Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of the Upland Cress Extract
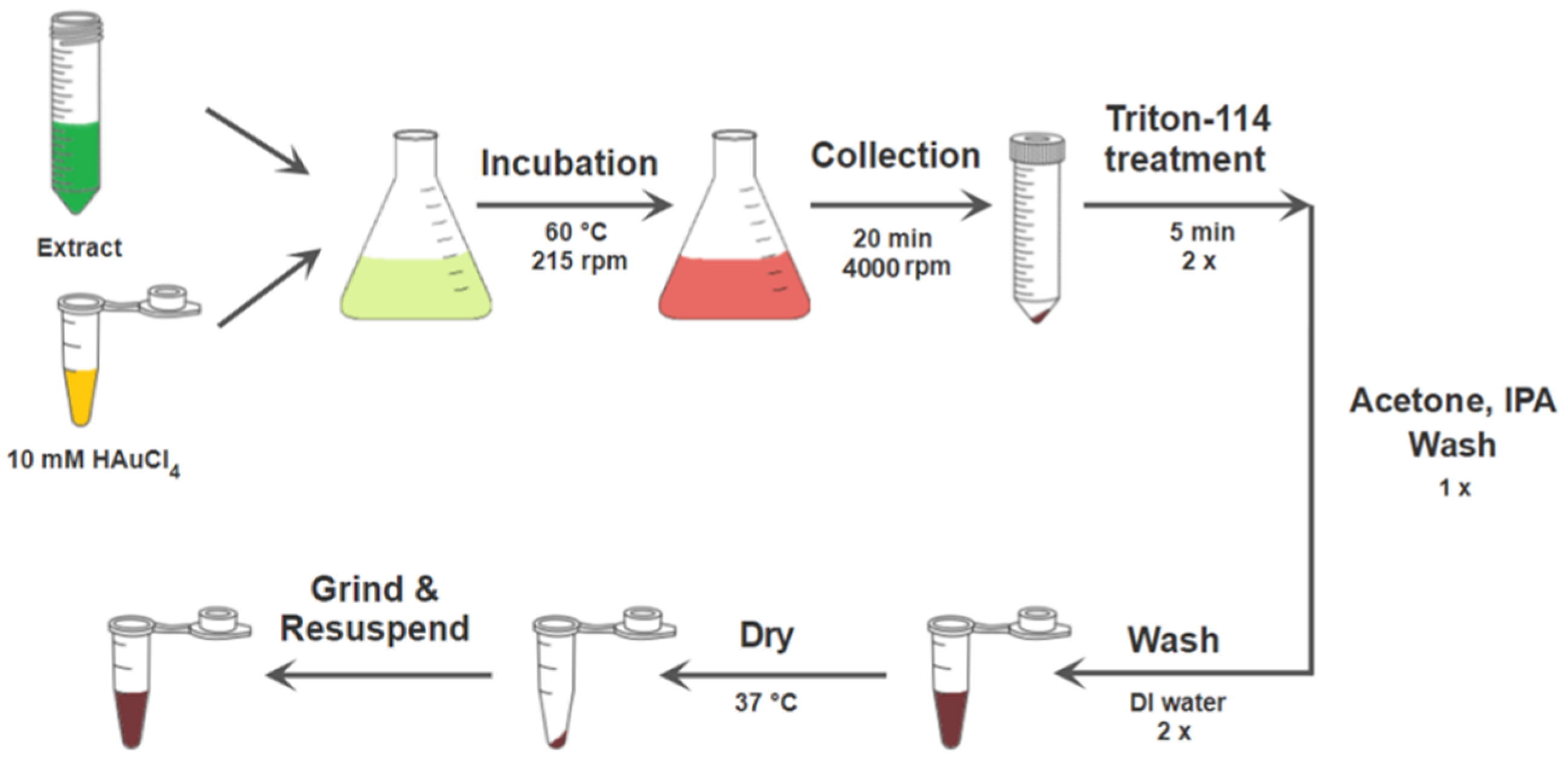
2.3. Green Synthesis of Au NPs
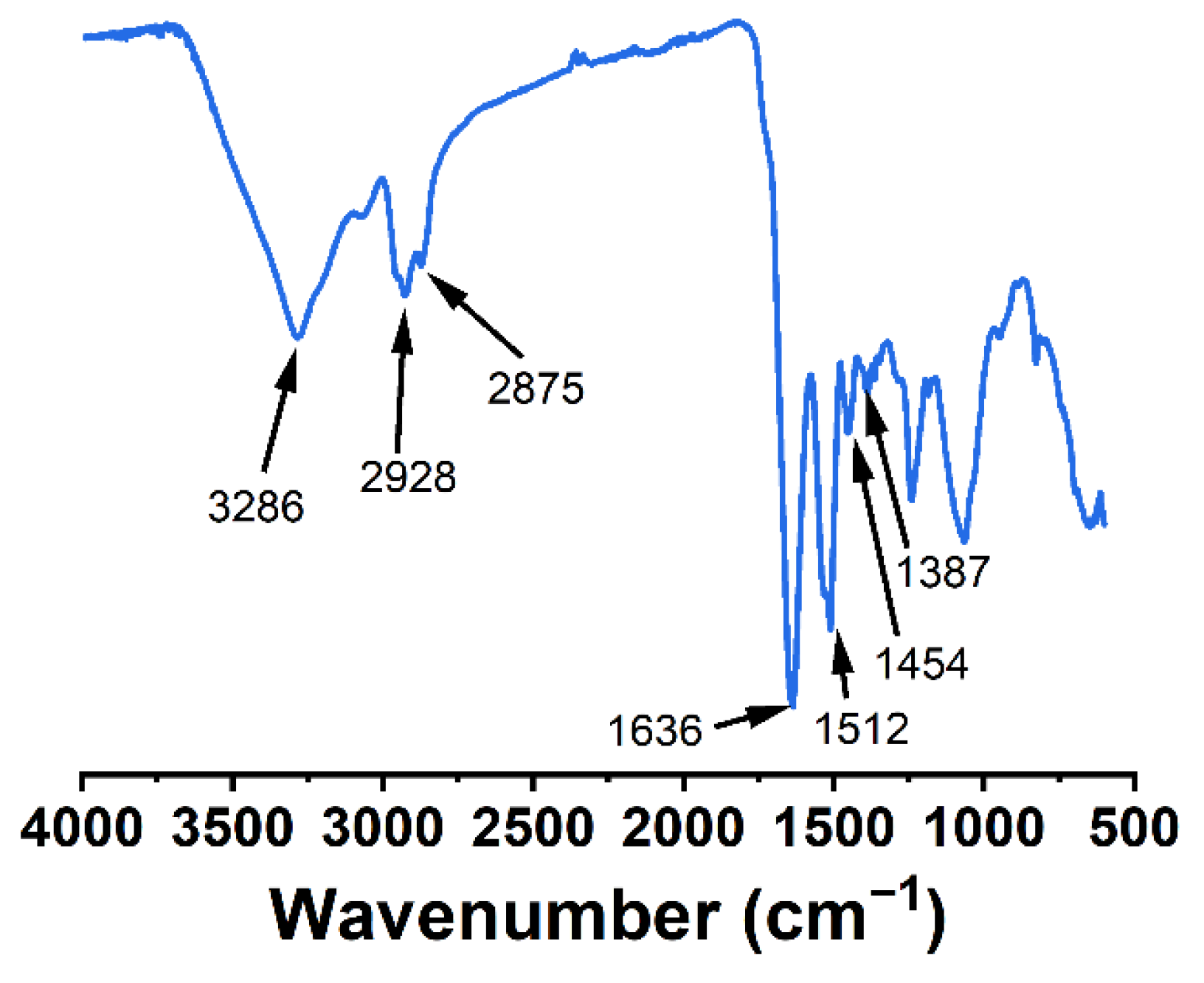
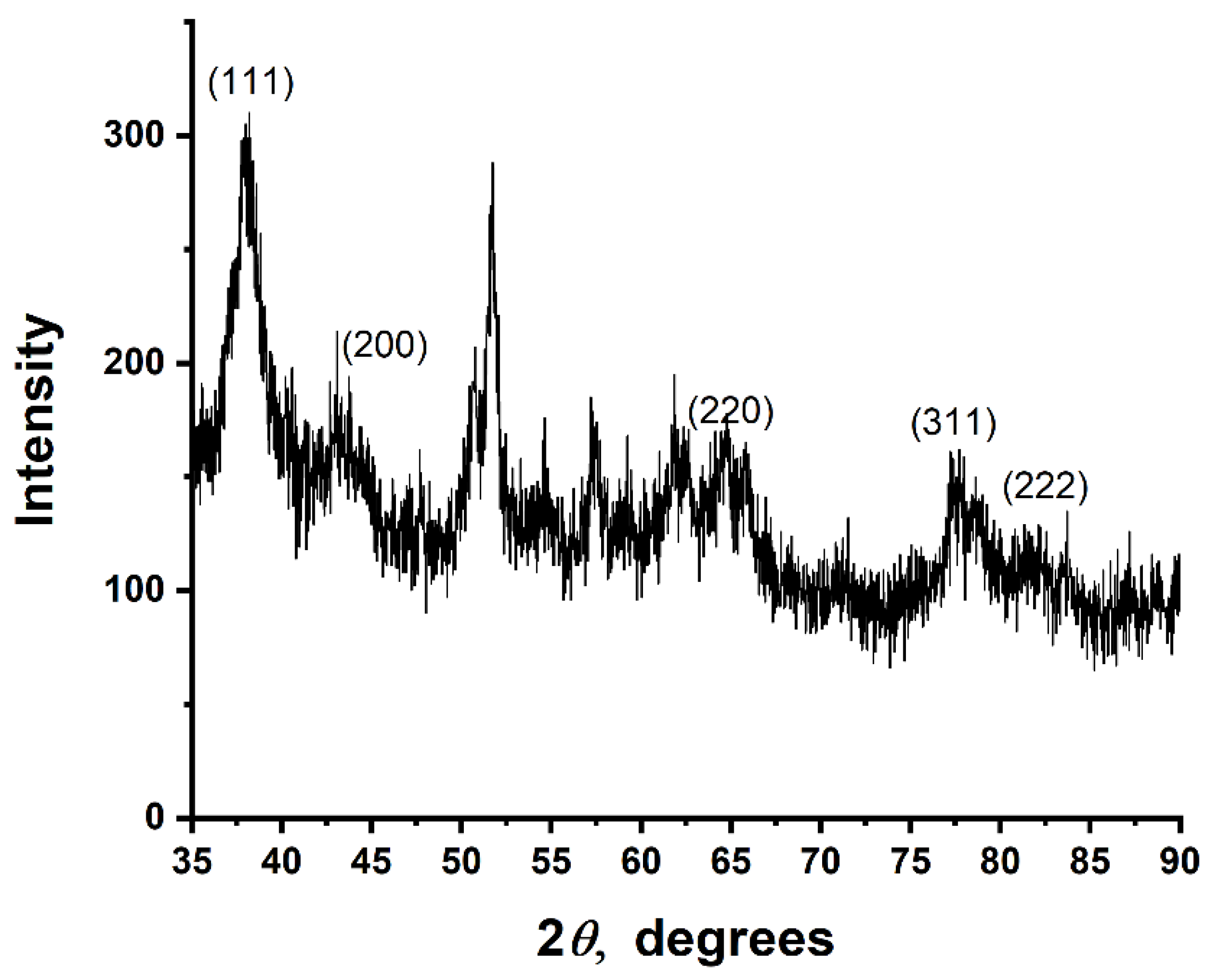
2.4. Characterization of Au NPs
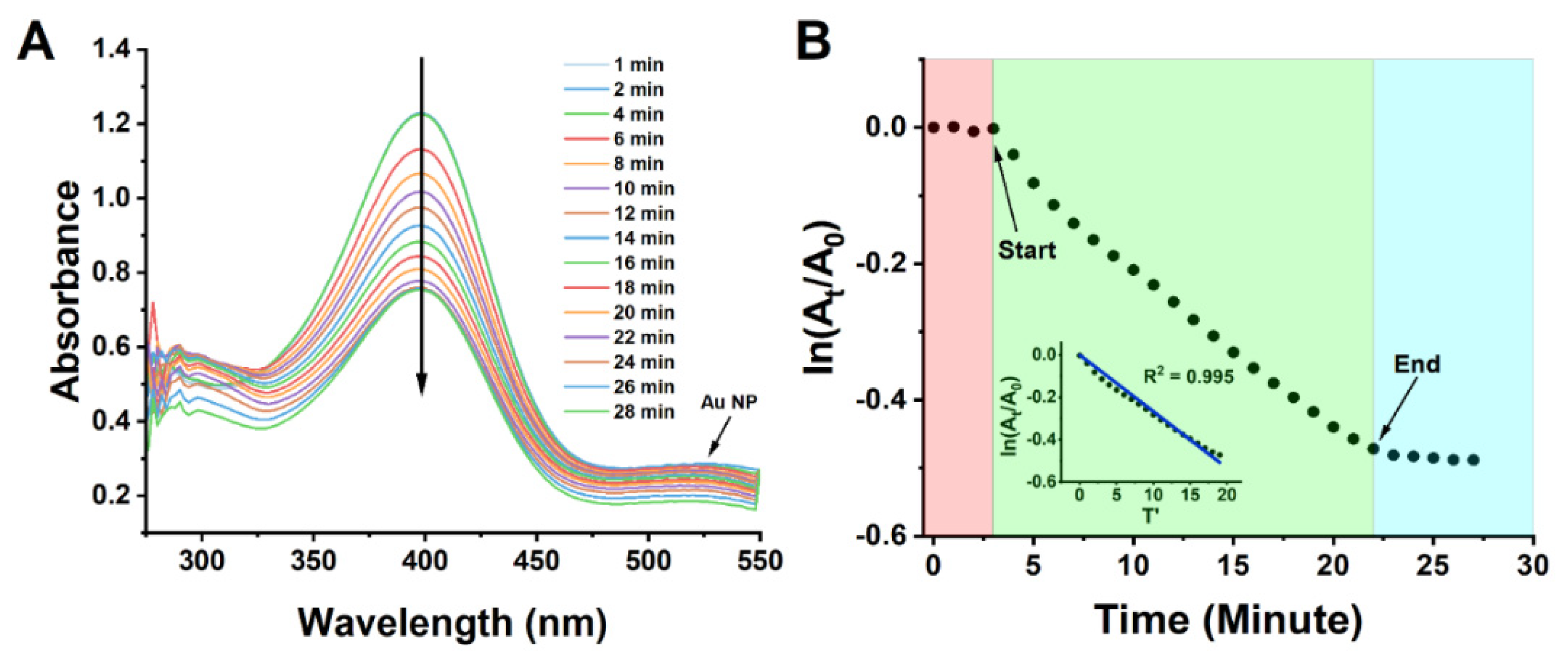
2.5. Catalysis of the Reduction of 4-Nitrophenol
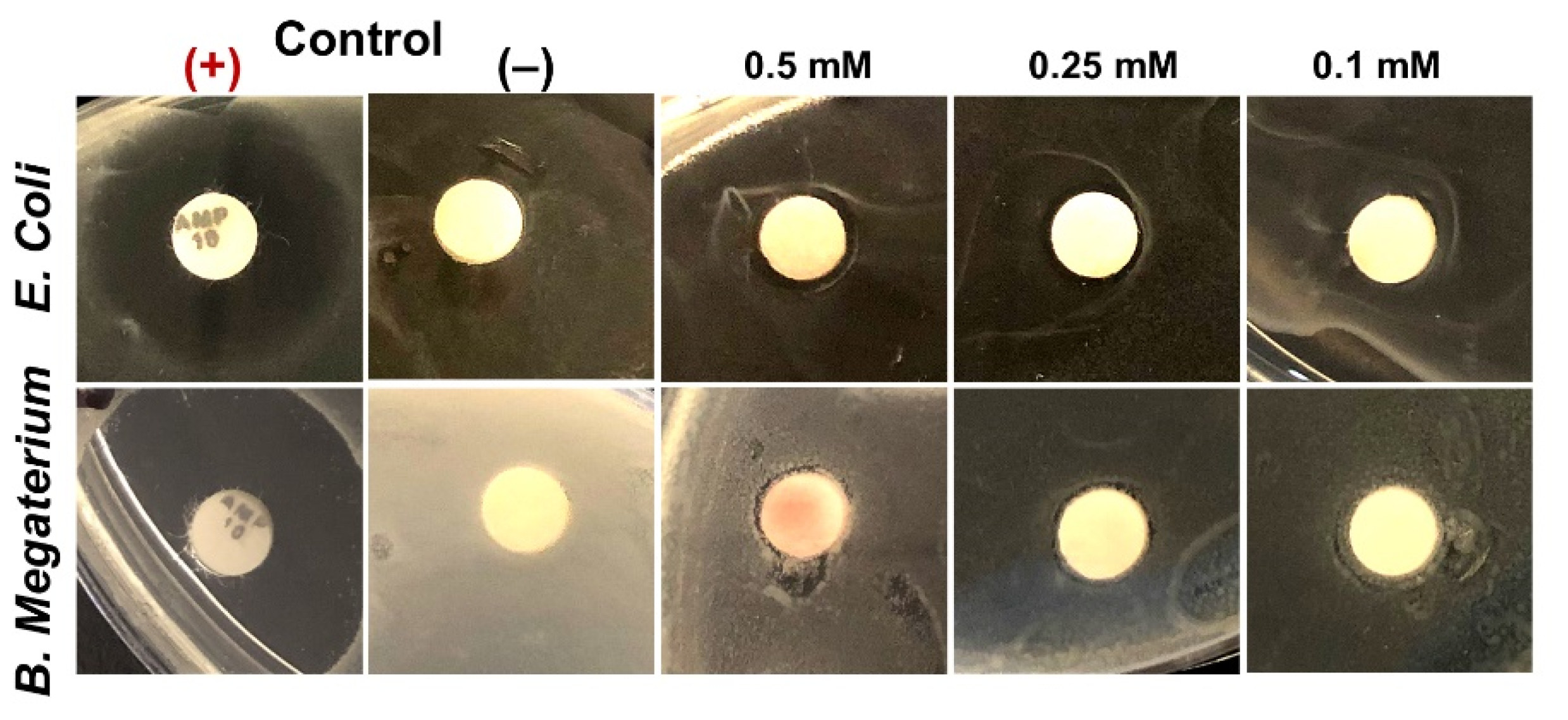
2.6. Antibacterial Activity Testing
2.7. Cytotoxicity and Cellular Uptake Studies
3. Results and Discussion
3.1. Total Phenolic and Ascorbic Acid Content
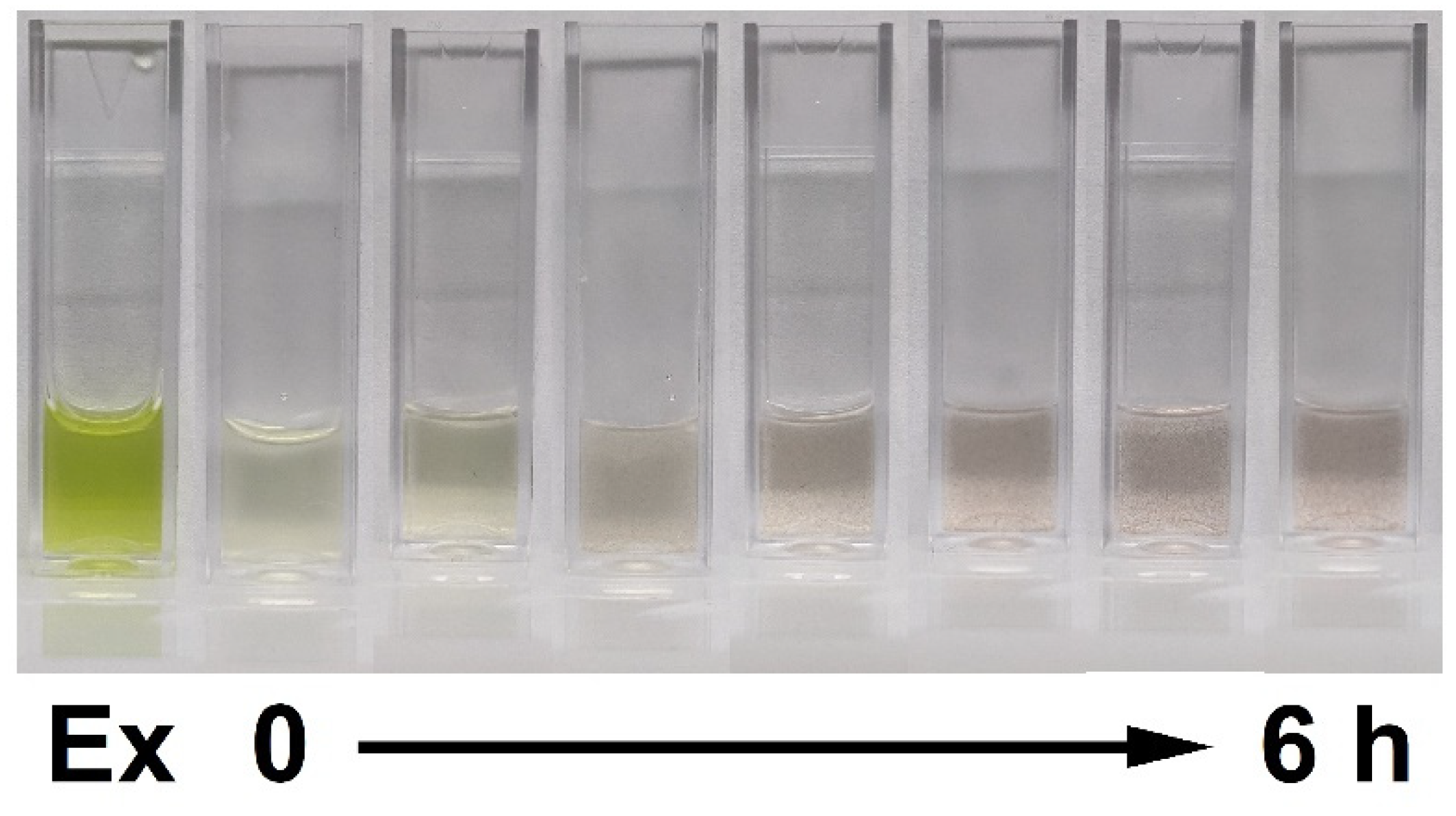
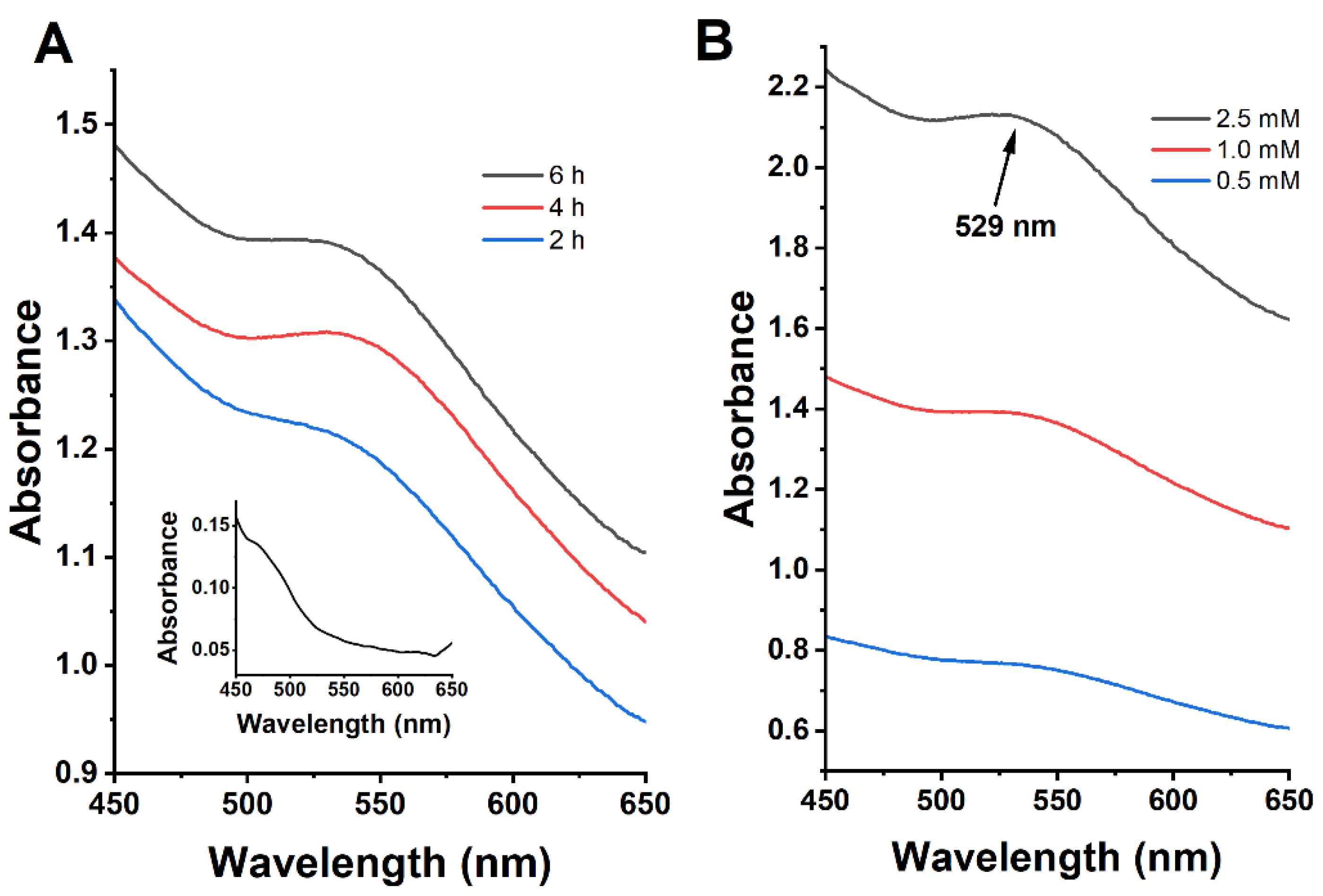
3.2. Effects of Incubation Time on the Green Synthesis Process
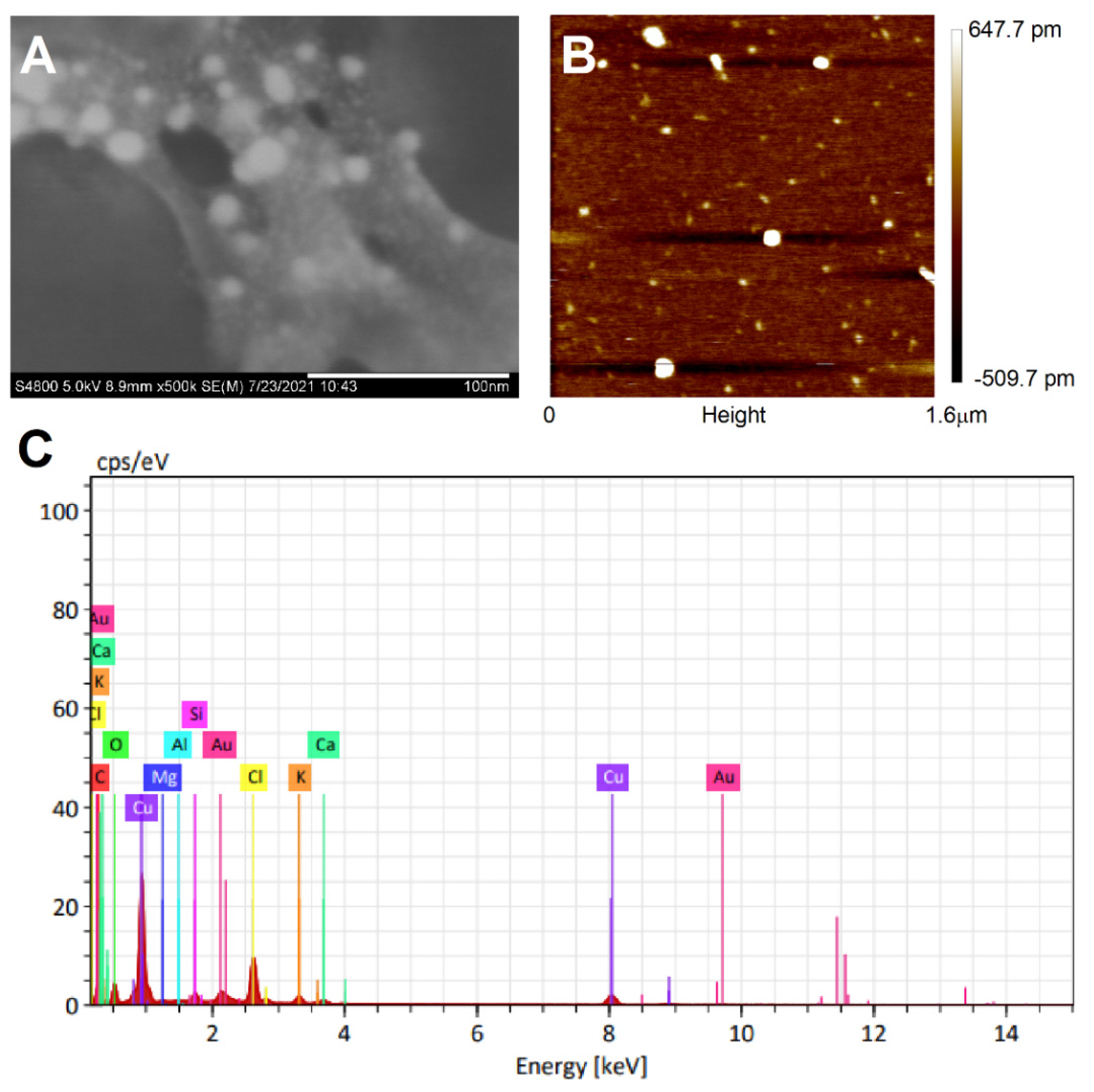
3.3. Morphology, Chemical Composition, and Surface Charge
3.4. Crystal Structure of Au NPs
3.5. Catalysis of the Reduction of 4-Nitrophenol
3.6. Antibacterial Activity
3.7. Cytotoxicity and Cellular Uptake
