B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
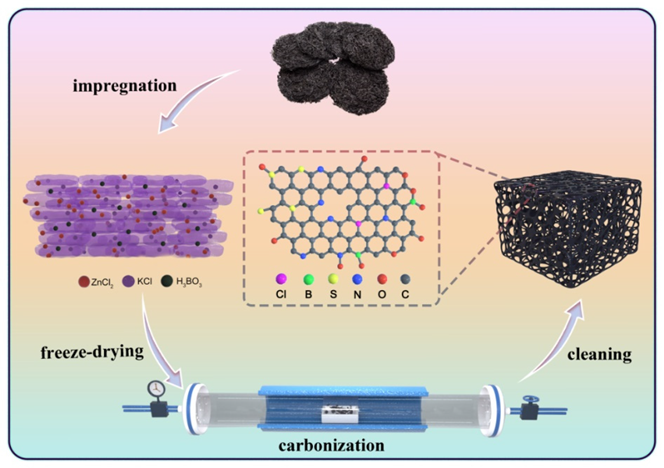
2.2. Materials Preparation
2.3. Characterization
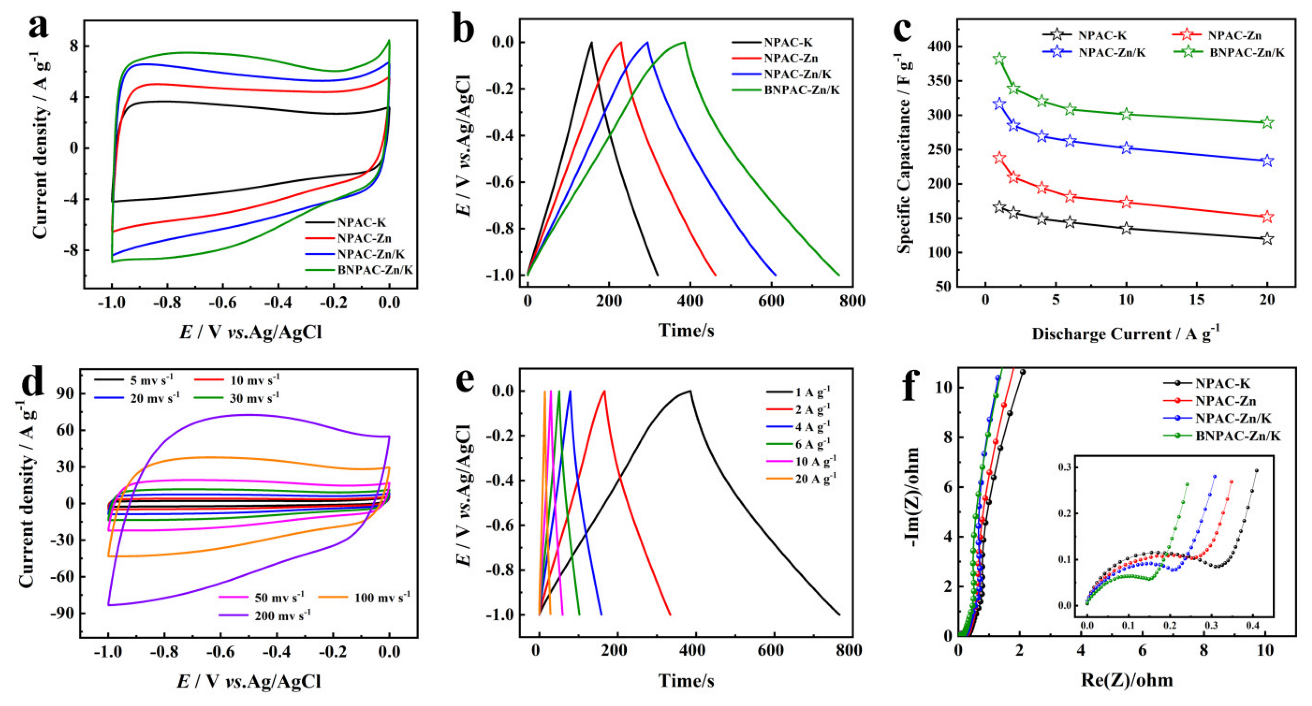
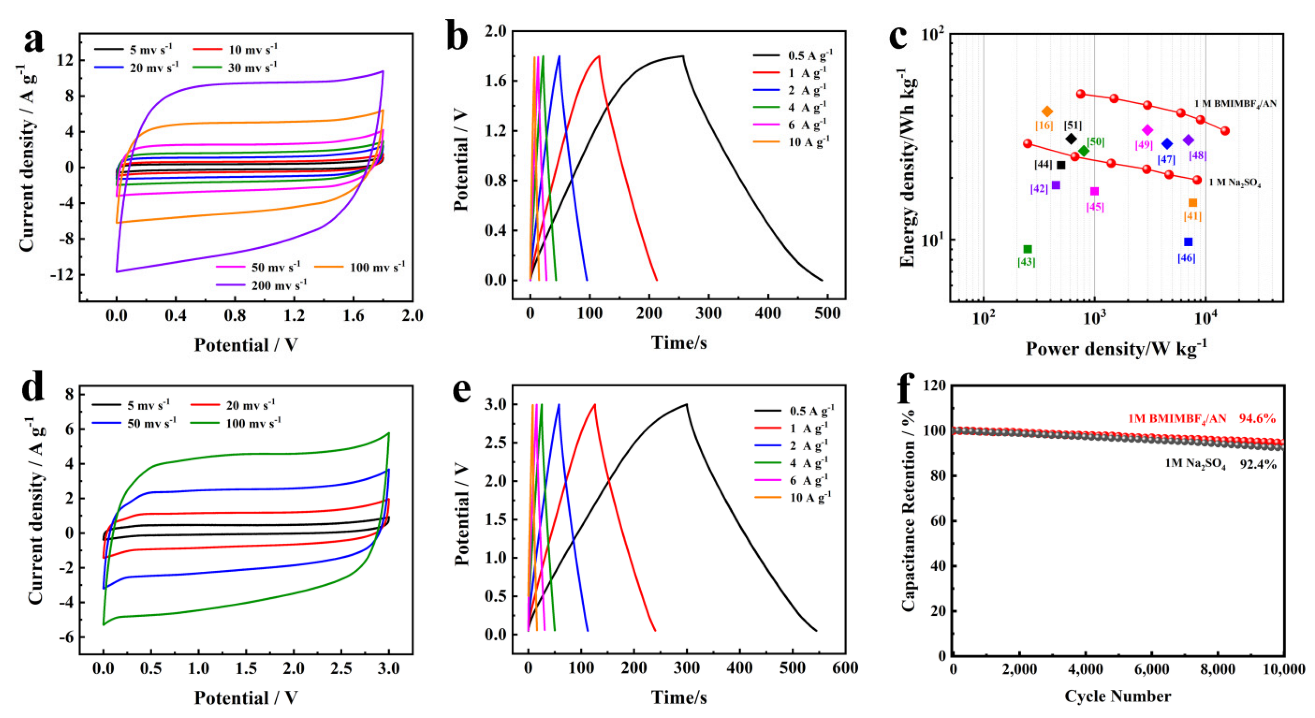
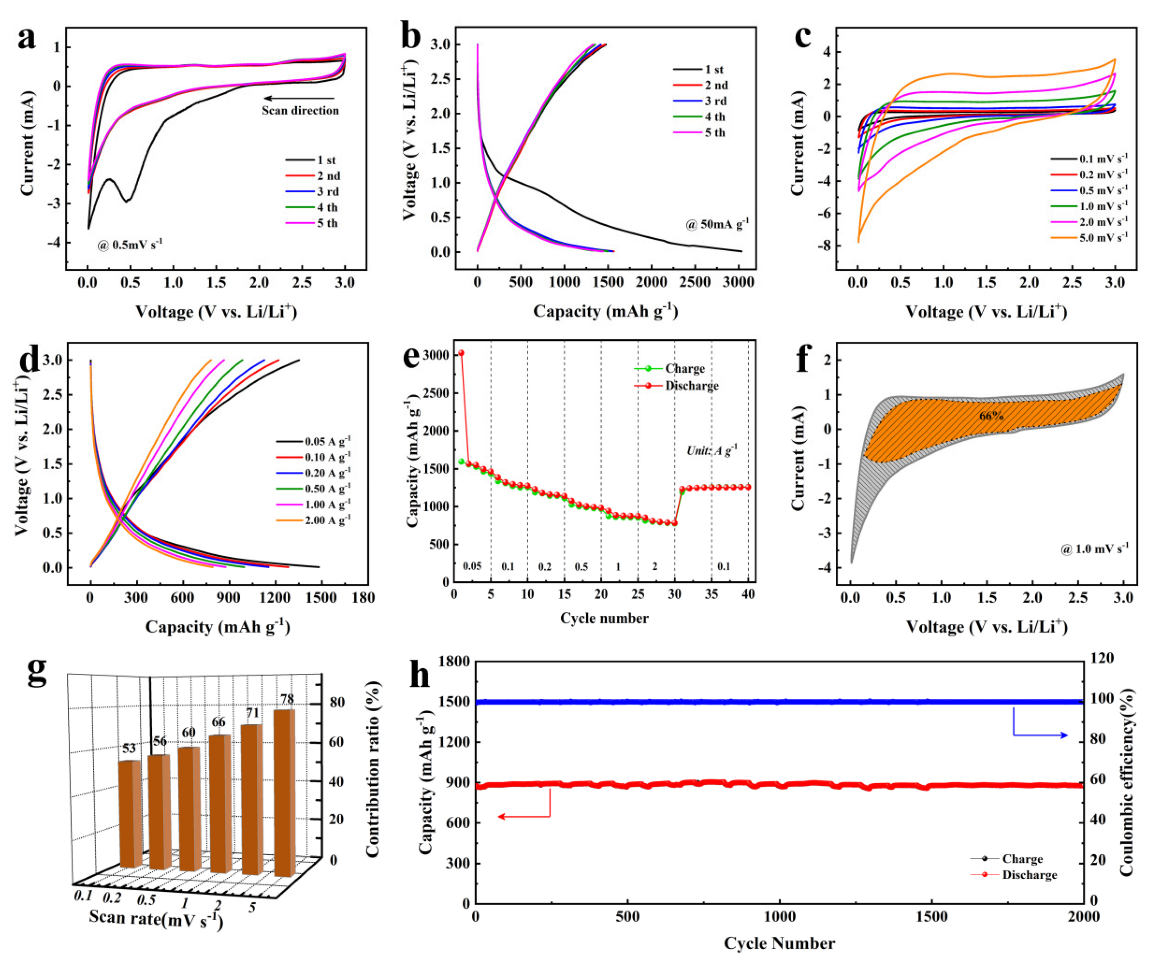
2.4. Electrochemical Characterization
3. Results
| Materials | Activation Methods | Electrolytes | Working Window of Electrolyte | Mass Loading (mg cm−2) | Current Density (A g−1) | Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| laver | ZnCl2, H3BO3, KCl | 6 M KOH | −1–0 V | 2.0 | 1 | 382.5 | this work |
| rapeseed cake | K2CO3, phytic acid, melamine | 6 M KOH | 0–1 V | - | 0.05 | 358 | [15] |
| peanut shells | CoCl2, ZnCl2 | 6 M KOH | −1–0 V | 2.0 | 0.5 | 343 | [16] |
| Walnut shell | H3PO4, KOH | 6 M KOH | −1–0 V | 1.2 | 0.5 | 332 | [18] |
| bean shell | H3BO3 | 6 M KOH | −1–0 V | 3.0 | 0.5 | 119 | [21] |
| sedum spectabile stalk | CH4N2S, H3BO3, ZnCl2 | 6 M KOH | −1–0 V | 3.0 | 0.5 | 290.7 | [24] |
| glucose | ZnCl2, H3BO3, NaCl | 3 M KOH | −1–0 V | 2.7 | 1 | 379.9 | [32] |
| fir bark | NH4B5O84H2O | 6 M KOH | −1–0 V | 1.5 | 0.5 | 188 | [40] |
| soybean straw | KOH | 6 M KOH | −1–0 V | 4.0 | 0.5 | 325 | [41] |
| Rice straw | KHCO3 | 6 M KOH | −1–0 V | 4.0 | 1 | 317 | [45] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Ang, E.H.; Yang, Y.; Ye, M.; Du, W.; Li, C.C. Interlayer Chemistry of Layered Electrode Materials in Energy Storage Devices. Adv. Funct. Mater. 2021, 31, 2007358. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Du, C.; Ma, X.; Cao, C. Advances and challenges in metal-organic framework derived porous materials for batteries and electrocatalysis. J. Mater. Chem. A 2020, 8, 24895–24919. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Xie, J.; Liu, X.; Lu, X. Recent progress and challenges of carbon materials for Zn-ion hybrid supercapacitors. Carbon Energy 2020, 2, 521–539. [Google Scholar] [CrossRef]
