Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Light Scattering Assay
2.3. Cell Cultures
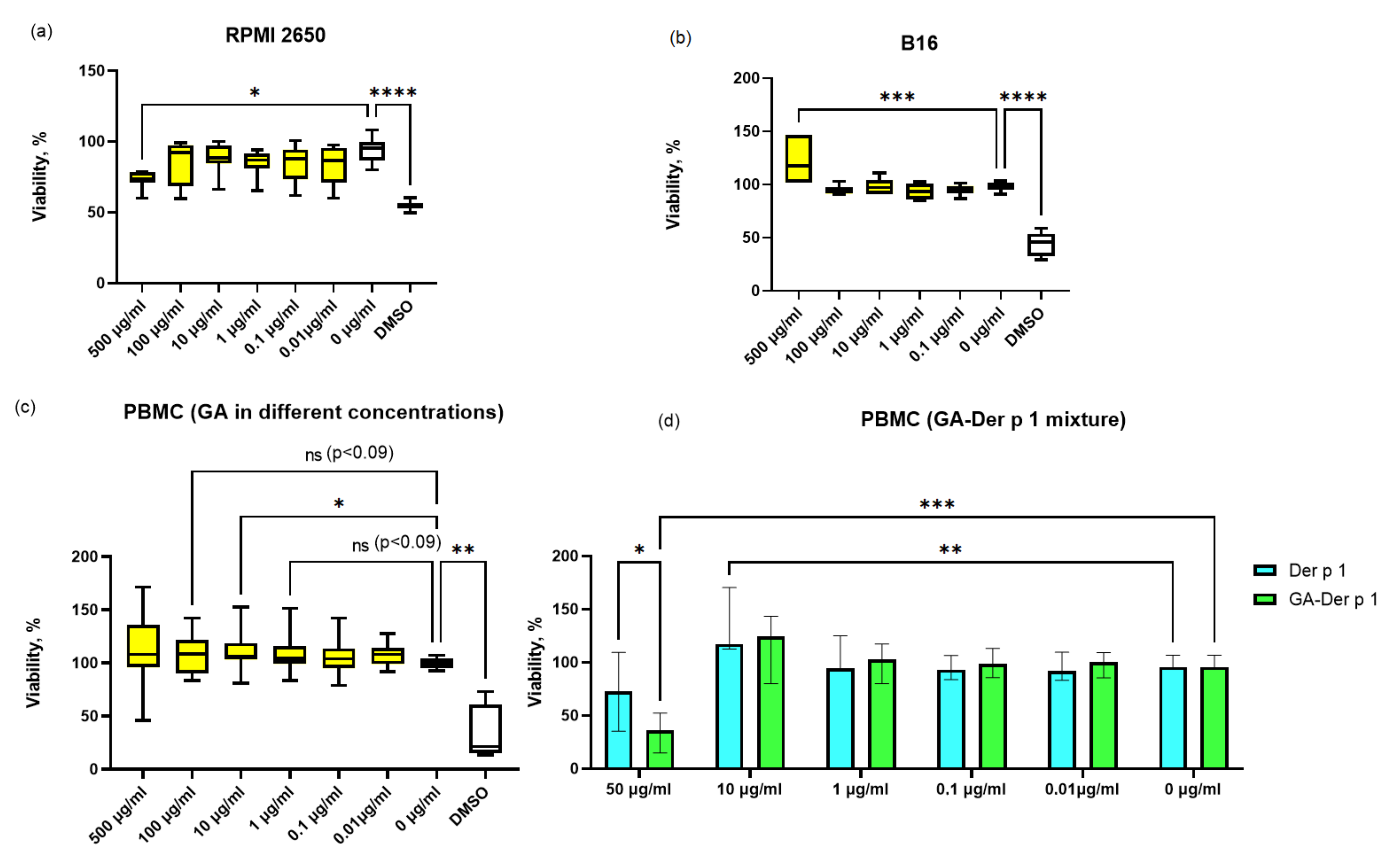
2.4. Viability Assay
2.5. Transport Assay
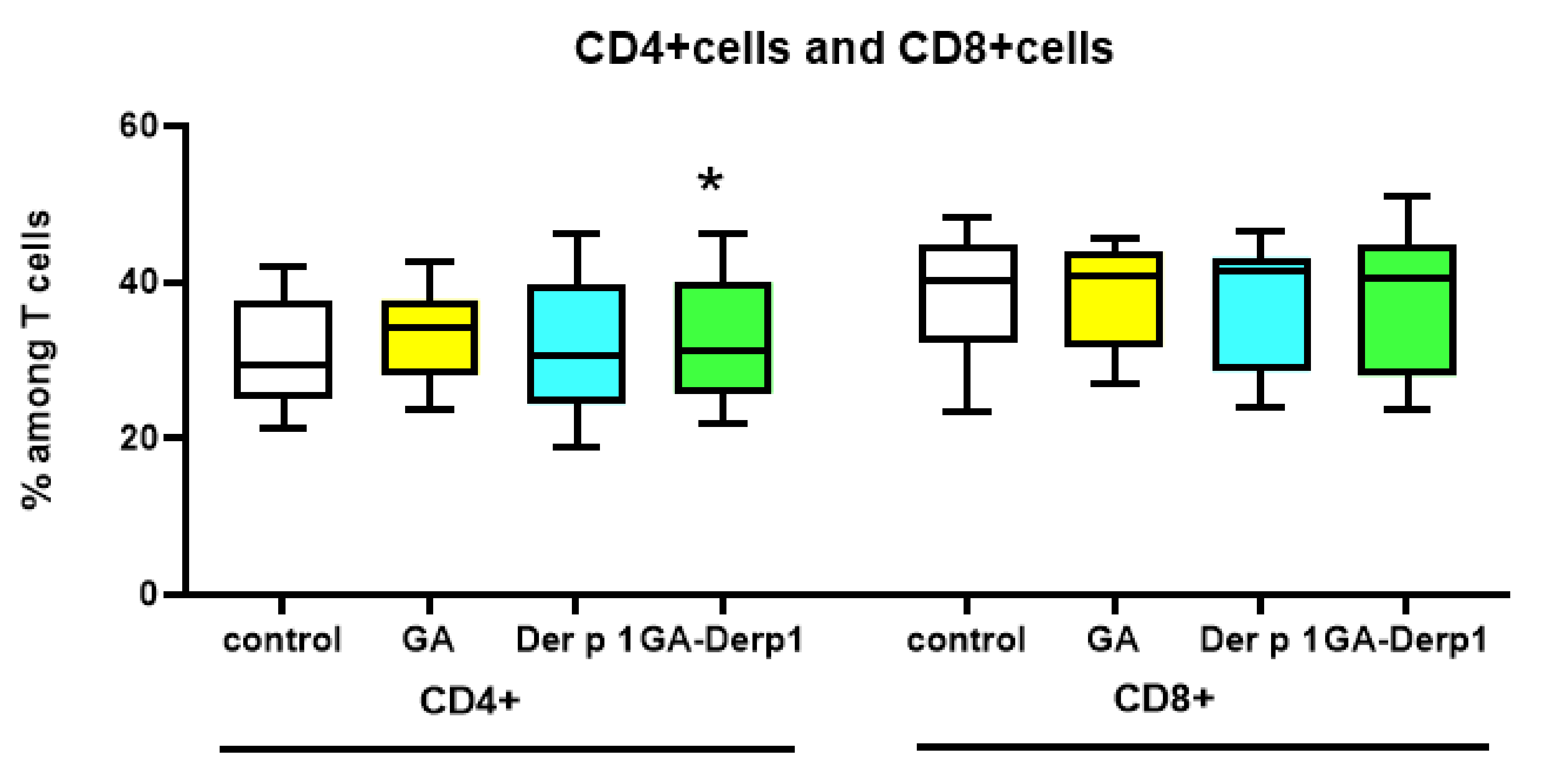
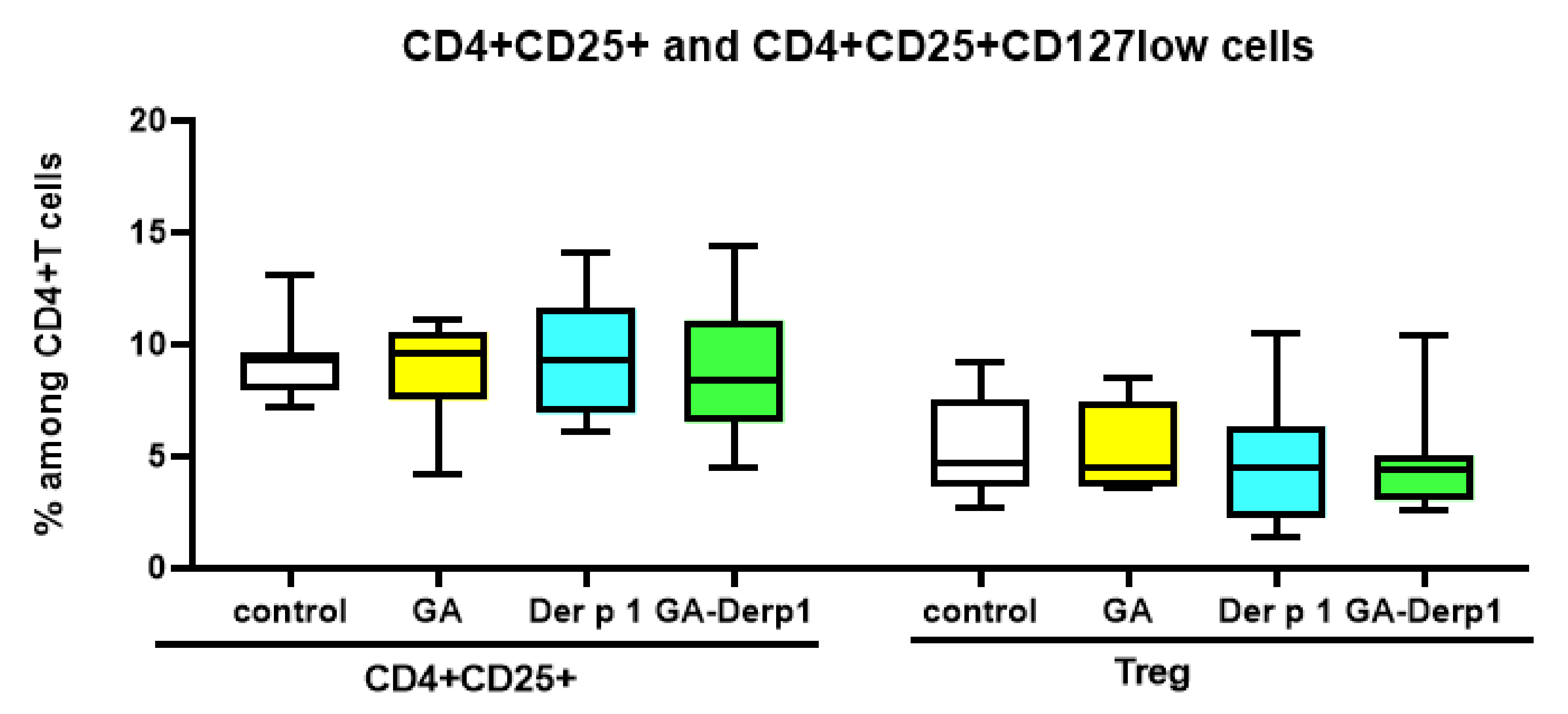
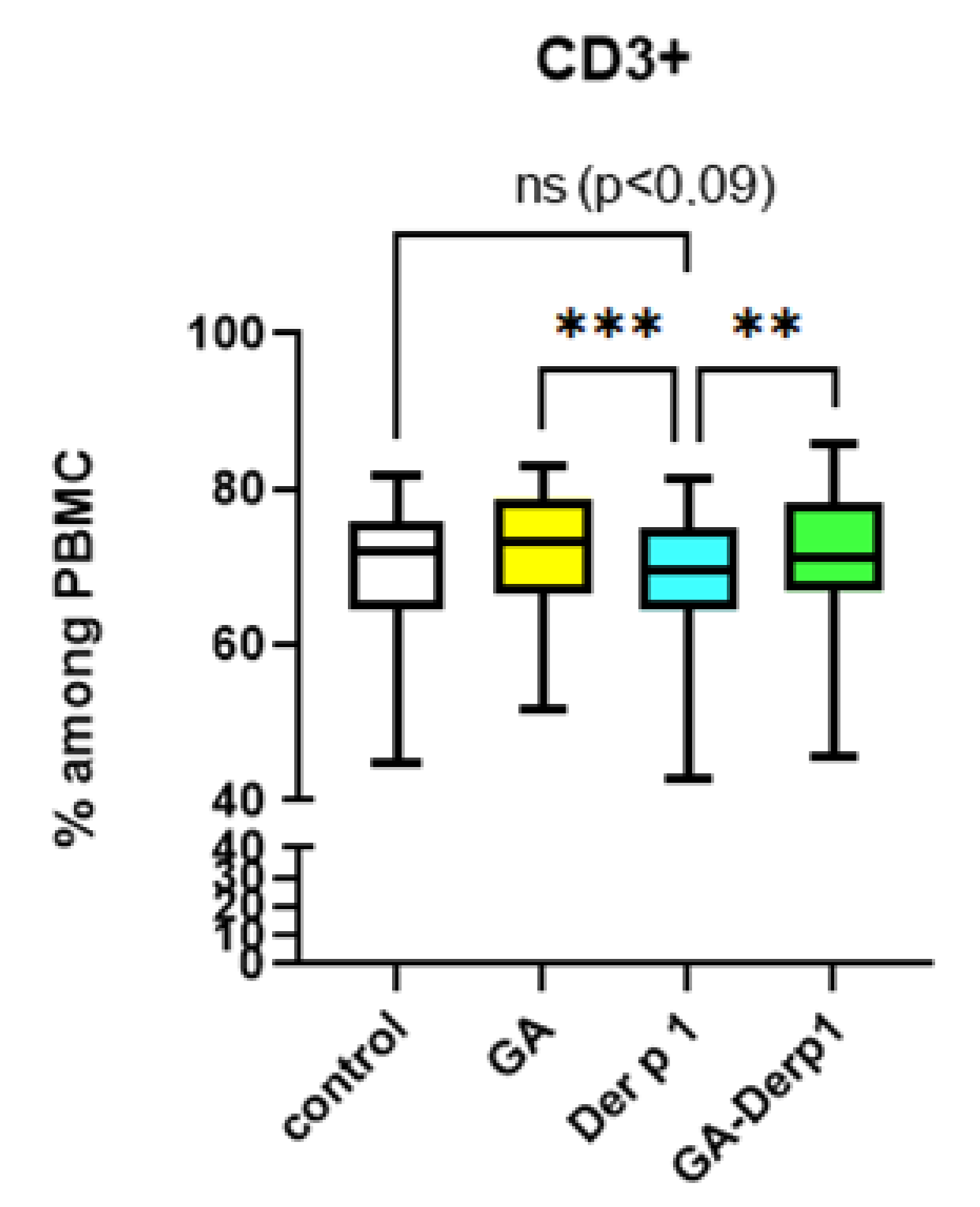
2.6. T Cell Analysis
2.7. ELISA Assay
2.8. Statistical Analysis
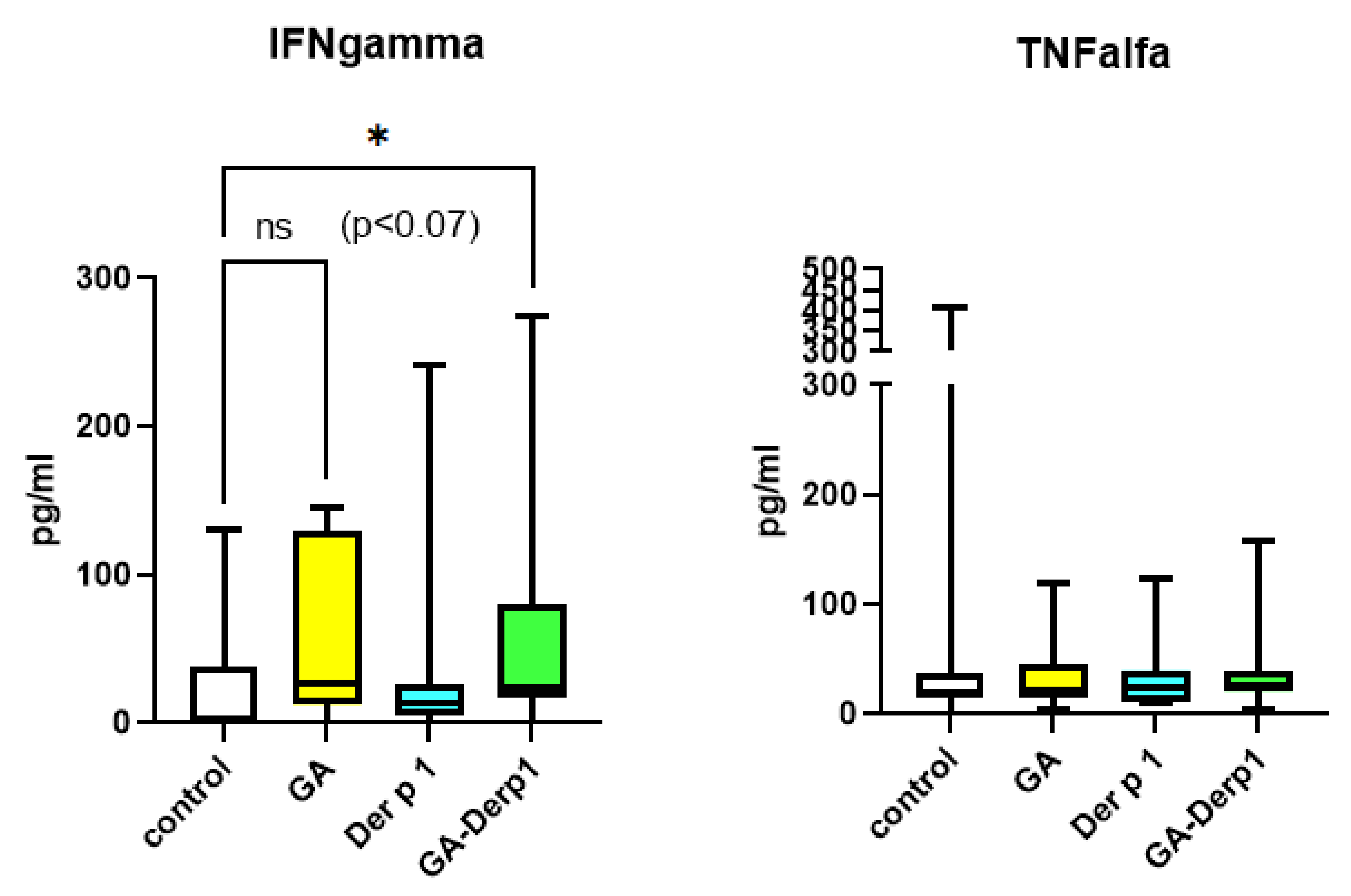
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Shi, Q.; Liu, Z.; Li, R.J.; Pan, P.W.; Hou, Y.Y.; Lu, W.G.; Bai, G. The synergistic anti-asthmatic effects of glycyrrhizin and salbutamol. Acta Pharmacol. Sin. 2010, 31, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Bagriyanik, A.; Uzuner, N. Glycyrrhizin and long-term histopathologic changes in a murine model of asthma. Curr. Ther. Res. Clin. Exp. 2011, 72, 250–561. [Google Scholar] [CrossRef] [PubMed]
- Cavone, L.; Cuppari, C.; Manti, S.; Grasso, L.; Arrigo, T.; Calamai, L.; Salpietro, C.; Chiarugi, A. Increase in the Level of Proinflammatory Cytokine HMGB1 in Nasal Fluids of Patients With Rhinitis and its Sequestration by Glycyrrhizin Induces Eosinophil Cell Death. Clin. Exp. Otorhinolaryngol. 2015, 8, 123–128. