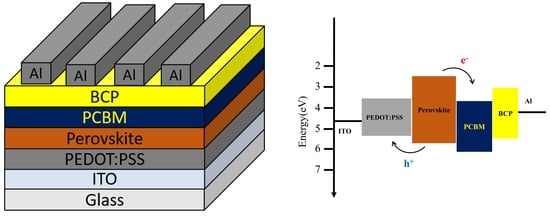
Properties of Halide Perovskite Photodetectors with Little Rubidium Incorporation
Abstract
:1. Introduction
2. Materials and Methods
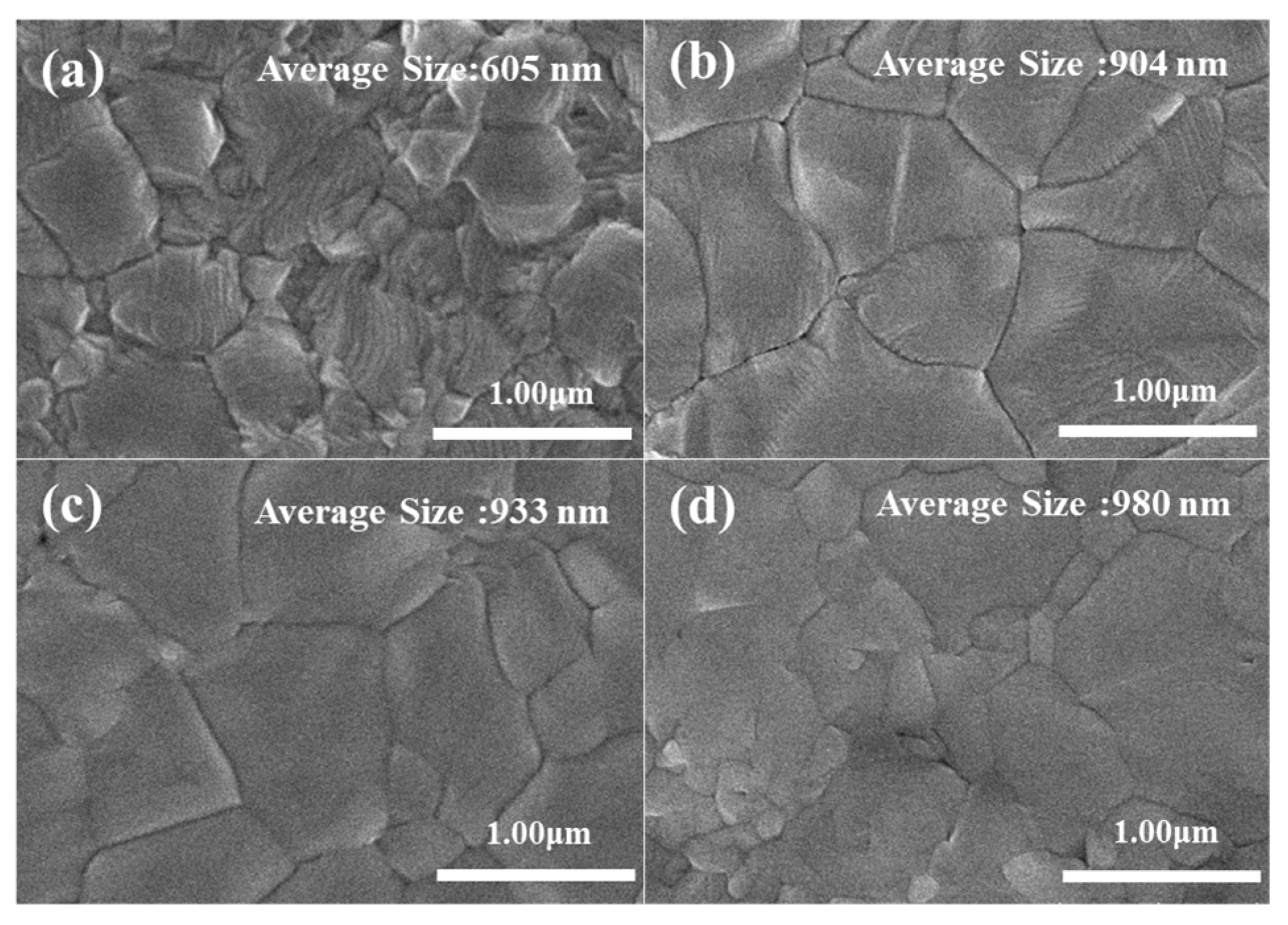
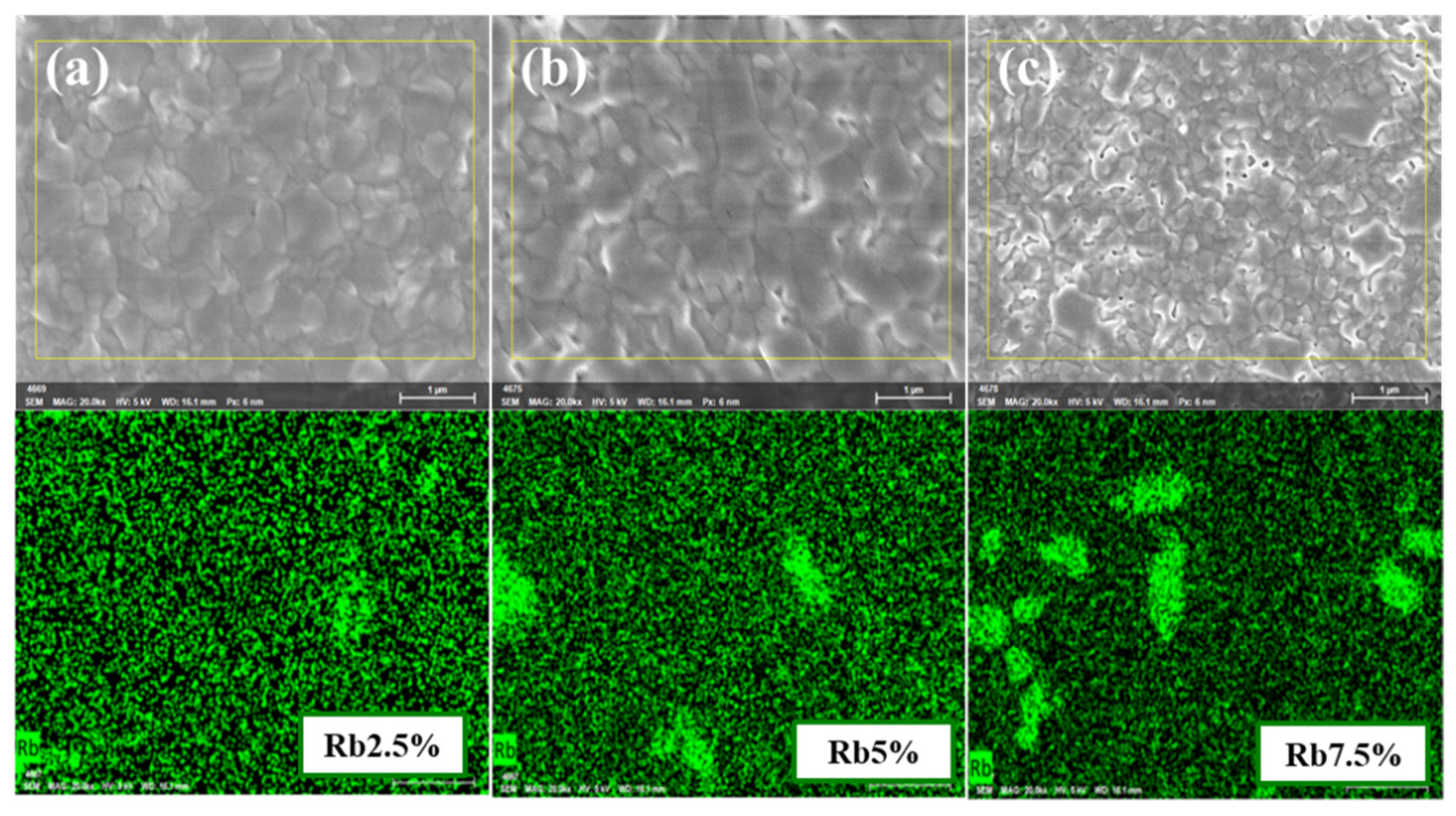
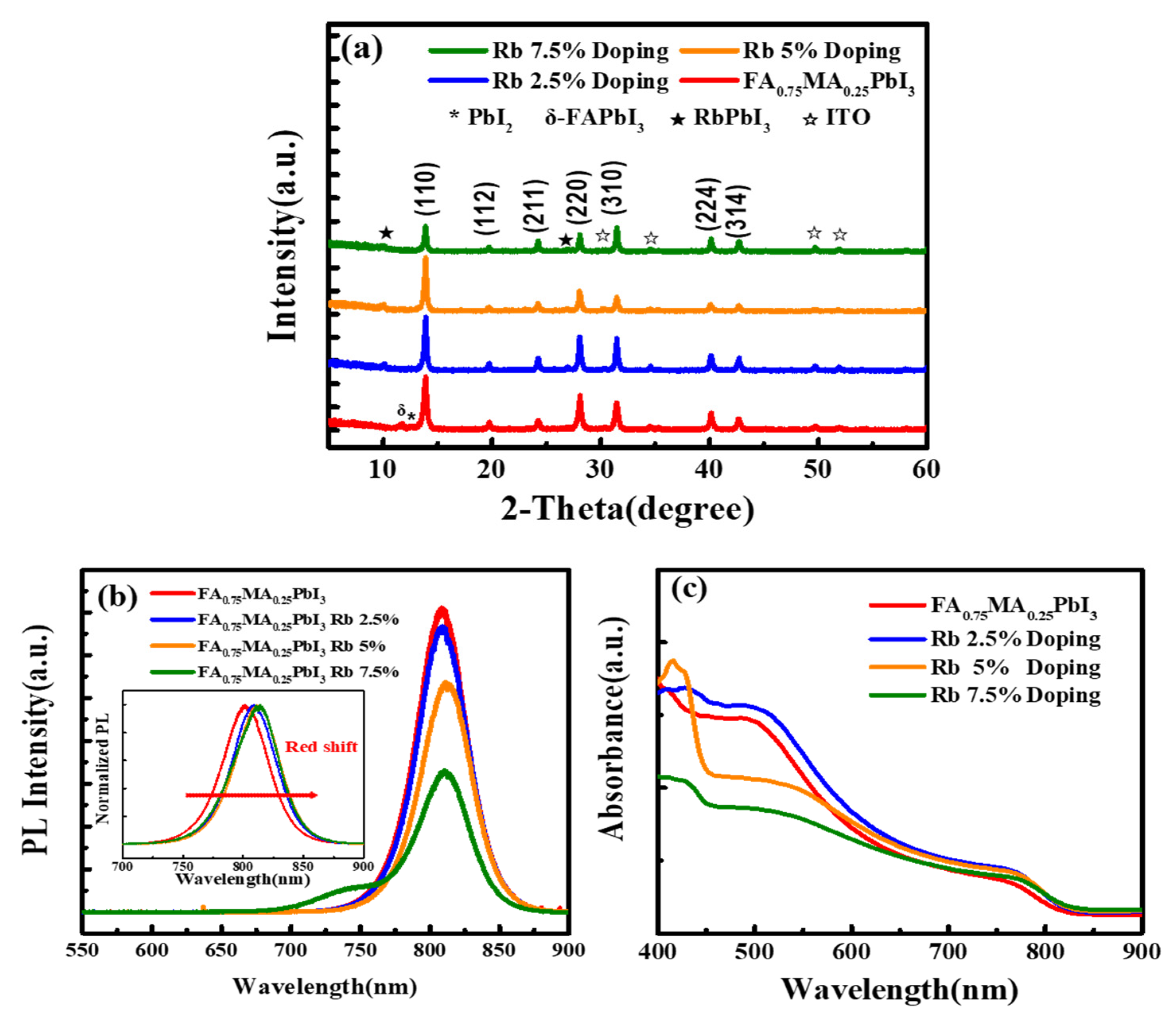
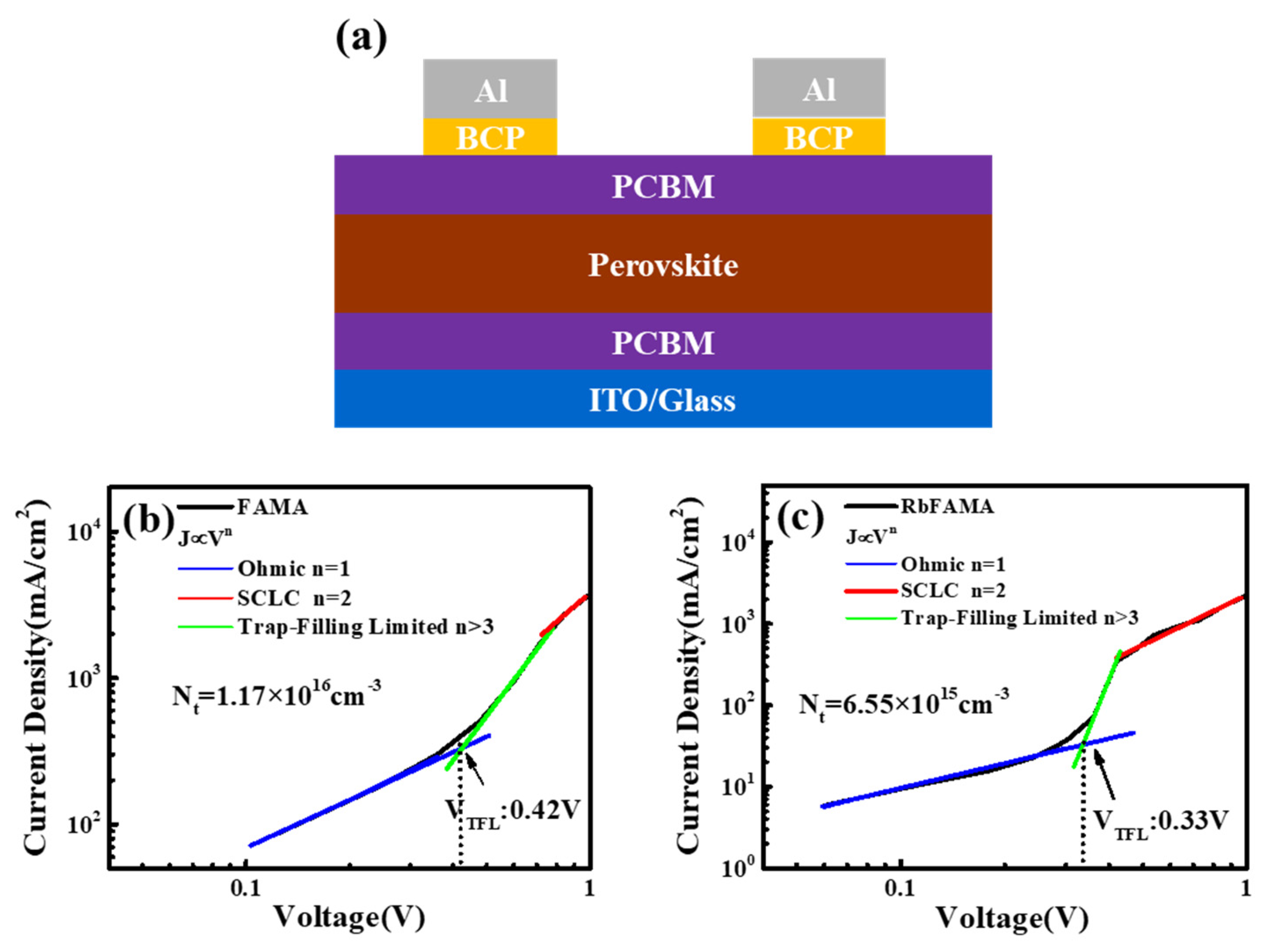
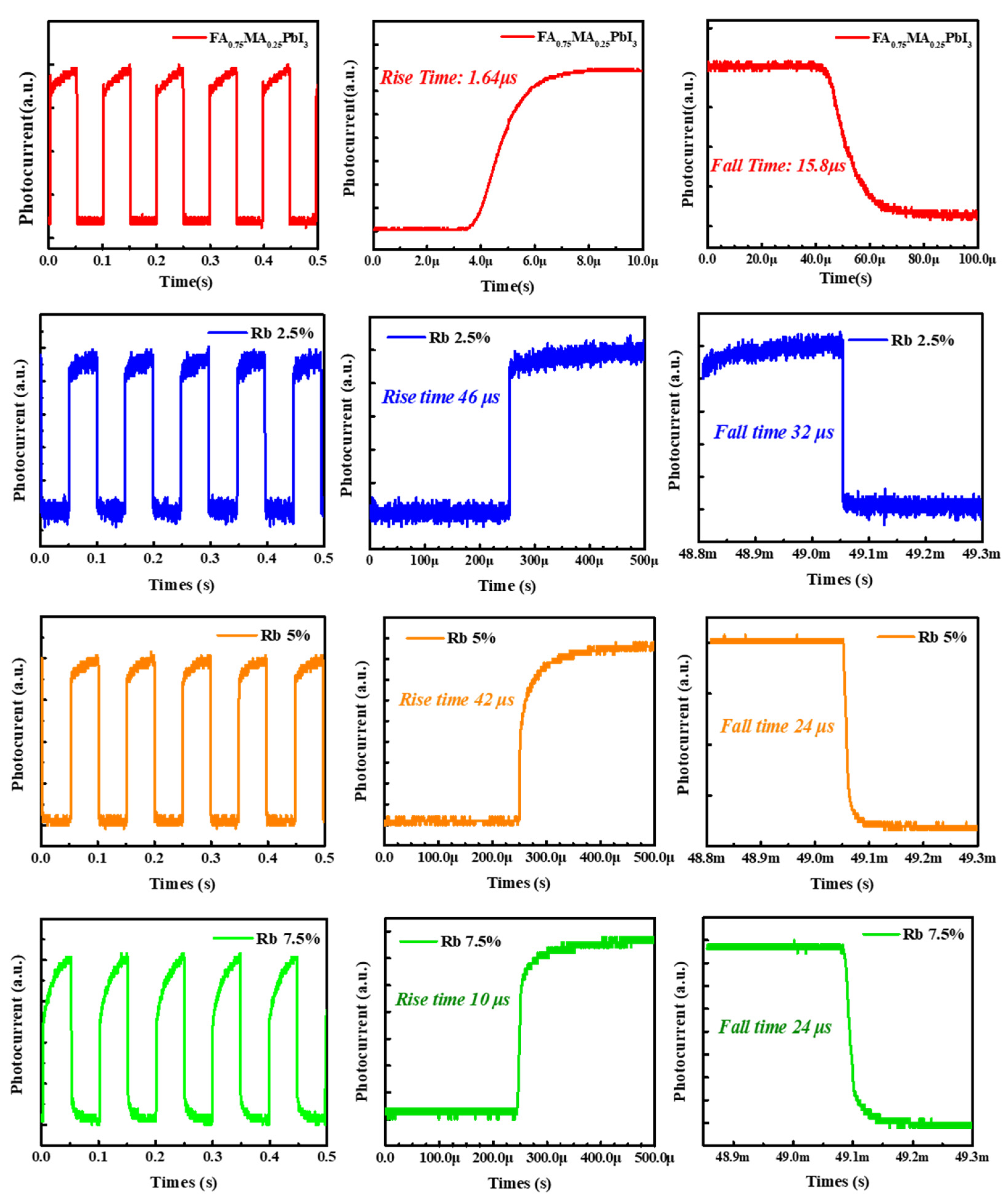
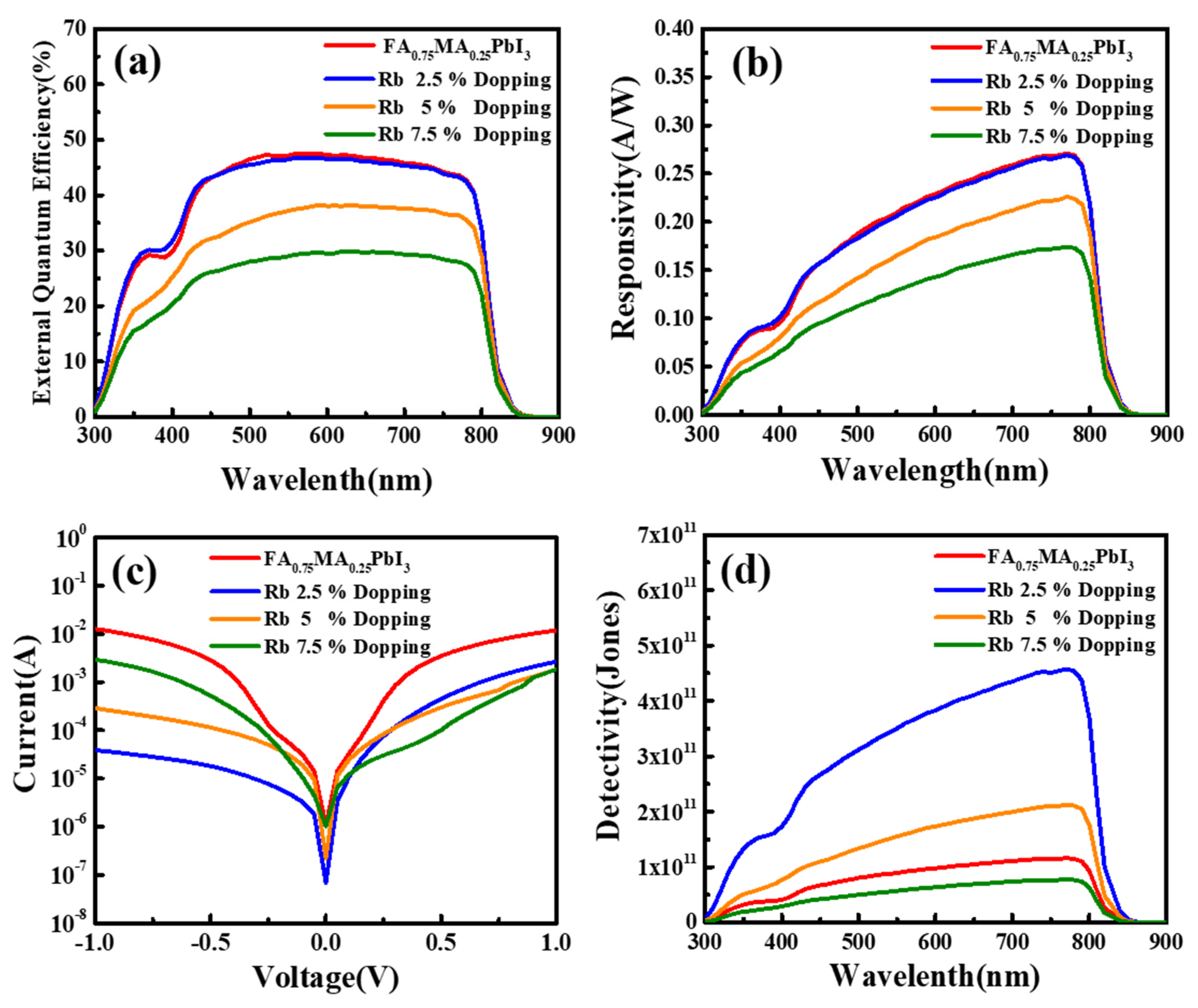
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, H.; Che, F.; Wei, M.; Zhao, Y.; Saidaminov, M.I.; Todorović, P.; Broberg, D.; Walters, G.; Tan, F.; Zhuang, T.; et al. Dipolar cations confer defect tolerance in wide-bandgap metal halide perovskites. Nat. Commun. 2018, 9, 3100. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Yao, F.; Gui, P.; Zhang, Q.; Lin, Q. Molecular engineering of perovskite photodetectors: Recent advances in materials and devices. Mol. Syst. Des. Eng. 2018, 3, 702–716. