Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials
Abstract
1. Introduction
2. Synthesis of Nanocrystal Heterostructures: Fundamental Concepts and Formation Mechanisms
2.1. Synthesis of Single-Material Nanocrystals
2.2. Growth Thermodynamics of a Nanocrystal Heterostructure
- (i)
- is the volume free energy earned () in the formation of the new crystalline domain upon the incorporation of moles of the relevant monomer constituents previously existing in the original liquid phase, and is the difference in chemical potential between the monomer species in the lattice and those in the solution environment.
- (ii)
- is the total surface-interface energy cost ( required for:
- -
- the creation of the solid/solution heterointerface of area and specific interfacial energy (also simply referred to as surface energy) , associated with the solid surface of the newly deposited material (2) exposed to the solution medium;
- -
- the creation of the solid/solid heterointerface of area and specific interfacial energy (or simply, interface energy), between the substrate (1) and the new material (2);
- -
- the elimination of a corresponding solid/solution heterointerface) of area (with ) and specific interfacial energy (surface energy) from the solution-exposed solid surface of the substrate (1).
2.3. Liquid-Phase Epitaxy via Seeded Growth
3. Heterostructures with Core@Shell Configurations
3.1. Direct Heterogeneous Deposition
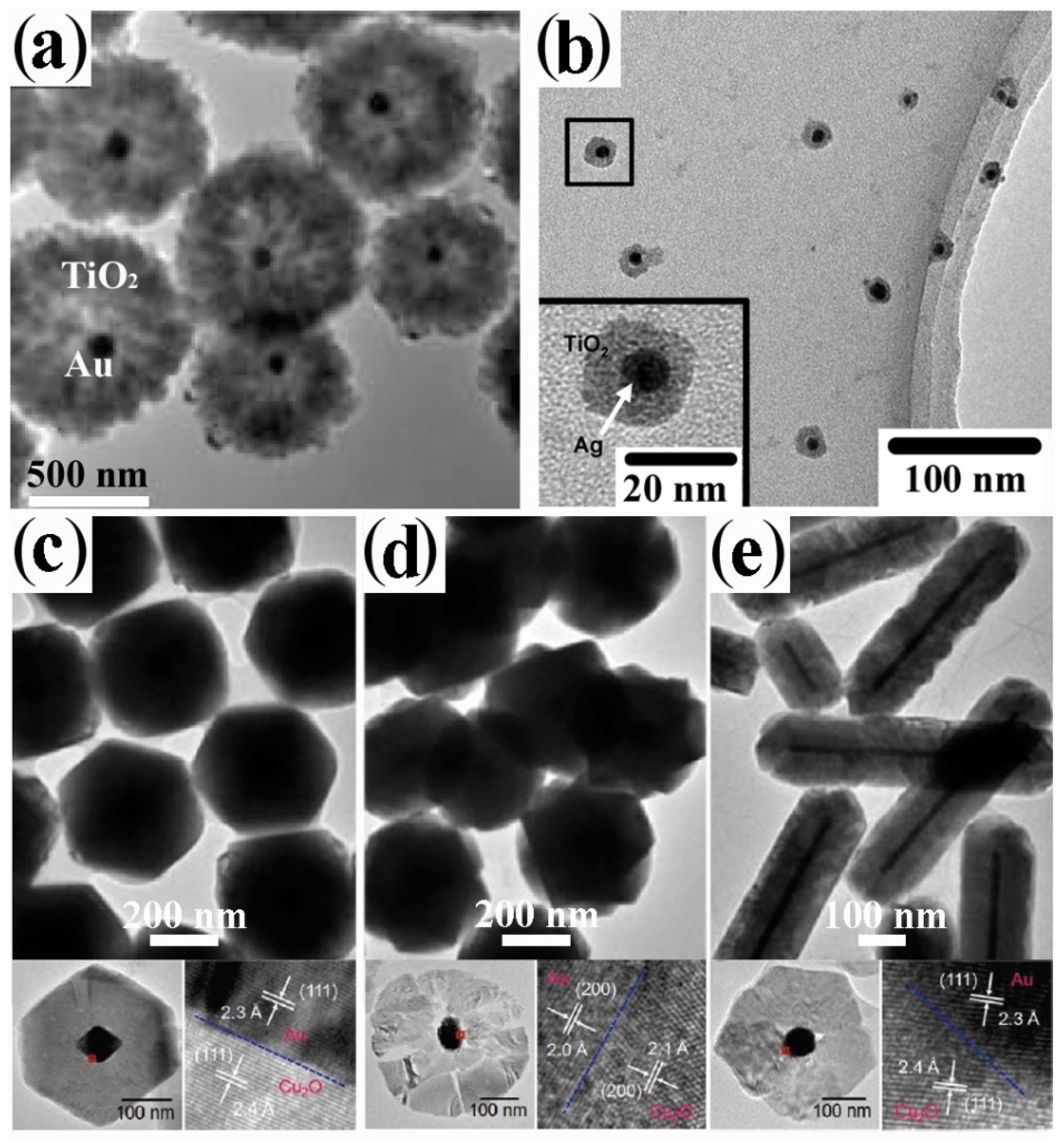
3.2. Silica Coating
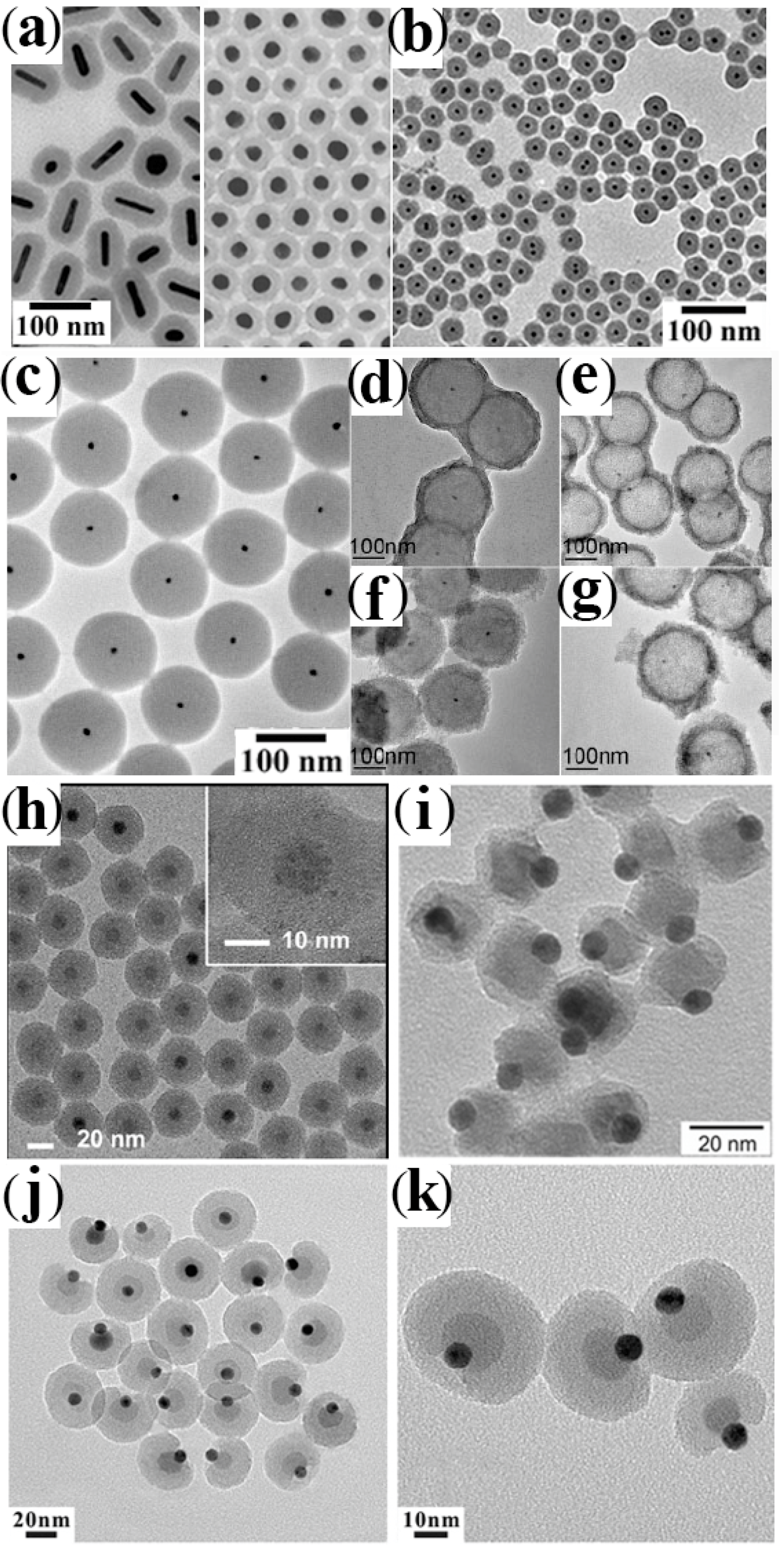
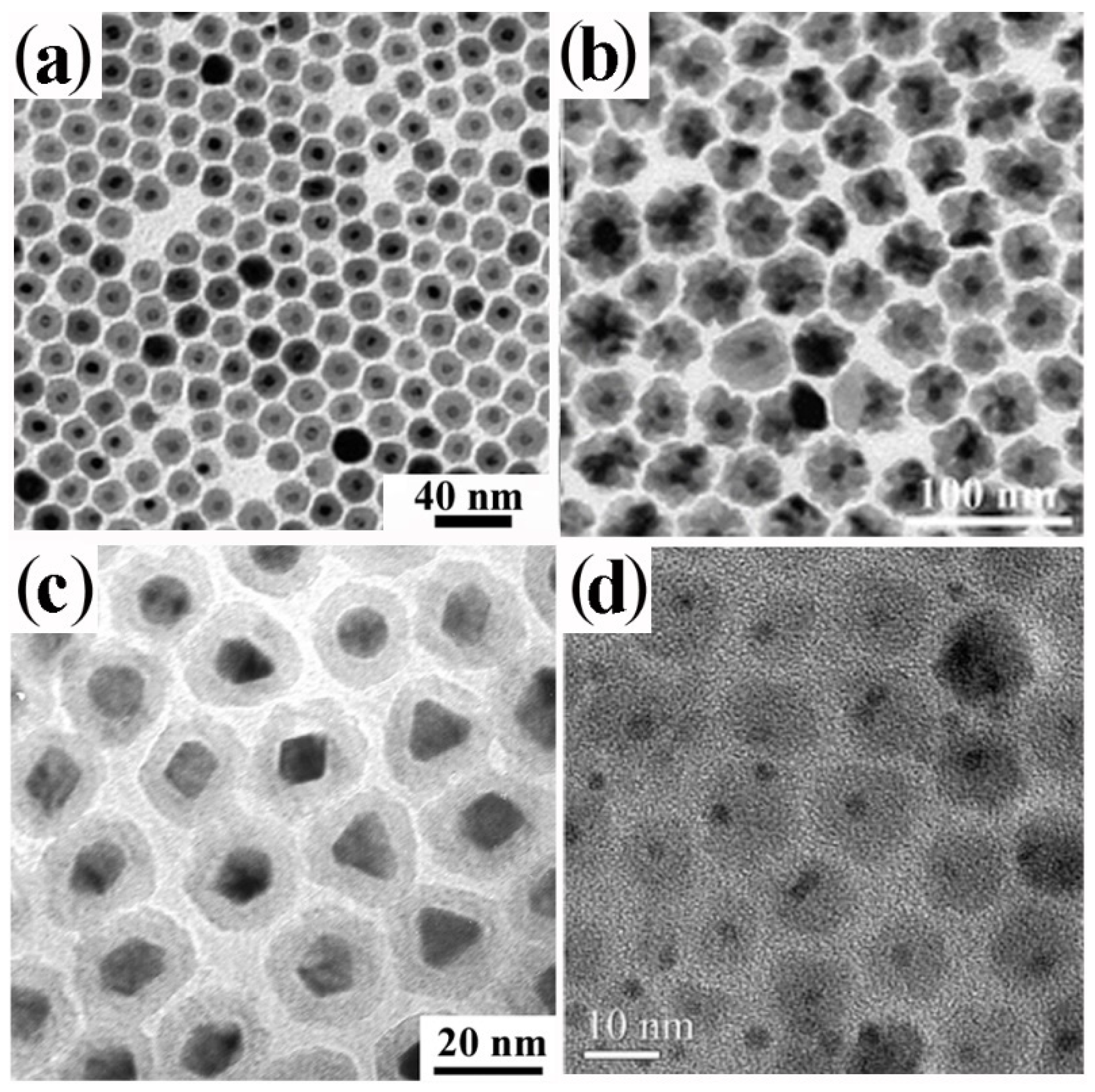
3.3. Shell Formation by Galvanic Replacement and Transformative Reactions
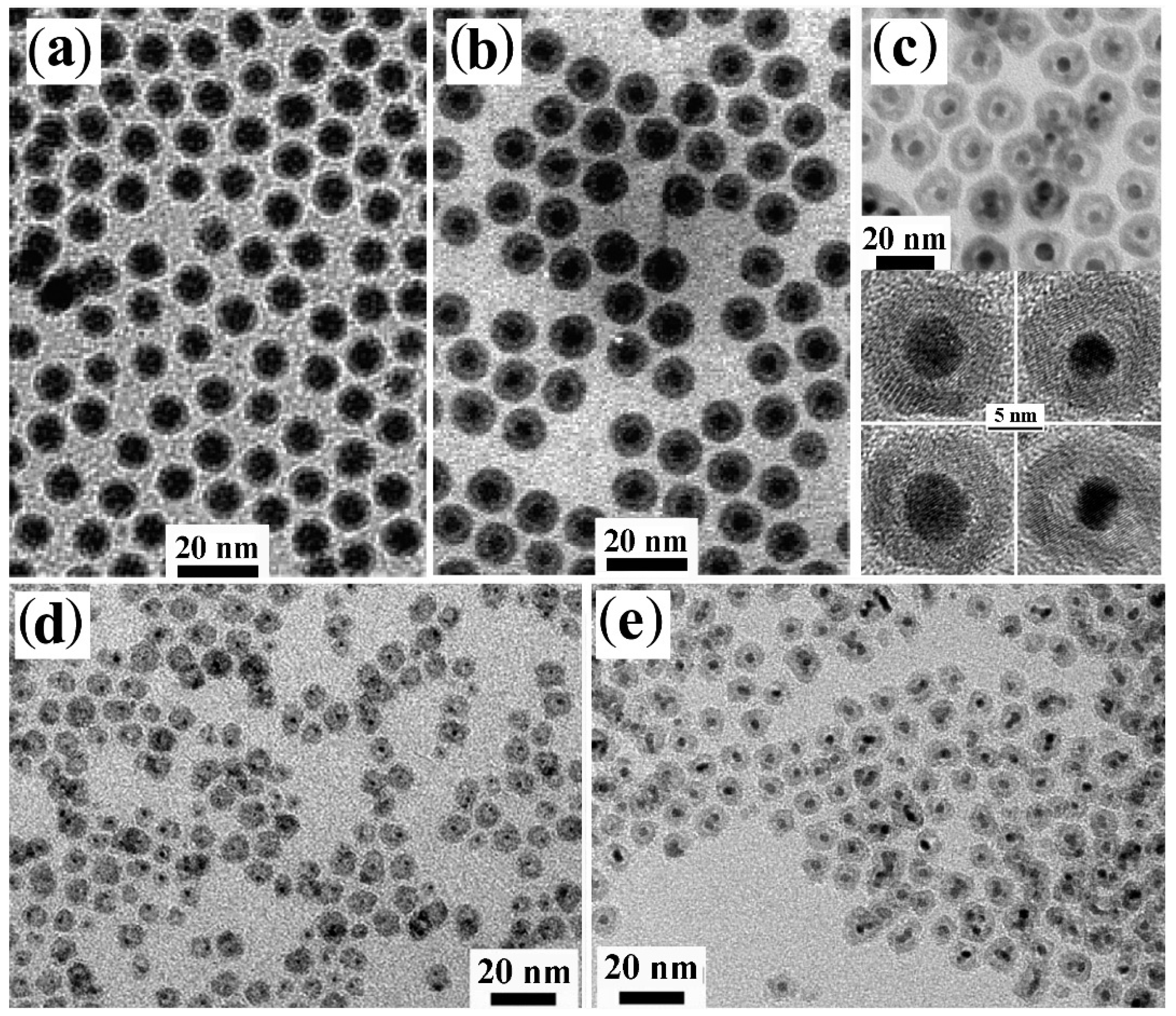
3.4. Shell Transformation via Cation-Exchange Reactions
3.5. Self-Regulated Nucleation and Growth
3.6. Thermally Induced Atomic Diffusion and Crystal-Phase Segregation
4. Non-Core@Shell Heteromeric Architectures
4.1. Heteromers Based on Nearly Isotropic-Shaped Material Domains
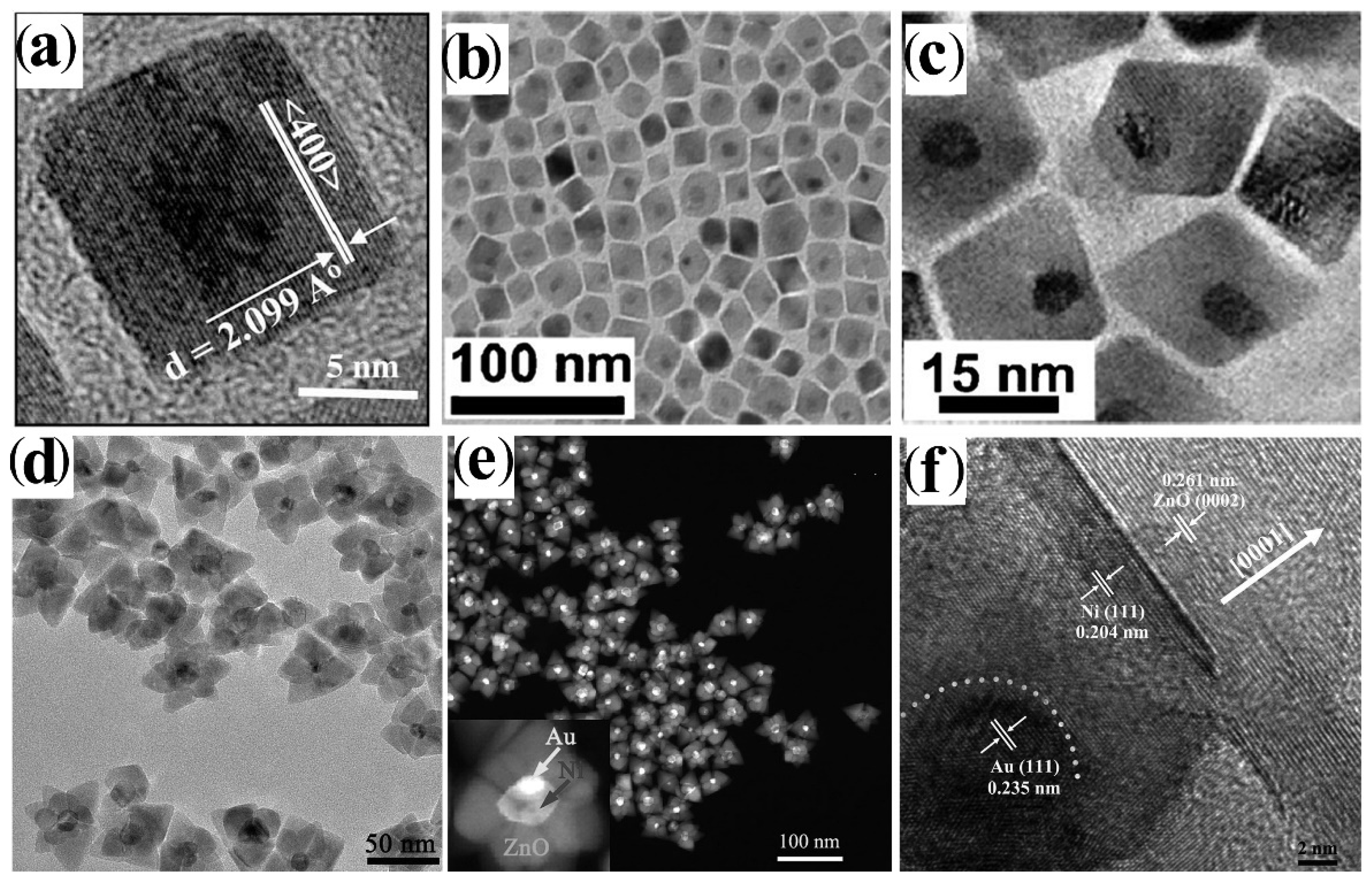
4.1.1. Direct Heterogeneous Nucleation-Growth (and De-Wetting)
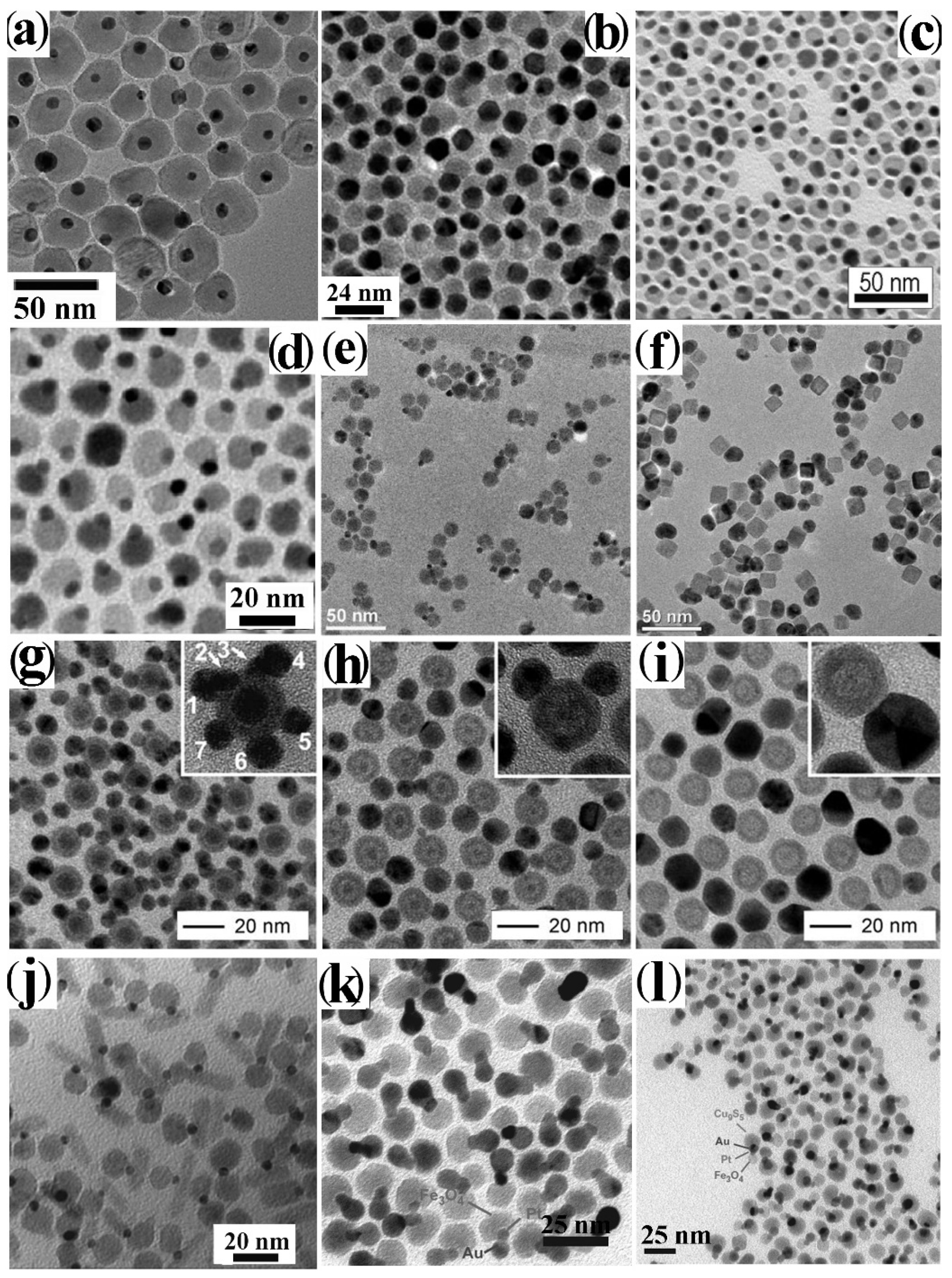
4.1.2. Heterogeneous Nucleation-Growth at the Interface between Immiscible Liquids
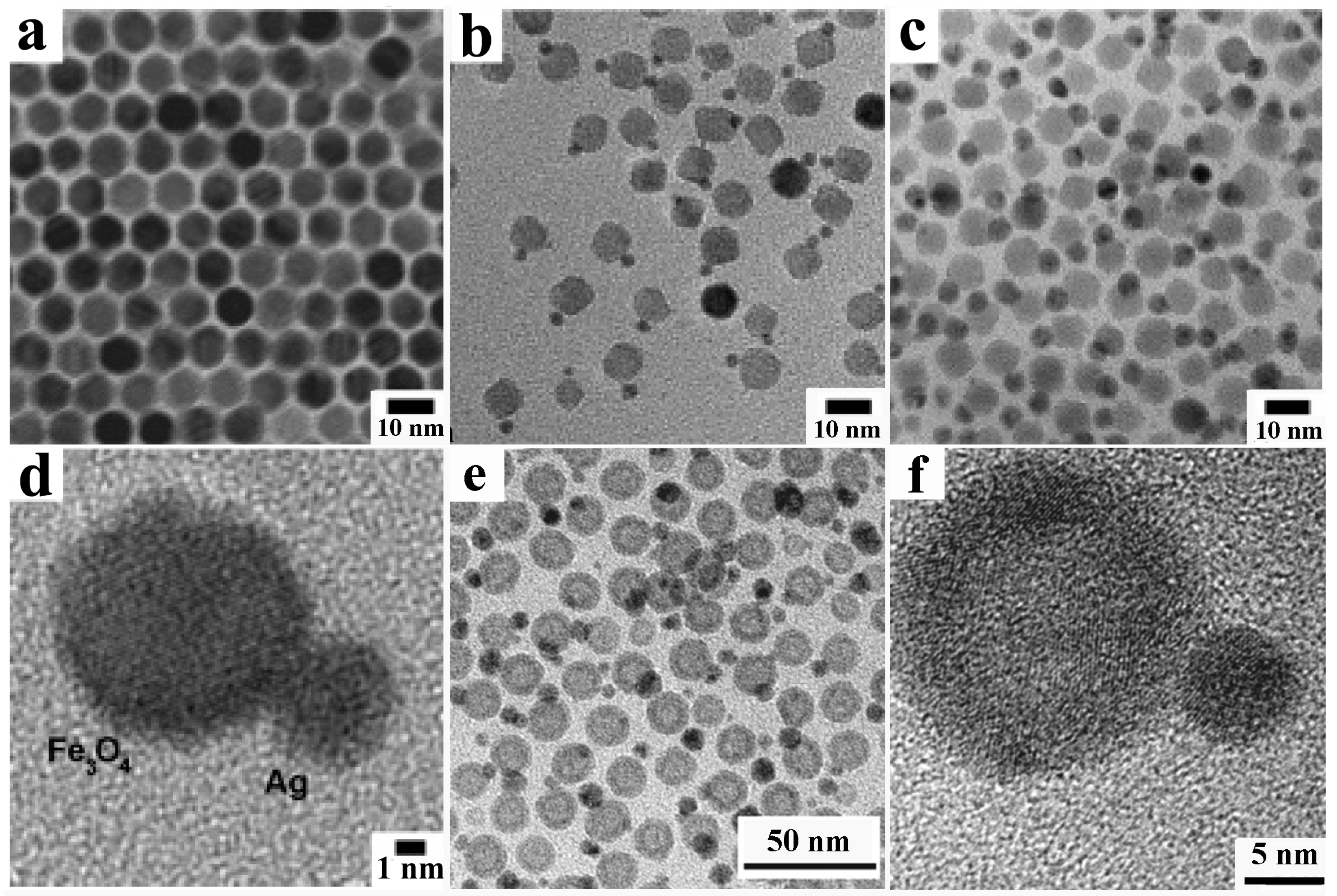
4.1.3. Self-Regulated Homogeneous and Heterogeneous Nucleation-Growth
4.1.4. Solid-State Diffusion and Phase Segregation
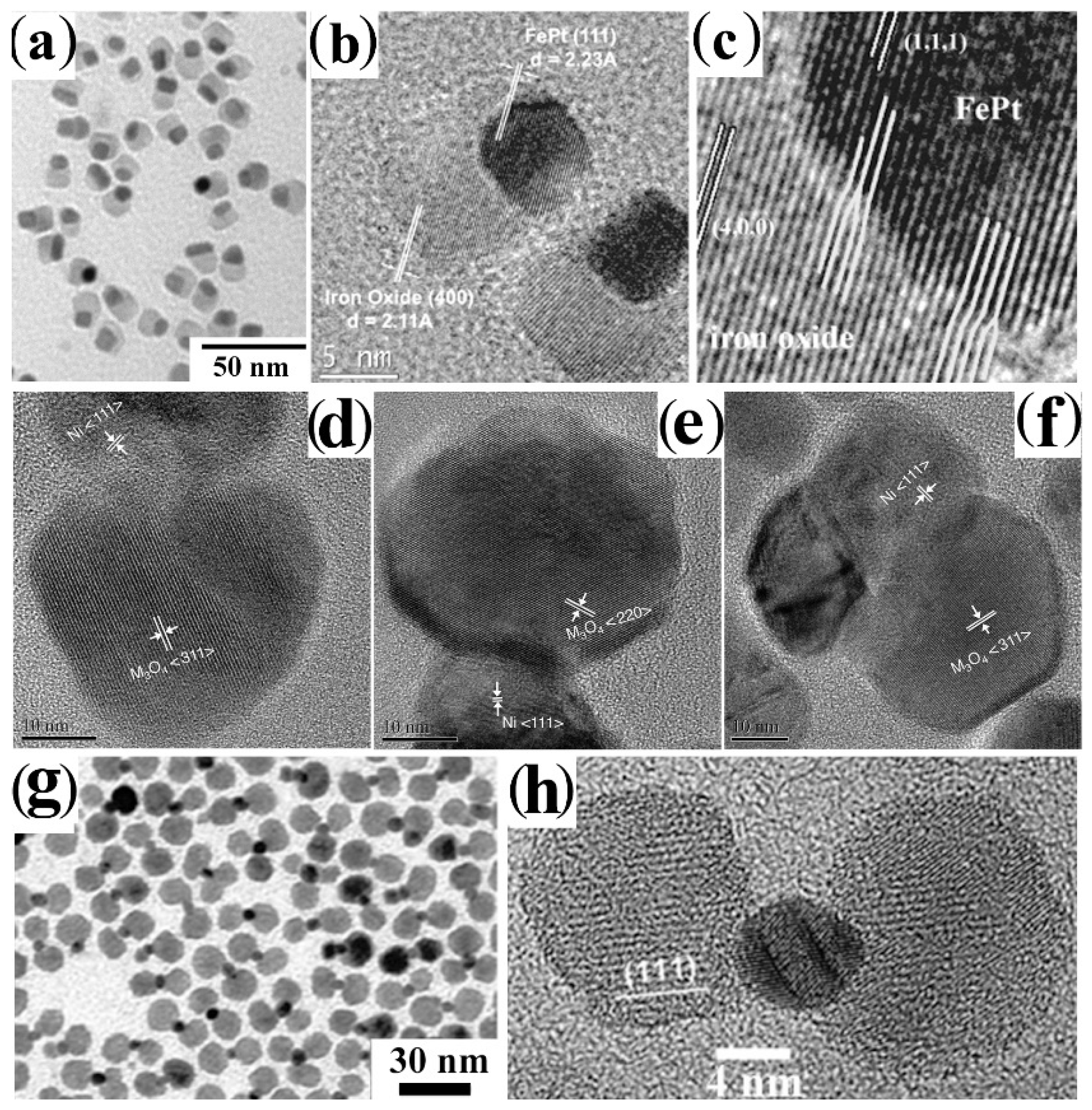
4.1.5. Induced Welding between Preformed Nanocrystal Hetero-Dimers
4.2. Nanoheterostructures Based on Anisotropic-Shaped Domains
4.2.1. Regioselective Heterogeneous Nucleation
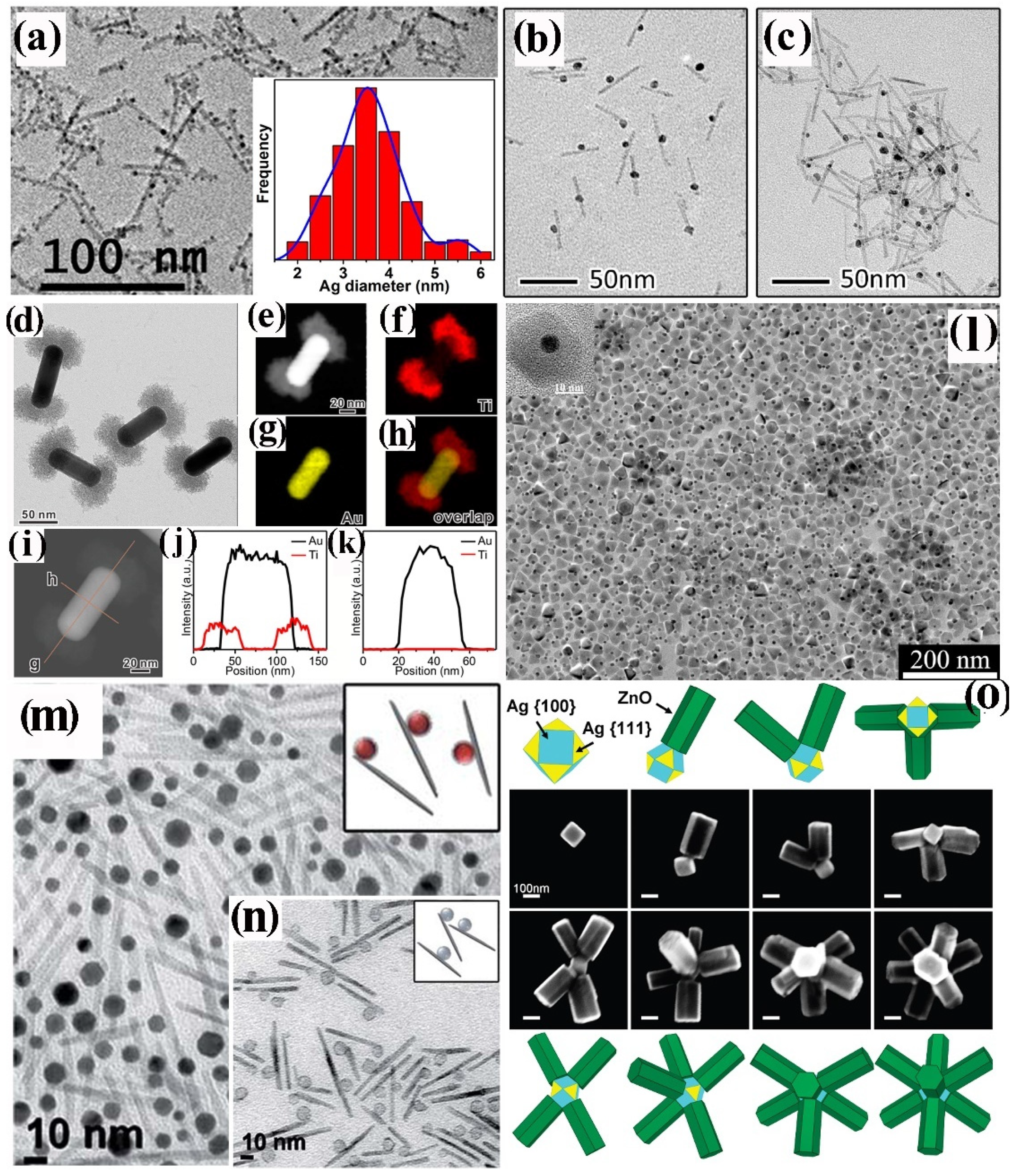
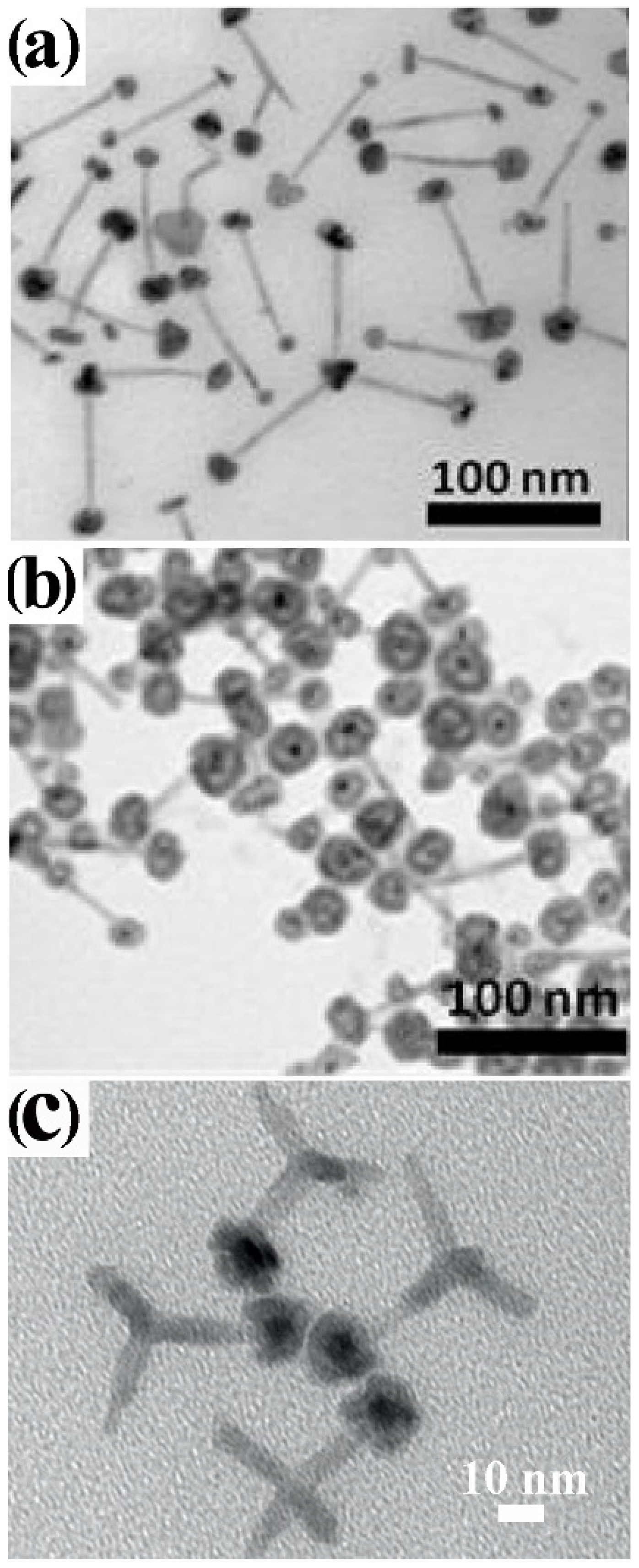
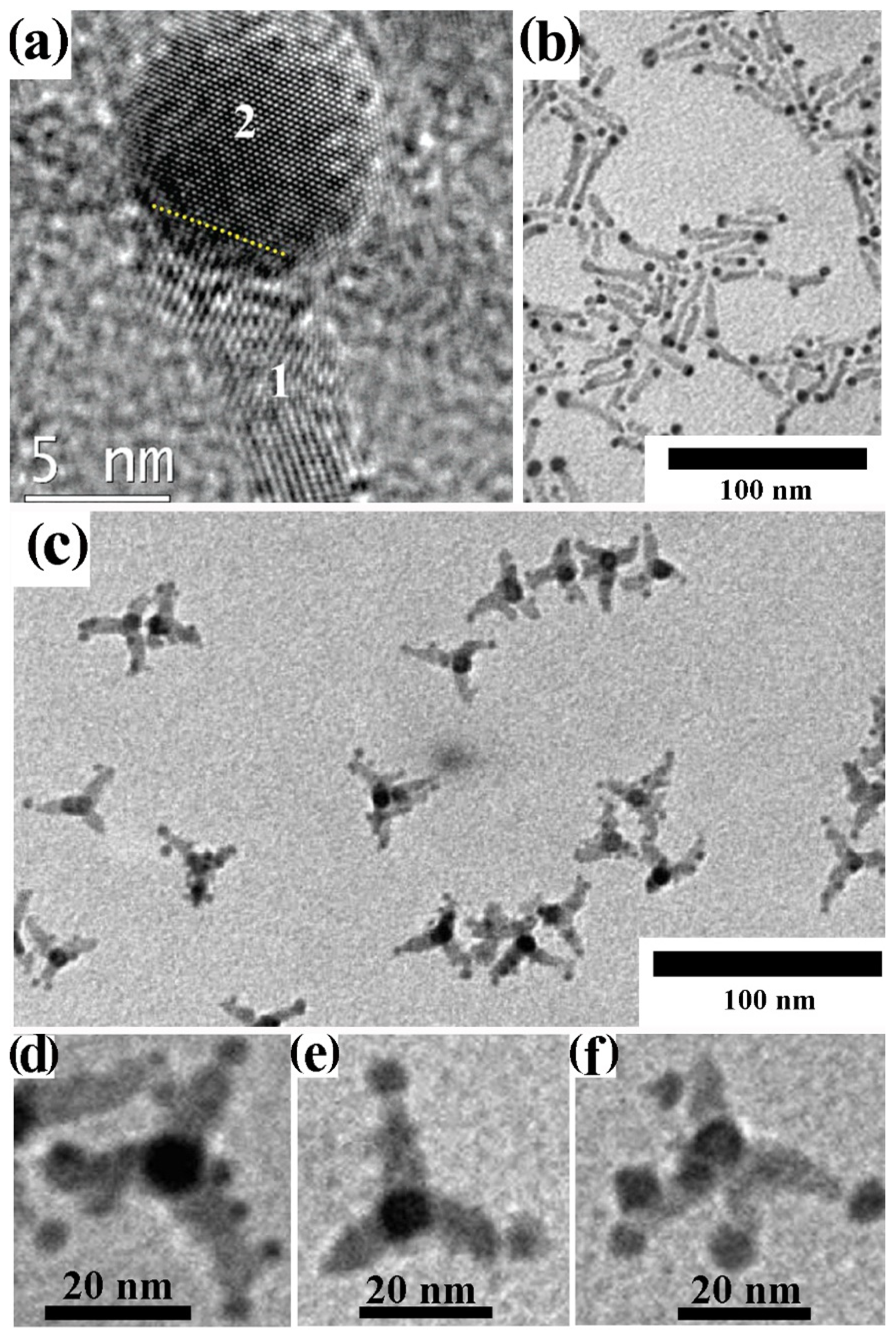
4.2.2. Surfactant-Controlled Facet-Selective Heterogeneous Nucleation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burda, C.; Chen, X.B.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D. (Ed.) Advanced Wet-Chemical Synthetic Approaches to Inorganic Nanostructures; Transworld Research Network: Kerala, India, 2008; p. 453. [Google Scholar]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents—Synthesis, Formation, Assembly and Application; Springer: London, UK, 2009; p. 217. [Google Scholar]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Rogach, A.L. (Ed.) Semiconductor Nanocrystal Quantum Dots: Synthesis, Assembly, Spectroscopy and Applications; Springer: Vienna, Austria; New York, NY, USA, 2008; p. 372. [Google Scholar]
- Chan, W.C.W. (Ed.) Bio-Applications of Nanoparticles. In Experimental Medicine and Biology; Landes Bioscience and Pringer Science+Business Media, LLC: New York, NY, USA, 2007; Volume 620, p. 222. [Google Scholar]
- Jun, Y.-W.; Choi, J.-S.; Cheon, J. Shape Control of Semiconductor and Metal Oxide Nanocrystals through Nonhydrolytic Colloidal Routes. Angew. Chem. Int. Ed. 2006, 45, 3414–3439. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Xia, Y. Metal Nanocrystals with Highly Branched Morphologies. Angew. Chem. Int. Ed. 2011, 50, 76–85. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xia, Y. Noble-Metal Nanocrystals with Concave Surfaces: Synthesis and Applications. Angew. Chem. Int. Ed. 2012, 51, 7656–7673. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- Kwon, S.G.; Hyeon, T. Colloidal Chemical Synthesis and Formation Kinetics of Uniformly Sized Nanocrystals of Metals, Oxides, and Chalcogenides. Acc. Chem. Res. 2008, 41, 1696–1709. [Google Scholar] [CrossRef]
- An, K.; Hyeon, T. Synthesis and biomedical applications of hollow nanostructures. Nano Today 2009, 4, 359–373. [Google Scholar]
- Wang, W.S.; Dahl, M.; Yin, Y.D. Hollow Nanocrystals through the Nanoscale Kirkendall Effect. Chem. Mater. 2013, 25, 1179–1189. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Goebl, J.; Yin, Y. Self-templated synthesis of hollow nanostructures. Nano Today 2009, 4, 494–507. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 116, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Bouet, C.; Tessier, M.D.; Ithurria, S.; Mahler, B.; Nadal, B.; Dubertret, B. Flat Colloidal Semiconductor Nanoplatelets. Chem. Mater. 2013, 25, 1262–1271. [Google Scholar] [CrossRef]
- Yang, J.; Son, J.S.; Yu, J.H.; Joo, J.; Hyeon, T. Advances in the Colloidal Synthesis of Two-Dimensional Semiconductor Nanoribbons. Chem. Mater. 2013, 25, 1190–1198. [Google Scholar] [CrossRef]
- Lhuillier, E.; Pedetti, S.; Ithurria, S.; Nadal, B.; Heuclin, H.; Dubertret, B. Two-Dimensional Colloidal Metal Chalcogenides Semiconductors: Synthesis, Spectroscopy, and Applications. Acc. Chem. Res. 2015, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Choi, J.-S.; Cheon, J. Heterostructured magnetic nanoparticles: Their versatility and high performance capabilities. Chem. Commun. 2007, 12, 1203–1214. [Google Scholar] [CrossRef]
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Bigall, N.C.; Parak, W.J.; Dorfs, D. Fluorescent, magnetic and plasmonic—Hybrid multifunctional colloidal nano objects. Nano Today 2012, 7, 282–296. [Google Scholar] [CrossRef]
- Lim, J.; Majetich, S.A. Composite magnetic-plasmonic nanoparticles for biomedicine: Manipulation and imaging. Nano Today 2013, 8, 98–113. [Google Scholar] [CrossRef]
- Buonsanti, R.; Casavola, M.; Caputo, G.; Cozzoli, P.D. Advances in the Chemical Fabrication of Complex Multimaterial Nanocrystals. Recent Pat. Nanotechnol. 2007, 1, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal Strategies for Preparing Oxide-Based Hybrid Nanocrystals. Eur. J. Inorg. Chem. 2008, 2008, 837–854. [Google Scholar] [CrossRef]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, G.; Wang, X. Rational synthesis and the structure-property relationships of nanoheterostructures: A combinative study of experiments and theory. NPG Asia Mater. 2015, 7, e164. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.R.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. Yolk/shell nanoparticles: Classifications, synthesis, properties, and applications. Nanoscale 2015, 7, 19789–19873. [Google Scholar] [CrossRef]
- de Mello Donegà, C. Synthesis and properties of colloidal heteronanocrystals. Chem. Soc. Rev. 2011, 40, 1512–1546. [Google Scholar] [CrossRef]
- Buck, M.R.; Schaak, R.E. Emerging Strategies for the Total Synthesis of Inorganic Nanostructures. Angew. Chem. Int. Ed. 2013, 52, 6154–6178. [Google Scholar] [CrossRef]
- Sitt, A.; Hadar, I.; Banin, U. Band-gap engineering, optoelectronic properties and applications of colloidal heterostructured semiconductor nanorods. Nano Today 2013, 8, 494–513. [Google Scholar] [CrossRef]
- Melinon, P.; Begin-Colin, S.; Duvail, J.L.; Gauffre, F.; Boime, N.H.; Ledoux, G.; Plain, J.; Reiss, P.; Silly, F.; Warot-Fonrose, B. Engineered inorganic core/shell nanoparticles. Phys. Rep. 2014, 543, 163–197. [Google Scholar] [CrossRef]
- Banin, U.; Ben-Shahar, Y.; Vinokurov, K. Hybrid Semiconductor–Metal Nanoparticles: From Architecture to Function. Chem. Mater. 2014, 26, 97–110. [Google Scholar] [CrossRef]
- Qi, J.; Lai, X.; Wang, J.; Tang, H.; Ren, H.; Yang, Y.; Jin, Q.; Zhang, L.; Yu, R.; Ma, G.; et al. Multi-shelled hollow micro-/nanostructures. Chem. Soc. Rev. 2015, 44, 6749–6773. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef]
- Armelles, G.; Cebollada, A.; Garcia-Martin, A.; Gonzalez, M.U. Magnetoplasmonics: Combining Magnetic and Plasmonic Functionalities. Adv. Opt. Mater. 2013, 1, 10–35. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Maccaferri, N. Coupling phenomena and collective effects in resonant meta-atoms supporting both plasmonic and (opto-)magnetic functionalities: An overview on properties and applications. J. Opt. Soc. Am. B Opt. Phys. 2019, 36, E112–E131. [Google Scholar] [CrossRef]
- Yin, P.H.; Radovanovic, P.V. Magnetoplasmon Resonances in Semiconductor Nanocrystals: Potential for a New Information Technology Platform. ChemSusChem 2019, 13, 4885–4893. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Xie, W. Plasmonic metal/semiconductor hybrid nanomaterials for solar to chemical energy conversion. J. Energy Chem. 2021, 63, 40–53. [Google Scholar]
- Zhang, J.; Wang, Y.; Li, D.; Sun, Y.; Jiang, L. Engineering Surface Plasmons in Metal/Nonmetal Structures for Highly Desirable Plasmonic Photodetectors. ACS Mater. Lett. 2022, 4, 343–355. [Google Scholar] [CrossRef]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured Catalysts for Organic Transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Rawalekar, S.; Mokari, T. Rational Design of Hybrid Nanostructures for Advanced Photocatalysis. Adv. Energy Mater. 2013, 3, 12–27. [Google Scholar] [CrossRef]
- Song, H. Metal Hybrid Nanoparticles for Catalytic Organic and Photochemical Transformations. Acc. Chem. Res. 2015, 48, 491–499. [Google Scholar] [CrossRef]
