Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetite Nanoparticles’ Synthesis
2.2. PEI Coating Procedure
2.3. Transmission Electron Microscopy and X-ray Diffraction
2.4. Magnetic Properties
2.5. Zeta Potential
2.6. Microalgae Strains and Cultivation
2.7. Magnetite Harvesting Procedure
2.8. Adsorption Experiments
2.9. Statistical Analysis
3. Results
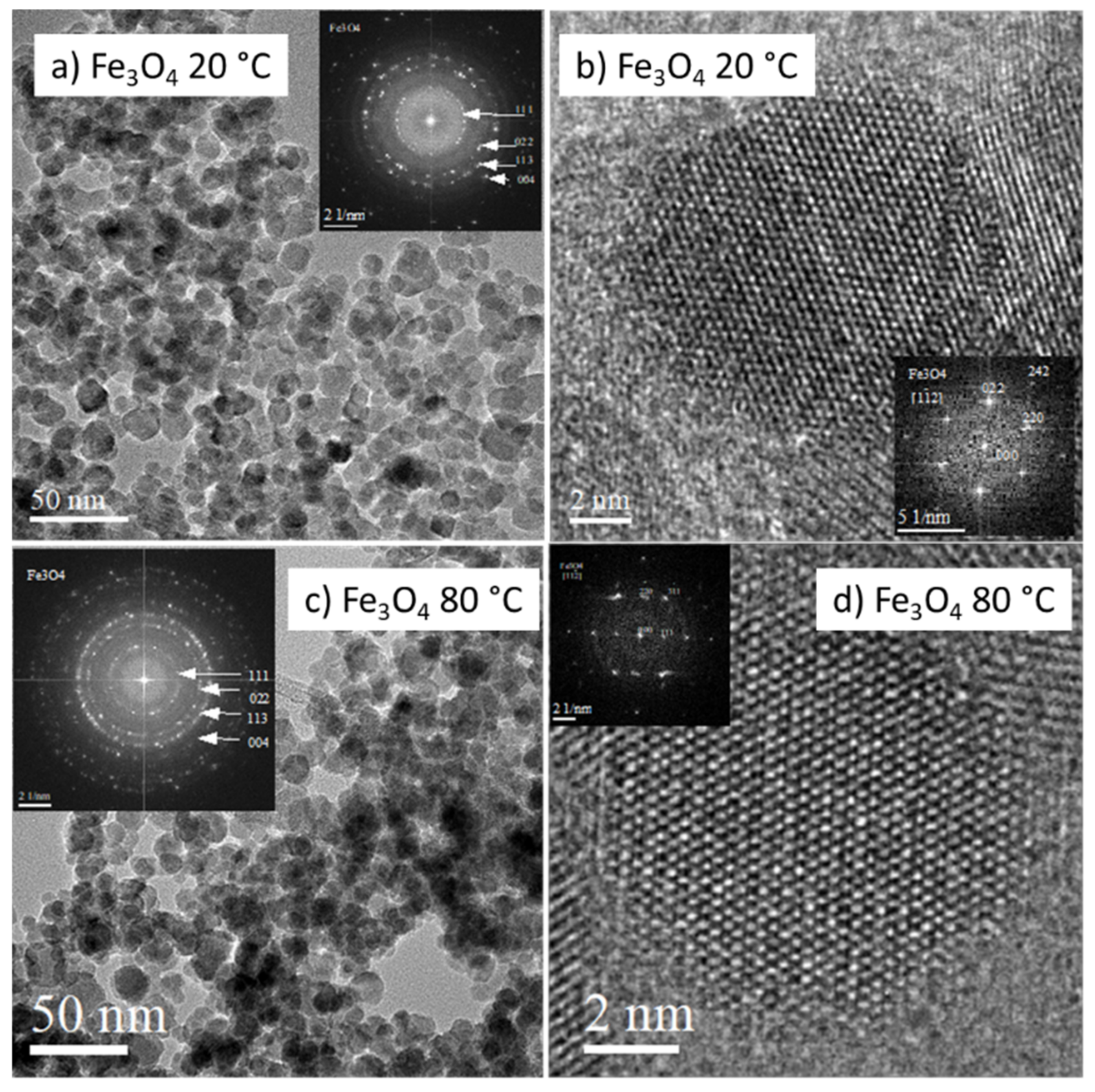
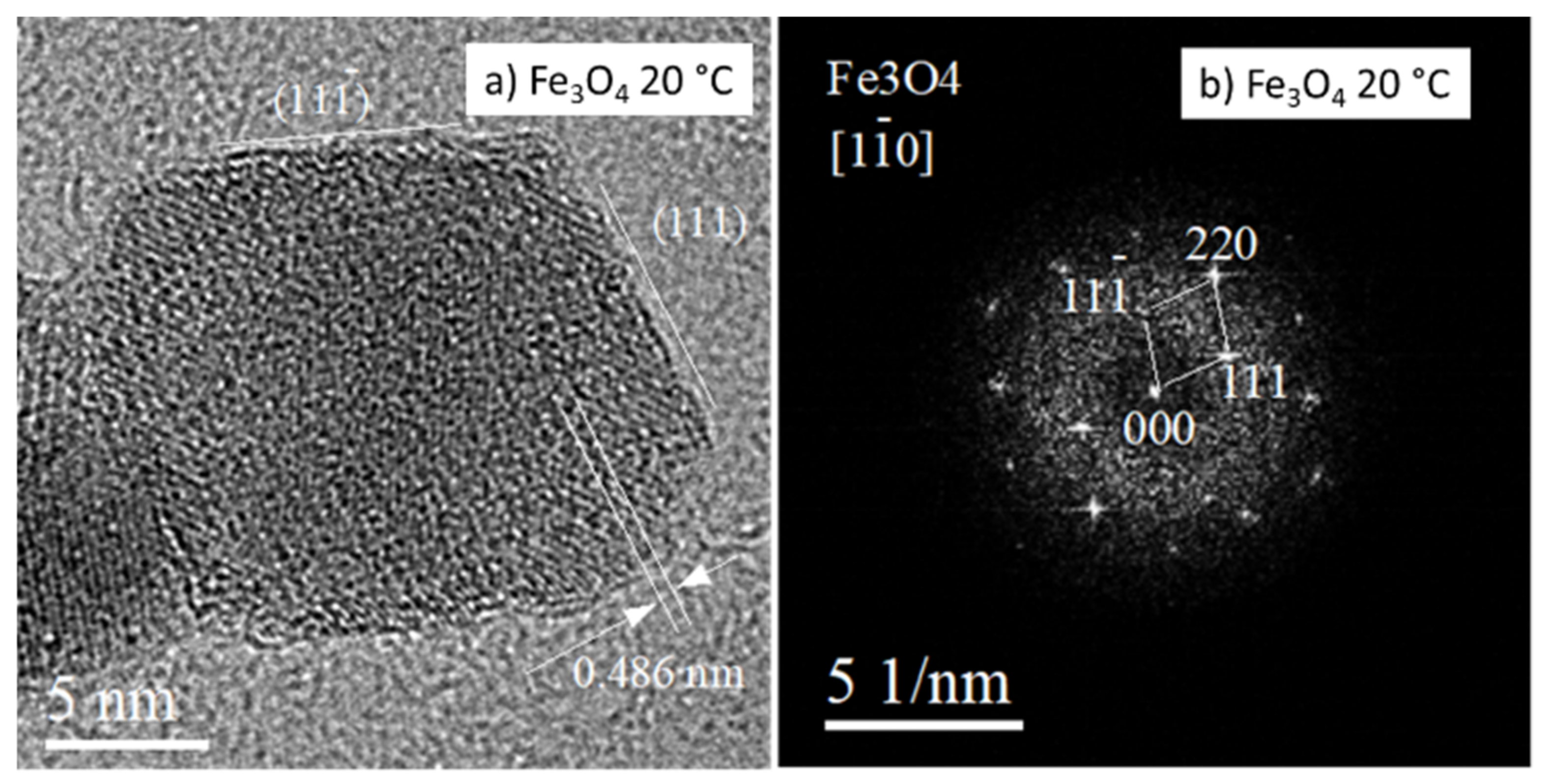
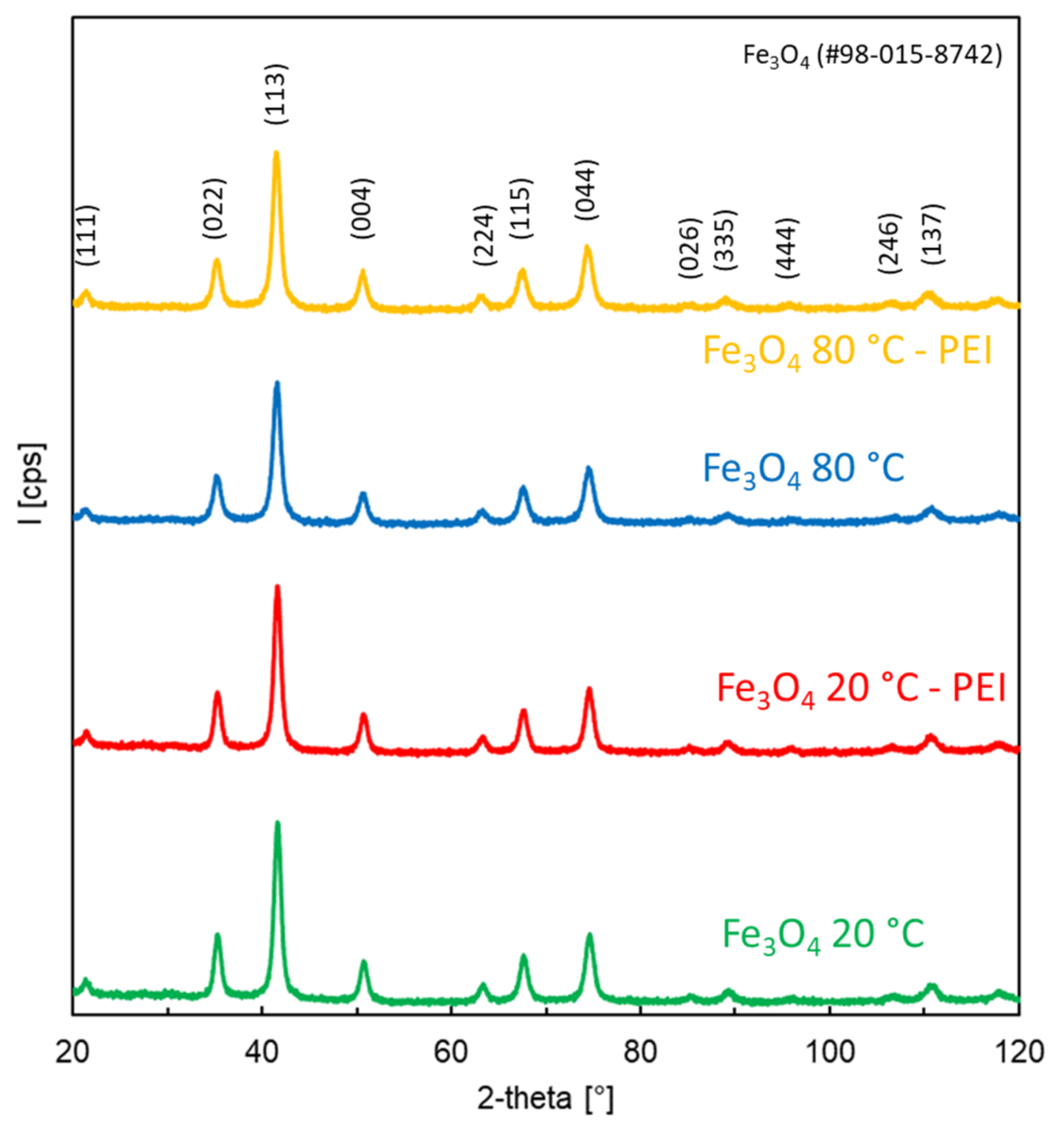
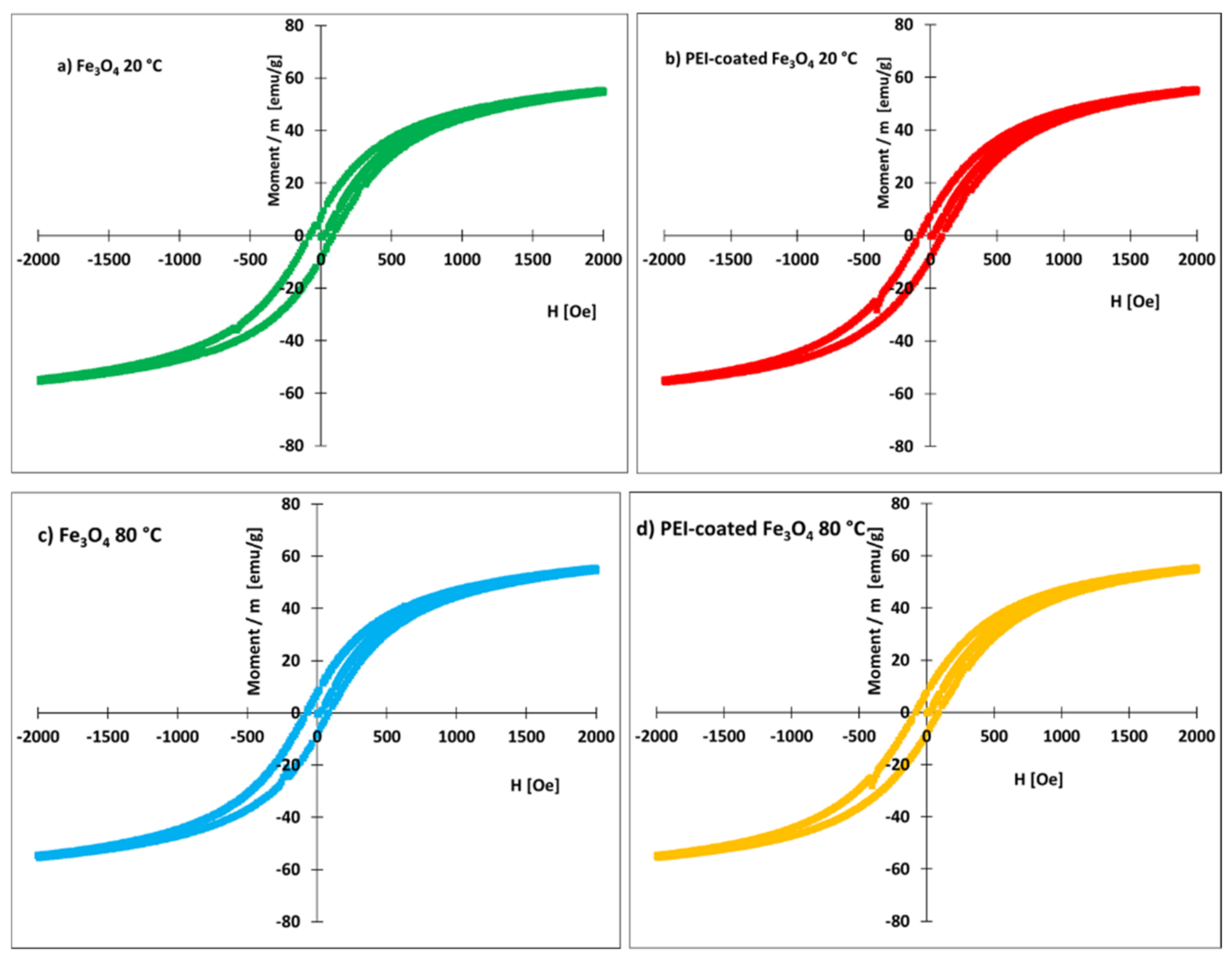
3.1. Characterization of Prepared Fe3O4 and Fe3O4-PEI NPs
3.2. Zeta Potential
3.3. Magnetic Harvesting of Microalgae
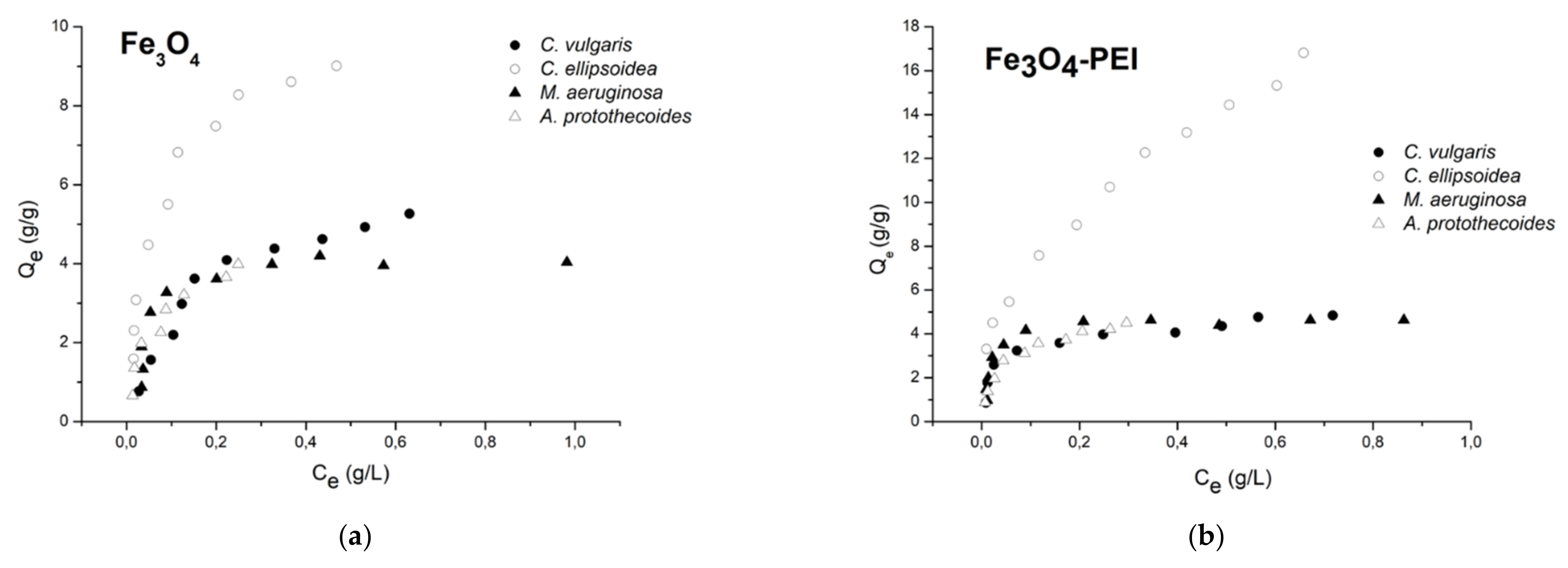
3.4. Adsorption Isotherms
3.5. Adsorption Mechanism
4. Conclusions
- The prepared NPs were spherical. The particle size distribution was relatively uniform and an average particle diameter of ~10 nm was found. The NPs prepared at 20 °C were smaller. However, the difference in nanoparticle diameter between materials prepared at 20 and 80 °C was not significant. The crystal structure of magnetite was confirmed by electron diffraction.
- The zeta potential of the uncoated nanoparticles was negative within the investigated pH range (4–9). The zeta potentials of the PEI-coated nanoparticles were positive at pH 4–9. The presence of PEI thus brings a positive charge to magnetite.
- The algae species displayed a negative zeta potential within the investigated pH range. Negative values decreased as pH increased due to deprotonation.
- Microalgae harvesting was studied at different pH levels and different flocculant doses. Higher efficiencies, close to 100%, were obtained for PEI-coated Fe3O4 NPs.
- The adsorption of magnetic flocculants on harvested microalgal cells has been studied by Langmuir and Freundlich isotherms. A better fit was found for the Langmuir isotherm, indicating a monolayer adsorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seo, J.Y.; Kim, M.G.; Lee, K.; Lee, Y.C.; Na, J.G.; Jeon, S.G.; Park, S.B.; Oh, Y.K. Multifunctional Nanoparticle Applications to Microalgal Biorefinery. In Nanotechnology for Bioenergy and Biofuel Production, 2nd ed.; Rai, M., Silva, S.S.D., Eds.; Springer: Cham, Switzerland, 2017; pp. 59–87. [Google Scholar] [CrossRef]
- Gangl, D.; Zedler, J.A.Z.; Rajakumar, P.D.; Martinez, E.M.R.; Riseley, A.; Włodarczyk, A.; Purton, S.; Sakuragi, Y.; Howe, C.J.; Jensen, P.E.; et al. Biotechnological exploitation of microalgae. J. Exp. Bot. 2015, 66, 6975–6990. [Google Scholar] [CrossRef] [PubMed]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Bioresour. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Alalwan, H.A.; Alminshid, A.