Aromatic Dipeptide Homologue-Based Hydrogels for Photocontrolled Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Boc-Dipeptides
2.4. Self-Assembly Protocol
2.5. Gel Preparation
2.6. Incorporation of Oxidized CNTs or GO
2.7. Drug Loading
2.8. Release of l-Ascorbic Acid
3. Results and Discussion
3.1. Peptide Self-Assembly in Water
3.2. Formation of the Hydrogels
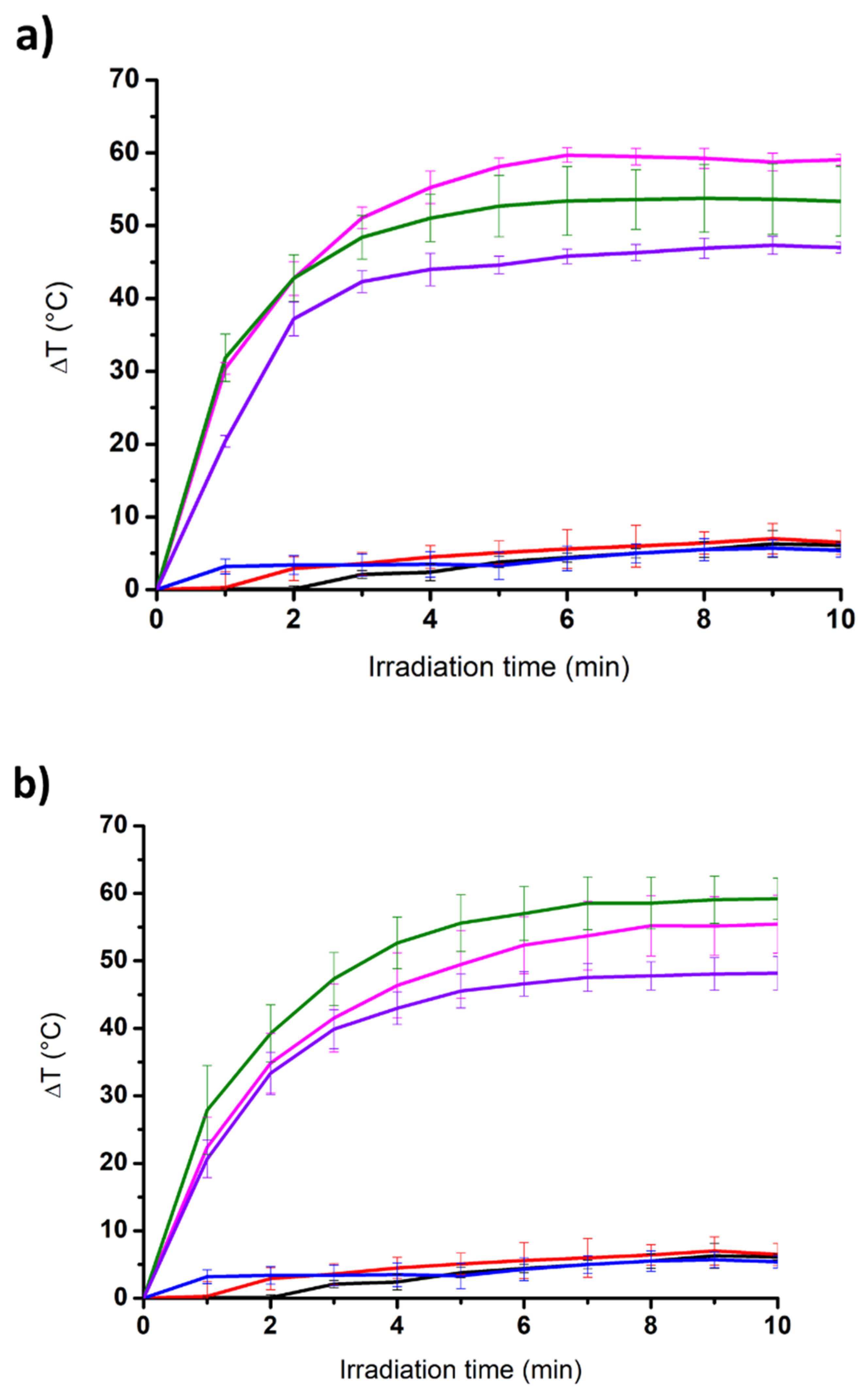
3.3. Incorporation of Carbon Nanomaterials and Photothermal Properties
3.4. Morphological Study
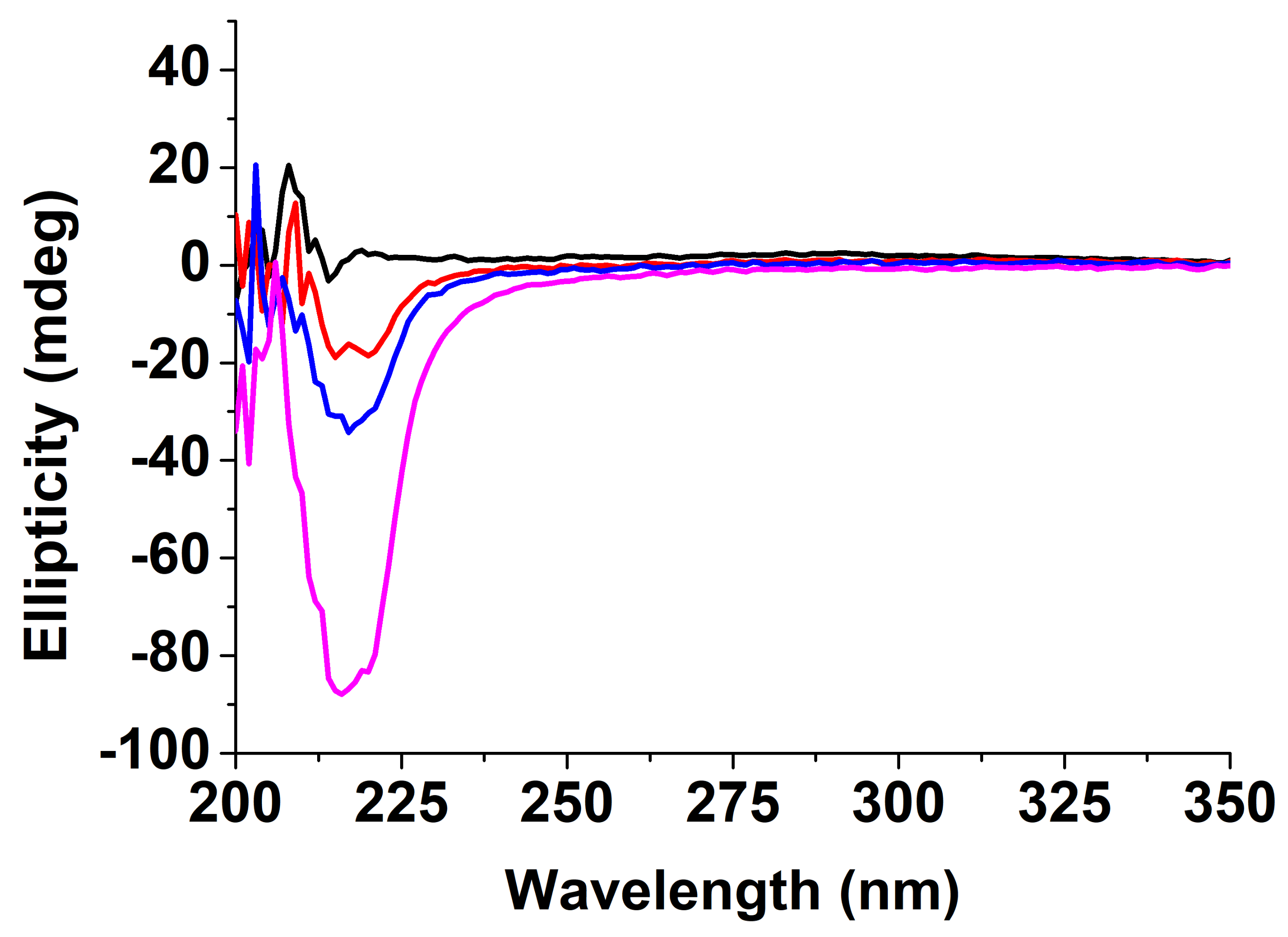
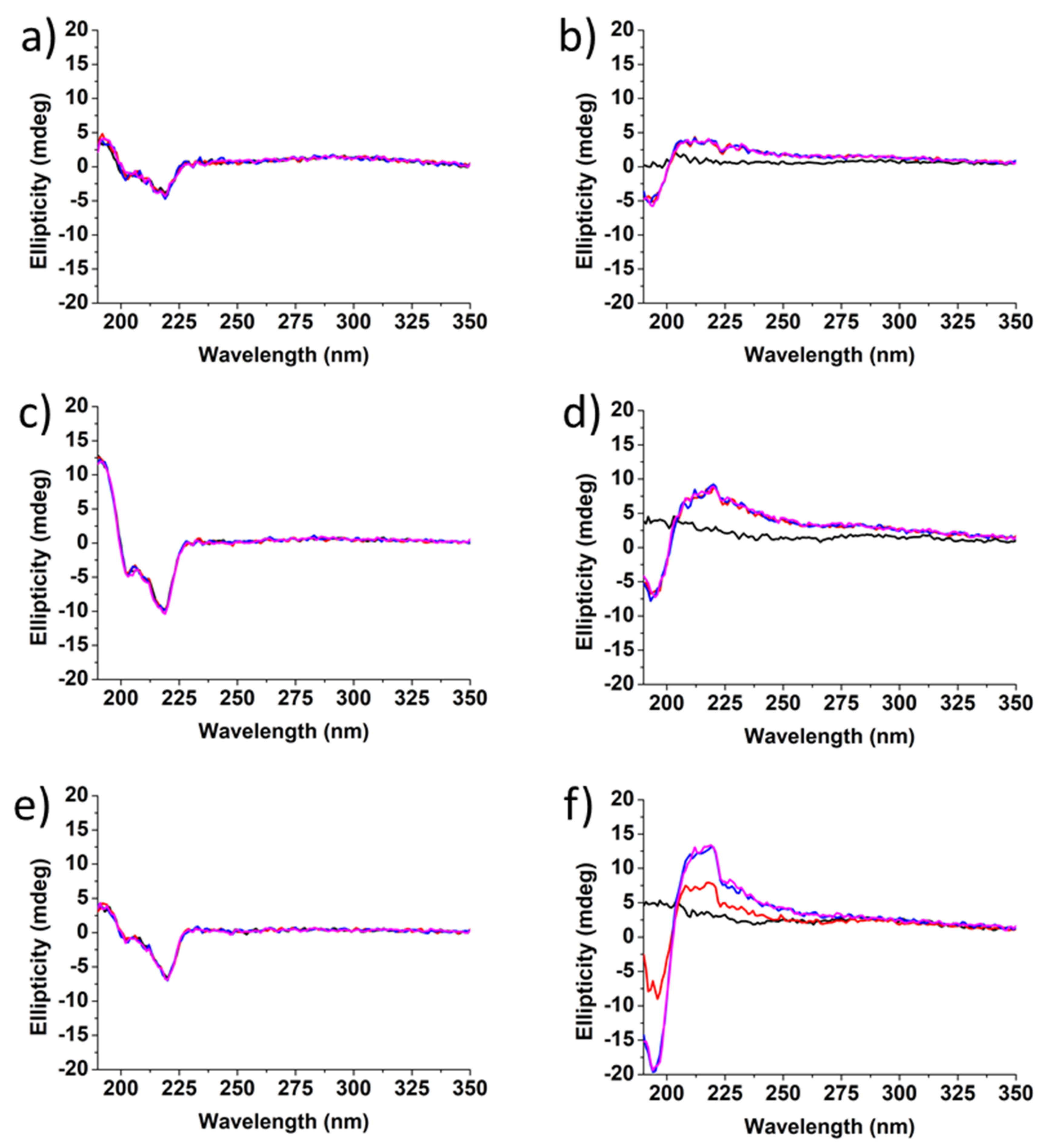
3.5. Conformational Studies
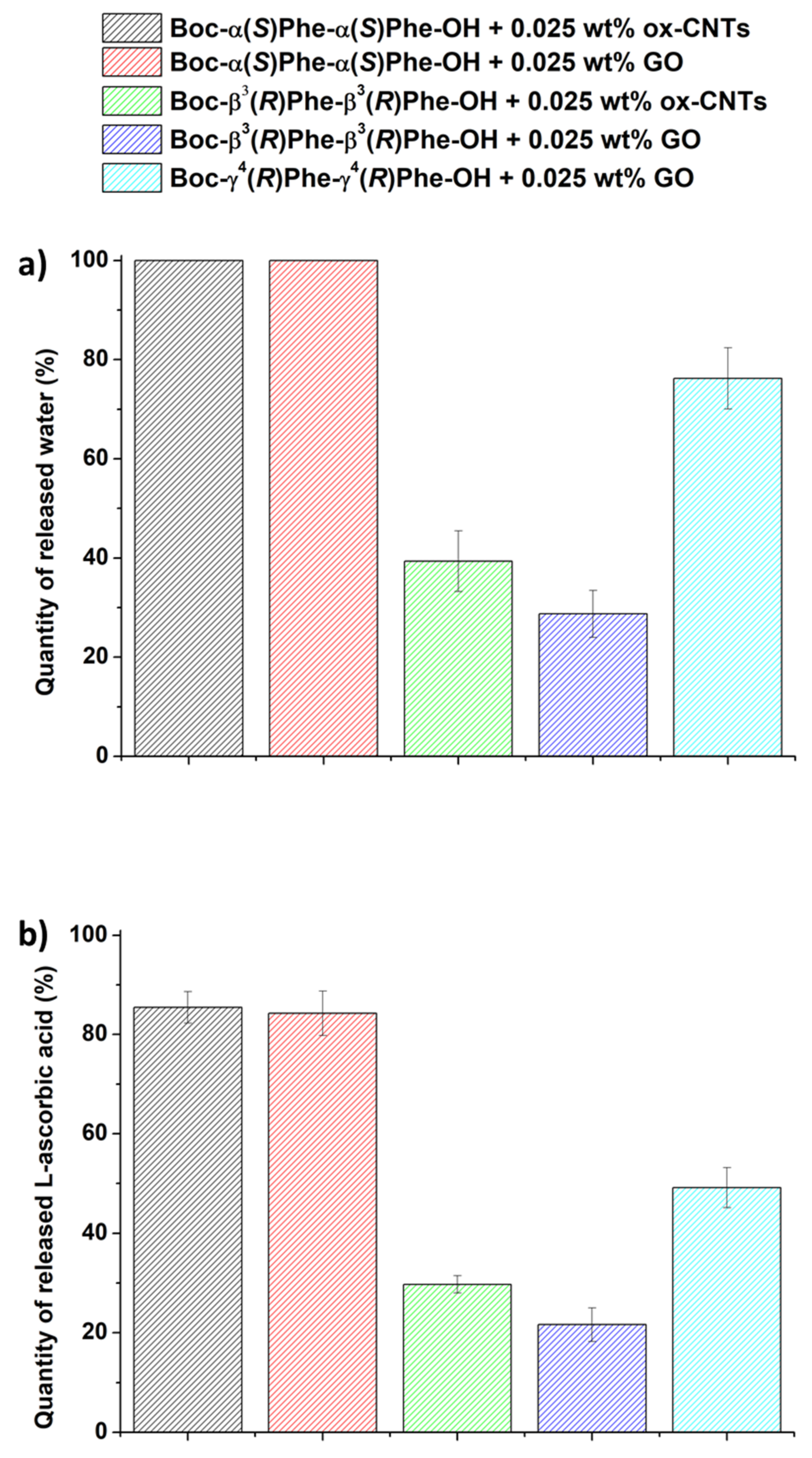
3.6. Drug Loading and Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine as a suitable building block for the preparation of hybrid materials and their potential applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi-Joshaghani, M.H.; Seidi, K.; Azizi, M.; Jaymand, M.; Javaheri, T.; Jahanban-Esfahlan, R.; Hamblin, M.R. Potential Applications of Advanced Nano/Hydrogels in Biomedicine: Static, Dynamic, Multi-Stage, and Bioinspired. Adv. Funct. Mater. 2020, 30, 2004098. [Google Scholar] [CrossRef]
- Fu, W.; Sabet, Z.F.; Liu, J.; You, M.; Zhou, H.; Wang, Y.; Gao, Y.; Li, J.; Ma, X.; Chen, C. Metal ions modulation of the self-assembly of short peptide conjugated nonsteroidal anti-inflammatory drugs (NSAIDs). Nanoscale 2020, 12, 7960–7968. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Guo, Y.; Li, X.; Xue, H.; Fei, J.; Li, J. Co-assembled Supramolecular Gel of Dipeptide and Pyridine Derivatives with Controlled Chirality. Angew. Chem. Int. Ed. 2021, 60, 2099–2103. [Google Scholar] [CrossRef]
