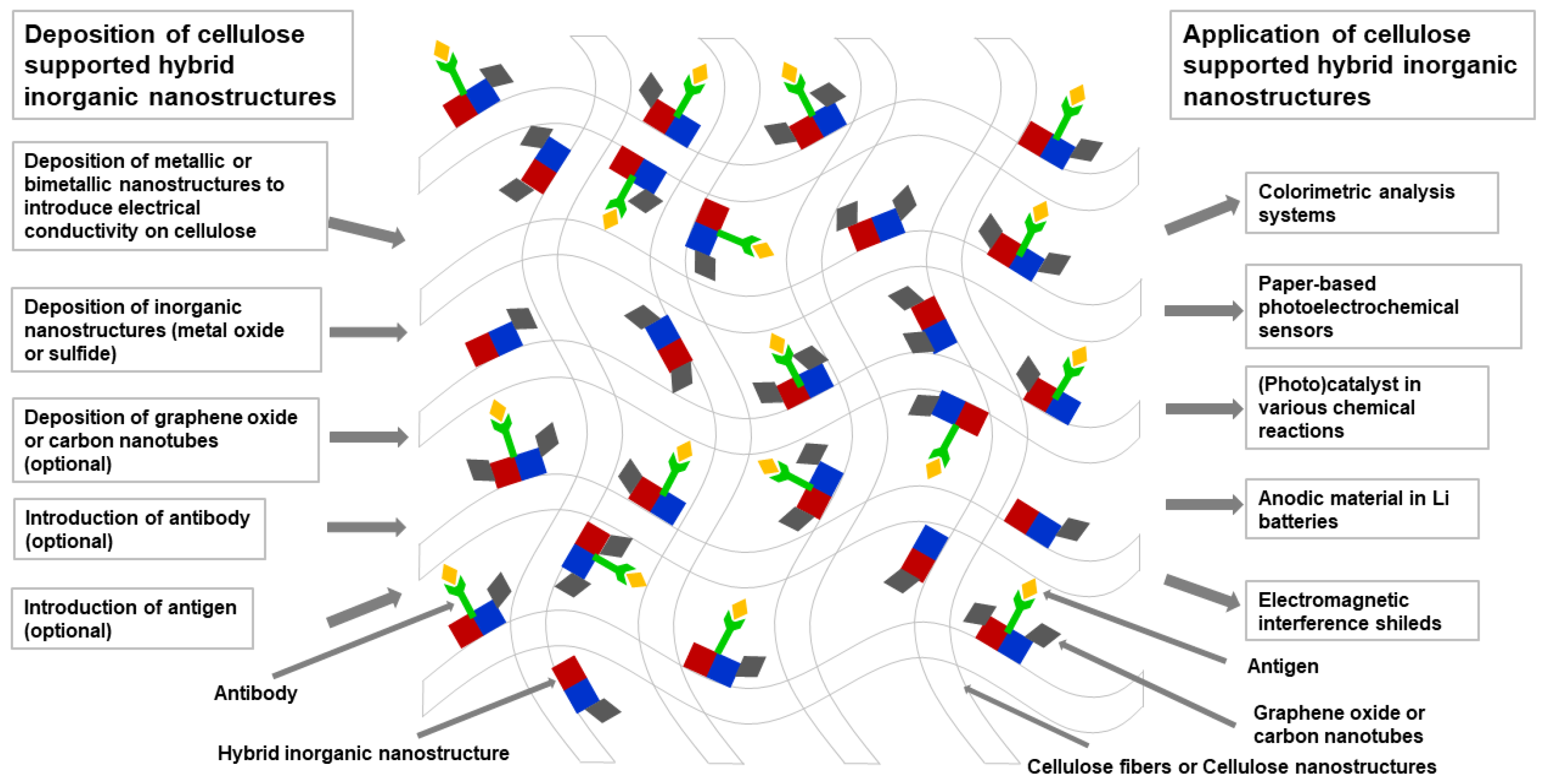
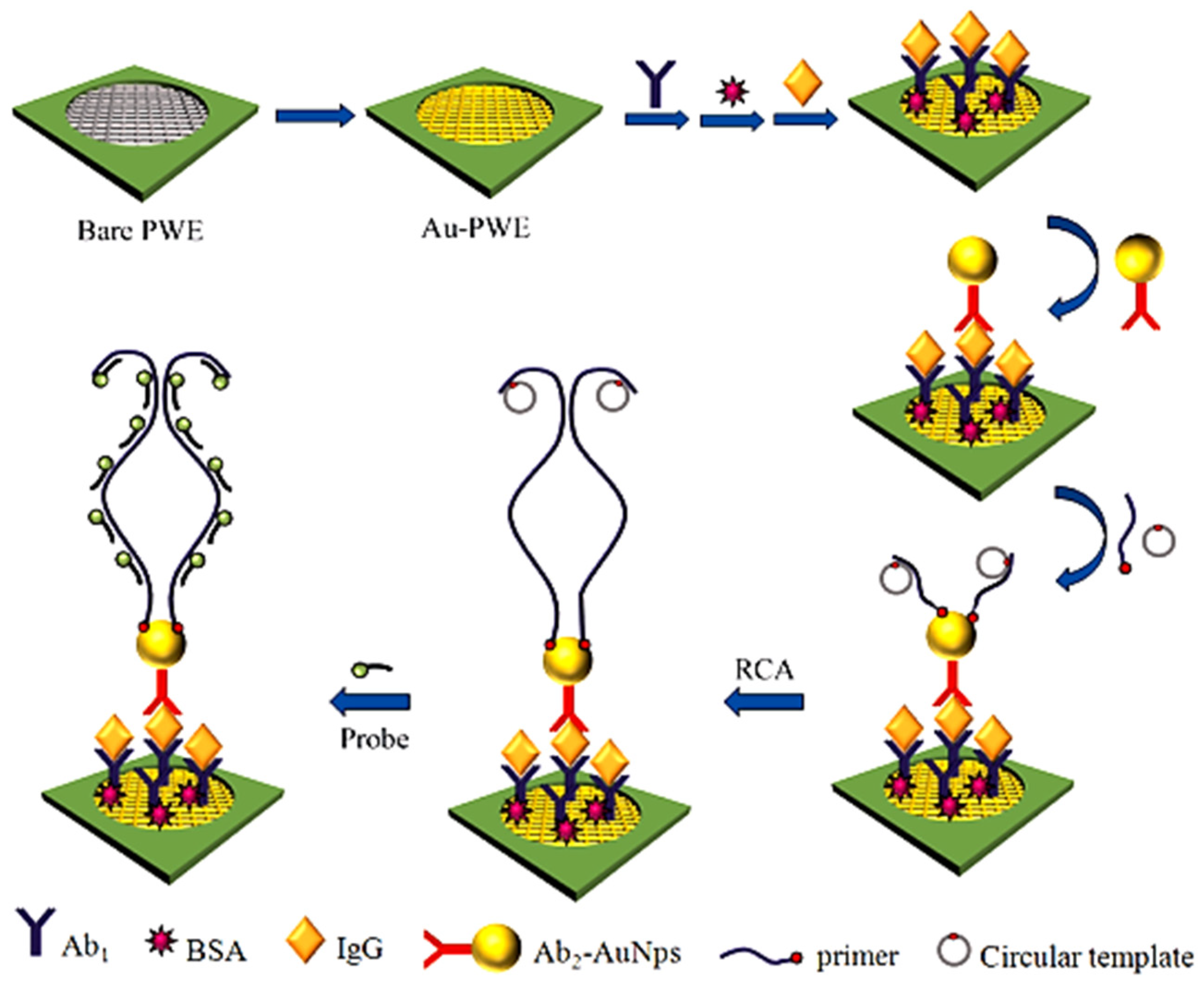
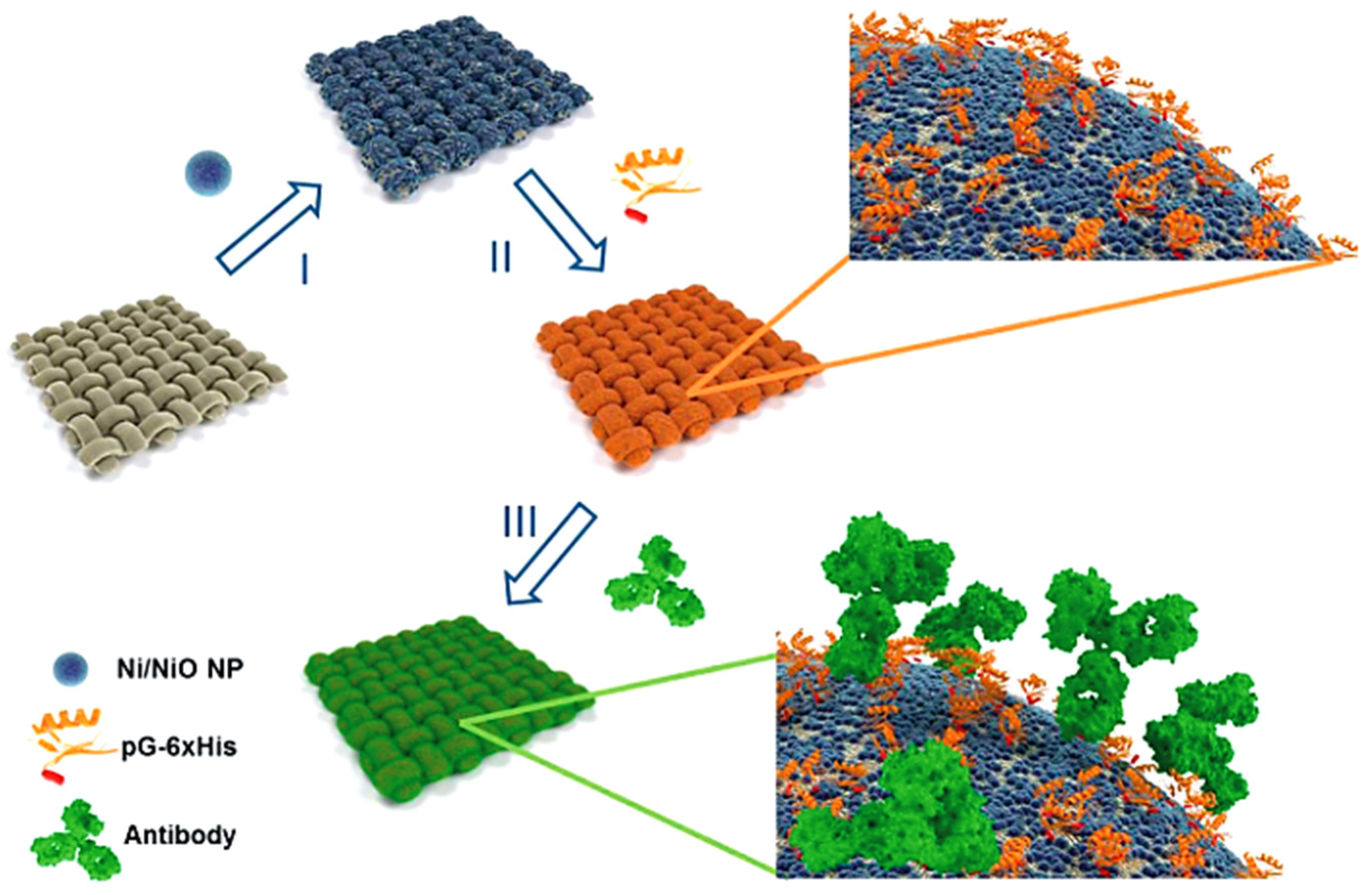
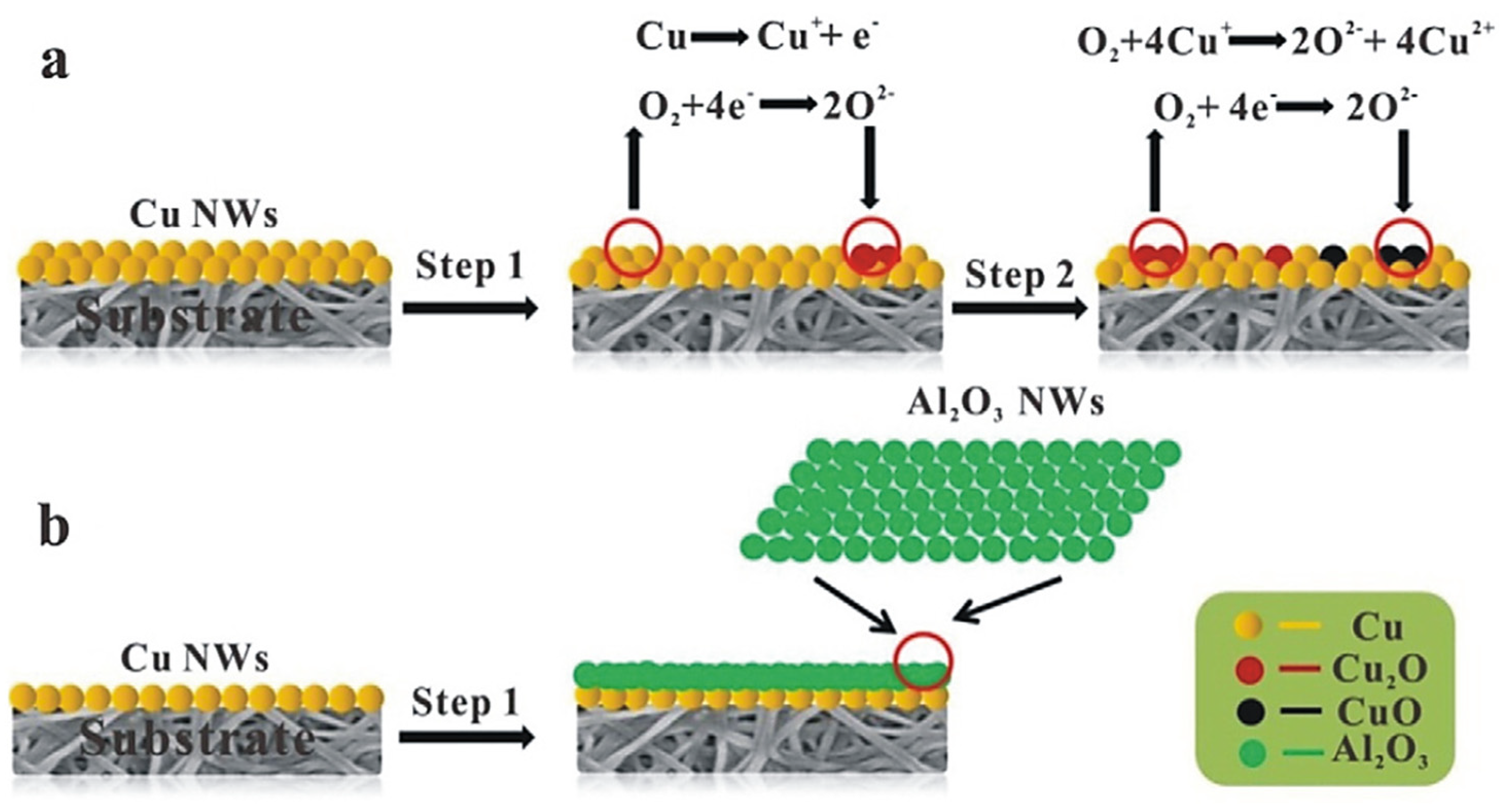
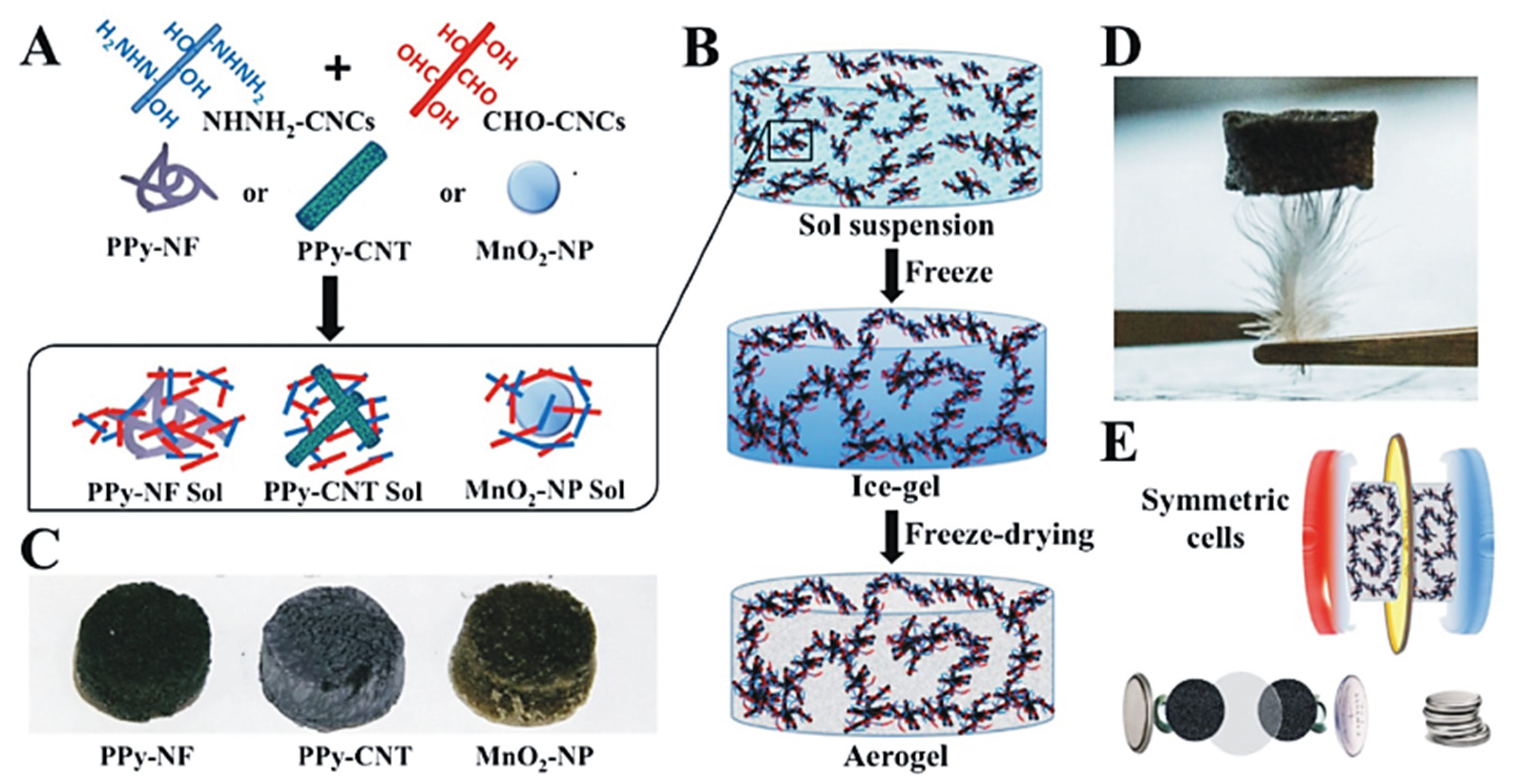
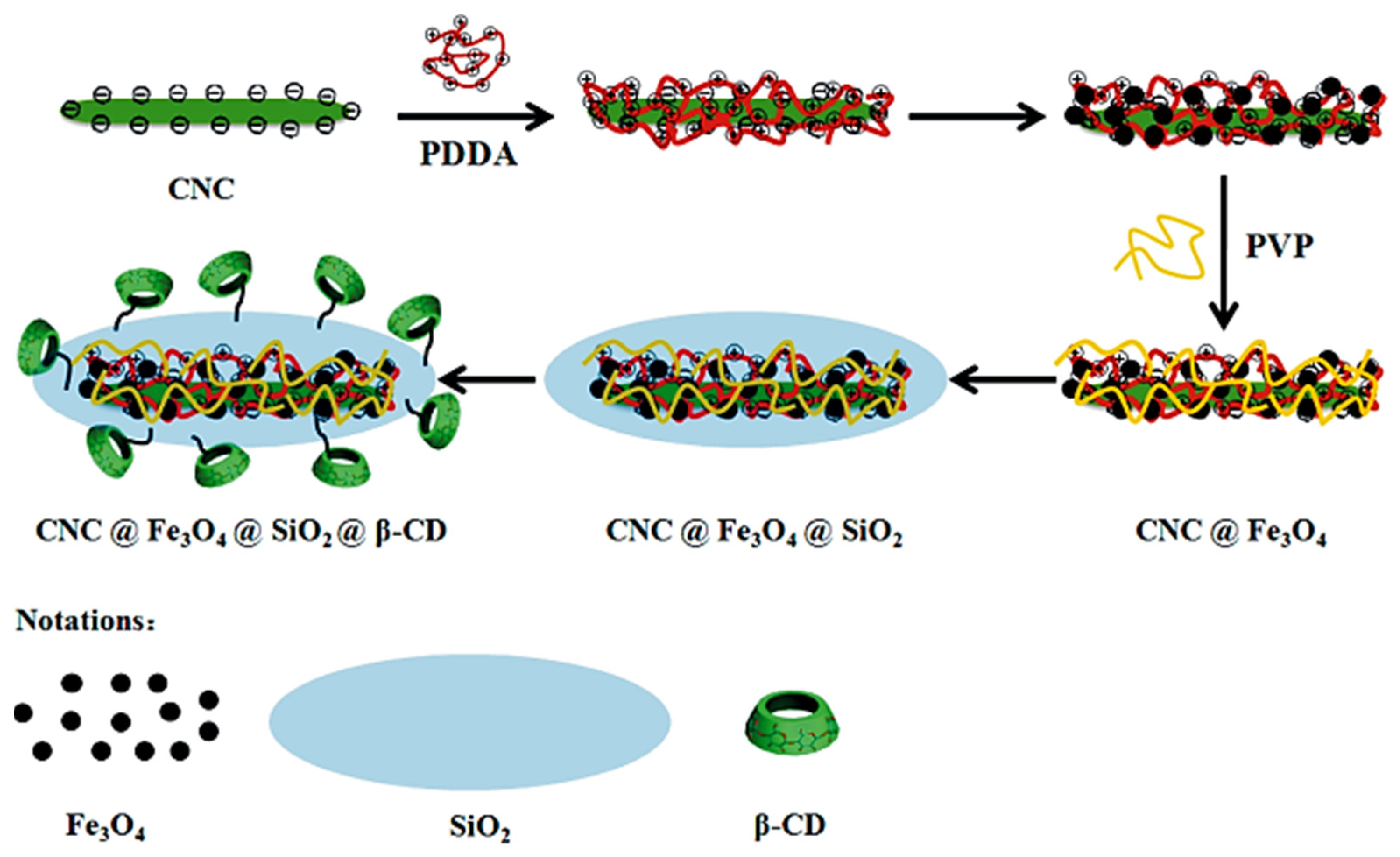
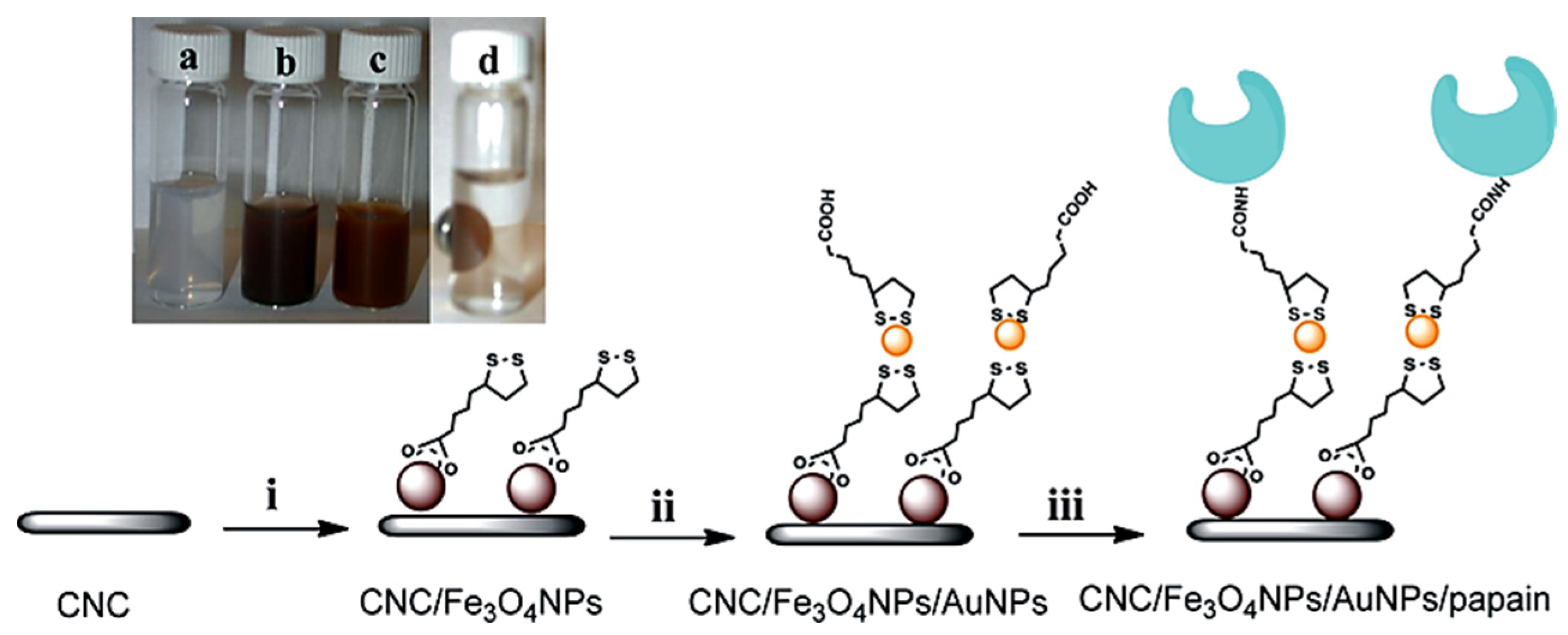
| Inorg. Particle (Size—nm) | Cellulose Used | Precursor (Synthetic Path) | Application | Reference |
|---|---|---|---|---|
| Precious Metallic Cellulose Supported Nanostructures | ||||
| Au NPs (~2 nm) | CF | Bis(ethylenediamine) Au(III)Cl3, (CH3)2Au(III) acetylacet.,(red.by NaBH4) | Catalyst (glucose oxidation) | [88] |
| Ag NPs (8–20 nm) | RC | AgNO3 (hydrothermal reduction) | Catalyst (reduction of 4-nitrophenol) | [89] |
| Au NPs (<5 nm) | MFC | HAuCl4 (reduction by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [90] |
| Au–Ag NPs | MFC | Conductive nanofiller | [91] | |
| Au–Pd NPs (4–9 nm) | MFC | HAuCl4, [Pd(NH3)4]·Cl2 (reduction by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [92] |
| Au/Ag NPs | BNC | HAuCl4, AgNO3 (reduc. by poly(ethyleneimine) | - | [93] |
| Au NPs | BNC | HAuCl4 (reduction by cellulose) | Conductive nanofiller | [94] |
| AuNR@AgNCs | BNC | HAuCl4, NaBH4, AgNO3, CTAC | SERS–detection of TNT | [95] |
| Au NPs (2–10 nm) | CNCs | HAuCl4, trisodium citrate, oleylamine, mercaptocation | Chiral photonic materials | [96] |
| Au NPs (20–30 nm) | CNCs | HAuCl4, NaOH (reduction by CNCs) | Photothermal nanocomposite materials | [97] |
| Au NPs (30.5 nm) | CNCs | HAuCl4 (reduction by cellulose) | Catalyst (reduction of 4-nitrophenol) | [98] |
| Au NPs (4.5–7.1 nm) | CNCs | HAuCl4 (reduction by NaBH4) | Biosensor (for 2-mercaptoethanol) | [99] |
| Au NPs (2–3 nm) | CNCs | HAuCl4 (reduction by—HS groups on the CNC surface) | Catalyst (alkyne–aldehyde–amine-coupling) | [100] |
| Au NPs (2–4 nm) | CNCs | HAuCl4 (reduced with no and by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [101] |
| Au NPs (~3 nm) | CNCs | HAuCl4 (reduction by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [102] |
| Au, Ag NPs | CNCs | HAuCl4, AgNO3, ascorbic acid | Catalyst (reduction of 4-nitrophenol, 4-aminophenol) | [103] |
| Au NPs(30–80 nm) | CNF | HAuCl4, Na3Cit·2H2O | Substrate–SERS spectroscopy | [104] |
| Au NPs (30–80 nm) | CNCs | HAuCl4, K2CO3, NaOH | Seeds for Au coating of CNCs with tunable optical properties | [105] |
| Au NPs (35 nm) | CNCs | HAuCl4 (reduction by trisodium citrate) | Sorption and detection of Au nanoparticles in H2O | [106] |
| Pt NPs (1–5 nm) | CF | H2PtCl6, Na rhodizonate | Catalyst (reduction of 4-nitrophenol, methyl orange) | [107] |
| Pt NPs | CNF | H2PtCl6, (reduction by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [108] |
| Pt NPs (5–30 nm) | CNCs | H2PtCl6 (reduction by CNCs) | — | [109] |
| Pt NPs (11–101 nm) | CNCs | H2PtCl6 (reduction by wood nanomaterial) | Catalyst (reduction of 4-nitrophenol) | [110] |
| Pt NPs (~2 nm); | CNCs | H2PtCl6 (reduction by CNCs) | Electrocatalyst (oxygen reduction) | [111] |
| Pd NPs (~20 nm) | BNC | PdCl2 (reduction by KBH4) | Catalyst (Heck reaction) | [112] |
| Pd NPs (~20 nm) | BNC | K2PdCl4 (reduction by NaBH4) | Catalyst (Suzuki–Miyaura reaction) | [113] |
| Pd NPs (3.6 nm) | CNCs | PdCl2 (reduction by H2) | Catalyst (hydrogenation of phenol; Heck Coupling) | [114] |