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Winckelmann, D.; Bleeke, F.; Thomas, B.; Elle, C.; Klöck, G. Open pond cultures of indigenous algae grown on non-arable land in an arid desert using wastewater. Int. Aquat. Res. 2015, 7, 221–233. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; García-Ulloa, J.; Ghazoul, J.; Schenk, P.M. Freeing land from biofuel production through microalgal cultivation in the Neotropical region. Environ. Res. Lett. 2020, 15, 94094. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing carbon footprint via microalgae as a biological capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2022, 21, 1–28. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Tang, Y.; Rosenberg, J.N.; Bohutskyi, P.; Yu, G.; Betenbaugh, M.J.; Wang, F. Microalgae as a Feedstock for Biofuel Precursors and Value-Added Products: Green Fuels and Golden Opportunities. BioResources 2016, 11, 2850–2885. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Pinto, L.F.R.; Filho, R.M.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Almanza, V.; Parra, O.; Bicudo, C.E.D.M.; Baeza, C.; Beltran, J.; Figueroa, R.; Urrutia, R. Occurrence of toxic blooms of Microcystis aeruginosa in a central Chilean (36° Lat. S) urban lake. Rev. Chil. Hist. Nat. 2016, 89, 1349. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Tian, L.-L.; Ren, C.-Y.; Xu, C.-Y.; Wang, Y.-Y.; Li, L. Extracellular polysaccharide synthesis in a bloom-forming strain of Microcystis aeruginosa: Implications for colonization and buoyancy. Sci. Rep. 2019, 9, 1251. [Google Scholar] [CrossRef]
- Abed, R.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Rós, P.D.; Silva, C.S.; Stenico, M.E.; Fiore, M.; Castro, H.F.D. Microcystis aeruginosa lipids as feedstock for biodiesel synthesis by enzymatic route. J. Mol. Catal. B Enzym. 2012, 84, 177–182. [Google Scholar] [CrossRef]
- Upendar, G.; Singh, S.; Chakrabarty, J.; Ghanta, K.C.; Dutta, S.; Dutta, A. Sequestration of carbon dioxide and production of biomolecules using cyanobacteria. J. Environ. Manag. 2018, 218, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ataeian, M.; Liu, Y.; Canon-Rubio, K.A.; Nightingale, M.; Strous, M.; Vadlamani, A. Direct capture and conversion of CO2 from air by growing a cyanobacterial consortium at pH up to 11.2. Biotechnol. Bioeng. 2019, 116, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2020, 137, 110498. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Bajpai, P. Harvesting and Drying of Algal Biomass. In Third Generation Biofuels; Springer: Singapore, 2019; pp. 29–36. [Google Scholar]