- Liao, F.; Lo, B.T.W.; Tsang, E. The Applications of Nano-Hetero-Junction in Optical and Thermal Catalysis. Eur. J. Inorg. Chem. 2016, 2016, 1924–1938. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Liu, C.; Chong, W.H.; Chen, H. Thermodynamics versus Kinetics in Nanosynthesis. Angew. Chem. Int. Ed. 2015, 54, 2022–2051. [Google Scholar] [CrossRef]
- De Mello Donegà, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical evaluation of the hot-injection method, a synthesis route for monodisperse nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F.; Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale 2010, 2, 18–34. [Google Scholar] [CrossRef]
- Li, H.B.; Kanaras, A.G.; Manna, L. Colloidal Branched Semiconductor Nanocrystals: State of the Art and Perspectives. Acc. Chem. Res. 2013, 46, 1387–1396. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X. Ultrathin nanostructures: Smaller size with new phenomena. Chem. Soc. Rev. 2013, 42, 5577–5594. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, R.; Chen, Q. Synthesis and assembly of nanomaterials under magnetic fields. Nanoscale 2014, 6, 14064–14105. [Google Scholar] [CrossRef]
- Wang, F.; Richards, V.N.; Shields, S.P.; Buhro, W.E. Kinetics and Mechanisms of Aggregative Nanocrystal Growth. Chem. Mater. 2014, 26, 5–21. [Google Scholar] [CrossRef]
- Markov, I.V. Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth, and Epitaxy; World Scientific: Singapore, 2003. [Google Scholar]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Gentili, D.; Foschi, G.; Valle, F.; Cavallini, M.; Biscarini, F. Applications of dewetting in micro and nanotechnology. Chem. Soc. Rev. 2012, 41, 4430–4443. [Google Scholar] [CrossRef] [PubMed]
- Jerison, E.R.; Xu, Y.; Wilen, L.A.; Dufresne, E.R. Deformation of an Elastic Substrate by a Three-Phase Contact Line. Phys. Rev. Lett. 2011, 106, 186103. [Google Scholar] [CrossRef] [PubMed]
- Scarfiello, R.; Nobile, C.; Cozzoli, P.D. Colloidal Magnetic Heterostructured Nanocrystals with Asymmetric Topologies: Seeded-Growth Synthetic Routes and Formation Mechanisms. Front. Mater. 2016, 3, 56. [Google Scholar] [CrossRef][Green Version]
- Oldfield, G.; Ung, T.; Mulvaney, P. Au@SnO2 Core-Shell Nanocapacitors. Adv. Mater. 2000, 12, 1519–1522. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.C. Size Tuning, Functionalization, and Reactivation of Au in TiO2 Nanoreactors. Angew. Chem. Int. Ed. 2005, 44, 4342–4345. [Google Scholar] [CrossRef]
- Sakai, H.; Kanda, T.; Shibata, H.; Ohkubo, T.; Abe, M. Preparation of Highly Dispersed Core/Shell-type Titania Nanocapsules Containing a Single Ag Nanoparticle. J. Am. Chem. Soc. 2006, 128, 4944–4945. [Google Scholar] [CrossRef]
- Bayles, A.; Tian, S.; Zhou, J.; Yuan, L.; Yuan, Y.; Jacobson, C.R.; Farr, C.; Zhang, M.; Swearer, D.F.; Solti, D.; et al. Al@TiO2 Core–Shell Nanoparticles for Plasmonic Photocatalysis. ACS Nano 2022, 16, 5839–5850. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Hua, T.-E.; Huang, M.H. Au Nanocrystal-Directed Growth of Au-Cu2O Core-Shell Heterostructures with Precise Morphological Control. J. Am. Chem. Soc. 2009, 131, 17871–17878. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Y.; Liu, F.; Tong, Z.; Sha, J.; Zhao, W.; Liu, M.; Zheng, Y. Seeded Growth of Au@CuxO Core–Shell Mesoporous Nanospheres and Their Photocatalytic Properties. Front. Chem. 2021, 9, 671220. [Google Scholar] [CrossRef] [PubMed]
- Sundarapandi, M.; Shanmugam, S.; Ramaraj, R. Tuning Cu2O Shell on Gold Nanocube Core Employing Amine-Functionalized Silane for Electrocatalytic Nitrite Detection. ACS Appl. Nano Mater. 2022, 5, 1674–1682. [Google Scholar] [CrossRef]
- Piella, J.; Gónzalez-Febles, A.; Patarroyo, J.; Arbiol, J.; Bastús, N.G.; Puntes, V. Seeded-Growth Aqueous Synthesis of Colloidal-Stable Citrate-Stabilized Au/CeO2 Hybrid Nanocrystals: Heterodimers, Core@Shell, and Clover- and Star-Like Structures. Chem. Mater. 2019, 31, 7922–7932. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Green Emission to Probe Photoinduced Charging Events in ZnO-Au Nanoparticles. Charge Distribution and Fermi-Level Equilibration. J. Phys. Chem. B 2003, 107, 7479–7485. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Influence of Metal/Metal Ion Concentration on the Photocatalytic Activity of TiO2-Au Composite Nanoparticles. Langmuir 2003, 19, 469–474. [Google Scholar] [CrossRef]
- Dawson, A.; Kamat, P.V. Semiconductor-Metal Nanocomposites. Photoinduced Fusion and Photocatalysis of Gold-Capped TiO2 (TiO2/Gold) Nanoparticles. J. Phys. Chem. B 2001, 105, 960–966. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Dawson, A. Metal-metal and metal-semiconductor composite nanoclusters. Colloid Surf. A Physicochem. Eng. Asp. 2002, 202, 269–279. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bysakh, S.; Mishra, P.M.; De, G. In Situ Synthesis of Mesoporous TiO2 Nanofibers Surface-Decorated with AuAg Alloy Nanoparticles Anchored by Heterojunction Exhibiting Enhanced Solar Active Photocatalysis. Langmuir 2019, 35, 14364–14375. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Giersig, M.; Mulvaney, P. Fermi level equilibration in quantum dot-metal nanojunctions. J. Phys. Chem. B 2001, 105, 8810–8815. [Google Scholar] [CrossRef]
- Hikov, T.; Schroeter, M.-K.; Khodeir, L.; Chemseddine, A.; Muhler, M.; Fischer, R.A. Selective photo-deposition of Cu onto the surface of monodisperse oleic acid capped TiO2 nanorods probed by FT-IR CO-adsorption studies. Phys. Chem. Chem. Phys. 2006, 8, 1550–1555. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Qian, K.; Xu, W.; Lu, Y.; Huang, W.; Yu, S. Large scale photochemical synthesis of M@TiO2 nanocomposites (M = Ag, Pd, Au, Pt) and their optical properties, CO oxidation performance, and antibacterial effect. Nano Res. 2010, 3, 244–255. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Shafiee, A.; Rabiee, N.; Ahmadi, S.; Baneshi, M.; Khatami, M.; Iravani, S.; Varma, R.S. Core–Shell Nanophotocatalysts: Review of Materials and Applications. ACS Appl. Nano Mater. 2022, 5, 55–86. [Google Scholar] [CrossRef]
- Jiang, A.; Wang, Z.; Li, Q.; Dong, M. An efficient ruthenium-based dual-electrocatalyst towards hydrogen evolution and oxygen reduction reactions. Mater. Today Phys. 2021, 16, 100300. [Google Scholar] [CrossRef]
- Wang, L.Y.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.H.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed core-shell Fe3O4@Au nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and Antibacterial Activity of Fe3O4@Ag Nanoparticles. Nanotechnology 2007, 18, 285604–285610. [Google Scholar] [CrossRef]
- Yano, K.; Nandwana, V.; Chaubey, G.S.; Poudyal, N.; Kang, S.; Arami, H.; Griffis, J.; Liu, J.P. Synthesis and Characterization of Magnetic FePt/Au Core/Shell Nanoparticles. J. Phys. Chem. C 2009, 113, 13088–13091. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Upadhyay, R.V.; Mehta, R.V. Enhanced Antibacterial Activity of Bifunctional Fe3O4-Ag Core-Shell Nanostructures. Nano Res. 2009, 2, 955–965. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Park, H.-Y.; Schadt, M.J.; Wang, L.-Y.; Lim, I.-I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.-Y.; Luo, J.; Zhong, C.-J. Fabrication of Magnetic Core@Shell Fe Oxide@Au Nanoparticles for Interfacial Bioactivity and Bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Miao, X.; Wang, T.; Chai, F.; Zhang, X.; Wang, C.; Sun, W. A facile synthetic route for the preparation of gold nanostars with magnetic cores and their reusable nanohybrid catalytic properties. Nanoscale 2011, 3, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pang, X.; He, Y.; Wang, Y.; Chen, G.; Wang, W.; Lin, Z. Precisely Size-Tunable Magnetic/Plasmonic Core/Shell Nanoparticles with Controlled Optical Properties. Angew. Chem. Int. Ed. 2015, 54, 12091–12096. [Google Scholar] [CrossRef] [PubMed]
- Encina, E.R.; Coronado, E.A. Size Optimization of Iron Oxide@Noble Metal Core–Shell Nanohybrids for Photothermal Applications. J. Phys. Chem. C 2016, 120, 5630–5639. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mammeri, F.; Ammar, S. Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials 2018, 8, 149. [Google Scholar] [CrossRef]
- Hui, W.; Shi, F.; Yan, K.; Peng, M.; Cheng, X.; Luo, Y.; Chen, X.; Roy, V.A.L.; Cui, Y.; Wang, Z. Fe3O4/Au/Fe3O4 nanoflowers exhibiting tunable saturation magnetization and enhanced bioconjugation. Nanoscale 2012, 4, 747–751. [Google Scholar] [CrossRef]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as A Robust Anchor to Immobilize Functional Molecules on the Iron Oxide Shell of Magnetic Nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Sun, S.; Liu, J.P.; Wang, Z.L. Analyzing the Structure of CoFe-Fe3O4 Core-Shell Nanoparticles by Electron Imaging and Diffraction. J. Phys. Chem. B 2004, 108, 14005–14008. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Peng, S.; Rong, C.; Liu, J.P.; Sun, S. A Facile Synthesis of SmCo5 Magnets from Core/Shell Co/Sm2O3 Nanoparticles. Adv. Mater. 2007, 19, 3349–3352. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, M.; Zhang, Z.; Gong, H.; Chin, W.S.; Liu, B. Synthesis and Characterization of Multifunctional FePt/ZnO Core/Shell Nanoparticles. Adv. Mater. 2010, 22, 403–406. [Google Scholar] [CrossRef]
- Teng, X.W.; Black, D.; Watkins, N.J.; Gao, Y.L.; Yang, H. Platinum-maghemite core-shell nanoparticles using a sequential synthesis. Nano Lett. 2003, 3, 261–264. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Wang, Z.L.; Liu, J.P.; Sun, S.H. Bimagnetic core/shell FePt/Fe3O4 nanoparticles. Nano Lett. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, S.H.; Li, J.; Wang, Z.L.; Liu, J.P. Tailoring magnetic properties of core/shell nanoparticles. Appl. Phys. Lett. 2004, 85, 792–794. [Google Scholar] [CrossRef]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A General Approach to Binary and Ternary Hybrid Nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.R.; Bondi, J.F.; Schaak, R.E. A total-synthesis framework for the construction of high-order colloidal hybrid nanoparticles. Nat. Chem. 2012, 4, 37–44. [Google Scholar] [CrossRef]