- Ji, W.; Yuan, C.; Chakraborty, P.; Makam, P.; Bera, S.; Rencus-Lazar, S.; Li, J.; Yan, X.; Gazit, E. Coassembly-Induced Transformation of Dipeptide Amyloid-Like Structures into Stimuli-Responsive Supramolecular Materials. ACS Nano 2020, 14, 7181–7190. [Google Scholar] [CrossRef]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef]
- Ren, P.; Li, J.; Zhao, L.; Wang, A.; Wang, M.; Li, J.; Jian, H.; Li, X.; Yan, X.; Bai, S. Dipeptide Self-assembled Hydrogels with Shear-Thinning and Instantaneous Self-healing Properties Determined by Peptide Sequences. ACS Appl. Mater. Interfaces 2020, 12, 21433–21440. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Reches, M.; Gazit, E. Formation of Closed-Cage Nanostructures by Self-Assembly of Aromatic Dipeptides. Nano Lett. 2004, 4, 581–585. [Google Scholar] [CrossRef]
- Guo, C.; Luo, Y.; Zhou, R.; Wei, G. Probing the Self-Assembly Mechanism of Diphenylalanine-Based Peptide Nanovesicles and Nanotubes. ACS Nano 2012, 6, 3907–3918. [Google Scholar] [CrossRef]
- Mason, T.O.; Chirgadze, D.Y.; Levin, A.; Adler-Abramovich, L.; Gazit, E.; Knowles, T.P.J.; Buell, A.K. Expanding the solvent chemical space for self-assembly of dipeptide nanostructures. ACS Nano 2014, 8, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler-Abramovich, L.; Reches, M.; Sedman, V.L.; Allen, S.; Tendler, S.J.B.; Gazit, E. Thermal and chemical stability of diphenylalanine peptide nanotubes: Implications for nanotechnological applications. Langmuir 2006, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular Hydrogels Respond to Ligand–Receptor Interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Palui, G.; Banerjee, A. Pentapeptide based organogels: The role of adjacently located phenylalanine residues in gel formation. Soft Matter 2008, 4, 1430–1437. [Google Scholar] [CrossRef]