- Fasaei, F.; Bitter, J.; Slegers, P.; Boxtel, A.V. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Tran, D.-T.; Le, B.-H.; Lee, D.-J.; Chen, C.-L.; Wang, H.-Y.; Chang, J.-S. Microalgae harvesting and subsequent biodiesel conversion. Bioresour. Technol. 2013, 140, 179–186. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Wang, S.-K.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015, 9, 178–185. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Yasin, N.H.M. Harvesting microalgal biomass and lipid extraction for potential biofuel production: A review. J. Environ. Chem. Eng. 2017, 5, 555–563. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of Nanoparticles on Algae: Adsorption, Distribution, Ecotoxicity and Fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Bui, V.K.H.; Oh, Y.K.; Lee, Y.C. Recent advanced applications of nanomaterials in microalgae biorefinery. Algal Res. 2019, 41, 101522. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Hao, J.J.; Chen, H.L.; Ren, C.L.; Yan, N.; Geng, H.J.; Chen, X.G. Synthesis of superparamagnetic Fe3O4 nanocrystals in reverse microemulsion at room temperature. Mater. Res. Innov. 2010, 14, 324–326. [Google Scholar] [CrossRef]
- Liang, X.; Jia, X.; Cao, L.; Sun, J.; Yang, Y. Microemulsion Synthesis and Characterization of Nano-Fe3O4 Particles and Fe3O4 Nanocrystalline. J. Dispers. Sci. Technol. 2010, 31, 1043–1049. [Google Scholar] [CrossRef]
- Ahmadi, S.; Chia, C.-H.; Zakaria, S.; Saeedfar, K.; Asim, N. Synthesis of Fe3O4 nanocrystals using hydrothermal approach. J. Magn. Magn. Mater. 2012, 324, 4147–4150. [Google Scholar] [CrossRef]
- Wu, X.; Tang, J.; Zhang, Y.; Wang, H. Low temperature synthesis of Fe3O4 nanocrystals by hydrothermal decomposition of a metallorganic molecular precursor. Mater. Sci. Eng. B 2009, 157, 81–86. [Google Scholar] [CrossRef]
- Woo, S.; Kim, S.; Kim, H.; Cheon, Y.W.; Yoon, S.; Oh, J.-H.; Park, J. Charge-Modulated Synthesis of Highly Stable Iron Oxide Nanoparticles for In Vitro and In Vivo Toxicity Evaluation. Nanomaterials 2021, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Spivakov, A.; Lin, C.-R.; Chang, Y.-C.; Wang, C.-C.; Sarychev, D. Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure. Nanomaterials 2020, 10, 1888. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and magnetic properties of magnetite nanoparticles by sol–gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- Takai, Z.I.; Mustafa, M.K.; Asman, S.; Sekak, K.A. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int. J. Nanoelectron. Mater. 2019, 12, 37–46. [Google Scholar]
- Gholizadeh, A. A comparative study of physical properties in Fe3O4 nanoparticles prepared by coprecipitation and citrate methods. J. Am. Ceram. Soc. 2017, 100, 3577–3588. [Google Scholar] [CrossRef]