- Pineider, F.; de Julián Fernández, C.; Videtta, V.; Carlino, E.; al Hourani, A.; Wilhelm, F.; Rogalev, A.; Cozzoli, P.D.; Ghigna, P.; Sangregorio, C. Spin-Polarization Transfer in Colloidal Magnetic-Plasmonic Au/Iron Oxide Hetero-Nanocrystals. ACS Nano 2013, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Innocenti, C.; Bertoni, G.; Sangregorio, C.; Pineider, F. Modulation of the magnetic properties of gold-spinel ferrite heterostructured nanocrystals. Nano Res. 2020, 13, 785–794. [Google Scholar] [CrossRef]
- Tancredi, P.; Moscoso Londoño, O.; Rivas Rojas, P.C.; Wolff, U.; Socolovsky, L.M.; Knobel, M.; Muraca, D. Strategies to tailor the architecture of dual Ag/Fe-oxide nano-heterocrystals—interfacial and morphology effects on the magnetic behavior. J. Phys. D Appl. Phys. 2018, 51, 295303. [Google Scholar] [CrossRef]
- Sanna Angotzi, M.; Mameli, V.; Cara, C.; Grillo, V.; Enzo, S.; Musinu, A.; Cannas, C. Defect-assisted synthesis of magneto-plasmonic silver-spinel ferrite heterostructures in a flower-like architecture. Sci. Rep. 2020, 10, 17015. [Google Scholar] [CrossRef]
- Teng, X.; Yang, H. Synthesis of magnetic nanocomposites and alloys from platinum-iron oxide core-shell nanoparticles. Nanotechnology 2005, 16, S554–S561. [Google Scholar] [CrossRef]
- Jang, S.; Hira, S.A.; Annas, D.; Song, S.; Yusuf, M.; Park, J.C.; Park, S.; Park, K.H. Recent Novel Hybrid Pd-Fe3O4 Nanoparticles as Catalysts for Various C-C Coupling Reactions. Processes 2019, 7, 422. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, K.; Tian, Y.; Li, X.Z.; Kramer, M.J.; Sellmyer, D.J.; Shield, J.E.; Sun, S. One-Pot Synthesis of Urchin-like FePd–Fe3O4 and Their Conversion into Exchange-Coupled L10–FePd–Fe Nanocomposite Magnets. Nano Lett. 2013, 13, 4975–4979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.W.; Liu, W.; Yang, X.L.; Jiang, Z.; Yao, T.; Wei, S.Q.; Peng, X.G. Pt/Fe3O4 Core/Shell Triangular Nanoprisms by Heteroepitaxy: Facet Selectivity at the Pt-Fe3O4 Interface and the Fe3O4 Outer Surface. ACS Nano 2015, 9, 10950–10960. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, Z.; Li, Z.; Tian, L.; Ngai, T.; Wang, J. Plasmonic Gold-Superparamagnetic Hematite Heterostructures. Langmuir 2011, 27, 5071–5075. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Liu, H.-L.; Liu, X.; Fang, N.; Wang, X.-H.; Wu, J.-H. Synthesis of bi-phase dispersible core-shell FeAu@ZnO magneto-opto-fluorescent nanoparticles. Sci. Rep. 2015, 5, 16384. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-C.; Li, W.-P.; Luo, C.-H.; Su, C.-H.; Yeh, C.-S. Rattle-Type Fe3O4@CuS Developed to Conduct Magnetically Guided Photoinduced Hyperthermia at First and Second NIR Biological Windows. Adv. Funct. Mater. 2015, 25, 6527–6537. [Google Scholar] [CrossRef]
- Xie, Y.; Carbone, L.; Nobile, C.; Grillo, V.; D’Agostino, S.; Della Sala, F.; Giannini, C.; Altamura, D.; Oelsner, C.; Kryschi, C.; et al. Metallic-like Stoichiometric Copper Sulfide Nanocrystals: Phase- and Shape-Selective Synthesis, Near-Infrared Surface Plasmon Resonance Properties, and Their Modeling. ACS Nano 2013, 7, 7352–7369. [Google Scholar] [CrossRef]
- Masala, O.; Seshadri, R. Spinel Ferrite/MnO Core/Shell Nanoparticles: Chemical Synthesis of All-Oxide Exchange Biased Architectures. J. Am. Chem. Soc. 2005, 127, 9354–9355. [Google Scholar] [CrossRef]
- Skoropata, E.; Desautels, R.D.; Chi, C.C.; Ouyang, H.; Freeland, J.W.; van Lierop, J. Magnetism of iron oxide based core-shell nanoparticles from interface mixing with enhanced spin-orbit coupling. Phys. Rev. B 2014, 89, 024410. [Google Scholar] [CrossRef]
- Lee, K.S.; Anisur, R.M.; Kim, K.W.; Kim, W.S.; Park, T.J.; Kang, E.J.; Lee, I.S. Seed Size-Dependent Formation of Fe3O4/MnO Hybrid Nanocrystals: Selective, Magnetically Recyclable Catalyst Systems. Chem. Mater. 2012, 24, 682–687. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.J. Controlled Synthesis and Magnetic Properties of Bimagnetic Spinel Ferrite CoFe2O4 and MnFe2O4 Nanocrystals with Core–Shell Architecture. J. Am. Chem. Soc. 2012, 134, 10182–10190. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.-W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotech. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Estrader, M.; Lopez-Ortega, A.; Estrade, S.; Golosovsky, I.V.; Salazar-Alvarez, G.; Vasilakaki, M.; Trohidou, K.N.; Varela, M.; Stanley, D.C.; Sinko, M.; et al. Robust antiferromagnetic coupling in hard-soft bi-magnetic core/shell nanoparticles. Nat. Commun. 2013, 4, 2960. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Pichon, B.P.; Lefevre, C.; Ulhaq-Bouillet, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Bégin-Colin, S. High Exchange Bias in Fe3−δO4@CoO Core Shell Nanoparticles Synthesized by a One-Pot Seed-Mediated Growth Method. J. Phys. Chem. C 2013, 117, 11436–11443. [Google Scholar] [CrossRef]
- Krycka, K.L.; Borchers, J.A.; Salazar-Alvarez, G.; López-Ortega, A.; Estrader, M.; Estradé, S.; Winkler, E.; Zysler, R.D.; Sort, J.; Peiró, F.; et al. Resolving Material-Specific Structures within Fe3O4|γ-Mn2O3 Core|Shell Nanoparticles Using Anomalous Small-Angle X-ray Scattering. ACS Nano 2013, 7, 921–931. [Google Scholar] [CrossRef]
- Li, K.B.; Wu, Y.H.; Guo, Z.B.; Zheng, Y.K.; Han, G.C.; Qiu, J.J.; Luo, P.; An, L.H.; Zhou, T.J. Exchange coupling and its applications in magnetic data storage. J. Nanosci. Nanotechnol. 2007, 7, 13–45. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, J.; Yang, W.; Dong, Y.; Hou, Y.; Zhang, C.; Yin, H.; Sun, S. Building Nanocomposite Magnets by Coating a Hard Magnetic Core with a Soft Magnetic Shell. Angew. Chem. Int. Ed. 2014, 53, 2176–2180. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Zhelev, Z.; Ohba, H.; Bakalova, R. Single Quantum Dot-Micelles Coated with Silica Shell as Potentially Non-Cytotoxic Fluorescent Cell Tracers. J. Am. Chem. 2006, 128, 6324–6325. [Google Scholar] [CrossRef]
- Selvan, S.T.; Patra, P.K.; Ang, C.Y.; Ying, J.Y. Synthesis of Silica-Coated Semiconductor and Magnetic Quantum Dots and Their Use in the Imaging of Live Cells. Angew. Chem. Int. Ed. 2007, 46, 2448–2452. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Ow, H.; Wiesner, U. Fluorescent core-shell silica nanoparticles: Towards “Lab on a Particle” architectures for nanobiotechnology. Chem. Soc. Rev. 2006, 35, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.G.D.; Visconte, M.; Sun, J.; Schwartzberg, A.; Chen, S.; Zhang, J.Z. Silica-Coated CdTe Quantum Dots Functionalized with Thiols for Bioconjugation to IgG Proteins. J. Phys. Chem. B 2006, 110, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Han, M.Y. Synthesis, Functionalization, and Bioconjugation of Monodisperse, Silica-Coated Gold Nanoparticles: Robust Bioprobes. Adv. Funct. Mater. 2005, 15, 961–967. [Google Scholar] [CrossRef]
- Salgueirino-Maceira, V.; Correa-Duarte, M.A.; Farle, M.; Lopez-Quintela, A.; Sieradzki, K.; Diaz, R. Bifunctional Gold-Coated Magnetic Silica Spheres. Chem. Mater. 2006, 18, 2701–2706. [Google Scholar] [CrossRef]
- He, R.; You, X.; Shao, J.; Gao, F.; Pan, B.; Cui, D. Core/shell fluorescent magnetic silica-coated composite nanoparticles for bioconjugation. Nanotechnology 2007, 18, 315601. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally stable Pt/mesoporous silica core-shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-Temperature-Stable Catalysts by Hollow Sphere Encapsulation. Angew. Chem. Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, C.; Zuo, J.; Zheng, N.; Stucky, G.D. An Assembly Route to Inorganic Catalytic Nanoreactors Containing Sub-10-nm Gold Nanoparticles with Anti-Aggregation Properties. Small 2009, 5, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Guttel, R.; Paul, M.; Schuth, F. Ex-post size control of high-temperature-stable yolk-shell Au@ZrO2 catalysts. Chem. Commun. 2010, 46, 895–897. [Google Scholar] [CrossRef]
- Heitsch, A.T.; Smith, D.K.; Patel, R.N.; Ress, D.; Korgel, B.A. Multifunctional particles: Magnetic nanocrystals and gold nanorods coated with fluorescent dye-doped silica shells. J. Sol. State Chem. 2008, 181, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzan, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzan, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold-Silica Core-Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Mulvaney, P. The assembly of coated nanocrystals. J. Phys. Chem. B 2003, 107, 7312–7326. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Katakami, H.; Mine, E.; Nagao, D.; Konno, M.; Liz-Marzan, L.M. Silica coating of silver nanoparticles using a modified Stober method. J. Colloid Interface Sci. 2005, 283, 392–396. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Perez-Juste, J.; Liz-Marzan, L.M. Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chem. Mater. 2006, 18, 2465–2467. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Yu, K.N.; Kim, E.; Kim, J.S.; Kim, B.G.; Yun, S.-H.; Sohn, B.-H.; Cho, M.-H.; Lee, J.-K.; Park, S.B. Specific Targeting, Cell Sorting, and Bioimaging with Smart Magnetic Silica Core-Shell Nanomaterials. Small 2006, 2, 209–215. [Google Scholar] [CrossRef]
- Lee, D.C.; Mikulec, F.V.; Pelaez, J.M.; Koo, B.; Korgel, B.A. Synthesis and Magnetic Properties of Silica-Coated FePt Nanocrystals. J. Phys. Chem. B 2006, 110, 11160–11166. [Google Scholar] [CrossRef]