- Ma, M.; Kuang, Y.; Gao, Y.; Zhang, Y.; Gao, P.; Xu, B. Aromatic–Aromatic Interactions Induce the Self-Assembly of Pentapeptidic Derivatives in Water to Form Nanofibers and Supramolecular Hydrogels. J. Am. Chem. Soc. 2010, 132, 2719–2728. [Google Scholar] [CrossRef]
- Doran, T.M.; Ryan, D.M.; Nilsson, B.L. Reversible photocontrol of self-assembled peptide hydrogel viscoelasticity. Polym. Chem. 2013, 5, 241–248. [Google Scholar] [CrossRef]
- Orbach, R.; Mironi-Harpaz, I.; Adler-Abramovich, L.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Gazit, E.; Seliktar, D. The rheological and structural properties of Fmoc-peptide-based hydrogels: The effect of aromatic molecular architecture on self-assembly and physical characteristics. Langmuir 2012, 28, 2015–2022. [Google Scholar] [CrossRef]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-assembled Fmoc-peptides as a platform for the formation of nanostructures and hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef]
- Adams, D.J.; Topham, P.D. Peptide conjugate hydrogelators. Soft Matter 2010, 6, 3707–3721. [Google Scholar] [CrossRef] [Green Version]
- Baral, A.; Roy, S.; Ghosh, S.; Hermida-Merino, D.; Hamley, I.W.; Banerjee, A. A Peptide-Based Mechano-sensitive, Proteolytically Stable Hydrogel with Remarkable Antibacterial Properties. Langmuir 2016, 32, 1836–1845. [Google Scholar] [CrossRef]
- Ravarino, P.; Giuri, D.; Faccio, D.; Tomasini, C. Designing a Transparent and Fluorine Containing Hydrogel. Gels 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Overhand, M.; Kühnle, F.N.M.; Martinoni, B.; Oberer, L.; Hommel, U.; Widmer, H. β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a β-hexapeptide in solution and its stability towards pepsin. Helv. Chim. Acta 1996, 79, 913–941. [Google Scholar]