- Arévalo, P.; Isasi, J.; Caballero, A.C.; Marco, J.; Hernandez, F.M. Magnetic and structural studies of Fe3O4 nanoparticles synthesized via coprecipitation and dispersed in different surfactants. Ceram. Int. 2017, 43, 10333–10340. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef]
- Houshiar, M.; Zebhi, F.; Razi, Z.J.; Alidoust, A.; Askari, Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J. Magn. Magn. Mater. 2014, 371, 43–48. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Mumtaz, A.; Hasanain, S.; Liu, J.; Duan, J. Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J. Magn. Magn. Mater. 2008, 321, 1838–1842. [Google Scholar] [CrossRef]
- Borlido, L.; Azevedo, A.M.; Roque, A.; Aires-Barros, M. Magnetic separations in biotechnology. Biotechnol. Adv. 2013, 31, 1374–1385. [Google Scholar] [CrossRef]
- Eskandari, M.J.; Hasanzadeh, I. Size-controlled synthesis of Fe3O4 magnetic nanoparticles via an alternating magnetic field and ultrasonic-assisted chemical co-precipitation. Mater. Sci. Eng. B 2021, 266, 115050. [Google Scholar] [CrossRef]
- Shen, L.; Qiao, Y.; Guo, Y.; Meng, S.; Yang, G.; Wu, M.; Zhao, J. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles. Ceram. Int. 2014, 40, 1519–1524. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Bharte, S.; Desai, K. Harvesting Chlorella species using magnetic iron oxide nanoparticles. Phycol. Res. 2018, 67, 128–133. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, F.; Li, H.; Cui, L.; Qian, G.; Zhang, D.; Xu, Y. Application and mechanisms of microalgae harvesting by magnetic nanoparticles (MNPs). Sep. Purif. Technol. 2021, 265, 118519. [Google Scholar] [CrossRef]
- Egesa, D.; Chuck, C.J.; Plucinski, P. Multifunctional Role of Magnetic Nanoparticles in Efficient Microalgae Separation and Catalytic Hydrothermal Liquefaction. ACS Sustain. Chem. Eng. 2018, 6, 991–999. [Google Scholar] [CrossRef]
- Zhu, L.D.; Hiltunen, E.; Li, Z. Using magnetic materials to harvest microalgal biomass: Evaluation of harvesting and detachment efficiency. Environ. Technol. 2019, 40, 1006–1012. [Google Scholar] [CrossRef]
- Liu, P.; Wang, T.; Yang, Z.; Hong, Y.; Xie, X.; Hou, Y. Effects of Fe3O4 nanoparticle fabrication and surface modification on Chlorella sp. harvesting efficiency. Sci. Total Environ. 2020, 704, 135286. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F. Algal cells harvesting using cost-effective magnetic nano-particles. Sci. Total Environ. 2020, 720, 137621. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xu, Y.; Zhen, Z.; Fu, Y.; Liu, Y.; Li, W.; Luo, C.; Ding, A.; Zhang, D. Application, and reactivation of magnetic nanoparticles in Microcystis aeruginosa harvesting. Bioresour. Technol. 2015, 190, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Barizão, A.C.D.L.; Oliveira, J.P.D.; Gonçalves, R.F.; Cassini, S.T. Nanomagnetic approach applied to microalgae biomass harvesting: Advances, gaps, and perspectives. Environ. Sci. Pollut. Res. 2021, 28, 44795–44811. [Google Scholar] [CrossRef]