- Jo, C.; Voronina, N.; Sun, Y.; Myung, S. Gifts from Nature: Bio-Inspired Materials for Rechargeable Secondary Batteries. Adv. Mater. 2021, 33, 2006019. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Hong, D.P.; Mishra, S.; Dubal, D.P. Advances in bio-waste derived activated carbon for supercapacitors: Trends, challenges and prospective. Resour. Conserv. Recycl. 2021, 169, 105548. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Zhang, Y.; Lan, Y.; Cheng, K. Performance of lead ion removal by the three-dimensional carbon foam supported nanoscale zero-valent iron composite. J. Clean. Prod. 2021, 294, 125350. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Shen, X.; Wang, H.; Wang, H.; Xia, K.; Yin, Z.; Zhang, Y. Biomass-Derived Carbon Materials: Controllable Preparation and Versatile Applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Wu, C.W.; Hou, C.H. A critical re-view on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Awang, N.; Nasir, A.M.; Yajid, M.; Jaafar, J. A review on advancement and future perspective of 3D hierarchical porous aero-gels based on electrospun polymer nanofibers for electrochemical energy storage application. J. Environ. Chem. Eng. 2021, 9, 105437. [Google Scholar] [CrossRef]
- Zheng, G.; Huang, Z.; Liu, Z. Cooperative utilization of beet pulp and industrial waste fly ash to produce N/P/O self-co-doped hierarchically porous carbons for high-performance supercapacitors. J. Power Sources 2021, 482, 228935. [Google Scholar] [CrossRef]
- Kz, A.; Zg, A.; Yda, B.; Gc, C. Nitrogen-rich porous carbon in ultra-high yield derived from activation of biomass waste by a novel eutectic salt for high performance Li-ion capacitors. Carbon 2020, 161, 25–35. [Google Scholar]
- Qiu, S.; Chen, Z.; Zhuo, H.; Hu, Y.; Liu, Q.; Peng, X.; Zhong, L. Using FeCl3 as a Solvent, Template, and Activator to Prepare B, N Co-Doping Porous Carbon with Excellent Supercapacitance. ACS Sustain. Chem. Eng. 2019, 7, 15983–15994. [Google Scholar] [CrossRef]