| Pd NPs (1–7 nm) | CNCs | PdCl2 (reduction by CNCs) | Catalyst (red. of methylene blue and 4-nitrophenol) | [115] |
| Pd-Cu NPs | BNC | PdCl2 and CuCl2 (reduction by KBH4) | Catalyst (water denitrification) | [116] |
| Ru NPs (~8 nm) | MFC | RuCl3 (reduction by NaBH4) | Catalyst (aerobic oxidation of benzyl alcohol) | [117] |
| Ag-Au NPs (8–10 nm) | CF | AgNO3, HAuCl4, NaOH, urea | Antibacterial agent | [118] |
| Ag NPs | CF | RF sputtering | Antibacterial agent | [119] |
| Ag NPs (3–15 nm) | MCC | AgNO3, UV light reduction | Catalyst (reduction of p-nitrophen. to p-aminophen.) | [120] |
| Ag NPs (~6 nm) | MFC | AgNO3 (reduction by NaBH4) | Catalyst (reduction of rhodamine B) | [121] |
| Ag nanowires | MFC | Previously formed Ag nanowires | Conductor in transparent nanopaper | [122] |
| Ag NPs (3–4 nm) | MFC | AgNO3, UV light triggered reduction by MFC | Aerogels | [123] |
| Ag nanowires | MFC | Previously formed Ag nanowires | Conductive nanofiller | [124] |
| Ag NPs (~4 nm) | MFC | AgNO3 (reduction by NaBH4) | Catalyst (aza–Michael reaction) | [117] |
| Ag NPs (~6 nm) | MFC | AgNO3 (reduction by NaBH4) | Antibacterial agent | [125] |
| Ag nanowires | MFC | - | Conductive nanofiller | [126] |
| Ag NPs | BNC | AgNO3 (reduction by sodium citrate) | SERS substrate-pesticides detection | [127] |
| Ag NPs (var. sizes) | CF, MCC, CNCs | AgNO3, (reduction by cellulose) | - | [128] |
| Ag NPs (<10 nm) | BNC | AgNO3 (reduction by NaBH4) | Antibacterial agent | [129] |
| Ag NPs (17 nm) | BNC | AgNO3 (reduction by cellulose) | Antibacterial agent | [130] |
| Ag NPs (~30 nm) | BNC | AgNO3 (red. by NH2NH2, NH2OH, ascorbic acid) | Antibacterial agent | [131] |
| Ag NPs (8–15 nm) | BNC | AgNO3 (reduced by triethanolamine) | Antibacterial agent | [132] |
| Ag NPs (~16 nm) | BNC | AgNO3, (reduction by BNC) | Antibacterial agent | [133] |
| Ag NPs (5– 50 nm) | CNFs | AgNO3 (reduction by Na citrate) | Flusilazole adsorption and analysis | [134] |
| Ag NPs | CNFs | AgNO3 (reduction by Na citrate) | SERS probe for carbendiazim | [135] |
| Ag NPs (10–50 nm) | CNCs | AgNO3 (reduction by CNC) | Electrocatalyst (reduction of oxygen) | [136] |
| Ag NPs (<10 nm) | CNCs | AgNO3 (reduction by NaBH4) | DNA biosensor | [137] |
| Ag NPs (10–15 nm) | CNCs | AgNO3 (reduction by NaBH4) | Antibacterial agent | [138] |
| Ag NPs (1 nm–10 μm) | CNCs | AgNO3 (reduction by CNCs) | Antibacterial agent | [139] |
| Ag NPs (20–45 nm) | CNCs | AgNO3 (reduction by CNCs) | Antibacterial agent | [140] |
| Ag NPs (2–3 nm) | CNCs | AgNO3 (reduction by NaBH4) | — | [141] |
| Ag NPs (~10 nm) | CNCs | AgNO3 (reduction by dopamine) | Catalyst (reduction of 4-nitrophenol) | [142] |
| Ag NPs (~7 nm) | CNCs | AgNO3 (reduction with dopamine hydrochloride) | Antibacterial agent | [143] |
| Ag NPs (10–80 nm) | CNCs | AgNO3 (reduction by NaBH4) | — | [144] |
| Ag NPs (1–2 nm) | CNCs | Ag wire and AgNO3 (reduction by CNCs) | Catalyst (hydrogenation of aldehydes, nitrophenol, alkenes and alkynes) | [145] |
| Ag NPs (10–50 nm) | CNCs | AgNO3 (reduction by NaBH4) | Plasmonic activators for shape memory polymers | [146] |
| Semi- and non-precious cellulose supported metallic nanostructures | ||||
| Cu NPs | CF | CuSO4, poly(ethylenimine) | - | [147] |
| Cu NPs (~5 nm) | MFC | CuCl2 (reduction by ascorbic acid) | Catalyst for the reduction of 4-nitrophenol | [148] |
