Hydrothermal Synthesis of MnWO4@GO Composite as Non-Precious Electrocatalyst for Urea Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Electrocatalysts
2.2. Electrode Preparation
3. Results and Discussion
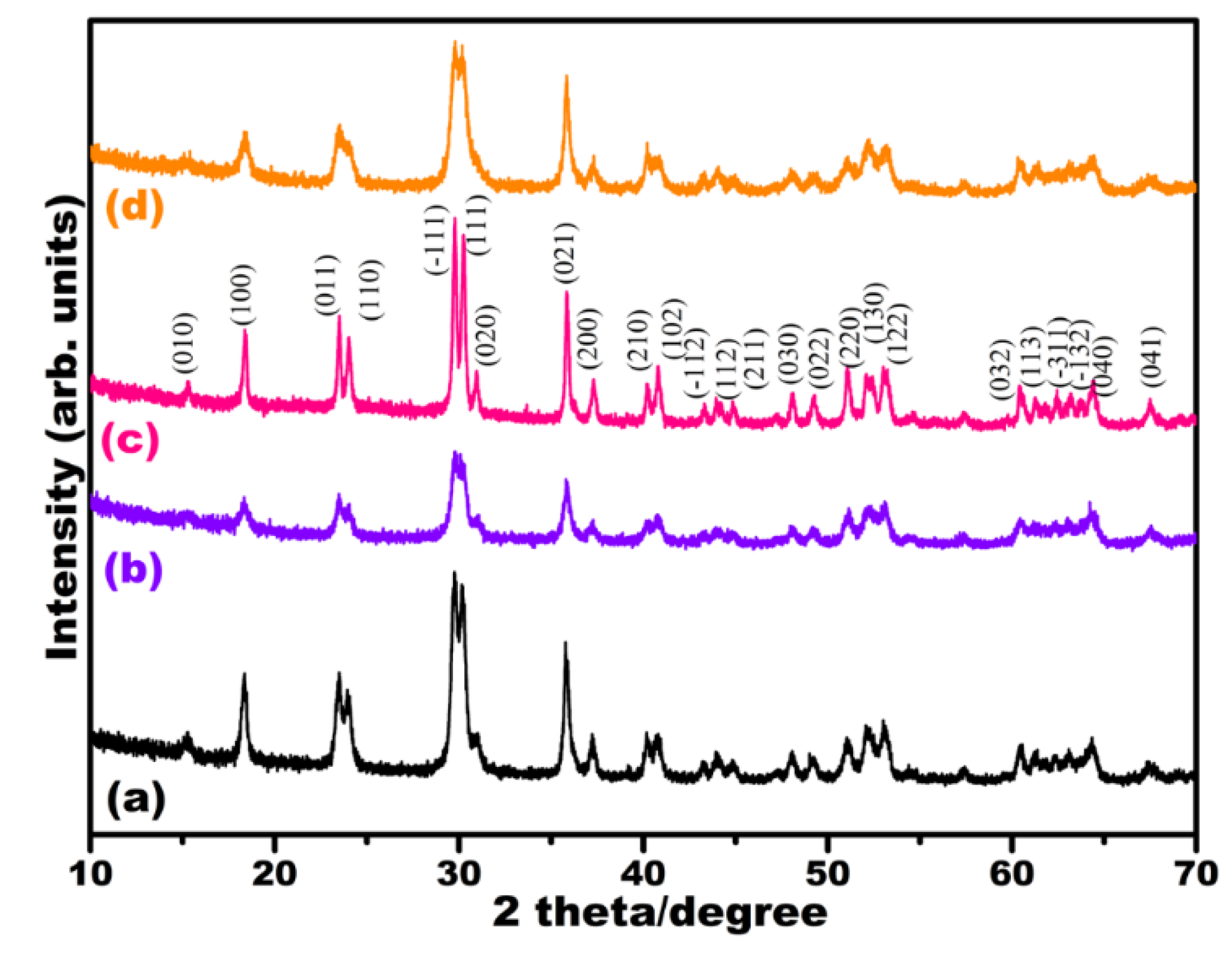
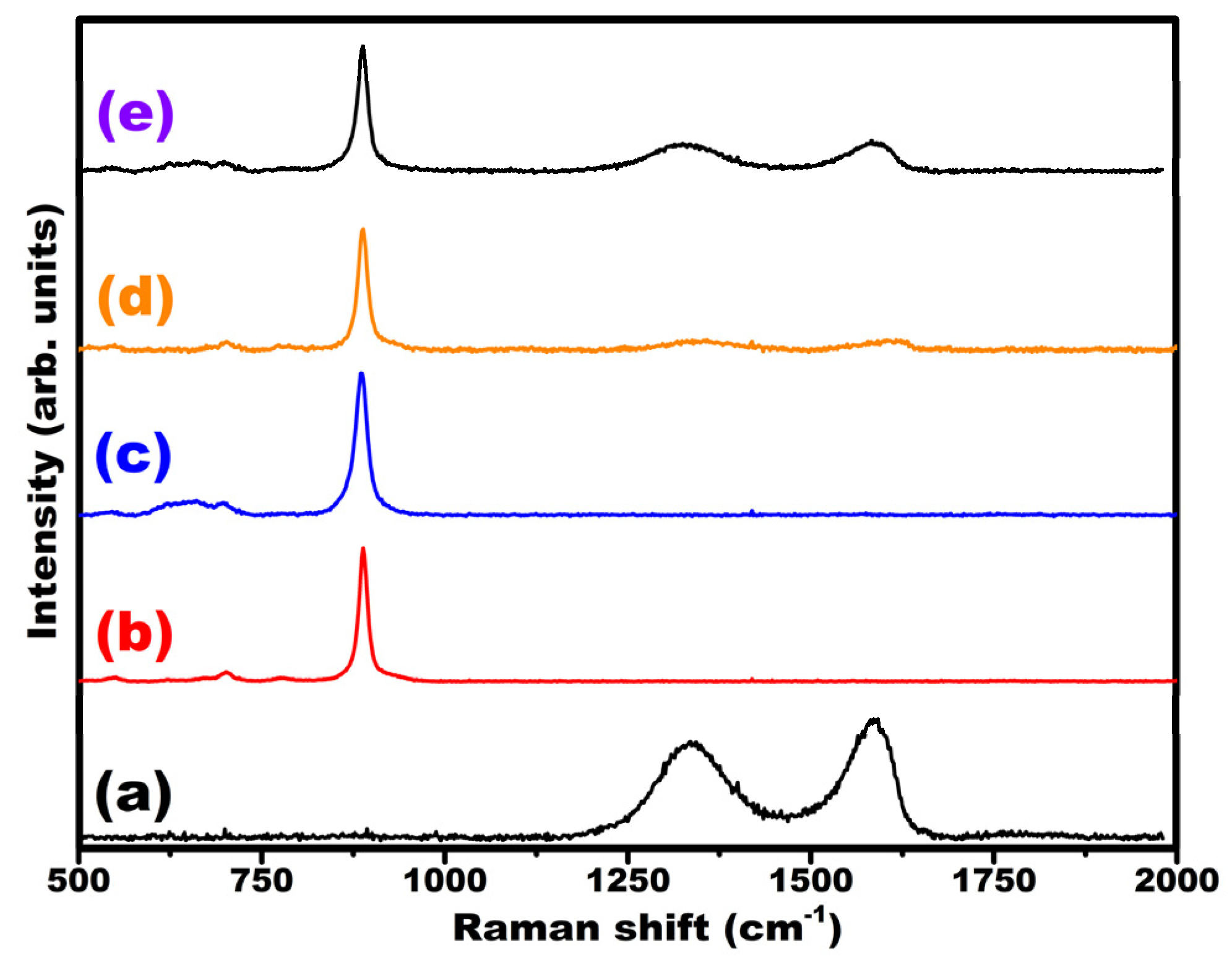
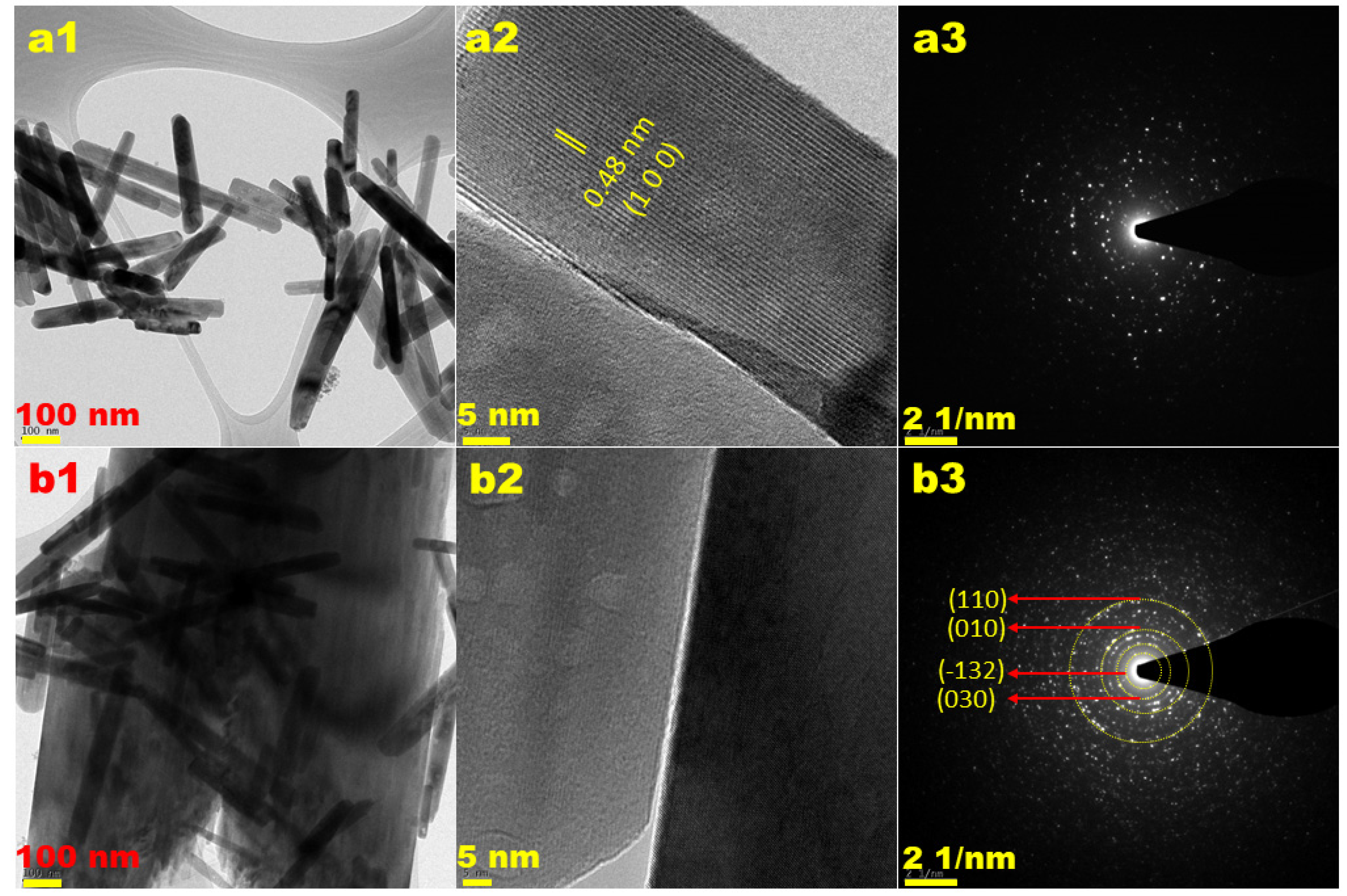
3.1. Characterization of Electrocatalysts
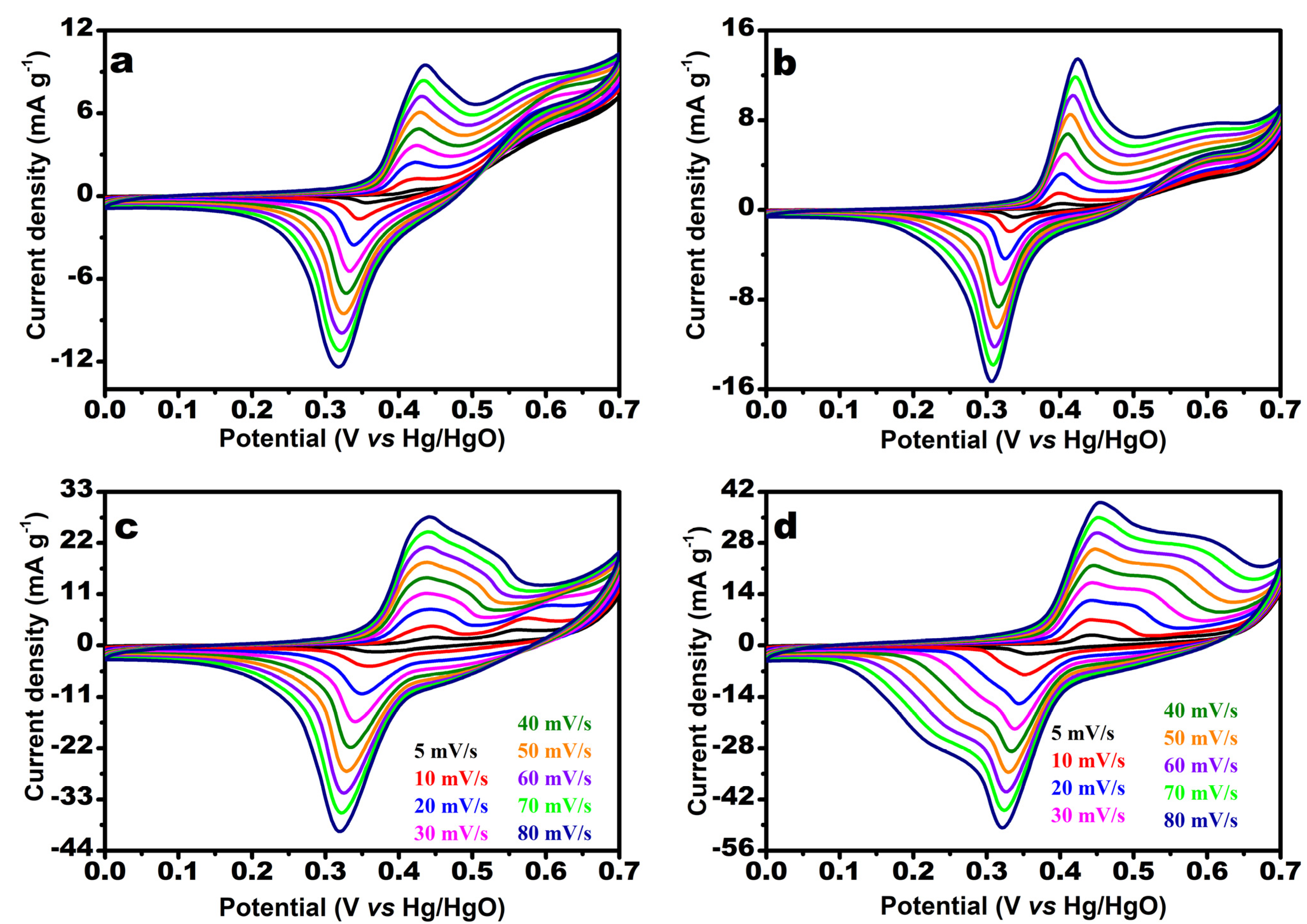
3.2. Urea Oxidation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Preparation of nickel nanowire arrays electrode for urea electrooxidation in alkaline medium. J. Power Sources 2015, 278, 562–568. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Prakasam, T. Electrocatalytic properties of 3D hierarchical graphitic carbon–cobalt nanoparticles for urea oxidation. ACS Omega 2020, 5, 26038–26048. [Google Scholar] [CrossRef]
- Wang, S.; Xu, P.; Tian, J.; Liu, Z.; Feng, L. Phase structure tuning of graphene supported Ni-NiO nanoparticles for enhanced urea oxidation performance. Electrochim. Acta 2021, 370, 137755. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.; Shi, W.; Xu, Q.; Wang, L.; Jiang, H.; Yang, Z.; Liu, E. Mesoporous Ni-P nanocatalysts for alkaline urea electrooxidation. Electrochim. Acta 2016, 222, 455–462. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a hydrogen carrier: A perspective on its potential for safe, sustainable and long-term energy supply. Energy Environ. Sci. 2014, 4, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, Z.; Ren, S.Y.; Xu, J.; Wang, C.; Hu, P.; Fu, J.J. Molecular engineering of a colorless, extremely tough, superiorly self-recoverable, and healable poly(urethane-urea) elastomer for impact-resistant applications. Mater. Horiz. 