- Bi, H.; He, X.; Zhang, H.; Li, H.; Qiu, J. N, P co-doped hierarchical porous carbon from rapeseed cake with enhanced supercapacitance. Renew. Energy 2021, 170, 188–196. [Google Scholar] [CrossRef]
- Wen, Y.; Chi, L.; Wen, X.; Chen, X.; Mijowska, E. Nitrogen/Oxygen Enriched Hierarchical Porous Carbons Derived from Waste Peanut Shells Boosting Performance of Supercapacitors. Adv. Electron. Mater. 2020, 6, 2000450. [Google Scholar] [CrossRef]
- Wan, L.; Wei, W.; Xie, M.; Zhang, Y.; Li, X.; Xiao, R.; Chen, J.; Du, C. Nitrogen, sulfur co-doped hierarchically porous carbon from rape pollen as high-performance supercapacitor electrode. Electrochim. Acta 2019, 311, 72–82. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, T.Y.; Wang, H.Z.; Zhu, K.; Cao, D.X. High-throughput fabrication of porous carbon by chemical foaming strategy for high performance supercapacitor. Chem. Eng. J. 2018, 18, 31230. [Google Scholar] [CrossRef]
- Kong, S.; Jin, B.; Quan, X.; Zhang, G.; Guo, X.; Zhu, Q.; Yang, F.; Cheng, K.; Wang, G.; Cao, D. MnO2 nanosheets decorated porous active carbon derived from wheat bran for high-performance asymmetric supercapacitor. J. Electroanal. Chem. 2019, 850, 113412. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Sun, Q.; Wang, P.; Li, Y. Durian shell-derived N, O, P-doped activated porous carbon materials and their electrochemical performance in supercapacitor. J. Mater. Sci. 2020, 55, 10142–10154. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W.; Yu, X.; Chen, T.; Wang, S.; Zhao, W. Boron and nitrogen co-doped porous carbon for supercapacitors: A comparison between a microwave-assisted and a conventional hydrothermal process. J. Energy Storage 2020, 32, 101706. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Meng, Z.; Xu, Z.; Liu, P.; Li, T. Multi-heteroatom doped porous carbon derived from insect feces for capacitance-enhanced sodium-ion storage. J. Energy Chem. 2021, 54, 482–492. [Google Scholar] [CrossRef]