| Cu NPs | CNCs | Cu(OCOCH3)2, NaOH (reduction by ascorb. acid) | Conductive nanofiller | [149] |
| Cu NPs (50 nm) | CNCs | CuSO4, (reduction by ascorbic acid and NaBH4) | Catalyst for C–N coupling reactions | [150] |
| Cu NPs (10–20 nm) | CNCs | CuSO4 (reduction by hydrazine) | Catalyst for oxidation of sulfides and alcohols | [151] |
| Fe NPs (200–300 nm) | CF | FeSO4 (reduction by NaBH4) | Adsorbent for Cd(II) ions | [152] |
| Entry [a] | SH Loading | Yield [b] | Cu Adsorbed |
|---|---|---|---|
| (mol%) | [%] | [%] | |
| 1 | 0 | <5 | 0 |
| 2 | 3.2 | 41 | 4 |
| 3 | 8 | 43 | 58 |
| 4 | 16 | 87 | 94 |
| 5 | 24 | 88 | 97 |
| 6 | 32 | 91 | 97.5 |
| Inorg. Particle (Size—nm) | Cellulose Used | Precursor (Synthetic Path) | Application | Reference |
|---|---|---|---|---|
| Cellulose Supported TiO2 Nanostructures | ||||
| TiO2 NPs (5–100 nm) | CF | Ti(OBu)4, HCl (hydrothermal reaction) | Photocatalyst for CO2 reduction | [176] |
| TiO2 NPs (40–250 nm) | CF | Commercial TiO2 (hydrothermal treatment) | Photocatalyst, antibacterial agent | [177] |
| TiO2 NPs (~4 nm) | CF | TiCl4 (hydrothermal reaction—ultrasonication) | Photocatalyst in self-cleaning fabric | [178] |
| TiO2 NPs (10–20 nm) | CF | Ti(IV) isopropoxide (hydrothermal reaction) | Photocatalyst and antibacterial agent | [179] |
| TiO2 NPs | CF | TiOSO4, urea | Adsorbent for phosphate removal | [180] |
| TiO2 NPs | MCC | TiCl4, EtOH (sol–gel process) | Adsorbent for Pb2+, Cd2+, Zn2+ ions | [181] |
| TiO2 NPs (300–900 nm) | MCC | TiCl3, NH3 (hydrothermal reaction) | Photocatalyst (H2 production) | [182] |
| TiO2 nanorods (l.: 26 nm; w.: 16 nm) | MFC | Commercial TiO2 NPs | — | [183] |
| TiO2 NPs | MFC | Commercial TiO2 NPs | Antibacterial agent | [184] |
| TiO2 NPs-anatase | BNC | TiO2 powder, laser vaporization | Photocatalyst, purification of drinking water | [185] |
| TiO2 NPs (5–15 nm) | BNC | Ti(IV) n-butoxide, sol–gel process | Detector for phosphopeptides | [186] |
| TiO2 NPs | BNC | Ti(IV) n-butoxide Solvothermal process | Photocatalyst (methyl orange degradation) | [187] |
| TiO2 NPs | CNF | Commercial TiO2 | Photoanodes | [188] |
| TiO2 NPs | BNC | Ti(IV) isopropoxide, Sol-Gel process | Antibacterial agent | [189] |
| TiO2 NPs | BNC | Commercial TiO2 | Adsorbent for water contaminants | [190] |
| Black TiO2 NPs | BNC | Ti[OCH(CH3)2]4, microwave-assist. sonochem. process | Interfacial solar evaporator | [191] |
| TiO2 NPs | CNCs | TiOSO4 (hydrolysis with sulfuric acid) | UV absorber in skin care products | [192] |
| TiO2 NPs (50–100 nm) | CNCs | Commercial nano TiO2 | Drug release system | [193] |
| Cellulose supported iron oxide nanostructures | ||||
| Fe3O4 NPs | CF | FeSO4, NH3, H2O2, PEG, previously formed Fe3O4 | Magnetic fibers | [194] |
| Fe2O3 NPs (50–200 nm) | CF | FeSO4, NaOH (ultrasonication) | Magnetic paper | [195] |
| Fe3O4 NPs | CF | FeCl3, FeCl2, NaOH | Cancer treatment with 5-Fluorouracil | [196] |
| Fe2O3 NPs | CF | (NH4)2Fe(SO4)2, NaH2PO2, | Encapsulation and delivery-biologically active compounds | [197] |
| Fe2O3 nanoplatelet (48 nm) | RC | FeCl2, FeCl3 (reaction with NaOH) | — | [198] |
| Fe3O4 NPs | CF | FeCl2, FeCl3 (reaction with NaOH) | Immobilization of prenyltransferase NovQ | [199] |
| Fe3O4 NPs | MCC | FeSO4, FeCl3, NH3 | Biotechnology, water purification | [200] |
| Fe3O4 NPs (~3 nm) | MCC | FeCl3, FeCl2, NH3 | Antibacterial and contrasting agent | [201] |