- Yang, D.; Hu, J.; Fu, S. Controlled Synthesis of Magnetite-Silica Nanocomposites via a Seeded Sol-Gel Approach. J. Phys. Chem. C 2009, 113, 7646–7651. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, L.; Lu, Q.; Fu, J.; Huang, X. Superparamagnetic submicro-megranates: Fe3O4 nanoparticles coated with highly cross-linked organic/inorganic hybrids. Chem. Commun. 2009, 42, 6370–6372. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, C.; Zhang, X. Synthesis of Fe3O4@SiO2@PMMA Core-Shell-Shell Magnetic Microspheres for Highly Efficient Enrichment of Peptides and Proteins for MALDI-ToF MS Analysis. Angew. Chem. Int. Ed. 2010, 49, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Cannas, C.; Musinu, A.; Ardu, A.; Orrù, F.; Peddis, D.; Casu, M.; Sanna, R.; Angius, F.; Diaz, G.; Piccaluga, G. CoFe2O4 and CoFe2O4/SiO2 Core/Shell Nanoparticles: Magnetic and Spectroscopic Study. Chem. Mater. 2010, 22, 3353–3361. [Google Scholar] [CrossRef]
- Zhang, F.; Braun, G.B.; Shi, Y.; Zhang, Y.; Sun, X.; Reich, N.O.; Zhao, D.; Stucky, G. Fabrication of Ag@SiO2@Y2O3: Er Nanostructures for Bioimaging: Tuning of the Upconversion Fluorescence with Silver Nanoparticles. J. Am. Chem. Soc. 2010, 132, 2850–2851. [Google Scholar] [CrossRef]
- Kim, T.; Momin, E.; Choi, J.; Yuan, K.; Zaidi, H.; Kim, J.; Park, M.; Lee, N.; McMahon, M.T.; Quinones-Hinojosa, A.; et al. Mesoporous Silica-Coated Hollow Manganese Oxide Nanoparticles as Positive T1 Contrast Agents for Labeling and MRI Tracking of Adipose-Derived Mesenchymal Stem Cells. J. Am. Chem. Soc. 2011, 133, 2955–2961. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ruwan, G.; Tamada, Y.; Kohara, K.; Kusano, Y.; Sasano, T.; Ohno, K.; Tsujii, Y.; Kageyama, H.; Ono, T.; et al. Transformation of Nano- to Mesosized Iron Oxide Cores to α-Fe within Organic Shells Preserved Intact. Chem. Mater. 2011, 23, 1564–1569. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.K.; Lee, D.-G.; Baek, K.; Oh, S.H.; Lee, I.S. Solid-State Conversion Chemistry of Multicomponent Nanocrystals Cast in a Hollow Silica Nanosphere: Morphology-Controlled Syntheses of Hybrid Nanocrystals. ACS Nano 2015, 9, 10719–10728. [Google Scholar] [CrossRef]
- Schick, I.; Lorenz, S.; Gehrig, D.; Schilmann, A.-M.; Bauer, H.; Panthöfer, M.; Fischer, K.; Strand, D.; Laquai, F.; Tremel, W. Multifunctional Two-Photon Active Silica-Coated Au@MnO Janus Particles for Selective Dual Functionalization and Imaging. J. Am. Chem. Soc. 2014, 136, 2473–2483. [Google Scholar] [CrossRef]
- Salgueirino-Maceira, V.; Caruso, F.; Liz-Marzan, L.M. Coated colloids with tailored optical properties. J. Phys. Chem. B 2003, 107, 10990–10994. [Google Scholar] [CrossRef]
- Chen, Y.J.; Gao, P.; Zhu, C.L.; Wang, R.X.; Wang, L.J.; Cao, M.S.; Fang, X.Y. Synthesis, magnetic and electromagnetic wave absorption properties of porous Fe3O4/Fe/SiO2 core/shell nanorods. J. Appl. Phys. 2009, 106, 054303. [Google Scholar] [CrossRef]
- Ung, T.; Liz-Marzan, L.M.; Mulvaney, P. Redox catalysis using Ag@SiO2 colloids. J. Phys. Chem. B 1999, 103, 6770–6773. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.C.; Song, H. A Nanoreactor Framework of a Au@SiO2 Yolk/Shell Structure for Catalytic Reduction of p-Nitrophenol. Adv. Mater. 2008, 20, 1523–1528. [Google Scholar] [CrossRef]
- Park, J.C.; Bang, J.U.; Lee, J.; Ko, C.H.; Song, H. Ni@SiO2 yolk-shell nanoreactor catalysts: High temperature stability and recyclability. J. Mater. Chem. 2010, 20, 1239–1246. [Google Scholar] [CrossRef]
- Salgueirino-Maceira, V.; Correa-Duarte, M.A.; Spasova, M.; Liz-Marzan, L.M.; Farle, M. Composite silica spheres with magnetic and luminescent functionalities. Adv. Func. Mater. 2006, 16, 509–514. [Google Scholar] [CrossRef]
- Song, Y.; Cao, X.; Guo, Y.; Chen, P.; Zhao, Q.; Shen, G. Fabrication of Mesoporous CdTe/ZnO@SiO2 Core/Shell Nanostructures with Tunable Dual Emission and Ultrasensitive Fluorescence Response to Metal Ions. Chem. Mater. 2009, 21, 68–77. [Google Scholar] [CrossRef]
- Wu, B.; Tang, S.; Chen, M.; Zheng, N. Amphiphilic modification and asymmetric silica encapsulation of hydrophobic Au-Fe3O4 dumbbell nanoparticles. Chem. Commun. 2014, 50, 174–176. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.G.S.; Souza, J.B.; Beck, W.; Varanda, L.C. Luminomagnetic Silica-Coated Heterodimers of Core/Shell FePt/Fe3O4 and CdSe Quantum Dots as Potential Biomedical Sensor. J. Nanomater. 2017, 2017, 2160278. [Google Scholar] [CrossRef]
- Shin, J.; Kim, H.; Lee, I.S. Synthesis of Fe3O4/PdO heterodimer nanocrystals in silica nanospheres and their controllable transformation into Fe3O4/Pd heterodimers and FePd nanocrystals. Chem. Commun. 2008, 43, 5553–5555. [Google Scholar] [CrossRef]
- Piao, Y.; Kim, J.; Na, H.B.; Kim, D.; Baek, J.S.; Ko, M.K.; Lee, J.H.; Shokouhimehr, M.; Hyeon, T. Wrap-bake-peel process for nanostructural transformation from b-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 2008, 7, 242. [Google Scholar] [CrossRef]
- Yeo, K.M.; Shin, J.; Lee, I.S. Reductive dissolution of Fe3O4 facilitated by the Au domain of an Fe3O4/Au hybrid nanocrystal: Formation of a nanorattle structure composed of a hollow porous silica nanoshell and entrapped Au nanocrystal. Chem. Commun. 2010, 46, 64–66. [Google Scholar] [CrossRef]
- Moon, G.D.; Ko, S.; Min, Y.; Zeng, J.; Xia, Y.N.; Jeong, U. Chemical transformations of nanostructured materials. Nano Today 2011, 6, 186–203. [Google Scholar] [CrossRef]
- Verelst, M.; Ely, T.O.; Amiens, C.; Snoeck, E.; Lecante, P.; Mosset, A.; Respaud, M.; Broto, J.M.; Chaudret, B. Synthesis and Characterization of CoO, Co3O4, and Mixed Co/CoO Nanoparticles. Chem. Mater. 1999, 11, 2702–2708. [Google Scholar] [CrossRef]
- Park, J.I.; Cheon, J. Synthesis of “solid solution” and “core-shell” type cobalt- platinum magnetic nanoparticles via transmetalation reactions. J. Am. Chem. Soc. 2001, 123, 5743–5746. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Kim, M.G.; Choi, J.R.; Park, J.I.; Ko, S.J.; Oh, S.J.; Cheon, J. Redox-transmetalation process as a generalized synthetic strategy for core-shell magnetic nanoparticles. J. Am. Chem. Soc. 2005, 127, 16090–16097. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Idrobo, J.-C.; Olamit, J.; Liu, K.; Browning, N.D.; Kauzlarich, S.M. Growth Mechanisms and Oxidation Resistance of Gold-Coated Iron Nanoparticles. Chem. Mater. 2005, 17, 3181–3186. [Google Scholar] [CrossRef]
- Lee, I.S.; Lee, N.; Park, J.; Kim, B.H.; Yi, Y.W.; Kim, T.; Kim, T.K.; Lee, I.H.; Paik, S.R.; Hyeon, T. Ni/NiO Core/Shell Nanoparticles for Selective Binding and Magnetic Separation of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2006, 128, 10658–10659. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and Stabilization of Monodisperse Fe nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef]
- Tracy, J.B.; Weiss, D.N.; Dinega, D.P.; Bawendi, M.G. Exchange biasing and magnetic properties of partially and fully oxidized colloidal cobalt nanoparticles. Phys. Rev. B 2005, 72, 064404. [Google Scholar] [CrossRef]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef]
- Peng, S.; Sun, S. Synthesis and Characterization of Monodisperse Hollow Fe3O4 Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4155–4158. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chiang, R.-K.; Lai, H.-Y.; Lin, C.-R. Characterization of Monodisperse Wüstite Nanoparticles Following Partial Oxidation. J. Phys. Chem. C 2010, 114, 4258–4263. [Google Scholar] [CrossRef]
- Wetterskog, E.; Tai, C.-W.; Grins, J.; Bergström, L.; Salazar-Alvarez, G. Anomalous Magnetic Properties of Nanoparticles Arising from Defect Structures: Topotaxial Oxidation of Fe1–xO|Fe3−δO4 Core|Shell Nanocubes to Single-Phase Particles. ACS Nano 2013, 7, 7132–7144. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Billotey, C.; Garofalo, A.; Ulhaq-Bouillet, C.; Lefèvre, C.; Taleb, J.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Lartigue, L.; et al. Mastering the Shape and Composition of Dendronized Iron Oxide Nanoparticles to Tailor Magnetic Resonance Imaging and Hyperthermia. Chem. Mater. 2014, 26, 5252–5264. [Google Scholar] [CrossRef]
- Li, K.; Ma, X.; He, S.; Wang, L.; Yang, X.; Zhang, G.; Guan, S.; Qu, X.; Zhou, S.; Xu, B. Ultrathin Nanosheet-Supported Ag@Ag2O Core–Shell Nanoparticles with Vastly Enhanced Photothermal Conversion Efficiency for NIR-II-Triggered Photothermal Therapy. ACS Biomater. Sci. Eng. 2022, 8, 540–550. [Google Scholar] [CrossRef]
- Kaur, M.; McCloy, J.S.; Jiang, W.; Yao, Q.; Qiang, Y. Size Dependence of Inter- and Intracluster Interactions in Core–Shell Iron–Iron Oxide Nanoclusters. J. Phys. Chem. C 2012, 116, 12875–12885. [Google Scholar] [CrossRef]
- Nemati, Z.; Khurshid, H.; Alonso, J.; Phan, M.H.; Mukherjee, P.; Srikanth, H. From core/shell to Hollow Fe/γ-Fe2O3 Nanoparticles: Evolution of the magnetic behavior. Nanotechnology 2015, 26, 405705. [Google Scholar] [CrossRef]
- Cheong, S.; Ferguson, P.; Feindel, K.W.; Hermans, I.F.; Callaghan, P.T.; Meyer, C.; Slocombe, A.; Su, C.-H.; Cheng, F.-Y.; Yeh, C.-S.; et al. Simple Synthesis and Functionalization of Iron Nanoparticles for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2011, 50, 4206–4209. [Google Scholar] [CrossRef]
- Famiani, S.; LaGrow, A.P.; Besenhard, M.O.; Maenosono, S.; Thanh, N.T.K. Synthesis of Fine-Tuning Highly Magnetic Fe@FexOy Nanoparticles through Continuous Injection and a Study of Magnetic Hyperthermia. Chem. Mater. 2018, 30, 8897–8904. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Bronstein, L.M.; Al-Hazmi, F.; Al-Noaiser, F.; Al-Ghamdi, A.A. Development of Fe/Fe3O4 Core–Shell Nanocubes as a Promising Magnetic Resonance Imaging Contrast Agent. Langmuir 2013, 29, 13095–13101. [Google Scholar] [CrossRef]