- Diez, N.; Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. Salt template-assisted chemical activation for the production of porous car-bons with enhanced power handling ability in supercapacitors. Batter. Supercaps 2019, 2, 701–711. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, D.; Li, Z.X.; Su, Q.; Wei, S.; Pang, S.F.; Zhao, X.F.; Liang, L.C.; Kang, L.H.; Cao, S.J. Preparation of boron/sulfur-co doped porous carbon derived from biological wastes and its application in a supercapacitor. Nanomaterials 2022, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Cheng, K.; Ouyang, T.; Gao, Y.; Ye, K.; Wang, G.; Cao, D. Facile electrodepositing processed of RuO2-graphene nanosheets-CNT composites as a binder-free electrode for electrochemical supercapacitors. Electrochim. Acta 2017, 246, 433–442. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, X.; Du, Y.; Jiang, Y.; Wan, J.; Ma, F. In-situ template cooperated with urea to construct pectin-derived hierarchical porous carbon with optimized pore structure for supercapacitor. Electrochim. Acta 2020, 355, 136801. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, J.; Hou, Q.; Sheng, W. Utilization of nutrient rich duckweed to create N, P Co-doped porous carbons for high performance supercapacitors. J. Alloys Compd. 2018, 711, 1009–1017. [Google Scholar]
- Wang, C.; Liu, T. Nori-based N, O, S, Cl co-doped carbon materials by chemical activation of ZnCl2 for supercapacitor. J. Alloys Compd. 2017, 696, 42–50. [Google Scholar] [CrossRef]
- Du, P.; Liu, L.; Dong, Y.; Li, W.; Wang, X. Synthesis of hierarchically porous boron-doped carbon material with enhanced sur-face hydrophobicity and porosity for improved supercapacitor performance. Electrochim. Acta 2021, 370, 137801. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, X.; Wang, H.; Xie, L.; Cheng, J.; Kong, Q.; Sun, G.; Chen, C.M. Structure evolution of oxygen removal from po-rous carbon for optimizing supercapacitor performance. J. Energy Chem. 2020, 51, 396–404. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Dai, Y.; Wang, H.