| Fe3O4 NPs (~9 nm) | BNC | Fe(III) acetylacetonate, (microwave sol–gel process) | Magnetic paper | [202] |
| Fe3O4 NPs | BNC | Fe2(SO4)3, FeSO4, reaction with NaOH | Absorbing agent (removal of Cr(VI) ions) | [203] |
| Fe3O4 NPs | CNFs | Fe(NO3)3, FeSO4, NaOH, citric acid, nano TiO2 | Recoverable catalyst-H2 photogeneration | [204] |
| Fe3O4 NPs (~10 nm) | CNCs | FeCl2, FeCl3, NH3 (precipitation) | Substrate for protein separation | [205] |
| Fe3O4 NPs | CNCs | FeCl2 and FeCl3 reaction with ammonia | Targeted delivery of doxorubicin | [206] |
| Fe3O4 NPs | CNCs | FeCl2, FeCl3 (reaction with ammonia) | Semiconductor in antistatic paper | [207] |
| Cellulose supported ZnO nanostructures | ||||
| ZnO nanorods | CF | (Zn(CH3COO)2, hexamethylenetetramine | Antibacterial agent in packaging | [208] |
| ZnO nanowires | CF | Previously synthesized ZnO NWs, Zn(CH3COO)2 (solvothermal hydrolysis) | Piezoelectric accelerometer | [209] |
| ZnO NPs (3–30 nm) | CF | Zn(CH3COO)2, reaction with NaOH | Antibacterial agent | [210] |
| ZnO nanorods | CF | Zn(NO3)2 (hydrothermal growth) | Semi-conductor | [211] |
| ZnO rods | CF | Zn(NO3)2, (CH2)6N4, (hydrothermal synthesis) | Antibacterial rubber | [212] |
| ZnO NPs | CF | Zn(NO3)2, urea (hydrothermal process) | Photocatalyst (methyl orange degradation) | [213] |
| ZnO nanorods | BNC | Zn(NO3)2, (CH2)6N4, (hydrothermal synthesis) | — | [214] |
| ZnO NPs | BNC | Zn(NO3)2, solution plasma process | Antibacterial agent | [215] |
| ZnO NPs (100–200 nm) | BNC | Zn(OOCCH3)2, solvothermal process | Antibacterial agent | [216] |
| ZnO NPs | BNC | Zn(NO3)2, NaOH | Antibacterial dressing for burn wounds | [217] |
| ZnO NPs | CNCs | Commercial nano ZnO | Semiconductor in flexible electronic applications | [218] |
| ZnO nanorod clusters | CNCs | Zn(CH3COO)2, reaction with NaOH | Antibacterial agent | [219] |
| ZnO NPs (10–50 nm) | CNCs | ZnCl2, NaOH | Catalyst in degrad. of tetracycline | [220] |
| Cellulose supported miscellaneous oxide nanostructures | ||||
| BiVO4 | CF | Bi(NO3)3·5H2O, citric acid | Photocatalyst for methyl orange | [221] |
| Ti3(PO4)4 | CF | Waste Ti metal, H2SO4, Na3PO4 | Photocatalyst for crystal and methyl violet | [222] |
| MnO2 (150 nm) | MCC | MnSO4, KMnO4 | Adsorbent of Pb2+, H2O purification | [223] |
| In2O3–10 wt.% SnO2 | MFC | RF sputtering | Semiconductive nanofiller | [128] |
| Ag2O NPs (2–20 nm) | MFC | AgNO3, NH3, NaOH | Adsorbent for Iˉ ions | [224] |
| H3PW12O40 | BNC | Commercial PTA | Photochromic agent | [225] |
| Mn3O4 (300 nm) | BNC | KMnO4, reduction by carboxymethyl cellulose | Electrode material | [226] |
| ZnO-CdS NPs | BNC | Zn(NO3)2, CdSO4 | Photocatalyst (degr. of methyl orange) | [227] |
| BaTiO3 | BNC | Ti(OC4H9-n)4, H4Ba6(O)(OCH2CH2OCH3)14, hydrolysis | Piezoelectric paper | [228] |
| BaTiO3 | CNCs | Commercial BaTiO3 | Piezoelectric energy harvester | [229] |
| La2CuO4 | BNC | La(NO3)3/Cu(NO3)2 hydrothermal process | Catalyst-methanol steam reforming | [230] |
| CoFe2O4 | BNC | FeCl3 and CoCl2, reaction with NaOH | Magnetic paper | [231] |
| V2O5 | BNC | Vanadium(V) oxytriisopropoxide hydrolysis with HCl | Semiconductive nanofiller | [232] |
| CuO NPs | BNC | Previously prepared CuO NPs | Antimicrobial agent | [233] |
| CuO NPs | BNC | Cu(OOCCH3)2, CH3COOH, ethylene glycol | Antibacterial agent | [234] |