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Nagiri, R.; Burn, P.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, L.; Liu, Z.; Huang, J.; Yun, J.S.; Zeng, Y.; Pu, A.; Sun, K.; Hameiri, Z.; et al. Exploring Inorganic Binary Alkaline Halide to Passivate Defects in Low-Temperature-Processed Planar-Structure Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800138. [Google Scholar] [CrossRef]
- Jia, Y.; Kerner, R.A.; Grede, A.J.; Rand, B.P.; Giebink, N.C. Continuous-wave lasing in an organic–inorganic lead halide perovskite semiconductor. Nat. Photonics 2017, 11, 784–788. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Meng, L.; Wang, M.; Tian, W.; Li, L. Gradient Energy Band Driven High-Performance Self-Powered Perovskite/CdS Photodetector. Adv. Mater. 2019, 31, e1806725. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, S.; Tang, G.; Li, G.; Liu, D.; Li, J.; Hu, X.; Liu, Y.; Li, J.; Yang, Z.; et al. High-Performance, Self-Powered Photodetectors Based on Perovskite and Graphene. ACS Appl. Mater. Interfaces 2017, 9, 42779–42787. [Google Scholar] [CrossRef]
- Zhou, H.; Mei, J.; Xue, M.; Song, Z.; Wang, H. High-Stability, Self-Powered Perovskite Photodetector Based on a CH3NH3PbI3/GaN Heterojunction with C60 as an Electron Transport Layer. J. Phys. Chem. C 2017, 121, 21541–21545. [Google Scholar] [CrossRef]
- Ghosh, J.; Natu, G.; Giri, P. Plasmonic hole-transport-layer enabled self-powered hybrid perovskite photodetector using a modified perovskite deposition method in ambient air. Org. Electron. 2019, 71, 175–184. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Bian, J.; Feng, Y.; Wang, C.; Huang, Y.; Zhang, Y.; Shi, Y. High-Performance and Stable Mesoporous Perovskite Solar Cells via Well-Crystallized FA0.85MA0.15Pb(I0.8Br0.2)3. ACS Appl. Mater. Interfaces 2018, 11, 2989–2996. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the Efficiency Beyond 20% with a Mixed Cation Perovskite Solar Cell Fabricated in Ambient Air under Controlled Humidity. Adv. Energy Mater. 2017, 8, 1700677. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Zhang, P.; Liu, D.; Zhang, T.; Ji, L.; Gu, X.; Chen, Z.D.; Li, S. Stitching triple cation perovskite by a mixed anti-solvent process for high performance perovskite solar cells. Nano Energy 2017, 39, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Eze, V.O.; Seike, Y.; Mori, T. Efficient planar perovskite solar cells using solution-processed amorphous WOx/fullerene C60 as electron extraction layers. Org. Electron. 2017, 46, 253–262. [Google Scholar] [CrossRef]
- Adams, G.R.; Eze, V.O.; Shohag, A.S.; Simpson, R.; Parker, H.; I Okoli, O. Fabrication of rapid response self-powered photodetector using solution-processed triple cation lead-halide perovskite. Eng. Res. Express 2020, 2, 015043. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Sa, R.; Liu, M.; Zha, W. Effect of rubidium incorporation on the structural, electronic and properties of MAPbI3. Chem. Phys. Lett. 2020, 743, 137179. [Google Scholar] [CrossRef]
- Erodici, M.P.; Pierone, P.J.; Hartono, N.T.P.; Hidalgo, J.; Lai, B.; Buonassisi, T.; Correa-Baena, J.-P.; Sher, M.-J. Enhanced charge carrier lifetime and mobility as a result of Rb and Cs incorporation in hybrid perovskite. Appl. Phys. Lett. 2021, 118, 063901. [Google Scholar] [CrossRef]
- Binyamin, T.; Pedesseau, L.; Remennik, S.; Sawahreh, A.; Even, J.; Etgar, L. Fully Inorganic Mixed Cation Lead Halide Perovskite Nanoparticles: A Study at the Atomic Level. Chem. Mater. 