2021, 8, 2238–2250. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Q.; Wang, K.; Lee, D.; Qiu, W.; Wang, J. Urea hydrolysis and recovery of nitrogen and phosphorus as MAP from stale human urine. J. Environ. Sci. 2008, 20, 1018–1024. [Google Scholar] [CrossRef]
- Kameda, T.; Horikoshi, K.; Kumagai, S.; Saito, Y.; Yoshioka, T. Adsorption of urea, creatinine, and uric acid from three solution types using spherical activated carbon and its recyclability. Chin. J. Chem. Eng. 2020, 28, 2993–3001. [Google Scholar] [CrossRef]
- Zaher, A.; Shehata, N. Recent advances and challenges in management of urea wastewater: A mini review. IOP Conf. Series Mater. Sci. Eng. 2021, 1046, 012021. [Google Scholar] [CrossRef]
- Rittstieg, K.; Robra, K.H.; Somitsch, W. Aerobic treatment of a concentrated urea wastewater with simultaneous stripping of ammonia. Appl. Microbiol. Biotechnol. 2001, 56, 820–825. [Google Scholar] [CrossRef]
- Zequine, C.; Wang, F.; Li, X.; Guragain, D.; Mishra, S.R.; Siam, K.; Kahol, P.K.; Gupta, R.K. Nanosheets of CuCo2O4 as a high-performance electrocatalyst in urea oxidation. Appli. Sci. 2019, 9, 793. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co-P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Muthamizh, S.; Suresh, R.; Giribabu, K.; Manigandan, R.; Praveen Kumar, S.; Munusamy, S.; Narayanan, V. MnWO4 nanocapsules: Synthesis, characterization and electrochemical sensing property. J. Alloys Compd. 2015, 619, 601–609. [Google Scholar] [CrossRef]