| CuO NPs (~7 nm) | CNCs | CuSO4 (reduction by NaBH4) | Catalyst (reduction of 4-nitrophenol) | [235] |
| FeMnOx (50–100 nm) | CF | KMnO4, FeSO4, NaOH, cetyltrimethyl ammonium Br | Adsorbent for As(III) and As(V) removal | [236] |
| CeO2 NPs (10–40 nm) | RC | Ce(NO3)3, NaOH | UV shielding | [237] |
| Inorg. Particle (Size—nm) | Cellulose Used | Precursor (Synthetic Path) | Application | Reference |
|---|---|---|---|---|
| Cellulose Templated Inorganic Oxides | ||||
| TiO2 | MCC | titanium isopropoxide, HNO3, H3PO4, NaOH, urea | Sorbent microextr. of organic compounds | [262] |
| TiO2 nanofibers | CNF | titanium isopropoxide, NH3 | Photocatalyst—degr. of methylene blue | [263] |
| TiO2 network | CNCs | Ti(N(CH3)2)4, atomic layer deposition | Photoelectrochemical water splitting | [250] |
| TiO2 | CNCs | titanium(IV) ethoxide | Sorbent for microextraction | [264] |
| TiO2 | CNCs | Ti-n-butoxide | Photocatalyst–(degradation of methylene blue) | [265] |
| TiO2 | CNCs | tetra-n-butyl titanium | Photocatalyst–degradation of Rhodamine B | [266] |
| α-Fe2O3 | CF | FeCl3, calcination | Catalyst (oxidation of alcohols with H2O2) | [267] |
| α-Fe2O3 | CF | FeCl3, calcination | Magnetic materials, catalyst | [268] |
| α-Fe2O3 | CF | FeCl3, calcination | – | [269] |
| Fe2O3 | RC | FeCl2, NaOH | – | [270] |
| Fe3O4 NPs (10 nm) | BNC | Fe(NO3)3, urea, hydrothermal reaction | Electrode in Li batteries | [271] |
| Fe3O4 NPs (10 nm) | BNC | Fe(NO3)3, urea, hydrothermal reaction | Flexible electrodes in Li batteries | [272] |
| Fe2O3 NPs | BNC | Fe(NO3)3, pyrolysis | Anode in Li-ion batteries | [273] |
| ZnO | CF | ZnCl2, NaOH, ethylenediamine | Photocatalyst (degradation of methyl orange) | [274] |
| ZnO | CF | Zn(NO3)2, urea, calcination | Photocatalyst (degradation of methyl orange) | [275] |
| ZnO (500–1000 nm) | MCC | Zn(NO3)2, NH3, microwaves, hydrothermal reaction | Photocatalyst (degr. of methylene blue and rhodamine B) | [276] |
| In2O3–SnO2 nanotubular (ITO) | CF | In(III) acetylacetonate, Sn(IV) isopropoxide | Active multilayers in photoanode | [277] |
| Co3O4 NPs (50–100 nm) | RC | CoCl2, calcination | Electrode in Li batteries | [278] |
| MnO2 NPs | MCC | Mn(CH3COO)2, CO(NH2)2, KOH, (CH2)6N4, NaOH, calcin | – | [279] |
| Co0.5Cu0.5Fe2O4 | CF | Cu(II) acetate, Co(II) acetate, Fe(NO3)3, sol-gel process, calcination | – | [280] |
| V2O5, V2O3, Fe3O4, WO3 (200–1000 nm) | CF | Vanadium(V) oxychloride, tungsten(VI) chloride, iron(III) chloride | – | [281] |
| CoFe2O4 NPs (5–10 nm) | BNC | Fe(NO3)3, Co(NO3)2, NH4OH | Catalyst in fuel cells | [282] |
| MnO2 NPs | BNC | KMnO4, K2SO4, reduction by C | Electrode in supercapacitors | [283] |
| MnFe2O4 | CF | Previously synthesized MnFe2O4 | Adsorbent for As(III) and As(V) | [284] |
| LaNiO3 | MCC | La nitrate, Ni nitrate, NH3, EDTA | Catalyst (steam CO2 reforming of methane) | [285] |
| SnO2 | CF | (Sn(OiPr)4, sol-gel process, calcination | Electrode Li batteries | [286] |
| SnO2 | BNC | SnCl2, pyrolysis | Anode in Li-ion batteries | [287] |
| Inorganic Nanostructures Supported by Carbon Fibers Formed from Cellulose | ||||
| Ge | BNC | GeBr2, pyrolysis | Anode in Li-ion batteries | [287] |
| Pd-Al | CF | Pd(NO3)2, Al(NO3)3 (decomposition in a reducing atm. H2/N2) | Catalyst–(hydrogenation of cyclohexene) | [288] |