-Q.; Zhang, H.; Wang, Q.; Hou, W.; Yan, H.; Li, W.; Zheng, J.-C. Nitrogen and phosphorus co-doped silkworm-cocoon-based self-activated porous carbon for high performance supercapacitors. J. Power Sources 2019, 438, 227045. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, N.; Bai, Z.; Mi, H.; Ji, C.; Sun, L. Acid-Assisted Strategy Combined with KOH Activation to Efficiently Optimize Carbon Architectures from Green Copolymer Adhesive for Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 14838–14846. [Google Scholar] [CrossRef]
- Ansari, M.S.; Edison, T.N.J.I.; Lee, Y.R. Enhanced electrocatalytic and supercapacitive performance using the synergistic effect of defect-rich N/S co-doped hierarchical porous carbon. Sustain. Energy Fuels 2020, 4, 5697–5708. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.O.; Yang, J.; Park, S.; Shanker, R.; Song, H.K.; Kim, Y.; Kwak, S.K.; Ko, H. N,S-doped carbon nanospheres from bio-inspired artificial melanosomes: A route to efficient air electrodes for seawater batteries. J. Mater. Chem. A 2018, 6, 24459–24467. [Google Scholar] [CrossRef]
- Lu, L.A.; Yz, A.; Wen, Y.A.; Xi, W.A.; Sw, B.; Wz, A. Two-step synthesis of B and N co-doped porous carbon composites by microwave-assisted hydrothermal and pyrolysis process for supercapacitor application-ScienceDirect. Electrochim. Acta 2020, 360, 137010. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Xu, R.-X.; Cao, J.-P.; Zhang, X.-Y.; Zhu, J.-S.; Wei, X.-Y. N/O co-doped interlinked porous carbon nanoflakes derived from soybean stalk for high-performance supercapacitors. J. Electroanal. Chem. 2020, 871, 114288. [Google Scholar] [CrossRef]
- Xu, L.; Guo, W.; Zeng, L.; Xia, X.; Wang, Y.; Xiong, P.; Chen, Q.; Zhang, J.; Wei, M.; Qian, Q. V3Se4 embedded within N/P co-doped carbon fibers for sodium/potassium ion batteries. Chem. Eng. J. 2021, 419, 129607. [Google Scholar] [CrossRef]