- Wang, C.; Baer, D.R.; Amonette, J.E.; Engelhard, M.H.; Antony, J.; Qiang, Y. Morphology and Electronic Structure of the Oxide Shell on the Surface of Iron Nanoparticles. J. Am. Chem. Soc. 2009, 131, 8824–8832. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, H.; Shao, H.; Weissleder, R. Highly Magnetic Core–Shell Nanoparticles with a Unique Magnetization Mechanism. Angew. Chem. Int. Ed. 2011, 50, 4663–4666. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, Y.; Yu, L.; Dong, L.; Shi, C.; Hu, M.-J.; Xu, Y.-J.; Wen, L.-P.; Yu, S.-H. Hydrophilic Co@Au Yolk/Shell Nanospheres: Synthesis, Assembly, and Application to Gene Delivery. Adv. Mater. 2010, 22, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, G.; Cheung, J.S.; Pan, Y.; Kuang, Y.; Zhao, F.; Zhang, B.; Zhang, X.; Wu, E.X.; Xu, B. Multifunctional Yolk-Shell Nanoparticles: A Potential MRI Contrast and Anticancer Agent. J. Am. Chem. Soc. 2008, 130, 11828–11833. [Google Scholar] [CrossRef]
- Yin, Y.D.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef]
- Gao, J.; Liang, G.; Zhang, B.; Kuang, Y.; Zhang, X.; Xu, B. FePt@CoS2 Yolk-Shell Nanocrystals as a Potent Agent to Kill HeLa Cells. J. Am. Chem. Soc. 2007, 129, 1428–1433. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Bodnarchuk, M.I.; Kovalenko, M.V.; Talapin, D.V.; Smith, R.K.; Aloni, S.; Heiss, W.; Alivisatos, A.P. Gold/Iron Oxide Core/Hollow-Shell Nanoparticles. Adv. Mater. 2008, 20, 4323–4329. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Li, H.; Li, L.; Hong, X.; Peng, Q.; Li, Y. Semiconductor-noble metal hybrid nanomaterials with controlled structures. J. Mater. Chem. A 2013, 1, 1587–1590. [Google Scholar] [CrossRef]
- Kim, S.Y.Y.; Alivisatos, A.P.; Somorjai, G.A.; Yates, J.T., Jr. IR Spectroscopic Observation of Molecular Transport through Pt@CoO Yolk-Shell Nanostructures. J. Am. Chem. Soc. 2007, 129, 9510–9513. [Google Scholar] [CrossRef]
- De Trizio, L.; Manna, L. Forging Colloidal Nanostructures via Cation Exchange Reactions. Chem. Rev. 2016, 116, 10852–10887. [Google Scholar] [CrossRef]
- Sytnyk, M.; Kirchschlager, R.; Bodnarchuk, M.I.; Primetzhofer, D.; Kriegner, D.; Enser, H.; Stangl, J.; Bauer, P.; Voith, M.; Hassel, A.W.; et al. Tuning the Magnetic Properties of Metal Oxide Nanocrystal Heterostructures by Cation Exchange. Nano Lett. 2013, 13, 586–593. [Google Scholar] [CrossRef]
- Lai, J.; Shafi, K.V.P.M.; Ulman, A.; Loos, K.; Popovitz-Biro, R.; Lee, Y.; Vogt, T.; Estournes, C. One-Step Synthesis of Core(Cr)/Shell(g-Fe2O3) Nanoparticles. J. Am. Chem. Soc. 2005, 127, 5730–5731. [Google Scholar] [CrossRef] [PubMed]
- Maryna, I.B.; Maksym, V.K.; Heiko, G.; Roland, R.; Michael, R.; Günter, H.; Rainer, T.L.; Walter, S.; Friedrich, S.; Wolfgang, H. Exchange-Coupled Bimagnetic Wüstite/Metal Ferrite Core/Shell Nanocrystals: Size, Shape, and Compositional Control. Small 2009, 5, 2247–2252. [Google Scholar]
- Hai, H.T.; Yang, H.T.; Kura, H.; Hasegawa, D.; Ogata, Y.; Takahashi, M.; Ogawa, T. Size control and characterization of wustite (core)/spinel (shell) nanocubes obtained by decomposition of iron oleate complex. J. Colloid Interface Sci. 2010, 346, 37–42. [Google Scholar] [CrossRef]
- Lak, A.; Kraken, M.; Ludwig, F.; Kornowski, A.; Eberbeck, D.; Sievers, S.; Litterst, F.J.; Weller, H.; Schilling, M. Size dependent structural and magnetic properties of FeO-Fe3O4 nanoparticles. Nanoscale 2013, 5, 12286–12295. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Y.; Zhang, Y.; Xuan, S.; Gong, X. Superparamagnetic Ag@Fe3O4 core-shell nanospheres: Fabrication, characterization and application as reusable nanocatalysts. Dalton Trans. 2012, 41, 4594–4601. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Liu, Y.; Pan, S.; Luo, Y.; Li, G. Facile one-step synthesis of plasmonic/magnetic core/shell nanostructures and their multifunctionality. J. Mater. Chem. 2012, 22, 10779–10786. [Google Scholar] [CrossRef]
- Nguyen, H.-Q.; Hwang, D.; Park, S.; Nguyen, M.-C.T.; Kang, S.S.; Tran, V.T.; Lee, J. One-Pot Synthesis of Magnetoplasmonic Au@FexOy Nanowires: Bioinspired Bouligand Chiral Stack. ACS Nano 2022, 16, 5795–5806. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavan, B.; Tahir, M.N.; Panthofer, M.; Gao, H.; Gasi, T.; Ksenofontov, V.; Branscheid, R.; Weber, S.; Kolb, U.; Schreiber, L.M.; et al. Controlling phase formation in solids: Rational synthesis of phase separated Co@Fe2O3 heteroparticles and CoFe2O4 nanoparticles. Chem. Commun. 2011, 47, 8898–8900. [Google Scholar] [CrossRef]
- Chai, Y.; Feng, F.; Li, Q.; Yu, C.; Feng, X.; Lu, P.; Yu, X.; Ge, M.; Wang, X.; Yao, L. One-Pot Synthesis of High-Quality Bimagnetic Core/Shell Nanocrystals with Diverse Exchange Coupling. J. Am. Chem. Soc. 2019, 141, 3366–3370. [Google Scholar] [CrossRef]
- Bastús, N.G.; Piella, J.; Perez, S.; Patarroyo, J.; Genç, A.; Arbiol, J.; Puntes, V. Robust one-pot synthesis of citrate-stabilized Au@CeO2 hybrid nanocrystals with different thickness and dimensionality. Appl. Mater. Today 2019, 15, 445–452. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, D.; Cortie, M.B.; Dowd, A.; Guo, H.; Wang, J.; Peng, D.-L. Seed-Induced Growth of Flower-Like Au–Ni–ZnO Metal–Semiconductor Hybrid Nanocrystals for Photocatalytic Applications. Small 2015, 11, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Deqian, Z.; Yuanzhi, C.; Jian, P.; Qingshui, X.; Dong-Liang, P. Synthesis and photocatalytic properties of multi-morphological AuCu3-ZnO hybrid nanocrystals. Nanotechnology 2015, 26, 415602. [Google Scholar]
- Zhou, W.; Zheng, K.; He, L.; Wang, R.; Guo, L.; Chen, C.; Han, X.; Zhang, Z. Ni/Ni3C Core-Shell Nanochains and Its Magnetic Properties: One-Step Synthesis at Low Temperature. Nano Lett. 2008, 8, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Carenco, S.; Le Goff, X.F.; Shi, J.; Roiban, L.; Ersen, O.; Boissière, C.; Sanchez, C.; Mézailles, N. Magnetic Core−Shell Nanoparticles from Nanoscale-Induced Phase Segregation. Chem. Mater. 2011, 23, 2270–2277. [Google Scholar] [CrossRef]
- Zhu, H.; Sigdel, A.; Zhang, S.; Su, D.; Xi, Z.; Li, Q.; Sun, S. Core/Shell Au/MnO Nanoparticles Prepared Through Controlled Oxidation of AuMn as an Electrocatalyst for Sensitive H2O2 Detection. Angew. Chem. Int. Ed. 2014, 43, 12508–12512. [Google Scholar]
- Duan, S.B.; Wang, R.M. Au/Ni12P5 core/shell nanocrystals from bimetallic heterostructures: In situ synthesis, evolution and supercapacitor properties. NPG Asia Mater. 2014, 6, e122. [Google Scholar] [CrossRef]
- Shams, S.F.; Ghazanfari, M.R.; Schmitz-Antoniak, C. Magnetic-Plasmonic Heterodimer Nanoparticles: Designing Contemporarily Features for Emerging Biomedical Diagnosis and Treatments. Nanomaterials 2019, 9, 97. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus Nanoparticles: From Fabrication to (Bio)Applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Klein, S.; Stiegler, L.M.S.; Harreiss, C.; Distel, L.V.R.; Neuhuber, W.; Spiecker, E.; Hirsch, A.; Kryschi, C. Understanding the Role of Surface Charge in Cellular Uptake and X-ray-Induced ROS Enhancing of Au–Fe3O4 Nanoheterodimers. ACS Appl. Bio Mater. 2018, 1, 2002–2011. [Google Scholar] [CrossRef]
- Klein, S.; Hubner, J.; Menter, C.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. A Facile One-Pot Synthesis of Water-Soluble, Patchy Fe3O4-Au Nanoparticles for Application in Radiation Therapy. Appl. Sci. 2019, 9, 15. [Google Scholar] [CrossRef]
- Choi, J.S.; Jun, Y.W.; Yeon, S.I.; Kim, H.C.; Shin, J.S.; Cheon, J. Biocompatible Heterostructured Nanoparticles for Multimodal Biological Detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gu, H.; Shao, H.; Devlin, E.; Papaefthymiou, G.C.; Ying, J.Y. Bifunctional Fe3O4–Ag Heterodimer Nanoparticles for Two-Photon Fluorescence Imaging and Magnetic Manipulation. Adv. Mater. 2008, 20, 4403–4407. [Google Scholar] [CrossRef]
- Choi, S.-H.; Na, H.B.; Park, Y.I.; An, K.; Kwon, S.G.; Jang, Y.; Park, M.-H.; Moon, J.; Son, J.S.; Song, I.C.; et al. Simple and Generalized Synthesis of Oxide-Metal Heterostructured Nanoparticles and their Applications in Multimodal Biomedical Probes. J. Am. Chem. Soc. 2008, 130, 15573–15580. [Google Scholar] [CrossRef] [PubMed]
- Schladt, T.D.; Shukoor, M.I.; Schneider, K.; Tahir, M.N.; Natalio, F.; Ament, I.; Becker, J.; Jochum, F.D.; Weber, S.; Köhler, O.; et al. Au@MnO Nanoflowers: Hybrid Nanocomposites for Selective Dual Functionalization and Imaging. Angew. Chem. Int. Ed. 2010, 49, 3976–3980. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xie, J.; Ho, D.; Wang, C.; Kohler, N.; Walsh, E.G.; Morgan, J.R.; Chin, Y.E.; Sun, S. Au-Fe3O4 Dumbbell Nanoparticles as Dual-Functional Probes. Angew. Chem. Int. Ed. 2008, 47, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Bodnarchuk, M.I.; Shevchenko, E.V.; Talapin, D.V. “Magnet-in-the-Semiconductor” FePt-PbS and FePt-PbSe Nanostructures: Magnetic Properties, Charge Transport, and Magnetoresistance. J. Am. Chem. Soc. 2010, 132, 6382–6391. [Google Scholar] [CrossRef]
- Umut, E.; Pineider, F.; Arosio, P.; Sangregorio, C.; Corti, M.; Tabak, F.; Lascialfari, A.; Ghigna, P. Magnetic, optical and relaxometric properties of organically coated gold-magnetite (Au-Fe3O4) hybrid nanoparticles for potential use in biomedical applications. J. Magn. Magn. Mater. 2012, 324, 2373–2379. [Google Scholar] [CrossRef]
- Velasco, V.; Munoz, L.; Mazario, E.; Menendez, N.; Herrasti, P.; Hernando, A.; Crespo, P. Chemically synthesized Au-Fe3O4 nanostructures with controlled optical and magnetic properties. J. Phys. D Appl. Phys. 2015, 48, 035502. [Google Scholar] [CrossRef]
- Kim, M.; Song, H. Precise adjustment of structural anisotropy and crystallinity on metal-Fe3O4 hybrid nanoparticles and its influence on magnetic and catalytic properties. J. Mater. Chem. C 2014, 2, 4997–5004. [Google Scholar] [CrossRef]
- Schick, I.; Lorenz, S.; Gehrig, D.; Tenzer, S.; Storck, W.; Fischer, K.; Strand, D.; Laquai, F.; Tremel, W. Inorganic Janus particles for biomedical applications. Beilstein J. Nanotechnol. 2014, 5, 2346–2362. [Google Scholar] [CrossRef]
- Comin, A.; Korobchevskaya, K.; George, C.; Diaspro, A.; Manna, L. Plasmon Bleaching Dynamics in Colloidal Gold-Iron Oxide Nanocrystal Heterodimers. Nano Lett. 2012, 12, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Korobchevskaya, K.; George, C.; Diaspro, A.; Manna, L.; Cingolani, R.; Comin, A. Ultrafast carrier dynamics in gold/iron-oxide nanocrystal heterodimers. Appl. Phys. Lett. 2011, 99, 011907. [Google Scholar] [CrossRef]