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Keijok, W.J.; Pereira, R.H.A.; Alvarez, L.A.C.; Prado, A.R.; da Silva, A.R.; Ribeiro, J.; de Oliveira, J.P.; Guimarães, M.C.C. Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci. Rep. 2019, 9, 16019. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arabian J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 14; pp. 411–446. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of gold nanoparticles using mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Navyatha, B.; Nara, S. Gold nanotheranostics: Future emblem of cancer nanomedicine. Nanobiomedicine 2021, 8, 18495435211053945. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.-D.; Thieu, A.T.; Nguyen, T.-D.; Nguyen, V.-C.; Cao, X.-T.; Nguyen, T.L.-H.; Le, V.T. Biosynthesis of gold nanoparticles using litsea cubeba fruit extract for catalytic reduction of 4-nitrophenol. J. Nanomater. 2020, 2020, 4548790. [Google Scholar] [CrossRef]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Aljabali, A.A.A.; Akkam, Y.; Al Zoubi, M.S.; Al-Batayneh, K.M.; Al-Trad, B.; Abo Alrob, O.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of gold nanoparticles using leaf extract of ziziphus zizyphus and their antimicrobial activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhassan-Moghaddam, M.; Zarghami, N.; Mohsenifar, A.; Rahmati-Yamchi, M.; Gholizadeh, D.; Akbarzadeh, A.; de la Guardia, M.; Nejati-Koshki, K. Watercress-based gold nanoparticles: Biosynthesis, mechanism of formation and study of their biocompatibility in vitro. Micro Nano Lett. 2014, 9, 345–350. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; diCenzo, G.C.; Gil, W.; Bojszczak, W.; Motyka, A.; Pogoda, D.; Pohl, P. Fermented juices as reducing and capping agents for the biosynthesis of size-defined spherical gold nanoparticles. J. Saudi Chem. Soc. 2018, 22, 767–776. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem. Lett. Rev. 2017, 10, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.L.; Wang, Y.; Stealey, S.T.; Alexander, A.K.; Kaltchev, M.G.; Chen, J.; Zhang, W. Biosynthesis of silver nanoparticles using upland cress: Purification, characterisation, and antimicrobial activity. Micro Nano Lett. 2020, 15, 110–113. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin c and antioxidant activity in wasted parts of sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Dioha, I.J.; Olugbemi, O.; Onuegbu, T.; Shahru, Z. Determination of ascorbic acid content of some tropical fruits by iodometric titration. Int. J. Biol. Chem. Sci. 2012, 5, 2180–2184. [Google Scholar] [CrossRef] [Green Version]
- Serrà, A.; Artal, R.; Pozo, M.; Garcia-Amorós, J.; Gómez, E. Simple environmentally-friendly reduction of 4-nitrophenol. Catalysts 2020, 10, 458. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter Inst. Phys. J. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from uv−vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Guo, M.; Li, W.; Yang, F.; Liu, H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 73–79. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Ningaraju, S.; Munawer, U.; Raghavendra, V.B.; Balaji, K.S.; Melappa, G.; Brindhadevi, K.; Pugazhendhi, A. Chaetomium globosum extract mediated gold nanoparticle synthesis and potent anti-inflammatory activity. Anal. Biochem. 2021, 612, 113970. [Google Scholar] [CrossRef] [PubMed]
- Amargeetha, A.; Velvan, S. X-ray diffraction (XRD) and Energy Dispersive Spectroscopy (eds) analysis of silver nanoparticles synthesized from Erythrina indica flowers. Nanosci. Technol. Open Access 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on powder x-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [Green Version]