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, A.G. Evaluation of the immunity activity of glycyrrhizin in AR mice. Molecules 2012, 17, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, Z.; Liao, X.L.; Liu, J.; Fu, Q.; Ma, S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+) CD25(+) Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013, 148, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, Y.; Hu, X.; Wang, Q.; Lei, W.; Zhou, L.; Huang, J. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology 2016, 21, 102–111. [Google Scholar] [CrossRef]
- Han, S.; Sun, L.; He, F.; Che, H. Anti-Allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci. Rep. 2017, 7, 7222. [Google Scholar] [CrossRef]
- Chen, D.; Bellussi, L.M.; Cocca, S.; Wang, J.; Passali, G.C.; Hao, X.; Chen, L.; Passali, D. Glycyrrhetinic acid suppressed hmgb1 release by up-regulation of Sirt6 in nasal inflammation. J. Biol. Regul. Homeost. Agents 2017, 31, 269–277. [Google Scholar]
- Fouladi, S.; Masjedi, M.; Ghasemi, R.; Hakemi, M.G.; Eskandari, N. The In Vitro Impact of Glycyrrhizic Acid on CD4+ T Lymphocytes through OX40 Receptor in the Patients with Allergic Rhinitis. Inflammation 2018, 41, 1690–1701. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, B.; Wang, S.; Liang, Q.; Cai, Y.; Yang, F.; Li, G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016, 23, 1623–1635. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Baltina, L.A.; Grankina, V.P.; Kondratenko, R.M.; Tolstikova, T.G. Licorice: Biodiversity, Chemistry, Medical Applications; Academic Publishing House: Novosibirsk, Russia, 2007; p. 311. ISBN 5-9747-0060-0. (In Russian) [Google Scholar]