| Ni | MCC | Ni(OOCCH3)2, | Catalyst (conv. of p-nitrophenol into p-aminophenol) | [289] |
| Ni/NiO NPs (2–100 nm) | CNCs | Ni(NO3)2, glycine, calcination | – | [290] |
| Ru NPs | BNC | RuCl3, pyrolysis | Electrode in Li–O2 batteries | [291] |
| Fe3C NPs (10–30 nm) | BNC | FeCl3, pyrolysis | Electrode in Li-ion or Na-ion batteries | [292] |
| MoS2 nanoleaves | BNC | Na2MoO4, CS(NH2)2, pyrolysis | Anode in Li-ion batteries | [293] |
| MoS2 NPs | BNC | (NH4)6Mo7O24, CH4N2S, hydrothermal process | Electrocatalyst in H2 evolution reaction | [294] |
| NiCo2S4 | CF | Ni(NO3)2, Co(NO3)2, hexamethylenetetramine | Positive electrode in supercapacitors | [295] |
| BiOBr | CNFs | Bi(NO3)3, NaBr, ethylene glycol | Adsorbent for rhodamine B and Cr(VI) | [296] |
| Inorg. Particle (Size—nm) | Cellulose Used | Precursor (Synthetic Path) | Application | Reference |
|---|---|---|---|---|
| Cellulose Supported Metal Sulfides | ||||
| CdS | CF | CdCl2, AgNO3, mercaptoacet. acid | Colorimetric paper test stripe for Hg | [307] |
| ZnS–MoS2 (200–1000 nm) | CF | Na2MoO4, ZnCl2, CH4N2S | Flexible Broadband Photodetector | [308] |
| AgInS2 NPs | CF | AgNO3, In(NO3)3, NaOH, cysteine | Nanodrug for hepatocellular carcinoma | [309] |
| CuS | MFC | CuSO4, Na2S, NaOH, NH3, xylan | Paper for ablation of pathogenic microorganisms | [310] |
| CuS (100–1000 nm) | BNC | CuSO4, Na2S | Electrode in supercapacitors | [311] |
| CdS | BNC | Cadmium(II) chloride, thiourea | Photocatalyst-methyl orange under UV | [312] |
| MoS2 | CF | Na2MoO4, thiourea, MnCl2 | Photocatalyst–degr. of RhB dye | [313] |
| ZnS | BNC | Zinc(II) acetate, thioacetamide | Fluorescent material | [314] |
| ZnxCdyS | BNC | Cd(II) chloride, thiourea | Photocatalyst-H2 prod.-visible light | [315] |
| ZnO-CdS | BNC | Zinc(II) nitrate, Cd(II) sulfate | Photocatalyst–methyl orange–UV | [236] |
| MoS2 NPs (10 nm) | BNC | (NH4)2MoS4, N2H4 | Electrocatalyst in H2 evolution react. | [316] |
| MoS2 NPs | BNC | (NH4)2MoO4, CH4N2S, | Electrode in Li-ion batteries | [317] |
| ZnS | CF | Zinc(II) nitrate hexahydrate, sodium sulfide | Photoluminescent material–security paper | [318] |
| In doped Mo(O,S)2 | CNFs | MoCl5, InCl3, CH3CSNH2,NH2NH2 | Photocatalyst for organic dyes | [319] |
| FeS NPs (10–20 nm) | CF | Iron(II) chloride, sodium sulfide | Uranium(VI) ions immobilization–water purification | [320] |
| Ni3S2 NPs | BNC | NiCl2, CN2H4S, NH3 | Electrode in supercapacitor | [321] |
| Cellulose supported hydroxyapatites | ||||
| Ca hydroxyapatite | MFC | NaH2PO4, CaCl2 | Adsorbent for Cr(VI) ions, H2O purification | [322] |
| Ca hydroxyapatite | BNC | NaH2PO4, CaCl2 | Cell proliferation–bone tissue engin. | [323] |
| Ca hydroxyapatite | CF | Ca(NO3)2,CO(NH2)2 (NH4)2HPO4, NaOH, | Cell proliferation–bone tissue engin. | [324] |
| Ca phosphate–Ca hydroxyapatite | CF | CaCO3, P2O5, MgO Ca hydroxyapatite | Injectable bone paste | [325] |
| Ca hydroxyapatite | CF | Ca(NO3)2, (NH4)2HPO4, NH3 | Adsorbent for Hg, H2O purification | [326] |
| Ca hydroxyapatite | CNFs | Ca(NO3)2, (NH4)2HPO4 | Fire resistant composite foams | [327] |
| Ca nanohydroxyapatite | CNCs | Commercial hydroxyapatite | Cell proliferation–bone tissue engin. | [328] |
| Ca hydroxyapatite | CNCs | Ca(NO3)2, (NH4)2HPO4 | Adsorbent for chlortetracycline | [329] |
| Cellulose supported miscellaneous inorganic nanostructures | ||||