- Iben Ayad, A.; Luart, D.; Ould Dris, A.; Guénin, E. Kinetic analysis of 4-nitrophenol reduction by “water-soluble” palladium nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Bag, B.G.; Ghosh, P. Mimusops elengi bark extract mediated green synthesis of gold nanoparticles and study of its catalytic activity. Appl. Nanosci. 2016, 6, 521–528. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.; Raghuwanshi, V.S.; Wendt, R.; Wollgarten, M.; Hoell, A.; Rademann, K. Gold nanoparticles in novel green deep eutectic solvents: Self-limited growth, self-assembly & catalytic implications. Z. Phys. Chem. 2015, 229, 221–234. [Google Scholar] [CrossRef]
- Thawarkar, S.R.; Thombare, B.; Munde, B.S.; Khupse, N.D. Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles. RSC Adv. 2018, 8, 38384–38390. [Google Scholar] [CrossRef] [Green Version]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thünemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in gold nanoparticle toxicity and uptake: Size, shape, capping ligand, and biological corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.-H.; Li, W.-T.; Hung, W.-I.; Chen, C.-P.; Yeh, J.-M. Cytotoxicity and differentiation effects of gold nanoparticles to human bone marrow mesenchymal stem cells. Biomed. Eng. Appl. Basis Commun. 2011, 23, 141–152. [Google Scholar] [CrossRef]
- Volkova, N.; Pavlovich, O.; Fesenko, O.; Budnyk, O.; Kovalchuk, S.; Goltsev, A. Studies of the influence of gold nanoparticles on characteristics of mesenchymal stem cells. J. Nanomater. 2017, 2017, 6934757. [Google Scholar] [CrossRef] [Green Version]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchinson, N.; Wu, Y.; Wang, Y.; Kanungo, M.; DeBruine, A.; Kroll, E.; Gilmore, D.; Eckrose, Z.; Gaston, S.; Matel, P.; et al. Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment. Nanomaterials 2022, 12, 28. https://doi.org/10.3390/nano12010028
Hutchinson N, Wu Y, Wang Y, Kanungo M, DeBruine A, Kroll E, Gilmore D, Eckrose Z, Gaston S, Matel P, et al. Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment. Nanomaterials. 2022; 12(1):28. https://doi.org/10.3390/nano12010028
Chicago/Turabian StyleHutchinson, Noah, Yuelin Wu, Yale Wang, Muskan Kanungo, Anna DeBruine, Emma Kroll, De’Jorra Gilmore, Zachary Eckrose, Stephanie Gaston, Phoebe Matel, and et al. 2022. "Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment" Nanomaterials 12, no. 1: 28. https://doi.org/10.3390/nano12010028
APA StyleHutchinson, N., Wu, Y., Wang, Y., Kanungo, M., DeBruine, A., Kroll, E., Gilmore, D., Eckrose, Z., Gaston, S., Matel, P., Kaltchev, M., Nickel, A.-M., Kumpaty, S., Hua, X., & Zhang, W. (2022). Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment. Nanomaterials, 12(1), 28. https://doi.org/10.3390/nano12010028









