Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus molle Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis and Characterization of Silver Nanoparticles
2.2. Cell Culture
2.3. MTT Cytotoxicity Assay
2.4. Lactate Dehydrogenase Cytotoxicity Assay
2.5. Cytomorphological Changes Analysis
2.6. Measurement of Intracellular Reactive Oxygen Species (ROS) Using Carboxy-H2DCFDA
2.7. Apoptotic Morphological Changes by Acridine Orange–Ethidium Bromide (AO-EB) Dual Staining Method
2.8. Flow Cytometry Analysis of Apoptosis
2.9. Detection of Autophagy by Acridine Orange Staining
2.10. Alkaline Single Cell Gel Electrophoresis
2.11. Statistical Analysis
3. Results and Discussion
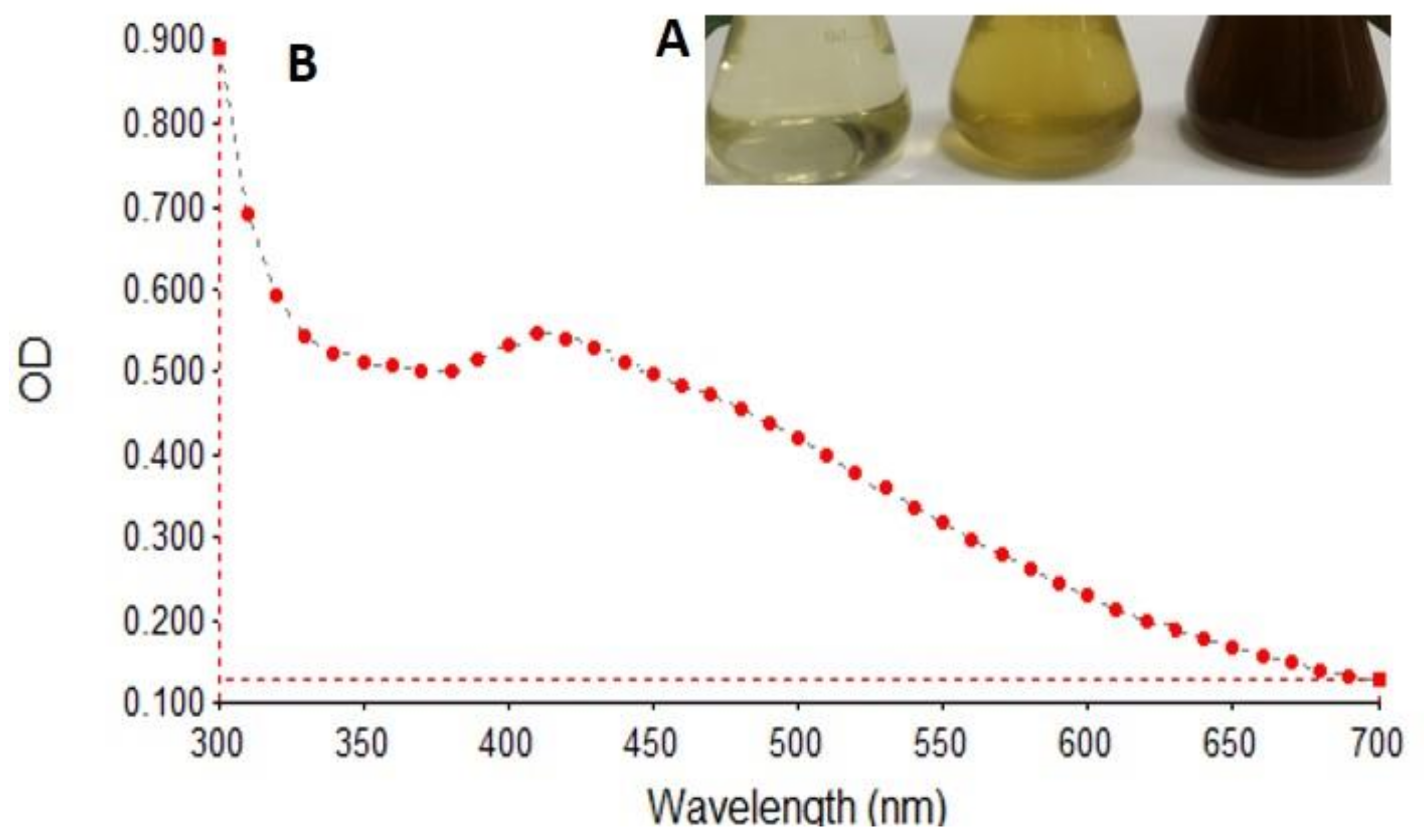
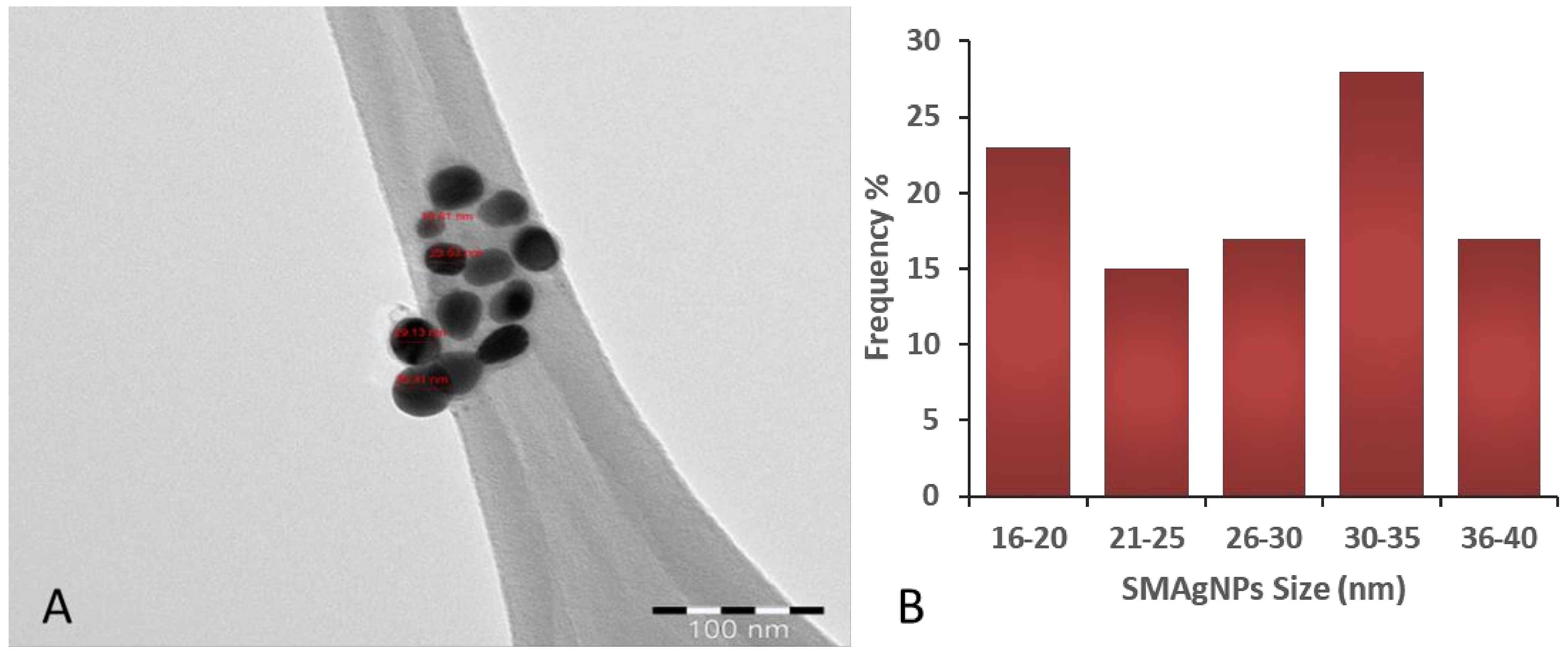
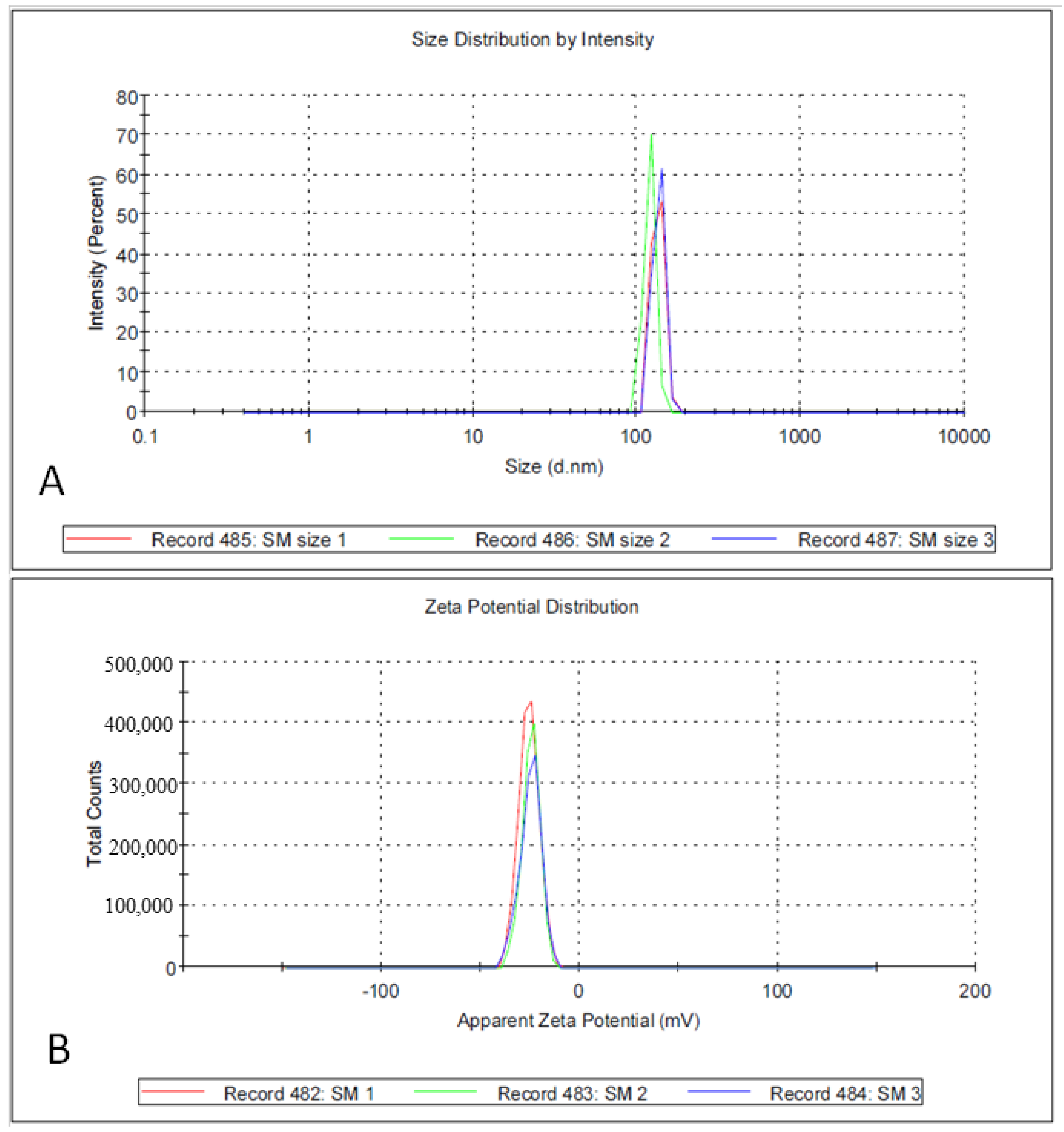
