Experimental Pore-Scale Study of a Novel Functionalized Iron-Carbon Nanohybrid for Enhanced Oil Recovery (EOR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Synthesis of Carbon Nanoparticle
2.1.3. Synthesis of Fe3O4/Carbon Nanohybrid
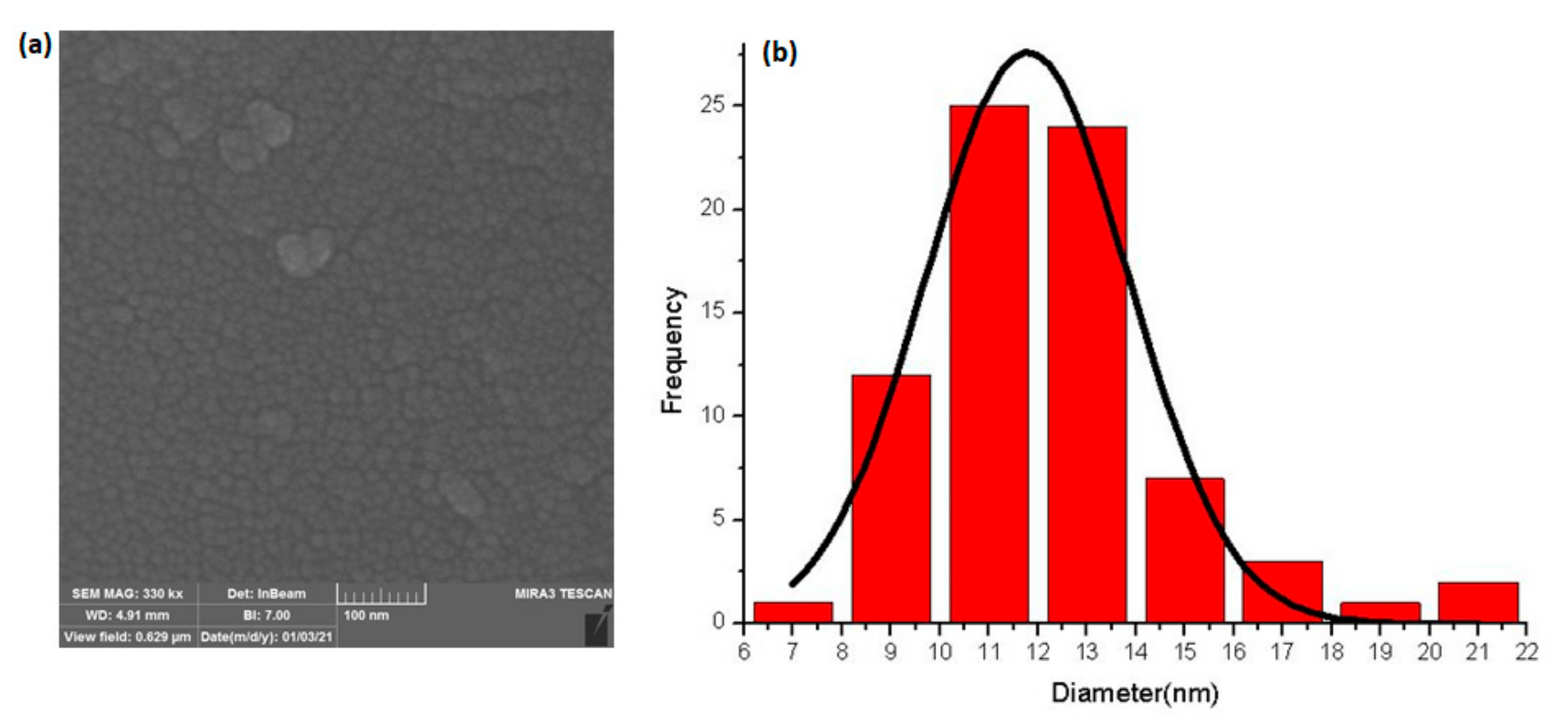
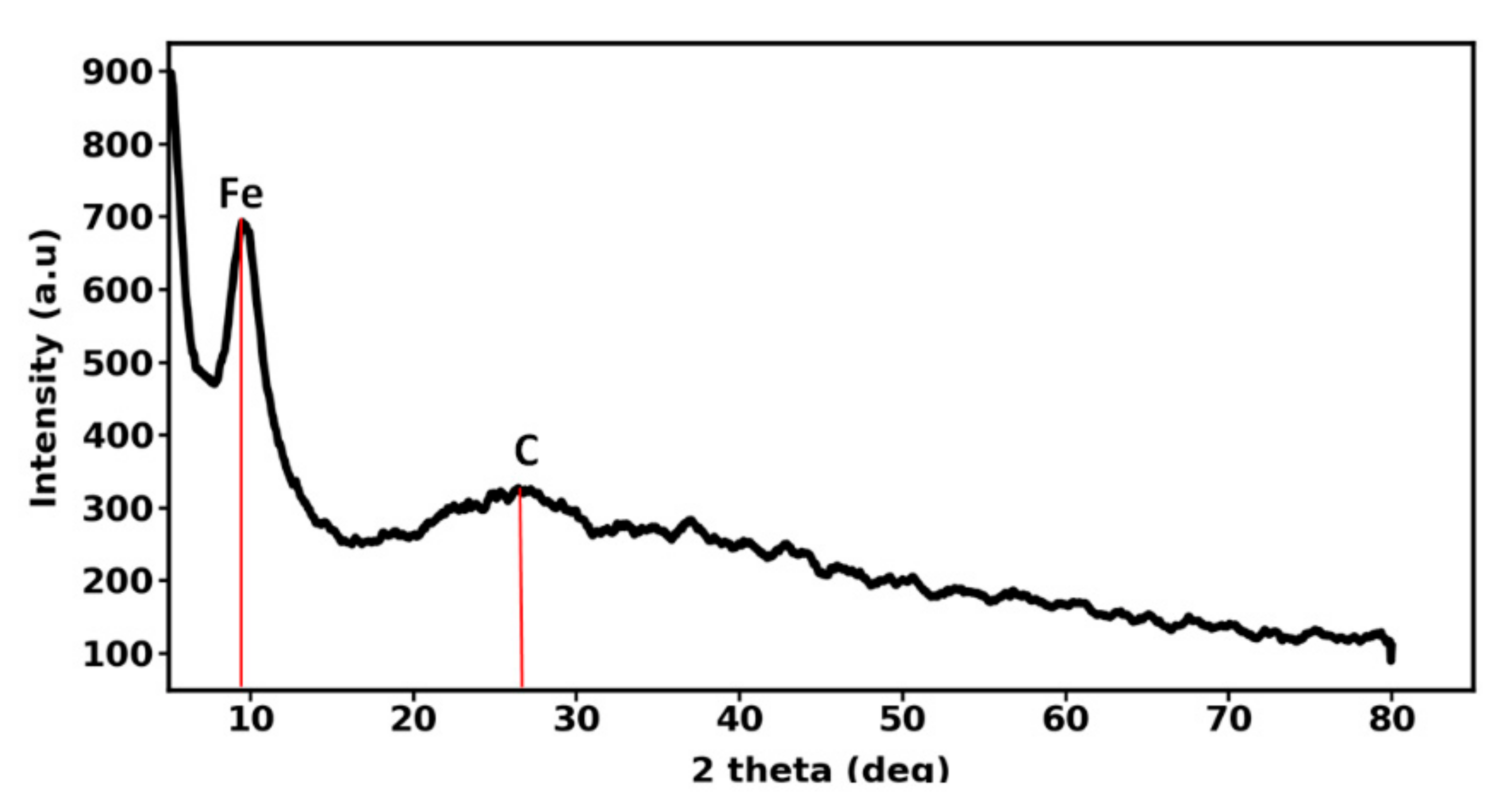
2.1.4. Nanoparticle Characterization