- Velu, D.; Sakkarapalayam, M.S.K. N and P dual heteroatom doped mesoporous hollow carbon as an efficient oxygen reduction reaction catalyst in alkaline electrolyte. Int. J. Hydrogen Energy 2022, 47, 17992–18006. [Google Scholar]
- Iyyamperumal, E.; Wang, S.; Dai, L. Vertically Aligned BCN Nanotubes with High Capacitance. ACS Nano 2012, 6, 5259–5265. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Cui, Z.; Chen, D.; Dong, L. Tailoring in-situ N, O, P, S-doped soybean-derived porous carbon with ultrahigh capacitance in both acidic and alkaline media. Renew. Energy 2021, 163, 375–385. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Tao, S.; Zu, X.; Li, S.; Qiao, L. Nitrogen/oxygen co-doped carbon nanofoam derived from bamboo fungi for high-performance supercapacitors. J. Power Sources 2020, 479, 228835. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Liang, Y.; Lin, H.; Zhang, S.; Liu, J.; Jin, C.; Choe, U.; Sheng, K. Green Synthesis of Nitrogen-doped Porous Carbon Derived from Rice Straw for High-performance Supercapacitor Application. Energy Fuels 2020, 34, 8966–8976. [Google Scholar] [CrossRef]
- Bai, Q.; Li, H.; Zhang, L.; Li, C.; Shen, Y.; Uyama, H. Flexible Solid-State Supercapacitors Derived from Biomass Konjac/Polyacrylonitrile-Based Nitrogen-Doped Porous Carbon. ACS Appl. Mater. Interfaces 2020, 12, 55913–55925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ding, Y.; Huang, Y.; Li, N.; Zhao, C. Soybean root-derived N, O co-doped hierarchical porous carbon for supercapacitors. Appl. Surf. Sci. 2021, 555, 149726. [Google Scholar] [CrossRef]
- Yan, J.; Fang, Y.Y.; Wang, S.W.; Wu, S.D.; Liu, F.J. Nitrogen-doped oxygen-rich activated carbon derived from longan shell for supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 1955–1982. [Google Scholar] [CrossRef]
- Guo, D.; Li, Z.; Liu, P.; Sun, M. N, P, S co-doped biomass-derived hierarchical porous carbon through simple phosphoric acid-assisted activation for high-performance electrochemical energy storage. Int. J. Hydrogen Energy 2021, 46, 8197–8209. [Google Scholar] [CrossRef]