- Kornievskaya, V.S.; Kruppa, A.I.; Leshina, T.V. NMR and photo-CIDNP investigations of the glycyrrhizinic acid micelles influence on solubilized molecules. J. Incl. Phenom. Macrocycl. Chem. 2007, 60, 123–130. [Google Scholar] [CrossRef]
- Tolstikova, T.G.; Khvostov, M.V.; Bryzgalov, A.O.; Dushkin, A.V.; Meteleva, E.S. Complex of nifedipine with glycyrrhizic acid as a novel water–Soluble antihypertensive and antiarrhythmic agent. Lett. Drug Des. Discov. 2009, 6, 155–158. [Google Scholar] [CrossRef]
- Dushkin, A.V.; Meteleva, E.S.; Tolstikova, T.G.; Khvostov, M.V.; Dolgikh, M.P.; Tolstikov, G.A. Complexing of Pharmacons with Glycyrrhizic Acid as a Route to the Development of the Preparations with Enhanced Efficiency. Chem. Sustain. Dev. 2010, 18, 437–444. [Google Scholar]
- Dushkin, A.V.; Tolstikova, T.G.; Khvostov, M.V.; Tolstikov, G.I. Complexes of polysaccharides and glycyrrhizic acid with drug molecules.Mechanochemical synthesis and pharmacological activity. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 573–602. [Google Scholar]
- Matsuoka, K.; Miyajima, R.; Ishida, I.; Karasawa, S.; Yoshimura, T. Aggregate formation of glycyrrhizic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 500, 112–117. [Google Scholar] [CrossRef]