- Levin, C.S.; Hofmann, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic-Plasmonic Core-Shell Nanoparticles. ACS Nano 2009, 3, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Nurmikko, A.V.; Sun, S. Enhanced Magnetooptical Response in Dumbbell-like Ag−CoFe2O4 Nanoparticle Pairs. Nano Lett. 2005, 5, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, C.; Zhu, H.; Overbury, S.H.; Sun, S.; Dai, S. Colloidal deposition synthesis of supported gold nanocatalysts based on Au-Fe3O4 dumbbell nanoparticles. Chem. Commun. 2008, 36, 4357–4359. [Google Scholar] [CrossRef][Green Version]
- Wu, B.; Zhang, H.; Chen, C.; Lin, S.; Zheng, N. Interfacial activation of catalytically inert Au (6.7 nm)-Fe3O4 dumbbell nanoparticles for CO oxidation. Nano Res. 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Aitbekova, A.; Goodman, E.D.; Wu, L.H.; Boubnov, A.; Hoffman, A.S.; Genc, A.; Cheng, H.; Casalena, L.; Bare, S.R.; Cargnello, M. Engineering of Ruthenium-Iron Oxide Colloidal Heterostructures: Improved Yields in CO2 Hydrogenation to Hydrocarbons. Angew. Chem. Int. Ed. 2019, 58, 17451–17457. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Sun, S. Dumbbell-like Pt-Fe3O4 Nanoparticles and Their Enhanced Catalysis for Oxygen Reduction Reaction. Nano Lett. 2009, 9, 1493–1496. [Google Scholar] [CrossRef]
- Wang, C.; Yin, H.; Dai, S.; Sun, S. A General Approach to Noble Metal-Metal Oxide Dumbbell Nanoparticles and Their Catalytic Application for CO Oxidation. Chem. Mater. 2010, 22, 3277–3282. [Google Scholar] [CrossRef]
- Lee, Y.; Garcia, M.A.; Frey Huls, N.A.; Sun, S. Synthetic Tuning of the Catalytic Properties of Au-Fe3O4 Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 1271–1274. [Google Scholar] [CrossRef]
- Chen, S.; Si, R.; Taylor, E.; Janzen, J.; Chen, J. Synthesis of Pd/Fe3O4 Hybrid Nanocatalysts with Controllable Interface and Enhanced Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2012, 116, 12969–12976. [Google Scholar] [CrossRef]
- Sun, X.; Guo, S.; Liu, Y.; Sun, S. Dumbbell-like PtPd–Fe3O4 Nanoparticles for Enhanced Electrochemical Detection of H2O2. Nano Lett. 2012, 12, 4859–4863. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Dorfs, D.; Bertoni, G.; Falqui, A.; Genovese, A.; Pellegrino, T.; Roig, A.; Quarta, A.; Comparelli, R.; Curri, M.L.; et al. A Cast-Mold Approach to Iron Oxide and Pt/Iron Oxide Nanocontainers and Nanoparticles with a Reactive Concave Surface. J. Am. Chem. Soc. 2011, 133, 2205–2217. [Google Scholar] [CrossRef]
- George, C.; Genovese, A.; Casu, A.; Prato, M.; Povia, M.; Manna, L.; Montanari, T. CO Oxidation on Colloidal Au0.80Pd0.20–FexOy Dumbbell Nanocrystals. Nano Lett. 2013, 13, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, S.; Sun, S.; You, X.-Z. Dumbbell-like Au-Fe3O4 nanoparticles: A new nanostructure for supercapacitors. Nanoscale 2015, 7, 4890–4893. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Fantechi, E.; Roca, A.G.; Sepúlveda, B.; Torruella, P.; Estradé, S.; Peiró, F.; Coy, E.; Jurga, S.; Bastús, N.G.; Nogués, J.; et al. Seeded Growth Synthesis of Au–Fe3O4 Heterostructured Nanocrystals: Rational Design and Mechanistic Insights. Chem. Mater. 2017, 29, 4022–4035. [Google Scholar] [CrossRef]
- Tancredi, P.; da Costa, L.S.; Calderon, S.; Moscoso-Londono, O.; Socolovsky, L.M.; Ferreira, P.J.; Muraca, D.; Zanchet, D.; Knobel, M. Exploring the synthesis conditions to control the morphology of gold-iron oxide heterostructures. Nano Res. 2019, 12, 1781–1788. [Google Scholar] [CrossRef]
- Nalench, Y.A.; Shchetinin, I.V.; Skorikov, A.S.; Mogilnikov, P.S.; Farle, M.; Savchenko, A.G.; Majouga, A.G.; Abakumov, M.A.; Wiedwald, U. Unravelling the nucleation, growth, and faceting of magnetite–gold nanohybrids. J. Mater. Chem. B. 2020, 8, 3886–3895. [Google Scholar] [CrossRef]
- Wei, Y.; Klajn, R.; Pinchuk, A.O.; Grzybowski, B.A. Synthesis, Shape Control, and Optical Properties of Hybrid Au/Fe3O4 “Nanoflowers”. Small 2008, 4, 1635–1639. [Google Scholar] [CrossRef]
- Lin, F.H.; Doong, R.A. Bifunctional Au-Fe3O4 Heterostructures for Magnetically Recyclable Catalysis of Nitrophenol Reduction. J. Phys. Chem. C 2011, 115, 6591–6598. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, J.; Kim, S.; Jun, S.W.; Kim, B.H.; Lee, D.W.; Kim, B.M.; Hyeon, T. Simple synthesis of Pd-Fe3O4 heterodimer nanocrystals and their application as a magnetically recyclable catalyst for Suzuki cross-coupling reactions. Phys. Chem. Chem. Phys. 2011, 13, 2512–2516. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Xuan, S.; Zhu, X.; Wang, D.; Chak, C.-P.; Lee, S.-F.; Ho, W.K.W.; Chung, B.C.T. Gold and iron oxide hybrid nanocomposite materials. Chem. Soc. Rev. 2012, 41, 1911–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Han, L.; Wang, P.; Li, G.; Ren, W.; Liu, L.; Wang, E.; Dong, S. Superparamagnetic Plasmonic Nanohybrids: Shape-Controlled Synthesis, TEM-Induced Structure Evolution, and Efficient Sunlight-Driven Inactivation of Bacteria. ACS Nano 2011, 5, 8562–8570. [Google Scholar] [PubMed]
- Wu, H.M.; Chen, O.; Zhuang, J.Q.; Lynch, J.; LaMontagne, D.; Nagaoka, Y.; Cao, Y.C. Formation of Heterodimer Nanocrystals: UO2/In2O3 and FePt/In2O3. J. Am. Chem. Soc. 2011, 133, 14327–14337. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavan, B.; Tahir, M.N.; Natalio, F.; Gao, H.; Schneider, K.; Schladt, T.; Ament, I.; Branscheid, R.; Weber, S.; Kolb, U.; et al. Phase separated Cu@Fe3O4 heterodimer nanoparticles from organometallic reactants. J. Mater. Chem. 2011, 21, 8605–8611. [Google Scholar] [CrossRef]
- Teranishi, T.; Wachi, A.; Kanehara, M.; Shoji, T.; Sakuma, N.; Nakaya, M. Conversion of Anisotropically Phase-Segregated Pd/γ-Fe2O3 Nanoparticles into Exchange-Coupled fct-FePd/α-Fe Nanocomposite Magnets. J. Am. Chem. Soc. 2008, 130, 4210–4211. [Google Scholar] [CrossRef]
- Yang, W.; Lei, W.; Yu, Y.; Zhu, W.; George, T.A.; Li, X.Z.; Sellmyer, D.J.; Sun, S. From FePt-Fe3O4 to L10-FePt-Fe nanocomposite magnets with a gradient interface. J. Mater. Chem. C 2015, 3, 7075–7080. [Google Scholar] [CrossRef]
- Sakuma, N.; Ohshima, T.; Shoji, T.; Suzuki, Y.; Sato, R.; Wachi, A.; Kato, A.; Kawai, Y.; Manabe, A.; Teranishi, T. Exchange Coupling Interaction in L10-FePd/α-Fe Nanocomposite Magnets with Large Maximum Energy Products. ACS Nano 2011, 5, 2806–2814. [Google Scholar] [CrossRef]
- Mezni, A.; Balti, I.; Mlayah, A.; Jouini, N.; Smiri, L.S. Hybrid Au–Fe3O4 Nanoparticles: Plasmonic, Surface Enhanced Raman Scattering, and Phase Transition Properties. J. Phys. Chem. C. 2013, 117, 16166–16174. [Google Scholar] [CrossRef]
- Vita, F.; Innocenti, C.; Secchi, A.; Albertini, F.; Grillo, V.; Fiore, A.; Cozzoli, P.D.; de Julián Fernández, C. Colloidal Au/iron oxide nanocrystal heterostructures: Magnetic, plasmonic and magnetic hyperthermia properties. J. Mater. Chem. C 2018, 6, 12329–12340. [Google Scholar] [CrossRef]
- Korobchevskaya, K.; George, C.; Manna, L.; Comin, A. Effect of Morphology on Ultrafast Carrier Dynamics in Asymmetric Gold–Iron Oxide Plasmonic Heterodimers. J. Phys. Chem. C 2012, 116, 26294–26298. [Google Scholar] [CrossRef]
- Zhu, L.; Deng, X.; Hu, Y.; Liu, J.; Ma, H.B.; Zhang, J.L.; Fu, J.C.; He, S.S.; Wang, J.; Wang, B.D.; et al. Atomic-scale imaging of the ferrimagnetic/diamagnetic interface in Au-Fe3O4 nanodimers and correlated exchange-bias origin. Nanoscale 2018, 10, 21499–21508. [Google Scholar] [CrossRef] [PubMed]
- Najafishirtari, S.; Kokumai, T.M.; Marras, S.; Destro, P.; Prato, M.; Scarpellini, A.; Brescia, R.; Lak, A.; Pellegrino, T.; Zanchet, D.; et al. Dumbbell-like Au0.5Cu0.5@Fe3O4 Nanocrystals: Synthesis, Characterization, and Catalytic Activity in CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 28624–28632. [Google Scholar] [CrossRef]
- Ye, X.C.; Hickey, D.R.; Fei, J.Y.; Diroll, B.T.; Paik, T.; Chen, J.; Murray, C.B. Seeded Growth of Metal-Doped Plasmonic Oxide Heterodimer Nanocrystals and Their Chemical Transformation. J. Am. Chem. Soc. 2014, 136, 5106–5115. [Google Scholar] [CrossRef]
- Patarroyo, J.; Delgado, J.A.; Merkoçi, F.; Genç, A.; Sauthier, G.; Llorca, J.; Arbiol, J.; Bastus, N.G.; Godard, C.; Claver, C.; et al. Hollow PdAg-CeO2 heterodimer nanocrystals as highly structured heterogeneous catalysts. Sci. Rep. 2019, 9, 18776. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Jiang, H.; Sun, S. Tug-of-War in Nanoparticles: Competitive Growth of Au on Au−Fe3O4 Nanoparticles. Nano Lett. 2009, 9, 4544–4547. [Google Scholar] [CrossRef]
- He, S.L.; Zhang, H.W.; Delikanli, S.; Qin, Y.L.; Swihart, M.T.; Zeng, H. Bifunctional Magneto-Optical FePt-CdS Hybrid Nanoparticles. J. Phys. Chem. C 2009, 113, 87–90. [Google Scholar] [CrossRef]
- Kwon, K.W.; Shim, M. g-Fe2O3/II-VI Sulfide Nanocrystal Heterojunctions. J. Am. Chem. Soc. 2005, 127, 10269–10275. [Google Scholar] [CrossRef]
- Kwon, K.-W.; Lee, B.H.; Shim, M. Structural Evolution in Metal Oxide/Semiconductor Colloidal Nanocrystal Heterostructures. Chem. Mater. 2006, 18, 6357–6363. [Google Scholar] [CrossRef]
- McDaniel, H.; Shim, M. Size and Growth Rate Dependent Structural Diversification of Fe3O4/CdS Anisotropic Nanocrystal Heterostructures. ACS Nano 2009, 3, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Randle, V. The Role of the Coincidence Site Lattice in Grain Boundary Engineering; Woodhead Publishing Limited: Cambridge, UK, 1997. [Google Scholar]
- Yang, J.; Ying, J.Y. A general phase-transfer protocol for metal ions and its application in nanocrystal synthesis. Nat. Mater. 2009, 8, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dou, Y.H.; Gu, H.C. Synthesis of Ag-Fe3O4 heterodimeric nanoparticles. J. Coll. Interface Sci. 2006, 297, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Sun, Y.H.; Huang, S.S.; Yu, K.; Zhao, Q.; Peng, F.; Yu, H.; Wang, H.J.; Yang, J. Crystal engineering and SERS properties of Ag-Fe3O4 nanohybrids: From heterodimer to core-shell nanostructures. J. Mater. Chem. 2011, 21, 17930–17937. [Google Scholar] [CrossRef]