- Ge, S.; Agbakpe, M.; Zhang, W.; Kuang, L. Heteroaggregation between PEI-coated magnetic nanoparticles and algae: Effect of particle size on algal harvesting efficiency. ACS Appl. Mater. Interfaces 2015, 7, 6102–6108. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Hou, J.; Wang, P.; Miao, L.; Wang, X. Optimization of cyanobacterial harvesting and extracellular organic matter removal utilizing magnetic nanoparticles and response surface methodology: A comparative study. Algal Res. 2020, 45, 101756. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, J.; Wang, P.; Wang, C.; Miao, L.; Ao, Y.; Xu, Y.; Wang, X.; Lv, B.; You, G.; et al. Interpretation of the disparity in harvesting efficiency of different types of Microcystis aeruginosa using polyethylenimine (PEI)-coated magnetic nanoparticles. Algal Res. 2018, 29, 257–265. [Google Scholar] [CrossRef]
- Hu, Y.R.; Guo, C.; Wang, F.; Wang, S.K.; Pan, F.; Liu, C.Z. Improvement of microalgae harvesting by magnetic nanocomposites coated with polyethylenimine. Chem. Eng. J. 2014, 242, 341–347. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Wang, F.; Wang, S.-K.; Liu, C.-Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef]
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef]
- Shaoxian, S.; Huang, R.; Li, Y.; Song, S. The effect of growth phase on the surface properties of three oleaginous microalgae (Botryococcus sp. FACGB-762, Chlorella sp. XJ-445 and Desmodesmus bijugatus XJ-231). PLoS ONE 2017, 12, e0186434. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Zhang, J.; Qiao, S.; Wang, X.; Zhang, X.; Miao, L.; Hou, J. A critical review on the interaction of iron-based nanoparticles with blue-green algae and their metabolites: From mechanisms to applications. Algal Res. 2022, 64, 102670. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Fu, Y.; Hu, F.; Qian, G.; Liu, Q.; Sun, Y. Interaction energy and detachment of magnetic nanoparticles-algae. Environ. Technol. 2019, 41, 2618–2624. [Google Scholar] [CrossRef]
- Gerulová, K.; Bartošová, A.; Blinová, L.; Bártová, K.; Dománková, M.; Garaiová, Z.; Palcut, M. Magnetic Fe3O4-polyethyleneimine nanocomposites for efficient harvesting of Chlorella zofingiensis, Chlorella vulgaris, Chlorella sorokiniana, Chlorella ellipsoidea and Botryococcus braunii. Algal Res. 2018, 33, 165–172. [Google Scholar] [CrossRef]