- Wu, K.; Fu, P.; Ruan, B.; Wu, M.; Wu, M.; Wu, R. Performances of MnWO4@AC mixed oxide composite materials as Pt-free counter electrodes for high efficiency dye sensitized solar cells. New J. Chem. 2021, 45, 1686–1694. [Google Scholar] [CrossRef]
- Ramasamy Raja, V.; Karthika, A.; Suganthi, A.; Rajarajan, M. Facile synthesis of MnWO4/BiOI nanocomposites and their efficient photocatalytic and photoelectrochemical activities under the visible-light irradiation. J. Sci. Adv. Mater. Devices 2018, 3, 331–341. [Google Scholar] [CrossRef]
- Mostafa, K.; Davar, M.B.; Mojtaba, A. Synthesis, structural characterization and reactivity of manganese tungstate nanoparticles in the oxidative degradation of methylene blue. Compts Rendus Chim. 2015, 18, 199–203. [Google Scholar]
- Wu, W.; Qin, W.; He, Y.; Wu, Y.; Wu, T. The effect of pH value on the synthesis and photocatalytic performance of MnWO4 nanostructure by hydrothermal method. J. Exp. NanoSci. 2012, 7, 390–398. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V.; Nagaiah, T.C. Tuning the MnWO4 morphology and its electrocatalytic activity towards oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2681–2692. [Google Scholar] [CrossRef]
- Trung, D.D.; Cuong, N.D.; Trung, K.Q.; Nguyen, T.D.; Toan, N.V.; Hung, C.M.; Hieu, N.V. Controlled synthesis of manganese tungstate nanorods for highly selective NH3 gas sensor. J. Alloys Compd. 2018, 735, 787–794. [Google Scholar] [CrossRef]