- Kong, R.; Zhu, X.; Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Polyakov, N.E.; Khovstov, M.V.; Baev, D.S.; Tolstikova, T.G.; Yu, J.; et al. Enhanced solubility and bioavailability of simvastatin mechanochemically obtained complexes. Int. J. Pharm. 2017, 534, 108–118. [Google Scholar] [CrossRef]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.V.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Zhu, X.; Meteleva, E.S.; Polyakov, N.E.; Khvostov, M.V.; Baev, D.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Atorvastatin calcium inclusion complexation with polysaccharide arabinogalactan and saponin disodium glycyrrhizate for increasing of solubility and bioavailability. Drug Deliv. Transl. Res. 2018, 8, 1200–1213. [Google Scholar] [CrossRef]
- Moingeon, P.; Lombardi, V.; Saint-Lu, N.; Tourdot, S.; Bodo, V.; Mascarell, L. Adjuvants and vector systems for allergy vaccines. Immunol. Allergy Clin N. Am. 2011, 31, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Gadermaier, E.; James, L.K.; Shamji, M.H.; Blatt, K.; Fauland, K.; Zieglmayer, P.; Garmatiuk, T.; Focke-Tejkl, M.; Villalba, M.; Beavil, R.; et al. Epitope specificity determines cross-protection of a SIT-induced IgG4 antibody. Allergy 2016, 71, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Moitra, S.; Datta, A.; Mondal, S.; Hazra, I.; Faruk, S.M.; Das, P.K.; Basu, A.K.; Tripathi, S.K.; Chaudhuri, S. Modulation of regulatory T cells by intranasal allergen immunotherapy in an experimental rat model of airway allergy. Int. Immunopharmacol. 2017, 47, 9–19. [Google Scholar] [CrossRef]
- Basomba, A.; Tabar, A.I.; de Rojas, D.H.; García, B.E.; Alamar, R.; Olaguíbel, J.M.; del Prado, J.M.; Martín, S.; Rico, P. Allergen vaccination with a liposome-encapsulated extract of Dermatophagoides pteronyssinus: A randomized, double-blind, placebo-controlled trial in asthmatic patients. J. Allergy Clin. Immunol. 2002, 109, 943–948. [Google Scholar] [CrossRef]
- Kitaoka, M.; Shin, Y.; Kamiya, N.; Kawabe, Y.; Kamihira, M.; Goto, M. Transcutaneous Peptide Immunotherapy of Japanese Cedar Pollinosis Using Solid-in-Oil Nanodispersion Technology. Aaps Pharmscitech 2015, 16, 1418–1424. [Google Scholar] [CrossRef]
- Tasaniyananda, N.; Chaisri, U.; Tungtrongchitr, A.; Chaicumpa, W.; Sookrung, N. Mouse Model of Cat Allergic Rhinitis and Intranasal Liposome-Adjuvanted Refined Fel d 1 Vaccine. PLoS ONE 2016, 11, e0150463. [Google Scholar] [CrossRef]
- Meechan, P.; Tungtrongchitr, A.; Chaisri, U.; Maklon, K.; Indrawattana, N.; Chaicumpa, W.; Sookrung, N. Intranasal, liposome-adjuvanted cockroach allergy vaccines made of refined major allergen and whole-body extract of Periplaneta americana. Int. Arch Allergy Immunol. 2013, 161, 351–362. [Google Scholar] [CrossRef]