| CaCO3 NPs | CF | CaCl2, Na2CO3 | Biomedical material | [330] |
| EuF3 NPs (250–600 nm) | CF | LiCl, EuF3 | Phosphor–flexible optical material | [331] |
| Se NPs | CNCs | H2SeO3, Na2SeO3, sorbitol | - | [332] |
| Bi NPs (2–10 nm) | CNFs | Bi(NO3)3, NaBH4 | Agent for enhanced radiation therapy | [333] |
| Ni(OH)2 NPs | BNC | NiSO4, NH4F, NH3 | Electrode in supercapacitors | [334] |
| AgCl NPs | CF | AgNO3, NaCl | Antibacterial agent | [335] |
| AgCl/Ag (~1200 nm) | CF | AgNO3, AlCl3, NaOH | Photocatalyst, antibacterial agent | [336] |
| FeOOH | BNC | - | Flexible electrode-supercapacitors | [337] |
| Inorg. Particle (Size—nm) | Cellulose Used | Precursor (Synthetic Path) | Application | Reference |
|---|---|---|---|---|
| Cellulose Supported Metallic Hybrid Nanostructures | ||||
| Au (~100 nm) | CF | HAuCl4, cetyltrimethyl ammonium chloride, H2O2 | Flexible electrode in environmental and biosensors | [357] |
| Au nanorods, Ag NPs, ZnO | CF | HAuCl4, H2NOH.HCl, ZnNO3, NH3, AgNO3, ethylene glycol | Electrode in electrochemical immunosensor | [358] |
| Au NPs (50–100 nm) | CF | HAuCl4, NaBH4, Na citrate, reduction | Flexible electrode sensor-oligonucleotides | [359] |
| Au NPs (30–100 nm) | CF | HAuCl4, trisodium citrate | Flexible plasmonic immunosensor for detection of sepsis | [360] |
| Au NPs, ZnO nanorods | CF | HAuCl4, cetyltrimethyl ammonium chloride, H2O2; Zn(NO3)2, (CH2)6N4, | Photoelectrochemical immunosensor-carcinoembryonic antigen antibodies | [361] |
| Au NPs, Mn2O3 | CF | HAuCl4, ascorbic acid, Mn(NO3)2, glucose | Paper-based biosensor for Pb2+ detection | [362] |
| Au-Ag NPs | CF | HAuCl4, AgNO3, NH3, ascorbic acid, reduction | Electrode in electrochemical immunosensor–carbohydrate antigen | [363] |
| Pt NPs (100–200 nm) | CF | H2PtCl6, ascorbic acid, reduction | Electrode in electrochemical immunodevice–tumor biomarker | [364] |
| Pt NPs–ZnO nanorods | CF | H2PtCl6, NaBH4, reduction; Zn(NO3)2, CH3COONH4, ethylenediamine | Electrode in H2O2 sensor–tumor cells detection | [365] |
| Pt NPs | CF | H2PtCl6, NaBH4, H2PtCl6, ascorbic acid, reduction; | Electrode in electrochemical sensor-DNA | [366] |
| Pt NPs | CF | H2PtCl6, ascorbic acid, reduction | Electrode in H2O2 sensor–cancer cells monitoring | [367] |
| Ag NPs | CF | AgNO3, ascorbic acid, reduction | Electrode in electrochemiluminescence sensor-antibodies | [368] |
| Ag NPs (20–100 nm) | CF | AgNO3, ascorbic acid, reduction | Electrode in immunodevice–cancer antigens | [369] |
| Ag NPs | CF | AgNO3, H2O2, NaOH, trisodium citrate | Self-healing superhydrophobicity | [370] |
| Pd-Au NPs (20–50 nm) | CF | Na2PdCl4 and HAuCl4, ascorbic acid, reduction | Electrode in H2O2 sensor–cancer cells monitoring | [371] |
| Au-Ag (100–500 nm) | CF | HAuCl4 and AgNO3, hydroxylammonium chloride, reduction | Electrode in cytodevice–glycan expression | [372] |
| Au-Pt (50–100 nm) | CF | HAuCl4 and H2PtCl6, ascorbic acid, reduction | Electrode in photoelectrochemical immunodevice-carcinoembryonic antigen | [373] |
| Pd-Au | CF | H2PdCl4 and HAuCl4, ascorbic acid, reduction | Electrode in electrochemical immunodevice-carcinoembryonic antigen | [374] |
| Ag NPs | CF | Previously formed Ag NPs, AgNO3, Na citrate | Paper-Based SERS Sensor for Pesticide Detection | [375] |