- Naik, K.K.; Gangan, A.S.; Pathak, A.; Chakraborty, B.; Nayak, S.K.; Rout, C.S. Facile hydrothermal synthesis of MnWO4 nanorods for non-enzymatic glucose sensing and supercapacitor properties with insights from density functional theory simulations. ChemistrySelect 2017, 2, 5707–5715. [Google Scholar] [CrossRef]
- Kang, R.; Zheng, L.; Tong, W.; Zhuangjun, F. Recent developments of transition metal compounds-carbon hybrid electrodes for high energy/power supercapacitors. Nano Micro Lett. 2021, 13, 129. [Google Scholar]
- Malkhandi, S.; Trinh, P.; Aswin, K.M.; Jayachandrababu, K.C.; Kindler, A.; Surya Prakash, G.K.; Narayanan, S.R. Electrocatalytic activity of transition metal oxide-carbon composites for oxygen reduction in alkaline batteries and fuel cells. J. Electrochem. Soc. 2013, 160, F943. [Google Scholar] [CrossRef]
- Hyun-Woo, S.; Ah-Hyeon, L.; Jae-Chen, K.; Gwang-Hee, L.; Dong-Wan, K. Hydrothermal realization of a hierarchical, flowerlike MnWO4@MWCNTs nanocomposites with enhanced reversible Li storage as a new anode material. Chem. Asian J. 2013, 8, 2851–2858. [Google Scholar]
- Anusha, V.; Eberechukwu, V.M.; Yingduo, C.; Chris, P. Carbon nanotube assembly and integration for applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar]
- Huang, L.; Yang, H.; Zhang, Y.; Xiao, W. Study on synthesis and antibacterial properties of AgNPs/GO nanocomposites. J. Nanomater. 2016, 2016, 5685967. [Google Scholar] [CrossRef] [Green Version]
- Mallick, S.; Samanta, A.; Retna Raj, C. Mesoporous carbon-supported manganese tungstate nanostructures for the development of zinc-air battery powered long lifespan asymmetric supercapacitor. Sustain. Energy Fuels 2020, 4, 4008–4017. [Google Scholar] [CrossRef]