- Baker, J.R., Jr.; Rasky, A.J.; Landers, J.J.; Janczak, K.W.; Totten, T.D.; Lukacs, N.W.; O’Konek, J.J. Intranasal delivery of allergen in a nanoemulsion adjuvant inhibits allergen-specific reactions in mouse models of allergic airway disease. Clin. Exp. Allergy 2021, 51, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Arebro, J.; Tengroth, L.; Razavi, R.; Kumlien Georén, S.; Winqvist, O.; Cardell, L.O. Antigen-Presenting epithelial cells can play a pivotal role in airway allergy. J. Allergy Clin. Immunol. 2016, 137, 957–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Q.; Suntsova, L.; Chistyachenko, Y.S.; Evseenko, V.; Khvostov, M.V.; Polyakov, N.E.; Dushkin, A.V.; Su, W. Preparation, physicochemical and pharmacological study of curcumin solid dispersion with an arabinogalactan complexation agent. Int. J. Biol. Macromol. 2019, 128, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Charbonnier, A.S.; Duez, C.; Jacquet, A.; Stewart, G.A.; Tonnel, A.B.; Pestel, J. Th2 polarization by Der p 1–pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood J. Am. Soc. Hematol. 2001, 98, 1135–1141. [Google Scholar] [CrossRef]
- Tu, C.T.; Li, J.; Wang, F.P.; Li, L.; Wang, J.Y.; Jiang, W. Glycyrrhizin regulates CD4+ T cell response during liver fibrogenesis via JNK, ERK and PI3K/AKT pathway. Int. Immunopharmacol. 2012, 14, 410–421. [Google Scholar] [CrossRef]