- Peng, S.; Lei, C.; Ren, Y.; Cook, R.E.; Sun, Y. Plasmonic/Magnetic Bifunctional Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 3158–3163. [Google Scholar] [CrossRef]
- Mao, Y.W.; Yi, P.W.; Deng, Z.W.; Ge, J.P. Fe3O4-Ag heterostructure nanocrystals with tunable Ag domains and magnetic properties. CrystEngComm 2013, 15, 3575–3581. [Google Scholar] [CrossRef]
- Mirtchev, P.; Liao, K.; Jaluague, E.; Qiao, Q.; Tian, Y.; Varela, M.; Burch, K.S.; Pennycook, S.J.; Perovic, D.D.; Ozin, G. Fe2O3/Cu2O heterostructured nanocrystals. J. Mater. Chem. A 2014, 2, 8525–8533. [Google Scholar] [CrossRef]
- Ivanchenko, M.; Nooshnab, V.; Myers, A.F.; Large, N.; Evangelista, A.J.; Jing, H. Enhanced dual plasmonic photocatalysis through plasmonic coupling in eccentric noble metal-nonstoichiometric copper chalcogenide hetero-nanostructures. Nano Res. 2022, 15, 1579–1586. [Google Scholar] [CrossRef]
- Franchini, I.R.; Bertoni, G.; Falqui, A.; Giannini, C.; Wang, L.W.; Manna, L. Colloidal PbTe-Au nanocrystal heterostructures. J. Mater. Chem. 2010, 20, 1357–1366. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Zhang, Q.; Peng, F.; Wang, H.; Yu, H. One-Step Synthesis and Characterization of Gold-Hollow PbSx Hybrid Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 3991–3995. [Google Scholar] [CrossRef]
- Wark, S.E.; Hsia, C.-H.; Son, D.H. Effects of Ion Solvation and Volume Change of Reaction on the Equilibrium and Morphology in Cation-Exchange Reaction of Nanocrystals. J. Am. Chem. Soc. 2008, 130, 9550–9555. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Levina, L.; Sargent, E.H.; Kelley, S.O. Heterogeneous deposition of noble metals on semiconductor nanoparticles in organic or aqueous solvents. J. Mater. Chem. 2006, 16, 4025–4028. [Google Scholar] [CrossRef]
- Yang, J.; Elim, H.I.; Zhang, Q.; Lee, J.Y.; Ji, W. Rational Synthesis, Self-Assembly, and Optical Properties of PbS-Au Heterogeneous Nanostructures via Preferential Deposition. J. Am. Chem. Soc. 2006, 128, 11921–11926. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Perez, N.; Gao, Y.; Hilgendorff, M.; Irsen, S.; Perez-Juste, J.; Spasova, M.; Farle, M.; Liz-Marzan, L.M.; Giersig, M. Magnetic-Noble Metal Nanocomposites with Morphology-Dependent Optical Response. Chem. Mater. 2007, 19, 4415–4422. [Google Scholar] [CrossRef]
- Mokari, T.; Sztrum, C.G.; Salant, A.; Rabani, E.; Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. Nat. Mater. 2005, 4, 855–863. [Google Scholar] [CrossRef]
- Zeng, J.; Jianliu, H.; Liu, C.; Wu, C.H.; Lin, Y.; Wang, X.; Zhang, S.; Hou, J.; Xia, Y. Gold-Based Hybrid Nanocrystals Through Heterogeneous Nucleation and Growth. Adv. Mater. 2010, 22, 1936–1940. [Google Scholar] [CrossRef]
- Mao, J.; Cao, X.; Zhen, J.; Shao, H.; Gu, H.; Lu, J.; Ying, J.Y. Facile synthesis of hybrid nanostructures from nanoparticles, nanorods and nanowires. J. Mater. Chem. 2011, 21, 11478–11481. [Google Scholar] [CrossRef]
- Wu, J.J.; Hou, Y.L.; Gao, S. Controlled synthesis and multifunctional properties of FePt-Au heterostructures. Nano Res. 2011, 4, 836–848. [Google Scholar] [CrossRef]
- Pellegrino, T.; Fiore, A.; Carlino, E.; Giannini, C.; Cozzoli, P.D.; Ciccarella, G.; Respaud, M.; Palmirotta, L.; Cingolani, R.; Manna, L. Heterodimers based on CoPt3-Au nanocrystals with tunable domain size. J. Am. Chem. Soc. 2006, 128, 6690–6698. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, J.; Liu, F.; Xing, R.; Zhang, C.; Yang, C.; Yin, H.; Hou, Y. Controlled synthesis of FePt-Au hybrid nanoparticles triggered by reaction atmosphere and FePt seeds. Nanoscale 2013, 5, 9141–9149. [Google Scholar] [CrossRef]
- Varandili, S.B.; Stoian, D.; Vavra, J.; Pankhurst, J.; Buonsanti, R. Ligand-mediated formation of Cu/metal oxide hybrid nanocrystals with tunable number of interfaces. Chem. Sci. 2020, 11, 13094–13101. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, X.; Chen, S.; Kang, H.; Tan, W. Facile synthesis of Ni/Au, Ni/Ag hybrid magnetic nanoparticles: New active substrates for surface enhanced Raman scattering. Coll. Surf. Sci. A 2012, 403, 148–154. [Google Scholar] [CrossRef]
- Yu, S.M.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic gold nanotriangles by microwave-assisted polyol synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef]
- Shi, W.; Sahoo, Y.; Zeng, H.; Ding, Y.; Swihart, M.T.; Prasad, P.N. Anisotropic Growth of PbSe Nanocrystals on Au-Fe3O4 Hybrid Nanoparticles. Adv. Mater. 2006, 18, 1889–1894. [Google Scholar] [CrossRef]
- Thomas, S.; Tresa Sunny, A.; Velayudhan, P. (Eds.) Colloidal Metal Oxide Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; p. 590. [Google Scholar]
- Hodges, J.M.; Morse, J.R.; Williams, M.E.; Schaak, R.E. Microscopic Investigation of Chemoselectivity in Ag–Pt–Fe3O4 Heterotrimer Formation: Mechanistic Insights and Implications for Controlling High-Order Hybrid Nanoparticle Morphology. J. Am. Chem. Soc. 2015, 137, 15493–15500. [Google Scholar] [CrossRef]
- Hodges, J.M.; Biacchi, A.J.; Schaak, R.E. Ternary Hybrid Nanoparticle Isomers: Directing the Nucleation of Ag on Pt–Fe3O4 Using a Solid-State Protecting Group. ACS Nano 2014, 8, 1047–1055. [Google Scholar] [CrossRef]
- Liu, Y.L.; Walker, A.R.H. Facile One-Pot Synthesis of Metal-Semiconductor Hybrid Nanocrystals via Chemical Transformation: The Case of Cu-CuxS Heterodimers and Hetero-Oligomers. J. Phys. Chem. C 2010, 114, 4264–4271. [Google Scholar] [CrossRef]
- Gu, H.W.; Yang, Z.M.; Gao, J.H.; Chang, C.K.; Xu, B. Heterodimers of nanoparticles: Formation at a liquid-liquid interface and particle-specific surface modification by functional molecules. J. Am. Chem. Soc. 2005, 127, 34–35. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, J.H.; Zhang, B.; Zhang, X.X.; Xu, B. Colloidosome-based Synthesis of a Multifunctional Nanostructure of Silver and Hollow Iron Oxide Nanoparticles. Langmuir 2010, 26, 4184–4187. [Google Scholar] [CrossRef]
- Figuerola, A.; Fiore, A.; Di Corato, R.; Falqui, A.; Giannini, C.; Micotti, E.; Lascialfari, A.; Corti, M.; Cingolani, R.; Pellegrino, T.; et al. One-Pot Synthesis and Characterization of Size-Controlled Bimagnetic FePt-Iron Oxide Heterodimer Nanocrystals. J. Am. Chem. Soc. 2008, 130, 1477–1487. [Google Scholar] [CrossRef]
- Eom, Y.; Kang, Y.M.; Kasturi, S.; Torati, S.R.; Kim, C. Phase controlled one-pot synthesis of heterostructured FePt-Fe3O4 nanocubes with excellent biocompatibility. RSC Adv. 2020, 10, 43480–43488. [Google Scholar] [CrossRef] [PubMed]
- Slaton, R.D.; Bae, I.-T.; Lutz, P.S.; Pathade, L.; Maye, M.M. The transformation of α-Fe nanoparticles into multi-domain FeNi–M3O4 (M = Fe, Ni) heterostructures by galvanic exchange. J. Mater. Chem. C. 2015, 3, 6367–6375. [Google Scholar] [CrossRef]
- Loiudice, A.; Niau, B.P.G.; Buonsanti, R. Crystal-Phase Control of Ternary Metal Oxides by Solid-State Synthesis with Nanocrystals. ACS Nanosci. Au 2022, in press. [Google Scholar] [CrossRef]
- Viswanath, B.; Kundu, P.; Halder, A.; Ravishankar, N. Mechanistic Aspects of Shape Selection and Symmetry Breaking during Nanostructure Growth by Wet Chemical Methods. J. Phys. Chem. C 2009, 113, 16866–16883. [Google Scholar] [CrossRef]
- Walsh, M.J.; Barrow, S.J.; Tong, W.; Funston, A.M.; Etheridge, J. Symmetry Breaking and Silver in Gold Nanorod Growth. ACS Nano 2015, 9, 715–724. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Peng, H.-C.; Yang, X.; Ruditskiy, A.; Xia, Y. Symmetry breaking during nanocrystal growth. Chem. Commun. 2017, 53, 4530–4541. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Site-Specific Photodeposition of Silver on ZnO Nanorods. Angew. Chem. Int. Ed. 2004, 43, 4774–4777. [Google Scholar] [CrossRef]
- Milliron, D.J.; Hughes, S.M.; Cui, Y.; Manna, L.; Li, J.B.; Wang, L.W.; Alivisatos, A.P. Colloidal nanocrystal heterostructures with linear and branched topology. Nature 2004, 430, 190–195. [Google Scholar] [CrossRef]
- Kudera, S.; Carbone, L.; Casula, M.F.; Cingolani, R.; Falqui, A.; Snoeck, E.; Parak, W.J.; Manna, L. Selective Growth of PbSe on One or Both Tips of Colloidal Semiconductor Nanorods. Nano Lett. 2005, 5, 445–449. [Google Scholar] [CrossRef]
- Wetz, F.; Soulantica, K.; Falqui, A.; Respaud, M.; Snoeck, E.; Chaudret, B. Hybrid Co-Au Nanorods: Controlling Au Nucleation and Location. Angew. Chem. Int. Ed. 2007, 46, 7079–7081. [Google Scholar] [CrossRef]
- Maynadiè, J.; Salant, A.; Falqui, A.; Respaud, M.; Shaviv, E.; Banin, U.; Soulantica, K.; Chaudret, B. Cobalt Growth on the Tips of CdSe nanorods. Angew. Chem. Int. Ed. 2009, 48, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Kudera, S.; Giannini, C.; Ciccarella, G.; Cingolani, R.; Cozzoli, P.D.; Manna, L. Selective reactions on the tips of colloidal semiconductor nanorods. J. Mater. Chem. 2006, 16, 3952–3956. [Google Scholar] [CrossRef]
- Deka, S.; Falqui, A.; Bertoni, G.; Sangregorio, C.; Poneti, G.; Morello, G.; De Giorgi, M.; Giannini, C.; Cingolani, R.; Manna, L.; et al. Fluorescent Asymmetrically Cobalt-Tipped CdSe@CdS Core@Shell Nanorod Heterostructures Exhibiting Room-Temperature Ferromagnetic Behavior. J. Am. Chem. Soc. 2009, 131, 12817–12828. [Google Scholar] [CrossRef] [PubMed]
- Dukovic, G.; Merkle, M.G.; Nelson, J.H.; Hughes, S.M.; Alivisatos, A.P. Photodeposition of Pt on Colloidal CdS and CdSe/CdS Semiconductor Nanostructures. Adv. Mater. 2008, 20, 4306–4311. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, K.F.; Cai, Y.; Fung, K.K.; Wang, N. Site-Specific Deposition of Titanium Oxide on Zinc Oxide Nanorods. J. Phys. Chem. C 2007, 111, 16712–16716. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Gozzo, F.; Garcia-Hernandez, M.; Garcia, M.A.; Cingolani, R.; Cozzoli, P.D. Architectural Control of Seeded-Grown Magnetic-Semicondutor Iron Oxide-TiO2 Nanorod Heterostructures: The Role of Seeds in Topology Selection. J. Am. Chem. Soc. 2010, 132, 2437–2464. [Google Scholar] [CrossRef]