- Sardar, K.; Thakur, S.; Maiti, S.; Besra, N.; Bairi, P.; Chandra, K.; Majumdhar, G.; Chattopadhyay, K.K. Amalgamation of MnWO4 nanorods with amorphous carbon nanotubes for highly stabilized energy efficient supercapacitor electrodes. Dalton Trans. 2021, 50, 5327–5347. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yixue, Z.; Puqun, X.; Mengqing, Z.; Huixia, W.; Shiping, Y. Graphene oxide/MnWO4 nanocomposite for magnetic resonance/photoacoustic dual-model imaging and tumor photothermochemotherapy. Carbon 2018, 138, 397–409. [Google Scholar]
- Jianhua, T.; Jianfeng, S.; Na, L.; Mingxin, Y. Facile synthesis of layered MnWO4/ reduced graphene oxide for supercapacitor applications. J. Alloys Compd. 2016, 666, 15–22. [Google Scholar]
- Dubale, A.A.; Su, W.N.; Tamirat, A.G.; Pan, C.J.; Aragaw, B.A.; Chen, H.M.; Chen, C.H.; Hwang, B.J. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting. J. Mater. Chem. A 2014, 2, 18383–18397. [Google Scholar] [CrossRef]
- Ahn, S. Changing the sp2 carbon clusters in graphene oxide during exfoliation. Trans. Electr. Electron. Mat. 2015, 16, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.T.; Van, N.N. rGO/persulfate metal-free catalytic system for the degradation of tetracycline: Effect of reaction parameters. Mater. Res. Express 2020, 7, 075501. [Google Scholar]
- Dai, R.C.; Ding, X.; Wang, Z.P.; Zhang, Z.M. Pressure and temperature dependence of Raman scattering of MnWO4. Chem. Phys. Lett. 2013, 586, 76–80. [Google Scholar] [CrossRef]