3.1. Biosynthesis and Characterization of AgNPs
3.2. Cytotoxicity Assessment of SMAgNPs
3.3. LDH Release Assay
3.4. The Cytomorphological Alterations
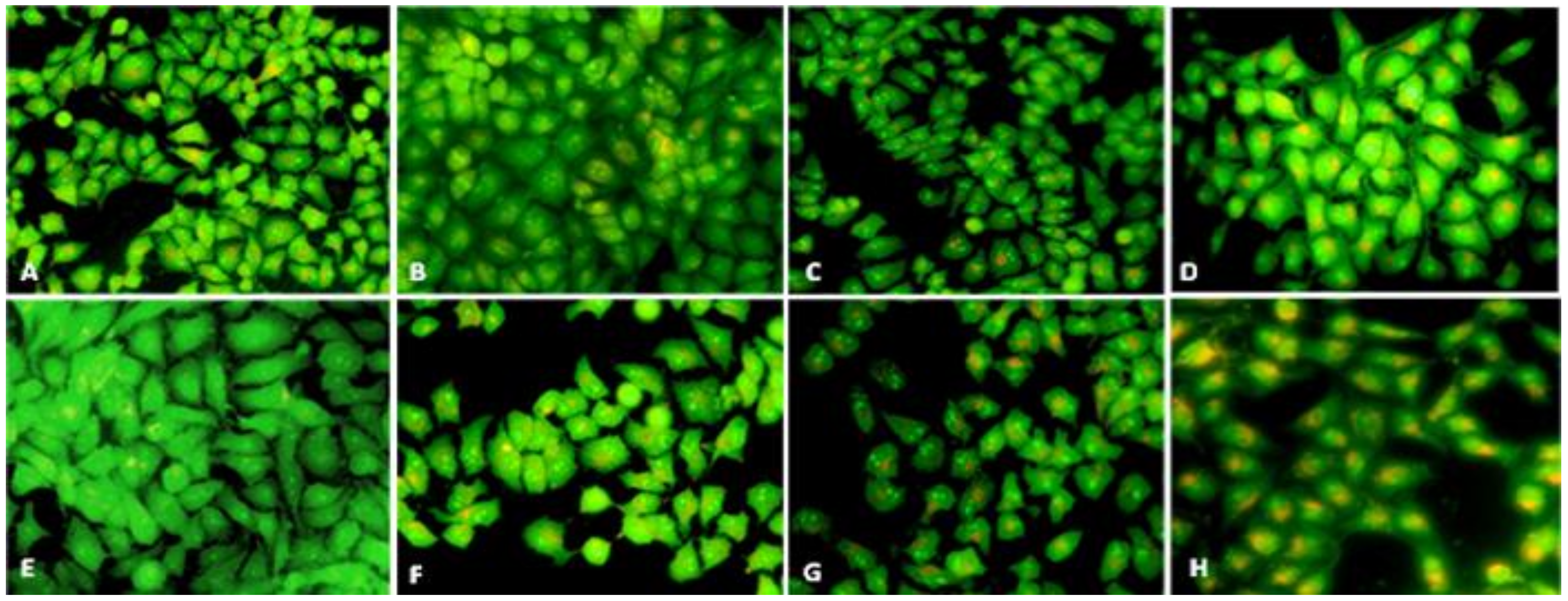
3.5. Estimation of Intracellular ROS Generation
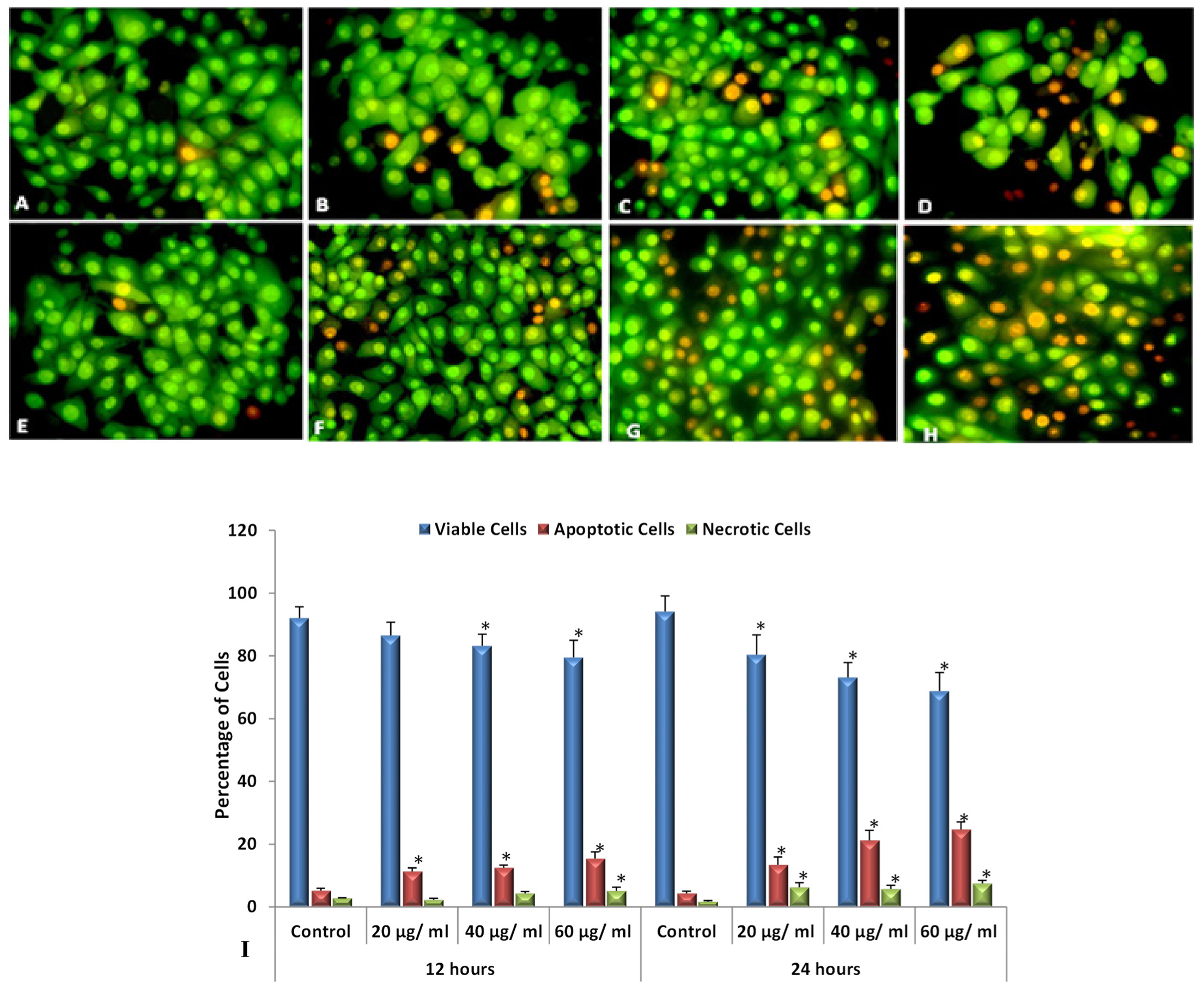
3.6. Apoptotic Morphological Changes by (AO-EB) Dual Staining Method
3.7. Annexin-V/PI Apoptosis Assay
3.8. Detection of Autophagy by Acidic Vesicular Organelles (AVOs)
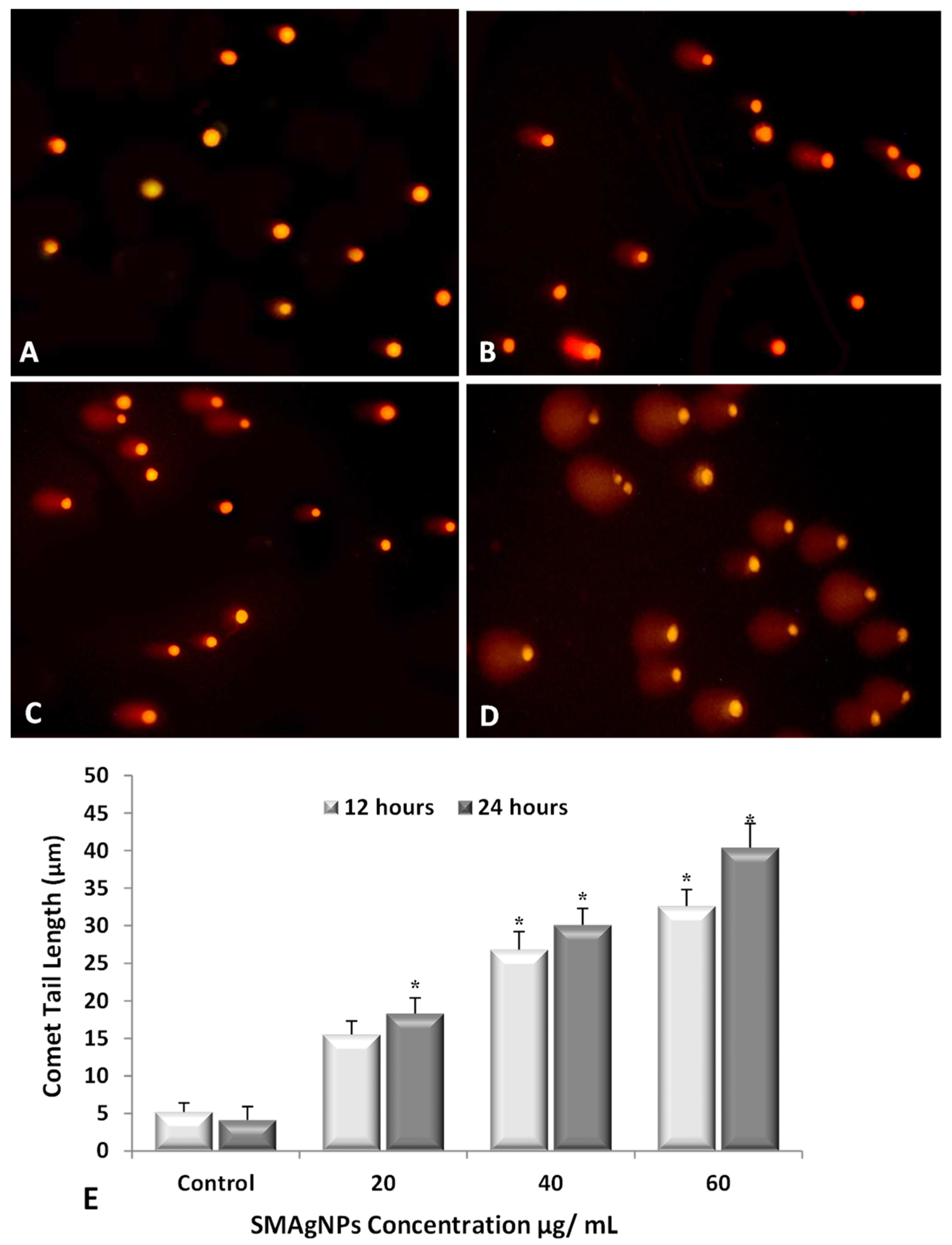
3.9. DNA Damage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Observatory; WHO: Geneva, Switzerland, 2018. Available online: who.int/gho/database/en/ (accessed on 8 August 2021).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, J.; Victor, D., III; Emad, H.A.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxid. Med. Cell. Longev. 