- Yao, Z.; Fu, Y. Glycyrrhizic acid restrains airway inflammation and remodeling in asthma via the TGF-β1/Smad signaling pathway. Exp. Ther. Med. 2021, 21, 461. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkina, E.; Evseenko, V.; Dumchenko, N.; Zelikman, M.; Aktanova, A.; Bykova, M.; Khvostov, M.; Dushkin, A.; Kozlov, V. Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy. Nanomaterials 2022, 12, 148. https://doi.org/10.3390/nano12010148
Pashkina E, Evseenko V, Dumchenko N, Zelikman M, Aktanova A, Bykova M, Khvostov M, Dushkin A, Kozlov V. Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy. Nanomaterials. 2022; 12(1):148. https://doi.org/10.3390/nano12010148
Chicago/Turabian StylePashkina, Ekaterina, Veronika Evseenko, Natalya Dumchenko, Maxim Zelikman, Alina Aktanova, Maria Bykova, Mikhail Khvostov, Aleksandr Dushkin, and Vladimir Kozlov. 2022. "Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy" Nanomaterials 12, no. 1: 148. https://doi.org/10.3390/nano12010148
APA StylePashkina, E., Evseenko, V., Dumchenko, N., Zelikman, M., Aktanova, A., Bykova, M., Khvostov, M., Dushkin, A., & Kozlov, V. (2022). Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy. Nanomaterials, 12(1), 148. https://doi.org/10.3390/nano12010148