- Iliev, M.N.; Gospodinov, M.M.; Litvinchuk, A.P. Raman spectroscopy of MnWO4. Phys. Rev. B 2009, 80, 212302. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yi, S.S.; Hwang, D.H.; Son, C.S. Structure, luminescence, and magnetic properties of crystalline manganese tungstate doped with rare earth ion. Materials 2021, 14, 3717. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, R.; Mariyappan, V.; Chen, S.M.; Keerthi, M.; Ramachandran, R. Electrochemical sensor for detection of tryptophan in the milk sample based on MnWO4 nanoplates encapsulated RGO nanocomposite. Colloid Surf. A 2021, 625, 126889. [Google Scholar] [CrossRef]
- Teeparthi, S.R.; Awin, E.W.; Kumar, R. Dominating role of crystal structure over defect chemistry in black and white zirconia on visible light photocatalytic activity. Sci. Rep. 2018, 8, 5541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulga, Y.M.; Baskakov, S.A.; Knerelman, E.I.; Davidova, G.I.; Badamshina, E.R.; Shulga, N.Y.; Skryleva, E.A.; Agapov, A.L.; Voylov, D.N.; Sokolov, A.P.; et al. Carbon nanomaterial produced by microwave exfoliation of graphite oxide: New insights. RSC Adv. 2014, 4, 587–592. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Tamilselvan, M.; Yoo, K.; Kim, J. Microwave-assisted in situ growth of VO2 nanoribbons on Ni foam as inexpensive bifunctional electrocatalyst for the methanol oxidation and oxygen evolution reactions. Appl. Surf. Sci. 2021, 570, 151119. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Zadeh, M.H.R.