2016, 33, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel Loaded Solid Lipid Nanoparticles: A Novel Drug Delivery System. IET Nanobiotechnol. 2017, 11, 621–629. [Google Scholar]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Deng, N. Silver nanoparticles induce HepG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res. Lett. 2016, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Chen, C.M.; Yang, W.Z.; Shi, S.S.; Chen, J. Enhancement effect of cytotoxicity response of silver nanoparticles combined with thermotherapy on C6 rat glioma cells. J. Nanosci. Nanotechnol. 2013, 13, 3851–3854. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; Kumar, M.R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa olezfera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Coll. Sur. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef]
- Farah, M.A.; Ali, M.A.; Chen, S.M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Coll. Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Ullah, A.A.; Banik, S.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Green Synthesized Silver Nanoparticles-Mediated Cytotoxic Effect in Colorectal Cancer Cells: NF-κB Signal Induced Apoptosis Through Autophagy. Biol. Trace Elem. Res. 2020, 199, 3272–3286. [Google Scholar] [CrossRef]
- Al-kawmani, A.A.; Alanazi, K.M.; Farah, M.A.; Ali, M.A.; Hailan, W.A.Q.; Al-Hemaid, F.M. Apoptosis-Inducing Potential of Biosynthesized Silver Nanoparticles in Breast Cancer Cells. J. King Saud Univ.-Sci. 2020, 32, 2480–2488. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Zaitoun, A.A.; Farag, M.A.; El Gayed, S.H.; Harraz, F.M. Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010, 3, 226–235. [Google Scholar] [CrossRef]
- Rocha, P.M.; Rodilla, J.M.; Díez, D.; Elder, H.; Guala, M.S.; Silva, L.A.; Pombo, E.B. Synergistic antibacterial activity of the essential oil of Aguaribay (Schinus molle L.). Molecules 2012, 17, 12023–12036. [Google Scholar] [CrossRef] [Green Version]
- Abderrahim, A.; Belhamel, K.; Chalard, P.; Figuérédo, G. Correlation between chemical composition and antioxidant activity of the essential oils from leaves and berries of Schinus molle L. growing in two areas of Bejaia (Algeria). J. Food Meas. Charact. 2018, 12, 1123–1134. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.I.; Al-Gendy, A.A.; El-Shazly, A.M. Chemical composition and cytotoxic activity of the essential oils of Schinus molle growing in Egypt. J. Pharm. Sci. Res. 2016, 8, 779. [Google Scholar]
- Barberia-Roque, L.; Gámez-Espinosa, E.; Viera, M.; Bellotti, N. Assessment of three plant extracts to obtain silver nanoparticles as alternative additives to control biodeterioration of coatings. Int. Biodeterior. Biodegrad. 2019, 141, 52–61. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Burd, J.F.; Usategui-Gomez, M. A colorimetric assay for serum lactate dehydrogenase. Clin. Chim. Acta 1973, 46, 223–227. [Google Scholar] [CrossRef]
- Baskić, D.; Popović, S.; Ristić, P.; Arsenijević, N.N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Swanner, J.; Mims, J.; Carroll, D.L.; Akman, S.A.; Furdui, C.M.; Torti, S.V.; Singh, R.N. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int. J. Nanomed. 2015, 10, 3937–3953. [Google Scholar]
- Mares-Briones, F.; Rosas, G. Structure and Stability of Gold Nanoparticles Synthesized Using Schinus molle L. Extract. J. Clust. Sci. 2017, 28, 1995–2003. [Google Scholar] [CrossRef]
- Ibrahim, M.T.; Haggag, E.G. Phenolics from Schinus molle stems and their anti-bacterial and anti-microalgal activity. Life Sci. J. 2013, 10, 1931–1937. [Google Scholar]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Hemlata, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.; Shim, Y.Y.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; Markossian, S., Grossman, A., Brimacombe, K., Eds.; Bethesda, Eli Lilly & Company: Indianapolis, IN, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53196/ (accessed on 22 August 2021).