2019, 32, 1467–1474. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.; Mulmudi, H.K.; Shen, H.; Wu, Y.; Barugkin, C.; Mayon, Y.O.; Nguyen, H.T.; Macdonald, D.; Peng, J.; Lockrey, M.; et al. Structural engineering using rubidium iodide as a dopant under excess lead iodide conditions for high efficiency and stable perovskites. Nano Energy 2016, 30, 330–340. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Kandada, A.R.S.; De Bastiani, M.; Gandini, M.; Petrozza, A. Tuning the Light Emission Properties by Band Gap Engineering in Hybrid Lead Halide Perovskite. J. Am. Chem. Soc. 2014, 136, 17730–17733. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, I.; Bae, S.; Son, H.J.; Lee, P.; Lee, J.; Lee, C.-H.; Ko, M.J. Inorganic Rubidium Cation as an Enhancer for Photovoltaic Performance and Moisture Stability of HC(NH2)2PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1605988. [Google Scholar] [CrossRef]
- Tong, S.; Wu, H.; Zhang, C.; Li, S.; Wang, C.; Shen, J.; Xiao, S.; He, J.; Yang, J.; Sun, J.; et al. Large-area and high-performance CH3NH3PbI3 perovskite photodetectors fabricated via doctor blading in ambient condition. Org. Electron. 2017, 49, 347–354. [Google Scholar] [CrossRef]
- Hun, C.-M.; Tien, C.-H.; Lee, K.-L.; Lai, H.-Y.; Chen, L.-C. The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application. Sensors 2021, 21, 4475. [Google Scholar] [CrossRef]
- Hsiao, Y.-W.; Song, J.-Y.; Wu, H.-T.; Hong, K.-T.; Leu, C.-C.; Shih, C.-F. Effects of cesium content on the triple-cation lead halide perovskite photodetectors with enhanced detectivity and response time. J. Alloy. Compd. 2021, 889, 161621. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-W.; Song, J.-Y.; Wu, H.-T.; Leu, C.-C.; Shih, C.-F. Properties of Halide Perovskite Photodetectors with Little Rubidium Incorporation. Nanomaterials 2022, 12, 157. https://doi.org/10.3390/nano12010157
Hsiao Y-W, Song J-Y, Wu H-T, Leu C-C, Shih C-F. Properties of Halide Perovskite Photodetectors with Little Rubidium Incorporation. Nanomaterials. 2022; 12(1):157. https://doi.org/10.3390/nano12010157
Chicago/Turabian StyleHsiao, Yuan-Wen, Jyun-You Song, Hsuan-Ta Wu, Ching-Chich Leu, and Chuan-Feng Shih. 2022. "Properties of Halide Perovskite Photodetectors with Little Rubidium Incorporation" Nanomaterials 12, no. 1: 157. https://doi.org/10.3390/nano12010157
APA StyleHsiao, Y.-W., Song, J.-Y., Wu, H.-T., Leu, C.-C., & Shih, C.-F. (2022). Properties of Halide Perovskite Photodetectors with Little Rubidium Incorporation. Nanomaterials, 12(1), 157. https://doi.org/10.3390/nano12010157