- Martínez-Mera, I.; Espinosa-Pesqueira, M.E.; Pérez-Hernández, R.; Arenas-Alatorre, J. Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Materials Letters 2007, 61, 4447–4451. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B.; Risdiana. The impact of synthesis temperature on magnetite nanoparticles size synthesized by co-precipitation method. J. Phys. Conf. Ser. 2018, 1013, 12190. [Google Scholar] [CrossRef]
- Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile Hydrothermal Synthesis and Surface Functionalization of Polyethyleneimine-Coated Iron Oxide Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2013, 5, 1722–1731. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Zhang, L.; Wei, X. One-pot solvothermal synthesis of Fe3O4–PEI composite and its further modification with Au nanoparticles. J. Nanoparticle Res. 2012, 15, 1338. [Google Scholar] [CrossRef]
- Félix, L.; Martínez, M.A.R.; Salazar, D.G.P.; Coaquira, J.A.H. One-step synthesis of polyethyleneimine-coated magnetite nanoparticles and their structural, magnetic, and power absorption study. RSC Adv. 2020, 10, 41807–41815. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Plaza, R.C.; Arias, J.L.; Espín, M.; Jiménez, M.L.; Delgado, A.V. Aging Effects in the Electrokinetics of Colloidal Iron Oxides. J. Colloid Interface Sci. 2002, 245, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.; Lee, J.; Na, K.; Park, S.; Hyun, J. Amphiphilic comb like polymers enhance the colloidal stability of Fe3O4 nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.G.; Dardavila, M.M.; Georgiopoulou, I.; Louli, V.; Stamatis, H.; Kekos, D.; Voutsas, E. Optimization of Microalga Chlorella vulgaris Magnetic Harvesting. Nanomaterials 2021, 11, 1614. [Google Scholar] [CrossRef]
- Baldassarre, F.; Cacciola, M.; Ciccarella, G. A predictive model of iron oxide nanoparticles flocculation tuning Z-potential in aqueous environment for biological application. J. Nanoparticle Res. 2015, 17, 377. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-Da-Silva, A.L. Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water. Molecules 2019, 24, 1958. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 117, 421–427. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Su, F.-W.; Forsling, W.; Samskog, P.-O. Surface Characteristics of Magnetite in Aqueous Suspension. J. Colloid Interface Sci. 1998, 197, 151–159. [Google Scholar] [CrossRef]
- Xu, L.; Guo, C.; Wang, F.; Zheng, S.; Liu, C.Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresour. Technol. 2011, 102, 10047–10051. [Google Scholar] [CrossRef]