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M. Oxidative stress and apoptotic responses elicited by nostoc-synthesized silver nanoparticles against different cancer cell lines. Cancers 2020, 12, 2099. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Abdullah, M.A. Biosynthesis, Mechanisms, and Biomedical Applications of Silver Nanoparticles. In Functional Bionanomaterials; Springer: Cham, Switzerland, 2020; pp. 313–332. [Google Scholar]
- Ali, A.Q.; Farah, M.A.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Ali, M.A.; Lee, J.; Mahmoud, A.H. Cytogenotoxic effects of Adenium obesum seeds extracts on breast cancer cells. Saudi J. Biol. Sci. 2019, 26, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lai, H.; Chen, J.; Li, L.; Wong, Y.S.; Chen, T.; Li, X. Natural borneol, a monoterpenoid compound, potentiates selenocystine-induced apoptosis in human hepatocellular carcinoma cells by enhancement of cellular uptake and activation of ROS-mediated DNA damage. PLoS ONE 2013, 8, e63502. [Google Scholar] [CrossRef]
- Asharani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Monera, A.A.; Albasher, G.; Alarifi, S. Effects of green silver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Huang, Z.; Zheng, W.; Fan, C.; Chen, T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomed: Nanotechnol. Biol. Med. 2013, 9, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.G.; Zhang, S.; Hwang, J.Y.; Kong, I.K. Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxid. Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Ahmadian, E.; Dizaj, S.M.; Rahimpour, E.; Hasanzadeh, A.; Eftekhari, A.; Halajzadeh, J.; Ahmadian, H. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater. Sci. Eng. C 2018, 93, 465–471. [Google Scholar] [CrossRef]
- Wilde, L.; Tanson, K.; Curry, J.; Martinez-Outschoorn, U. Autophagy in cancer: A complex relationship. Biochem. J. 2018, 475, 1939–1954. [Google Scholar] [CrossRef]
- Kim, D.K.; Yang, J.S.; Maiti, K.; Hwang, J.I.; Kim, K.; Seen, D.; Ahn, Y.; Lee, C.; Kang, B.C.; Kwon, H.B.; et al. Gonadotropin-releasing hormone-II antagonist induces autophagy of prostate cancer cells. Cancer Res. 2009, 69, 923–931. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef]
- Liu, J.; Fan, L.; Wang, H.; Sun, G. Autophagy, a double-edged sword in anti-angiogenesis therapy. Med. Oncol. 2016, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rosarin, F.S.; Arulmozhi, V.; Nagarajan, S.; Mirunalini, S. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac. J. Trop. Med. 2013, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sahu, S.C.; Roy, S.; Zheng, J.; Yourick, J.J.; Sprando, R.L. Comparative genotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells evaluated by fluorescent microscopy of cytochalasin B-blocked micronucleus formation. J. App. Toxicol. 2014, 34, 1200–1208. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, D.; Sun, L.; Huang, Z.; Zhang, X.; Ma, W.; Gu, N. Activation of autophagy by elevated reactive oxygen species rather than released silver ions promotes cytotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in hematopoietic cells. Nanoscale 2017, 9, 5489–5498. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hailan, W.A.; Al-Anazi, K.M.; Farah, M.A.; Ali, M.A.; Al-Kawmani, A.A.; Abou-Tarboush, F.M. Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus molle Extract. Nanomaterials 2022, 12, 161. https://doi.org/10.3390/nano12010161
Hailan WA, Al-Anazi KM, Farah MA, Ali MA, Al-Kawmani AA, Abou-Tarboush FM. Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus molle Extract. Nanomaterials. 2022; 12(1):161. https://doi.org/10.3390/nano12010161
Chicago/Turabian StyleHailan, Waleed Ali, Khalid Mashay Al-Anazi, Mohammad Abul Farah, Mohammad Ajmal Ali, Ahmed Ali Al-Kawmani, and Faisal Mohammed Abou-Tarboush. 2022. "Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus molle Extract" Nanomaterials 12, no. 1: 161. https://doi.org/10.3390/nano12010161
APA StyleHailan, W. A., Al-Anazi, K. M., Farah, M. A., Ali, M. A., Al-Kawmani, A. A., & Abou-Tarboush, F. M. (2022). Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus molle Extract. Nanomaterials, 12(1), 161. https://doi.org/10.3390/nano12010161