- Nanda, J.; Banerjee, A. β-Amino acid containing proteolitically stable dipeptide based hydrogels: Encapsulation and sustained release of some important biomolecules at physiological pH and temperature. Soft Matter 2012, 8, 3380–3386. [Google Scholar] [CrossRef]
- Dinesh, D.; Squillaci, M.A.; Ménard-Moyon, C.; Samorì, P.; Bianco, A. Self-assembly of diphenylalanine backbone homologues and their combination with functionalized carbon nanotubes. Nanoscale 2015, 7, 15873–15879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinesh, B.; Medelin, M.; Scaini, D.; Lareno Faccini, F.; Quici, F.; Ballerini, L.; Bianco, A. Hybrid Interfaces Made of Nanotubes and Backbone-Altered Dipeptides Tune Neuronal Network Architecture. ACS Chem. Neurosci. 2020, 11, 162–172. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Venkatesh, V.; Krishna, K.V.; Bonachera, F.; Verma, S.; Bianco, A. Self-Assembly of Tyrosine into Controlled Supramolecular Nanostructures. Chem. Eur. J. 2015, 21, 11681–11686. [Google Scholar] [CrossRef]
- Jang, H.-S.; Lee, J.-H.; Park, Y.-S.; Kim, Y.-O.; Park, J.; Yang, T.-Y.; Jin, K.; Lee, J.; Park, S.; You, J.M.; et al. Tyrosine-mediated two-dimensional peptide assembly and its role as a bio-inspired catalytic scaffold. Nat. Commun. 2014, 5, 3665. [Google Scholar] [CrossRef] [Green Version]