- Fraga-García, P.; Kubbutat, P.; Brammen, M.; Schwaminger, S.; Berensmeier, S. Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization. Nanomaterials 2018, 8, 292. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes, and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, L.; Hu, D.; Li, S.; Chu, R.; Liu, C.; Lv, Y.; Bao, J.; Xiang, M.; Zhu, L. Biocompatible magnetic flocculant for efficient harvesting of microalgal cells: Isotherms, mechanisms and water recycling. Sep. Purif. Technol. 2021, 279, 119679. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Toh, P.Y.; Ng, B.W.; Chong, C.H.; Ahmad, A.L.; Yang, J.W.; Chieh, D.C.J.; Lim, J.K. Magnetophoretic separation of microalgae: The role of nanoparticles and polymer binder in harvesting biofuel. RSC Adv. 2014, 4, 4114–4121. [Google Scholar] [CrossRef]
- Ge, S.; Agbakpe, M.; Wu, Z.; Kuang, L.; Zhang, W.; Wang, X. Influences of Surface Coating, UV Irradiation and Magnetic Field on the Algae Removal Using Magnetite Nanoparticles. Environ. Sci. Technol. 2015, 49, 1190–1196. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]





| Nonlinear Form | Plot | Linear Form | Plot | |
|---|---|---|---|---|
| Langmuir | ||||
| Freundlich | or |
| Microalgae/ Cyanobacteria | Algae DCW (g L−1) | NPs Type | Dosage g Floculant/g of Dry Algae | pH | Contact Time (s) | Harvesting Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| M. aeruginosa | 1.788 | Fe3O4 | 0.112 | 4 | 90 | 85.2 | This study |
| M. aeruginosa | n.a. | Fe3O4 | 0.58 | 3 | 300 | 99 | [65] |
| M. aeruginosa | 1.788 | Fe3O4-PEI | 0.112 | 4 | 90 | 89.4 | This study |
| M. aeruginosa | n.a. | Fe3O4-PEI | 0.14–0.18 | 3 | 70–95 | 93-97 | [68] |
| C. ellipsoidea | 1.128 | Fe3O4 | 0.177 | 4 | 90 | 82.9 | This study |
| C. ellipsoidea | n.a. | Fe3O4 | 0.3 | 4 | 60 | 90 | [92] |
| C. ellipsoidea | 1.128 | Fe3O4-PEI | 0.177 | 4 | 90 | 97.0 | This study |
| C. ellipsoidea | 0.75 | Fe3O4-PEI | 0.026 | 4 | 120 | 98 | [70] |
| C. vulgaris | 1.683 | Fe3O4 | 0.119 | 4 | 90 | 70.8 | This study |
| C. vulgaris | n.a. | Fe3O4 | 2.0 | 4 | 120 | >70 | [93] |
| C. vulgaris | 1.683 | Fe3O4-PEI | 0.119 | 4 | 90 | 87.8 | This study |
| A. protothecoides | 0.746 | Fe3O4 | 0.268 | 4 | 90 | 88.8 | This study |
| A. protothecoides | 0.746 | Fe3O4-PEI | 0.268 | 4 | 90 | 99.0 | This study |
| Microalgae Species | Chlorella vulgaris | Chlorella ellipsoidea | Microcystis aeruginosa | Auxenochlorella protothecoides | Chlorella vulgaris | Chlorella ellipsoidea | Microcystis aeruginosa | Auxenochlorella protothecoides |
|---|---|---|---|---|---|---|---|---|
| sorbent | Fe3O4 | Fe3O4 | Fe3O4 | Fe3O4 | Fe3O4-PEI | Fe3O4-PEI | Fe3O4-PEI | Fe3O4-PEI |
| dose (mg) | 10.0 | 5.0 | 10.0 | 5.0 | 10.0 | 2.5 | 10.0 | 5.0 |
| model | Langmuir linear | Langmuir linear | ||||||