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Dillip, G.R.; Nagajyothi, P.C.; Yoo, K.; Kim, J. Integration of Marigold 3D flower-like Ni-MOF self-assembled on MWCNTs via microwave irradiation for high-performance electrocatalytic alcohol oxidation and oxygen evolution reactions. Appl. Catal. B Environ. 2021, 285, 119793. [Google Scholar] [CrossRef]
- Imanzadeh, H.; Habibi, B. Electrodeposition of ternary CuNiPt alloy nanoparticles on graphenized pencil lead electrode as a new electrocatalyst for electro-oxidation of ethanol. Solid State Sci. 2020, 105, 106239. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagajyothi, P.C.; Yoo, K.; Ramaraghavulu, R.; Shim, J. Hydrothermal Synthesis of MnWO4@GO Composite as Non-Precious Electrocatalyst for Urea Oxidation. Nanomaterials 2022, 12, 85. https://doi.org/10.3390/nano12010085
Nagajyothi PC, Yoo K, Ramaraghavulu R, Shim J. Hydrothermal Synthesis of MnWO4@GO Composite as Non-Precious Electrocatalyst for Urea Oxidation. Nanomaterials. 2022; 12(1):85. https://doi.org/10.3390/nano12010085
Chicago/Turabian StyleNagajyothi, Patnamsetty Chidanandha, Kisoo Yoo, Rajavaram Ramaraghavulu, and Jaesool Shim. 2022. "Hydrothermal Synthesis of MnWO4@GO Composite as Non-Precious Electrocatalyst for Urea Oxidation" Nanomaterials 12, no. 1: 85. https://doi.org/10.3390/nano12010085
APA StyleNagajyothi, P. C., Yoo, K., Ramaraghavulu, R., & Shim, J. (2022). Hydrothermal Synthesis of MnWO4@GO Composite as Non-Precious Electrocatalyst for Urea Oxidation. Nanomaterials, 12(1), 85. https://doi.org/10.3390/nano12010085