- Song, Y.; Sw, A.; Xuan, L.A.; Li, L.B. Biomass derived interconnected hierarchical micro-meso-macro-porous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar]
- Ahirrao, D.J.; Tambat, S.; Pandit, A.B.; Jha, N. Sweet-lime-peels-derived activated-carbon-based electrode for highly efficient supercapacitor and flow-through water desalination. ChemistrySelect 2019, 4, 2619–2625. [Google Scholar] [CrossRef]
- Chang, C.; Wang, H.; Zhang, Y.; Wang, S.; Li, L. Fabrication of hierarchical porous carbon frameworks from metal-ion-assisted step-activation of biomass for supercapacitors with ultrahigh capacitance. ACS Sustain. Chem. Eng. 2019, 7, 10763–10772. [Google Scholar] [CrossRef]
- Niu, L.Y.; Shen, C.; Yan, L.J.; Zhang, J.H.; Lin, Y.; Gong, Y.Y.; Li, C.; Sun, C.Q.; Xu, S.Q. Waste bones derived nitrogen–doped carbon with high micropore ratio towards supercapacitor applications. J. Colloid Interface Sci. 2019, 547, 92–101. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Qian, W. Interlinked porous carbon nanoflakes derived from hydrolyzate residue during cellulosic bio-ethanol production for ultrahigh-rate supercapacitors in non-aqueous electrolytes. ACS Sustain. Chem. Eng. 2016, 5, 1297–1305. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Dou, M.; Li, Z.; Wang, F. Nitrogen and oxygen co-doped porous carbon nanosheets as high-rate and long-lifetime anode materials for high-performance Li-ion capacitors. Carbon 2019, 151, 28–35. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Liu, Y.; Meng, T.; Ma, L.; Zhang, H.; Kuang, M.; Jiang, J. Graphitic, porous and multi-heteroatoms co-doped car-bon microtubes made from hair wastes: A superb and sustained anode substitute for Li-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 13662–13669. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, C.; Dang, B.K.; Xiong, Y.; Jin, C.D.; Sun, Q.F.; Xu, M. Nitrogen, sulfur, phosphorous co-doped interconnect-ed porous carbon nanosheets with high defect density for enhancing supercapacitor and lithium-ion battery properties. Chemelectrochem 2018, 5, 2367–2375. [Google Scholar] [CrossRef]