- Delogu, L.G.; Venturelli, E.; Manetti, R.; Pinna, G.A.; Carru, C.; Madeddu, R.; Murgia, L.; Sgarrella, F.; Dumortier, H.; Bianco, A. Ex Vivo Impact of Functionalized Carbon Nanotubes on Human Immune Cells. Nanomedicine 2012, 7, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Marangon, I.; Ménard-Moyon, C.; Kolosnjaj-Tabi, J.; Béoutis, M.L.; Lartigue, L.; Alloyeau, D.; Pach, E.; Ballesteros, B.; Autret, G.; Ninjbadgar, T.; et al. Covalent Functionalization of Multi-Walled Carbon Nanotubes with a Gadolinium Chelate for Efficient T1-Weighted Magnetic Resonance Imaging. Adv. Funct. Mater. 2014, 24, 7173–7186. [Google Scholar]
- Jain, S.; Thakare, V.S.; Das, M.; Godugu, C.; Jain, A.K.; Mathur, R.; Chuttani, K.; Mishra, A.K. Toxicity of Multiwalled Carbon Nanotubes with End Defects Critically Depends on Their Functionalization Density. Chem. Res. Toxicol. 2011, 24, 2028–2039. [Google Scholar] [CrossRef]
- Jasim, D.A.; Murphy, S.; Newman, L.; Mironov, A.; Prestat, E.; McCaffrey, J.; Ménard-Moyon, C.; Rodrigues, A.F.; Bianco, A.; Haigh, S.; et al. The Effects of Extensive Glomerular Filtration of Thin Graphene Oxide Sheets on Kidney Physiology. ACS Nano 2016, 10, 10753–10767. [Google Scholar] [CrossRef] [PubMed]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelos, K. Purified Graphene Oxide Dispersions Lack In Vitro Cytotoxicity and in Vivo Pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.-C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical construction of a mechanically stable peptide–graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef]
- Smrcina, M.; Majer, P.; Majerová, E.; Guerassina, T.A.; Eissenstat, M.A. Facile stereoselective synthesis of γ-substituted γ-amino acids from the corresponding α-amino acids. Tetrahedron 1997, 53, 12867–12874. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Kol, N.; Yanai, I.; Barlam, D.; Shneck, R.Z.; Gazit, E.; Rousso, I. Self-Assembled Organic Nanostructures with Metallic-Like Stiffness. Angew. Chem. Int. Ed. 2010, 49, 9939–9942. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef] [Green Version]