| C0 (g L−1) | 1.6848 | 1.4979 | 1.7894 | 0.7463 | 1.6848 | 1.4979 | 1.7882 | 0.7463 |
| Qm (g g−1) | 6.700 | 10.222 | 4.369 | 4.874 | 4.932 | 18.612 | 4.735 | 4.871 |
| KL (L g−1) | 5.666 | 15.310 | 17.954 | 15.153 | 23.306 | 7.070 | 51.257 | 26.088 |
| R2 | 0.980 | 0.994 | 0.985 | 0.962 | 0.990 | 0.947 | 0.998 | 0.993 |
| model | Freundlich linear | Freundlich linear | ||||||
| C0 (g L−1) | 1.6848 | 1.4979 | 1.7894 | 0.7463 | 1.6848 | 1.4979 | 1.7882 | 0.7463 |
| KF (g g−1) | 8.154 | 15.078 | 5.698 | 8.763 | 5.830 | 18.252 | 5.926 | 7.956 |
| 1/nF | 0.577 | 0.454 | 0.350 | 0.511 | 0.303 | 0.382 | 0.269 | 0.405 |
| R2 | 0.905 | 0.909 | 0.637 | 0.862 | 0.823 | 0.986 | 0.709 | 0.953 |
| model | Langmuir nonlinear | Langmuir nonlinear | ||||||
| C0 (g L−1) | 1.6848 | 1.4979 | 1.7894 | 0.7463 | 1.6848 | 1.4979 | 1.7882 | 0.7463 |
| Qm (g g−1) | 6.519 | 10.105 | 4.548 | 4.683 | 4.601 | 19.523 | 4.843 | 4.753 |
| KL (L g−1) | 6.364 | 16.034 | 17.800 | 17.254 | 38.964 | 5.609 | 49.245 | 28.084 |
| R2 | 0.972 | 0.984 | 0.886 | 0.947 | 0.938 | 0.925 | 0.945 | 0.980 |
| χ2 | 0.0653 | 0.1178 | 0.2063 | 0.0692 | 0.1067 | 0.4899 | 0.1055 | 0.0309 |
| RL | 0.0853 | 0.0399 | 0.0304 | 0.0720 | 0.0150 | 0.1063 | 0.0112 | 0.0455 |
| model | Freundlich nonlinear | Freundlich nonlinear | ||||||
| C0 (g L−1) | 1.6848 | 1.4979 | 1.7894 | 0.7463 | 1.6848 | 1.4979 | 1.7882 | 0.7463 |
| KF (g g−1) | 6.757 | 12.908 | 4.720 | 28.084 | 5.369 | 19.268 | 5.319 | 6.986 |
| 1/nF | 0.434 | 0.370 | 0.255 | 0.426 | 0.245 | 0.424 | 0.199 | 0.345 |
| R2 | 0.912 | 0.934 | 0.699 | 0.935 | 0.914 | 0.990 | 0.755 | 0.964 |
| χ2 | 0.2027 | 0.0491 | 0.4497 | 0.0846 | 0.1467 | 0.0335 | 0.4702 | 0.0573 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerulová, K.; Kucmanová, A.; Sanny, Z.; Garaiová, Z.; Seiler, E.; Čaplovičová, M.; Čaplovič, Ľ.; Palcut, M. Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials 2022, 12, 1786. https://doi.org/10.3390/nano12111786
Gerulová K, Kucmanová A, Sanny Z, Garaiová Z, Seiler E, Čaplovičová M, Čaplovič Ľ, Palcut M. Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials. 2022; 12(11):1786. https://doi.org/10.3390/nano12111786
Chicago/Turabian StyleGerulová, Kristína, Alexandra Kucmanová, Zuzana Sanny, Zuzana Garaiová, Eugen Seiler, Mária Čaplovičová, Ľubomír Čaplovič, and Marián Palcut. 2022. "Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides" Nanomaterials 12, no. 11: 1786. https://doi.org/10.3390/nano12111786
APA StyleGerulová, K., Kucmanová, A., Sanny, Z., Garaiová, Z., Seiler, E., Čaplovičová, M., Čaplovič, Ľ., & Palcut, M. (2022). Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials, 12(11), 1786. https://doi.org/10.3390/nano12111786