- Jz, A.; Dz, A.; Kang, L.B.; Yt, A.; Yun, W.A.; Ts, A. N, O and S co-doped hierarchical porous carbon derived from a series of samara for lithium and sodium storage: Insights into surface capacitance and inner diffusion. J. Colloid Interface Sci. 2021, 598, 250–259. [Google Scholar]
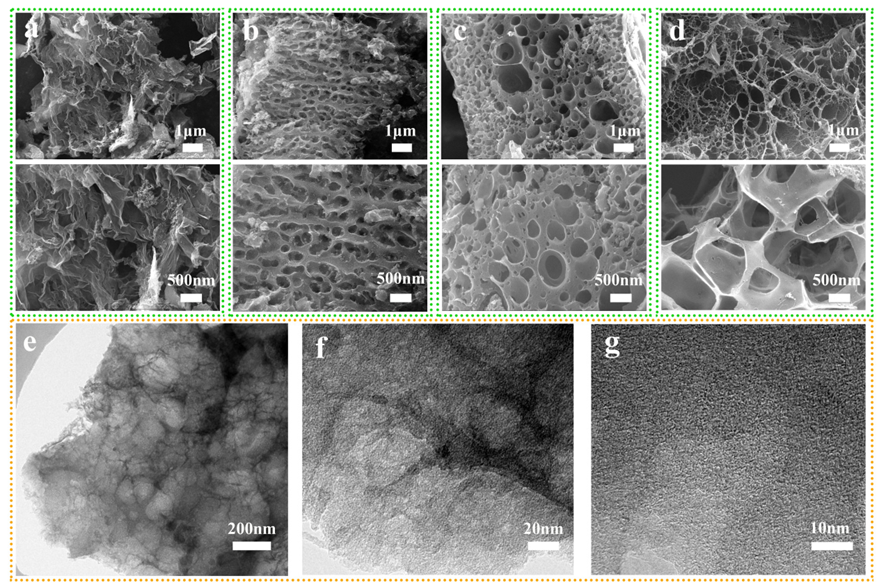


| Samples | SBETa [m2 g−1] | Vtotalb [cm3 g−1] | Vmicroc [cm3 g−1] | ID/IG | Elemental Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | N | S | Cl | B | |||||
| LHPC-K | 724 | 0.72 | 0.23 | 0.88 | 83.76 | 10.42 | 4.89 | 0.53 | 0.4 | 0 |
| LHPC-Zn | 925.7 | 0.6 | 0.36 | 0.89 | 85.8 | 9.01 | 4.19 | 0.65 | 0.35 | 0 |
| LHPC-Zn/K | 1394.6 | 0.92 | 0.49 | 0.9 | 85.27 | 9.58 | 4.32 | 0.4 | 0.43 | 0 |
| BLHPC-Zn/K | 1514.3 | 1.16 | 0.47 | 0.93 | 75.81 | 12.55 | 6.33 | 0.48 | 0.42 | 4.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, S.; Xiang, X.; Jin, B.; Guo, X.; Wang, H.; Zhang, G.; Huang, H.; Cheng, K. B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage. Nanomaterials 2022, 12, 1720. https://doi.org/10.3390/nano12101720
Kong S, Xiang X, Jin B, Guo X, Wang H, Zhang G, Huang H, Cheng K. B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage. Nanomaterials. 2022; 12(10):1720. https://doi.org/10.3390/nano12101720
Chicago/Turabian StyleKong, Shuying, Xinzhu Xiang, Binbin Jin, Xiaogang Guo, Huijun Wang, Guoqing Zhang, Huisheng Huang, and Kui Cheng. 2022. "B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage" Nanomaterials 12, no. 10: 1720. https://doi.org/10.3390/nano12101720
APA StyleKong, S., Xiang, X., Jin, B., Guo, X., Wang, H., Zhang, G., Huang, H., & Cheng, K. (2022). B, O and N Codoped Biomass-Derived Hierarchical Porous Carbon for High-Performance Electrochemical Energy Storage. Nanomaterials, 12(10), 1720. https://doi.org/10.3390/nano12101720