- Guilbaud-Chéreau, C.; Dinesh, B.; Schurhammer, R.; Collin, D.; Bianco, A.; Ménard-Moyon, C. Protected Amino Acid–Based Hydrogels Incorporating Carbon Nanomaterials for Near-Infrared Irradiation-Triggered Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 13147–13157. [Google Scholar] [CrossRef]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2019, 15, 487–496. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, L.; Cao, C.; Wei, P.; Yi, X.; Zhou, Y.; Lv, Q.; Zhou, D.; Yi, T. De novo design of self-assembly hydrogels based on Fmoc-diphenylalanine providing drug release. J. Mater. Chem. B 2021, 9, 8686–8693. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Yuran, S.; Razvag, Y.; Reches, M. Coassembly of Aromatic Dipeptides into Biomolecular Necklaces. ACS Nano 2012, 6, 9559–9566. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Sun, B.; Tao, K.; Jia, Y.; Yan, X.; Zou, Q.; Gazit, E.; Li, J. Photoactive properties of supramolecular assembled short peptides. Chem. Soc. Rev. 2019, 48, 4387–4400. [Google Scholar] [CrossRef]
- Farahani, A.D.; Martin, A.D.; Iranmanesh, H.; Bhadbhade, M.M.; Beves, J.E.; Thordarson, P. Gel-and Solid-State-Structure of Dialanine and Diphenylalanine Amphiphiles: Importance of C—H Interactions in Gelation. ChemPhysChem 2019, 20, 972–983. [Google Scholar] [CrossRef]