- Menagen, G.; Mocatta, D.; Salant, A.; Popov, I.; Dorfs, D.; Banin, U. Selective Gold Growth on CdSe Seeded CdS Nanorods. Chem. Mater. 2008, 20, 6900–6902. [Google Scholar] [CrossRef]
- Casavola, M.; Grillo, V.; Carlino, E.; Giannini, C.; Gozzo, F.; Fernandez Pinel, E.; Garcia, M.A.; Manna, L.; Cingolani, R.; Cozzoli, P.D. Topologically Controlled Growth of Magnetic-Metal-Functionalized Semiconductor Oxide Nanorods. Nano Lett. 2007, 7, 1386–1395. [Google Scholar] [CrossRef]
- Chakrabortty, S.; Yang, J.A.; Tan, Y.M.; Mishra, N.; Chan, Y. Asymmetric Dumbbells from Selective Deposition of Metals on Seeded Semiconductor Nanorods. Angew. Chem. Int. Ed. 2010, 49, 2888–2892. [Google Scholar] [CrossRef]
- Liakakos, N.; Gatel, C.; Blon, T.; Altantzis, T.; Lentijo-Mozo, S.; Garcia-Marcelot, C.; Lacroix, L.-M.; Respaud, M.; Bals, S.; Van Tendeloo, G.; et al. Co–Fe Nanodumbbells: Synthesis, Structure, and Magnetic Properties. Nano Lett. 2014, 14, 2747–2754. [Google Scholar] [CrossRef]
- Casavola, M.; Falqui, A.; Garcia, M.A.; Garcia-Hernandez, M.; Giannini, C.; Cingolani, R.; Cozzoli, P.D. Exchange-Coupled Bimagnetic Cobalt/Iron Oxide Branched Nanocrystal Heterostructures. Nano Lett. 2009, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Yang, Z.; Xu, J.; Wang, X.; Yang, J.; Hou, Y. Chemical synthesis, structure and magnetic properties of Co nanorods decorated with Fe3O4 nanoparticles. Sci. China Mater. 2018, 61, 1614–1622. [Google Scholar] [CrossRef]
- Chen, X.B.; Lou, Y.B.; Samia, A.C.; Burda, C. Coherency strain effects on the optical response of core/shell heteronanostructures. Nano Lett. 2003, 3, 799–803. [Google Scholar] [CrossRef]
- Koo, B.; Korgel, B.A. Coalescence and Interface Diffusion in Linear CdTe/CdSe/CdTe Heterojunction Nanorods. Nano Lett. 2008, 8, 2490–2496. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Habas, S.E.; Fakra, S.C.; Mokari, T. Probing Compositional Variation within Hybrid Nanostructures. ACS Nano 2009, 3, 3369–3376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadowski, T.; Ramprasad, R. Ab initio thermodynamic model to assess stability of heterostructure nanocrystals. Appl. Phys. Lett. 2010, 96, 101906. [Google Scholar] [CrossRef]
- Mokari, T.; Rothenberg, E.; Popov, I.; Costi, R.; Banin, U. Selective Growth of Metal Tips onto Semiconductor Quantum Rods and Tetrapods. Science 2004, 304, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Jen-La Plante, I.; Habas, S.E.; Yuhas, B.D.; Gargas, D.J.; Mokari, T. Interfacing Metal Nanoparticles with Semiconductor Nanowires. Chem. Mater. 2009, 21, 3662–3667. [Google Scholar] [CrossRef]
- Volokh, M.; Mokari, T. Metal/semiconductor interfaces in nanoscale objects: Synthesis, emerging properties and applications of hybrid nanostructures. Nanoscale Adv. 2020, 2, 930–961. [Google Scholar] [CrossRef]
- Habas, S.E.; Yang, P.; Mokari, T. Selective Growth of Metal and Binary Metal Tips on CdS Nanorods. J. Am. Chem. Soc. 2008, 130, 3294–3295. [Google Scholar] [CrossRef]
- Nakibli, Y.; Amirav, L. Selective Growth of Ni Tips on Nanorod Photocatalysts. Chem. Mater. 2016, 28, 4524–4527. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.Z.; Yang, M.; Fan, Y.J.; Zhan, J.H. Electroless Plating of Nickel Nanoparticles on CdS Nanowires. Eur. J. Inorg. Chem. 2009, 6, 897–902. [Google Scholar] [CrossRef]
- Haldar, K.K.; Pradhan, N.; Patra, A. Formation of Heteroepitaxy in Different Shapes of Au-CdSe Metal-Semiconductor Hybrid Nanostructures. Small 2013, 9, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO Heterostructure Nanocrystals: Synthesis, Characterization, and Photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Geng, B.; Wang, S. ZnO/Au Hybrid Nanoarchitectures: Wet-Chemical Synthesis and Structurally Enhanced Photocatalytic Performance. Environ. Sci. Technol. 2009, 43, 8968–8973. [Google Scholar] [CrossRef]
- Yao, T.; Yan, W.; Sun, Z.; Pan, Z.; He, B.; Jiang, Y.; Wei, H.; Nomura, M.; Xie, Y.; Xie, Y.; et al. High-Temperature Ferromagnetism of Hybrid Nanostructure Ag-Zn0.92Co0.08O Dilute Magnetic Semiconductor. J. Phys. Chem. C 2009, 113, 3581–3585. [Google Scholar] [CrossRef]
- Qiu, X.; Li, L.; Tang, C.; Li, G. Metal-Semiconductor Hybrid Nanostructure Ag-Zn0.9Co0.1O: Synthesis and Room-Temperature Ferromagnetism. J. Am. Chem. Soc. 2007, 129, 11908–11909. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, K.; Liu, Z.; Tao, R.; Sun, Z.; Zhang, H.; An, G. In Situ Controllable Loading of Ultrafine Noble Metal Particles on Titania. J. Am. Chem. Soc. 2009, 131, 6648–6649. [Google Scholar] [CrossRef]
- Su, C.; Liu, L.; Zhang, M.; Zhang, Y.; Shao, C. Fabrication of Ag/TiO2 nanoheterostructures with visible light photocatalytic function via a solvothermal approach. CrystEngComm 2012, 14, 3989–3999. [Google Scholar] [CrossRef]
- Liu, K.; Bi, Y.; Qu, S.C.; Tan, F.R.; Chi, D.; Lu, S.D.; Li, Y.P.; Kou, Y.L.; Wang, Z.G. Efficient hybrid plasmonic polymer solar cells with Ag nanoparticle decorated TiO2 nanorods embedded in the active layer. Nanoscale 2014, 6, 6180–6186. [Google Scholar] [CrossRef]
- Varapragasam, S.J.P.; Mia, S.; Wieting, C.; Balasanthiran, C.; Hossan, M.Y.; Baride, A.; Rioux, R.M.; Hoefelmeyer, J.D. Ag–TiO2 Hybrid Nanocrystal Photocatalyst: Hydrogen Evolution under UV Irradiation but Not under Visible-Light Irradiation. ACS Appl. Energy Mater. 2019, 2, 8274–8282. [Google Scholar] [CrossRef]
- Saunders, A.E.; Popov, I.; Banin, U. Synthesis of Hybrid CdS-Au Colloidal Nanostructures. J. Phys. Chem. B 2006, 110, 25421–25429. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lu, Z.; Lu, Y.; Lv, L.; Ning, Y.; Yu, H.; Hou, Y.; Yin, Y. Photocatalytic Synthesis and Photovoltaic Application of Ag-TiO2 Nanorod Composites. Nano Lett. 2013, 13, 5698–5702. [Google Scholar] [CrossRef]
- Wu, B.H.; Liu, D.Y.; Mubeen, S.; Chuong, T.T.; Moskovits, M.; Stucky, G.D. Anisotropic Growth of TiO2 onto Gold Nanorods for Plasmon-Enhanced Hydrogen Production from Water Reduction. J. Am. Chem. Soc. 2016, 138, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef]
- Herring, N.P.; AbouZeid, K.; Mohamed, M.B.; Pinsk, J.; El-Shall, M.S. Formation Mechanisms of Gold–Zinc Oxide Hexagonal Nanopyramids by Heterogeneous Nucleation using Microwave Synthesis. Langmuir 2011, 27, 15146–15154. [Google Scholar] [CrossRef]
- George, C.; Genovese, A.; Qiao, F.; Korobchevskaya, K.; Comin, A.; Falqui, A.; Marras, S.; Roig, A.; Zhang, Y.; Krahne, R.; et al. Optical and electrical properties of colloidal (spherical Au)-(spinel ferrite nanorod) heterostructures. Nanoscale 2011, 3, 4647–4654. [Google Scholar] [CrossRef]
- Fan, F.-R.; Ding, Y.; Liu, D.-Y.; Tian, Z.-Q.; Wang, Z.L. Facet-Selective Epitaxial Growth of Heterogeneous Nanostructures of Semiconductor and Metal: ZnO Nanorods on Ag Nanocrystals. J. Am. Chem. Soc. 2009, 131, 12036–12037. [Google Scholar] [CrossRef]
- Hill, L.J.; Bull, M.M.; Sung, Y.; Simmonds, A.G.; Dirlam, P.T.; Richey, N.E.; DeRosa, S.E.; Shim, I.-B.; Guin, D.; Costanzo, P.J.; et al. Directing the Deposition of Ferromagnetic Cobalt onto Pt-Tipped CdSe@CdS Nanorods: Synthetic and Mechanistic Insights. ACS Nano 2012, 6, 8632–8645. [Google Scholar] [CrossRef]
- Zhuang, T.-T.; Li, Y.; Gao, X.; Wei, M.; García de Arquer, F.P.; Todorović, P.; Tian, J.; Li, G.; Zhang, C.; Li, X.; et al. Regioselective magnetization in semiconducting nanorods. Nat. Nanotechnol. 2020, 15, 192–197. [Google Scholar] [CrossRef]
- Pavlopoulos, N.G.; Dubose, J.T.; Pinna, N.; Willinger, M.-G.; Char, K.; Pyun, J. Synthesis and Assembly of Dipolar Heterostructured Tetrapods: Colloidal Polymers with “Giant tert-butyl” Groups. Angew. Chem. Int. Ed. 2016, 55, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Costi, R.; Saunders, A.E.; Elmalem, E.; Salant, A.; Banin, U. Visible Light-Induced Charge Retention and Photocatalysis with Hybrid CdSe-Au Nanodumbbells. Nano Lett. 2008, 8, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Mokari, T.; Banin, U.; Millo, O. Electronic Structure of Metal-Semiconductor Nanojunctions in Gold CdSe Nanodumbbells. Phys. Rev. Lett. 2005, 95, 056805. [Google Scholar] [CrossRef] [PubMed]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Elmalem, E.; Saunders, A.E.; Costi, R.; Salant, A.; Banin, U. Growth of Photocatalytic CdSe-Pt Nanorods and Nanonets. Adv. Mater. 2008, 20, 4312–4317. [Google Scholar] [CrossRef]
- Deng, X.; Yang, D.; Tan, G.; Li, X.; Zhang, J.; Liu, Q.; Zhang, H.; Mellors, N.J.; Xue, D.; Peng, Y. Bimagnetic h-Co/h-CoO nanotetrapods: Preparation, nanoscale characterization, three-dimensional architecture and their magnetic properties. Nanoscale 2014, 6, 13710–13718. [Google Scholar] [CrossRef]
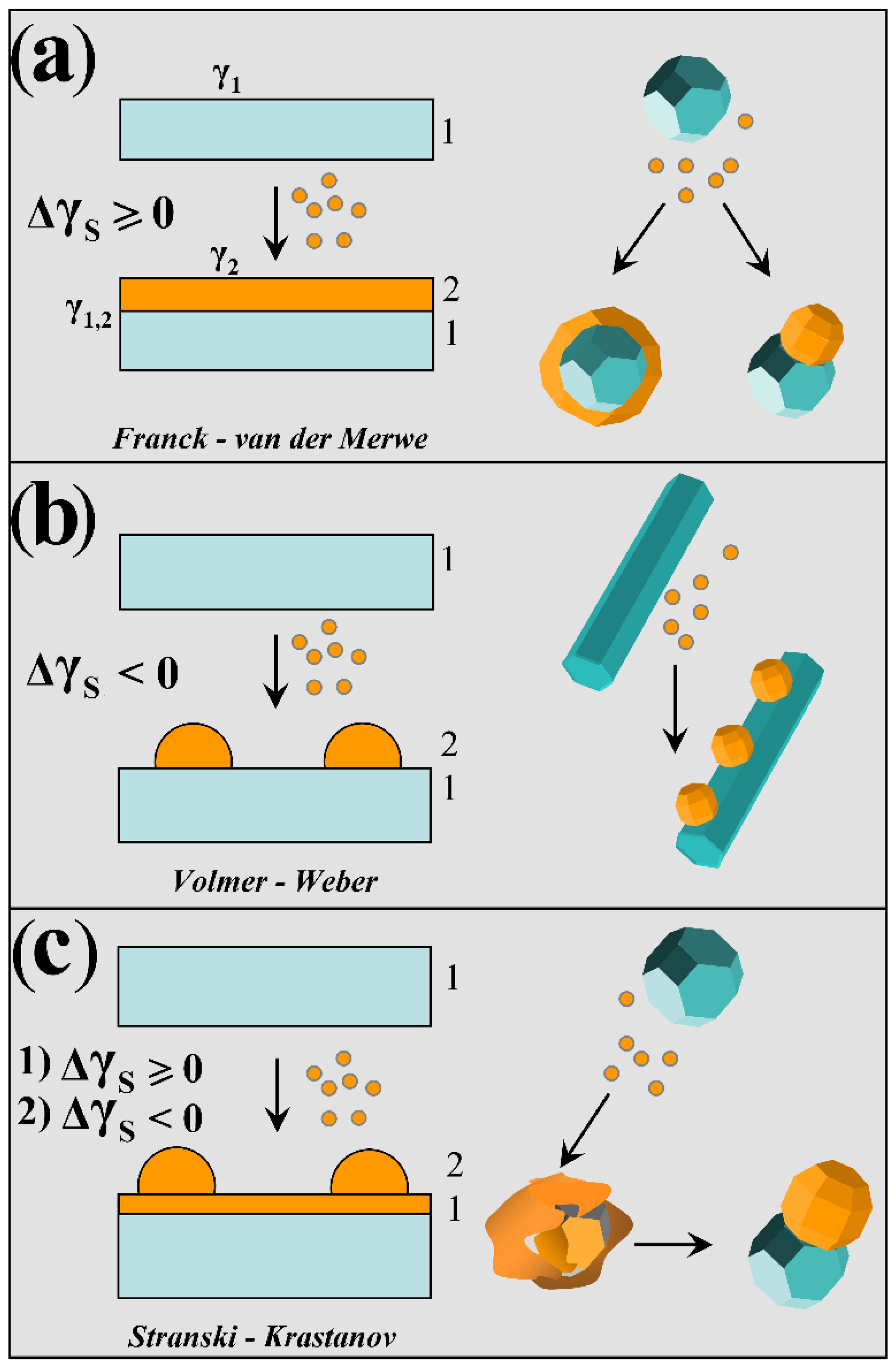
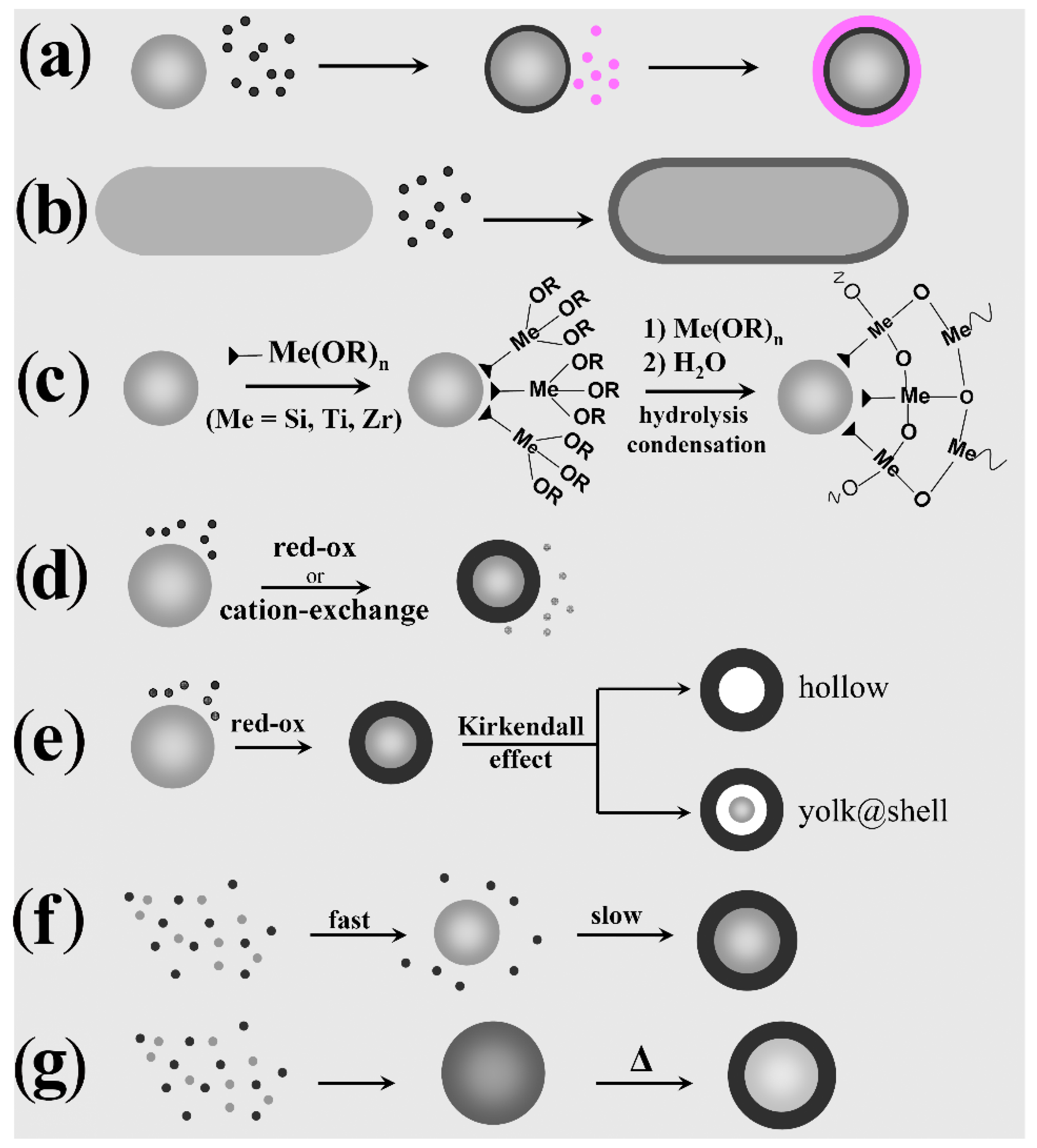
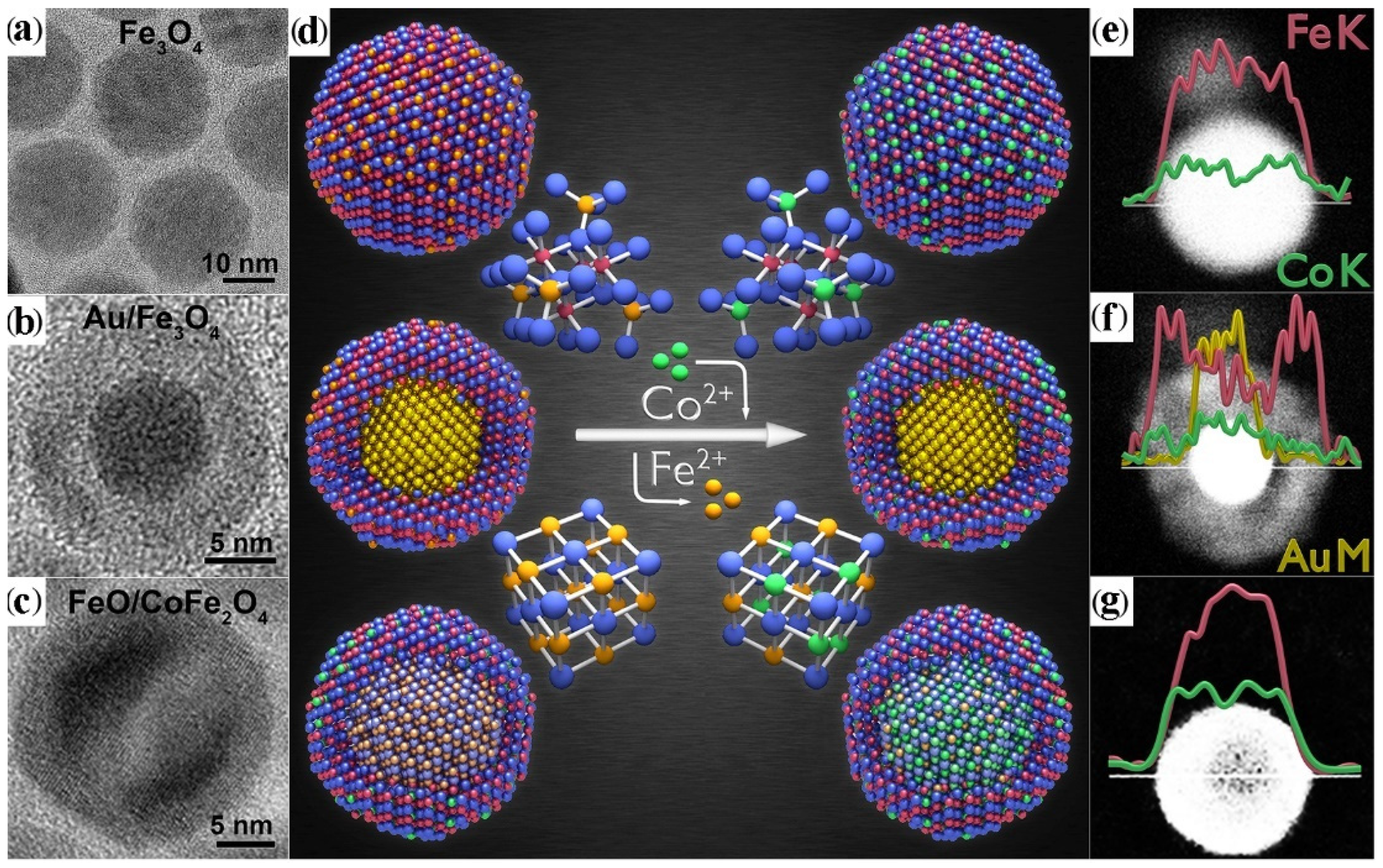
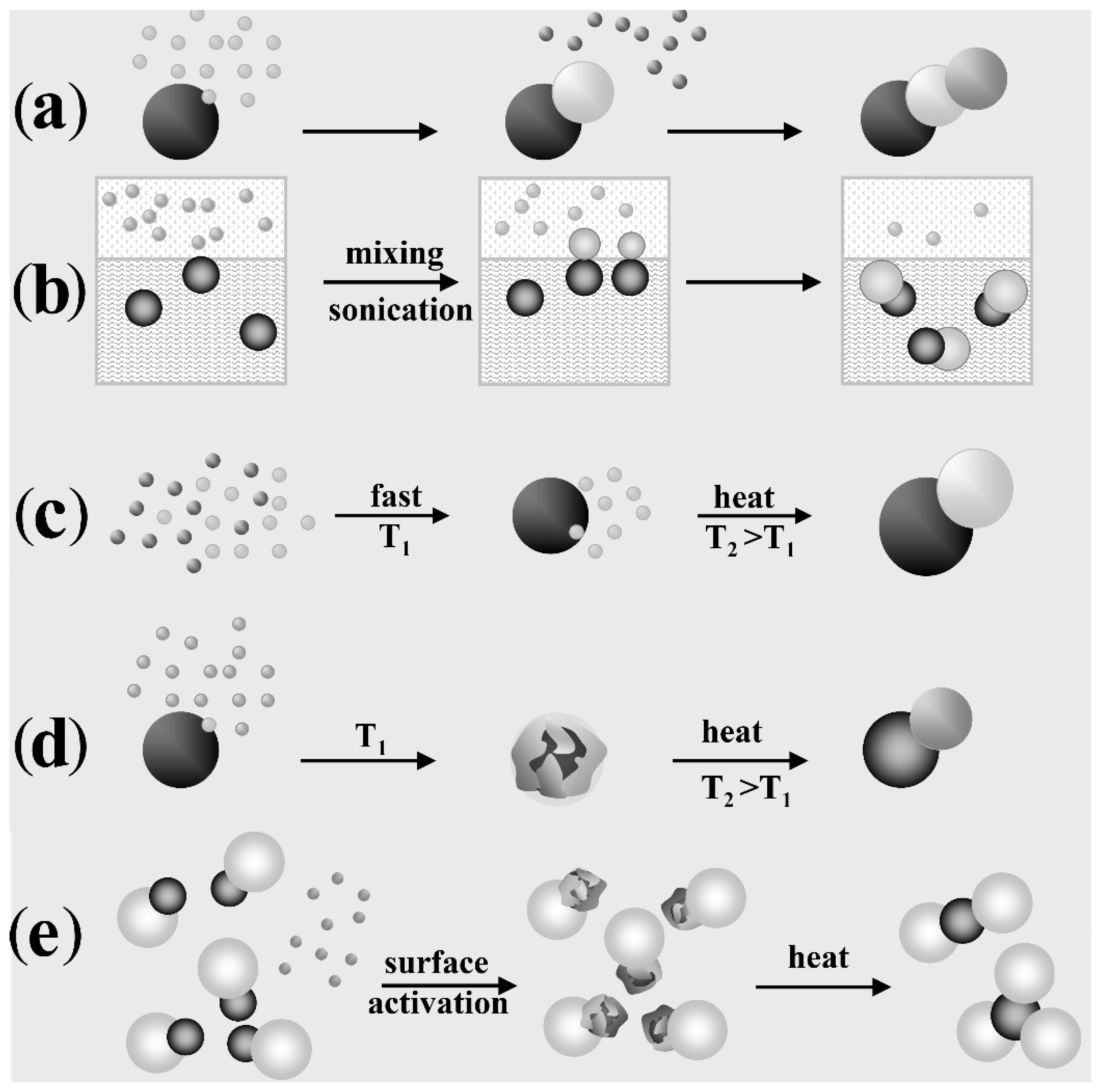
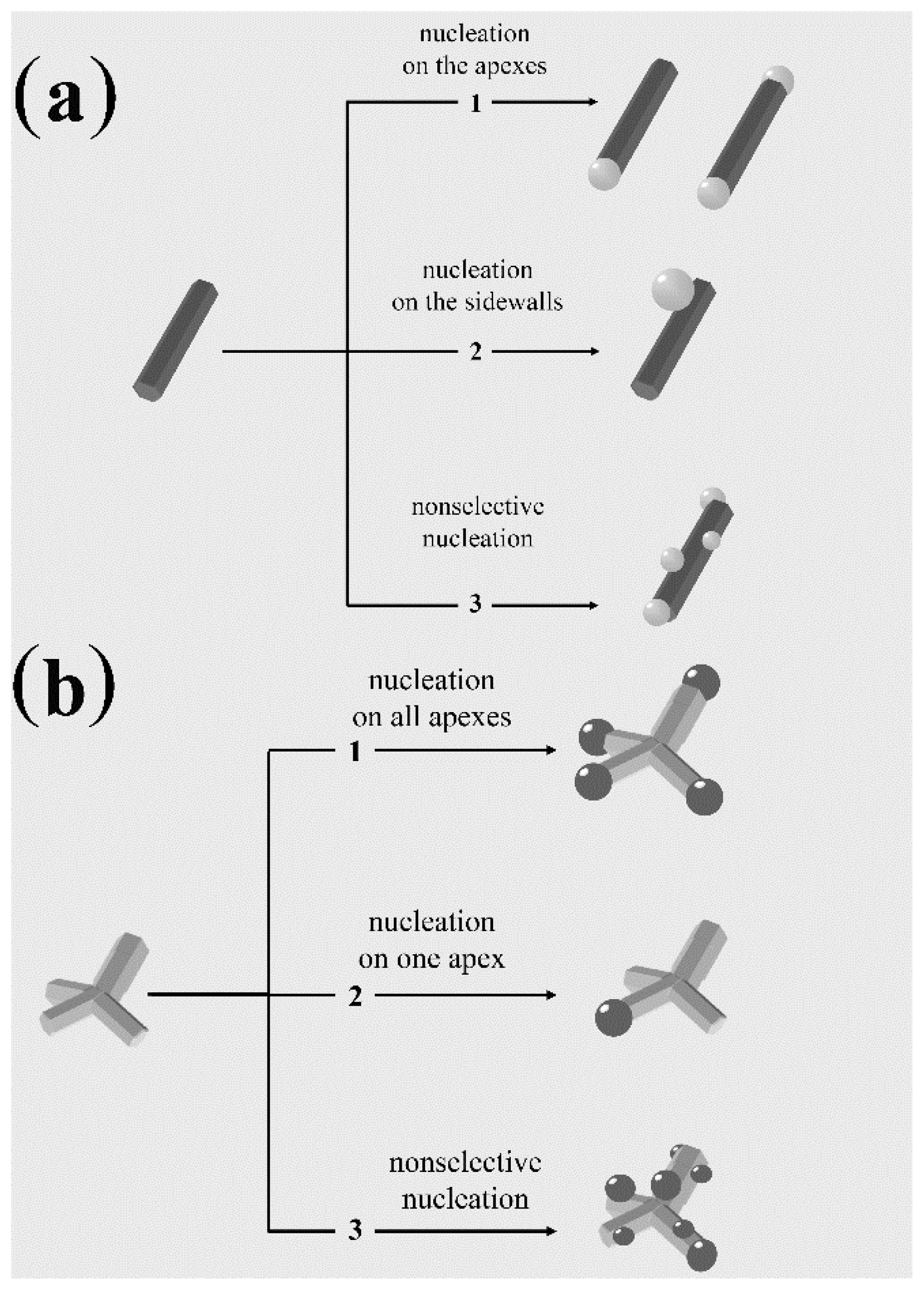
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, C.; Cozzoli, P.D. Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials. Nanomaterials 2022, 12, 1729. https://doi.org/10.3390/nano12101729
Nobile C, Cozzoli PD. Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials. Nanomaterials. 2022; 12(10):1729. https://doi.org/10.3390/nano12101729
Chicago/Turabian StyleNobile, Concetta, and Pantaleo Davide Cozzoli. 2022. "Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials" Nanomaterials 12, no. 10: 1729. https://doi.org/10.3390/nano12101729
APA StyleNobile, C., & Cozzoli, P. D. (2022). Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials. Nanomaterials, 12(10), 1729. https://doi.org/10.3390/nano12101729