| Ag NPs | CF | AgNO3, NaOH | Colorimetric assay for Hg2+ detection | [376] |
| Ag NPs | CF | AgNO3, dopamine hydrochloride | Antibacterial CA/TiO2/Ag NPs material | [377] |
| Au NPs (10–30 nm) | CF | HAuCl4, NaOH | Fluorescent probe for the detection of Hg(II) ions | [378] |
| Au-Pt NPs | CF | HAuCl4 and H2PtCl6, ascorbic acid, reduction | Electrode in cyto-device–H2S from cancer cells | [379] |
| Au NPs | CF | HAuCl4, NH3, trisodium citrate | Electrode in electrochemical biosensor for Pb2+ ions | [380] |
| Au NPs | CF | HAuCl4, cetyltrimethyl ammonium chloride, H2O2 | Electrode in photoelectrochemical sensor-pentachlorophenol | [381] |
| Au NPs | BNC | - | Electrode in bioenzymatic sensor-glucose | [382] |
| Cellulose Supported Metal Oxide or Sulfide Hybrid Nanostructures | ||||
| ZnO platelet- superstructure | CF | Zn(NO3)2, trisodium citrate, NaOH | Electrode in photoelectrochemical immunodevice-carcinoembryonic antigen | [383] |
| ZnO nanorods | CF | Zn acetate, Zn(NO3)2, hexamethylenetetramine | Electrode in electrochemical immunosensor— α-fetoprotein | [384] |
| ZnO platelets, CuS NPs | CF | Zn(NO3)2, KCl, electrodeposition; Cu(NO3)2, NaS2 | Electrode in photoelectrochemical (PEC) immunosensor-carcinoembryonic antigen | [385] |
| ZnO nanorods | CF | Pulsed laser deposition | Electrode in LED-photoelectrochemical immunoassay | [386] |
| SnO2 NPs | CF | SnCl4, graphene oxide | Electrode in chemiluminescence photoelectrochemical aptamer device | [387] |
| MnO2 nanowires | CF | MnSO4, Na2SO4, electrodeposition | Electrode in electrochemical immunosensor-prostate protein antigen | [388] |
| CdS NPs (~5 nm) | CF | CdCl2, Na2S, thioglycolic acid | Electrode in photoelectrochemical analytical device-adenosine triphosphate | [389] |
| Cu1.3Mn1.7O4 NPs (~50 nm) | CF | Cu(NO3)2, Mn(NO3)2, sol-gel process | Li-ion batteries | [390] |
| MoO3 (100–1000 nm) | CF | Previously prepared MoO3, (NH4)6Mo7O24, calcination | Colorimetric analysis system for Fe(III) ions | [391] |
| CoS NPs | BNC | Co(NO3)2, Na2S | Flexible electrodes-supercapacitors | [392] |
| FeOOH (5–50 nm) | CNCs | FeCl3, NaOH | Water defluoridation | [393] |
| ZnO-Ag | CNCs | Zn(OOCCH3)2, AgNO3, NaOH | Antibacterial agent | [394] |
| SnO2-Ag | CF | (Sn(OiPr)4, AgNO3, NH3, NaOH | Anodic material in Li-ion batteries | [395] |
| Co(Ac)2–Fe3O4 | CNF | FeCl3, NH3, Co acetate | Nanocatalyst for 4H-pyrane and pyranopyrazole | [396] |
| TiO2 nanorods–Au NPs | CNCs | TiCl4, Ti(OBu)4, HCl, Au(Ac)3, 1-octadecene | Photocatalyst for degr. of Rhodamine B | [397] |
| AgNW-Fe3O4 | CNF | AgNO3, ethylene glycol, PVP, FeCl3, FeCl2, NaOH | Electromagnetic interference shielding | [398] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anžlovar, A.; Žagar, E. Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies. Nanomaterials 2022, 12, 1837. https://doi.org/10.3390/nano12111837
Anžlovar A, Žagar E. Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies. Nanomaterials. 2022; 12(11):1837. https://doi.org/10.3390/nano12111837
Chicago/Turabian StyleAnžlovar, Alojz, and Ema Žagar. 2022. "Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies" Nanomaterials 12, no. 11: 1837. https://doi.org/10.3390/nano12111837
APA StyleAnžlovar, A., & Žagar, E. (2022). Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies. Nanomaterials, 12(11), 1837. https://doi.org/10.3390/nano12111837