- Martin, A.D.; Robinson, A.B.; Thordarson, P. Biocompatible small peptide super-hydrogelators bearing carbazole functionalities. J. Mater. Chem. B 2015, 3, 2277–2280. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Q.; Dong, C.; Lee, S.S.; Gao, L.; Li, Y.; D’Ortenzio, M.; Wu, J. Self-Assembling Peptide Nanofibrous Hydrogel as a Versatile Drug Delivery Platform. Curr. Pharm. Des. 2015, 21, 4342–4354. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Cui, H. Peptide-Based Supramolecular Hydrogels for Delivery of Biologics. Bioeng. Transl. Med. 2016, 1, 306–322. [Google Scholar] [CrossRef]
- Lee, E.; Kim, K.; Choi, M.; Lee, Y.; Park, J.-W.; Kim, B. Development of smart delivery system for ascorbic acid using pH-responsive P(MAA-co-EGMA) hydrogel microparticles. Drug Deliv. 2010, 17, 573–580. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Park, W.H. Dual-crosslinked, self-healing and thermo-responsive methylcellulose/chitosan oligomer copolymer hydrogels. Carbohydr. Polym. 2021, 258, 117705. [Google Scholar] [CrossRef] [PubMed]
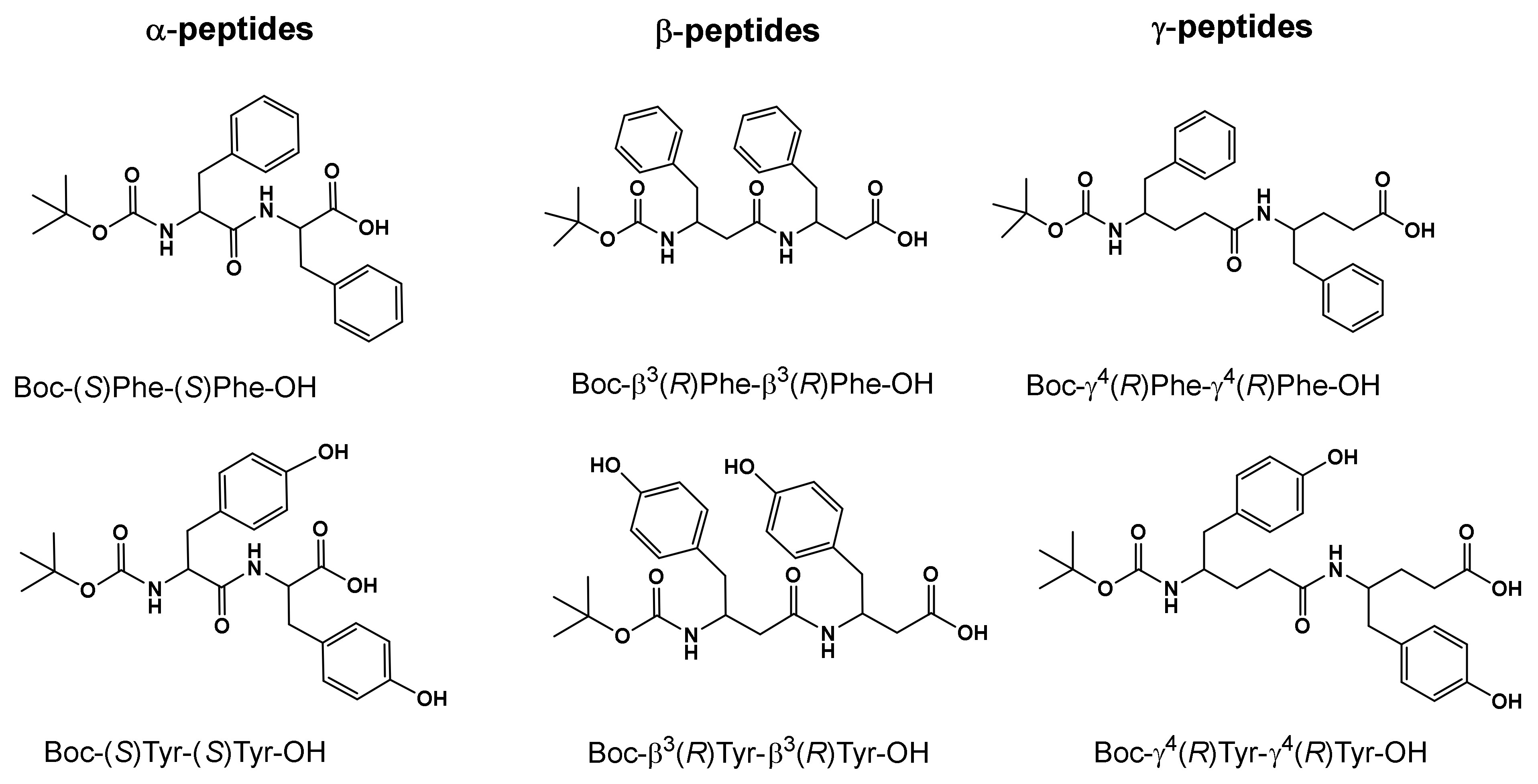



| Boc-α(S)Phe-α(S)Phe-OH | Boc-β3(R)Phe-β3(R)Phe-OH | Boc-γ4(R)Phe-γ4(R)Phe-OH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Aspect | Gelation duration | Stability in time | Aspect | Gelation duration | Stability in time | Aspect | Gelation duration | Stability in time | |
| T = 4 °C | Viscous liquid | / | / | Heterogenous gel | 13 h | yes | Homogenous gel | <5 min | yes |
| Ambient | no | ||||||||
| T = 37 °C | Homogenous gel | 15 h | no | ||||||
| pH = 4 | Heterogenous gel | 2 h | no | Heterogeneous gel | 24 h | yes | Homogenous gel | <5 min | yes |
| pH = 7 | 15 h | Heterogenous viscous liquid | / | / | |||||
| pH = 11 | 15 h | / | / | Viscous liquid | / | / | |||
| 2.45 mM | Homogenous gel | 21 h | no | Heterogenous liquid | / | / | Heterogenous liquid | / | / |
| 9.8 mM | Heterogenous gel | 15 h | no | Heterogeneous gel | 1 h | yes | Homogenous gel | <5 min | yes |
| 2% HFIP | Heterogenous liquid | / | / | Heterogenous liquid | / | / | Heterogenous liquid | / | / |
| 2% MeOH | Heterogenous liquid | / | / | Heterogenous gel | 24 h | yes | Heterogenous liquid | / | / |
| 5% DMSO | / | / | / | Heterogenous gel | 1 h | no | / | / | / |
| 10% DMSO | / | / | / | Heterogenous gel | 5 min | yes | / | / | / |
| NaCl | Heterogenous liquid | / | / | Heterogenous liquid | / | / | Heterogenous liquid | / | / |
| CaCl2 | / | / | / | / | / | ||||
| pH switch | Homogenous gel | 7 h | 1 day | Homogenous gel | <1 min | yes | Homogenous gel | <1 h | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilbaud-Chéreau, C.; Dinesh, B.; Wagner, L.; Chaloin, O.; Ménard-Moyon, C.; Bianco, A. Aromatic Dipeptide Homologue-Based Hydrogels for Photocontrolled Drug Release. Nanomaterials 2022, 12, 1643. https://doi.org/10.3390/nano12101643
Guilbaud-Chéreau C, Dinesh B, Wagner L, Chaloin O, Ménard-Moyon C, Bianco A. Aromatic Dipeptide Homologue-Based Hydrogels for Photocontrolled Drug Release. Nanomaterials. 2022; 12(10):1643. https://doi.org/10.3390/nano12101643
Chicago/Turabian StyleGuilbaud-Chéreau, Chloé, Bhimareddy Dinesh, Laurène Wagner, Olivier Chaloin, Cécilia Ménard-Moyon, and Alberto Bianco. 2022. "Aromatic Dipeptide Homologue-Based Hydrogels for Photocontrolled Drug Release" Nanomaterials 12, no. 10: 1643. https://doi.org/10.3390/nano12101643
APA StyleGuilbaud-Chéreau, C., Dinesh, B., Wagner, L., Chaloin, O., Ménard-Moyon, C., & Bianco, A. (2022). Aromatic Dipeptide Homologue-Based Hydrogels for Photocontrolled Drug Release. Nanomaterials, 12(10), 1643. https://doi.org/10.3390/nano12101643