2.1.5. Oil Specifications
2.1.6. Brine Solutions
2.2. Methods
2.2.1. Colloidal Stability
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.3. Viscosity of the Nanofluid
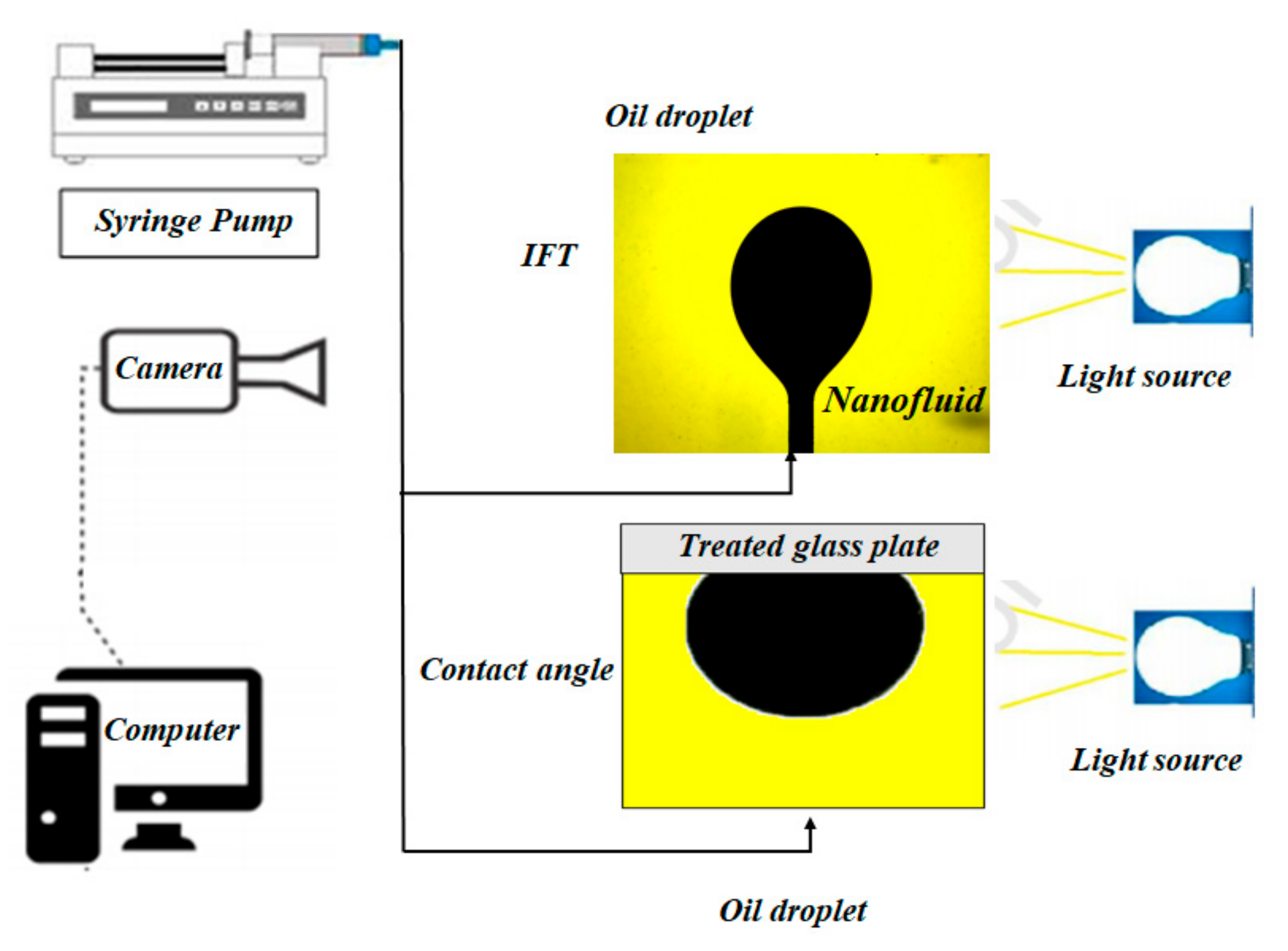
2.2.4. Interfacial Tension (IFT) Measurement
2.2.5. Contact Angle Measurement
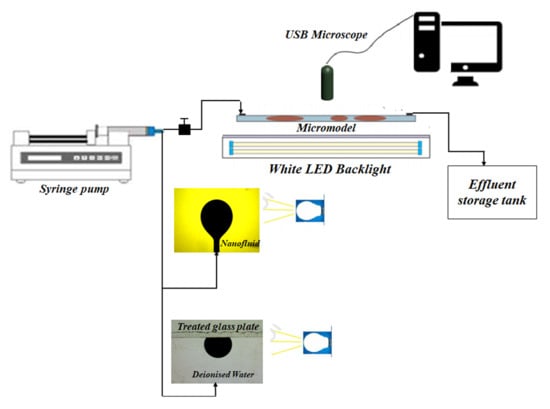
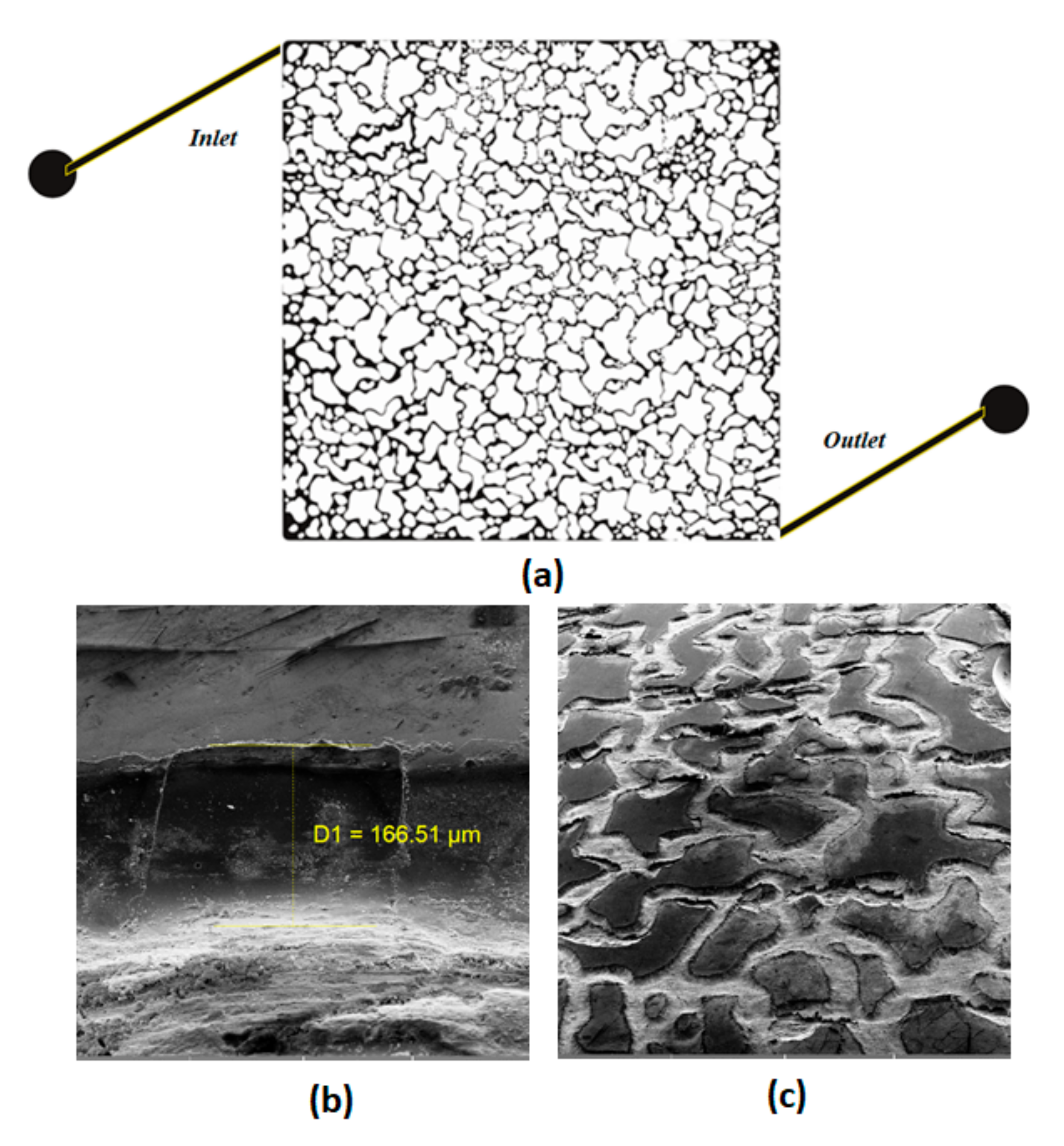
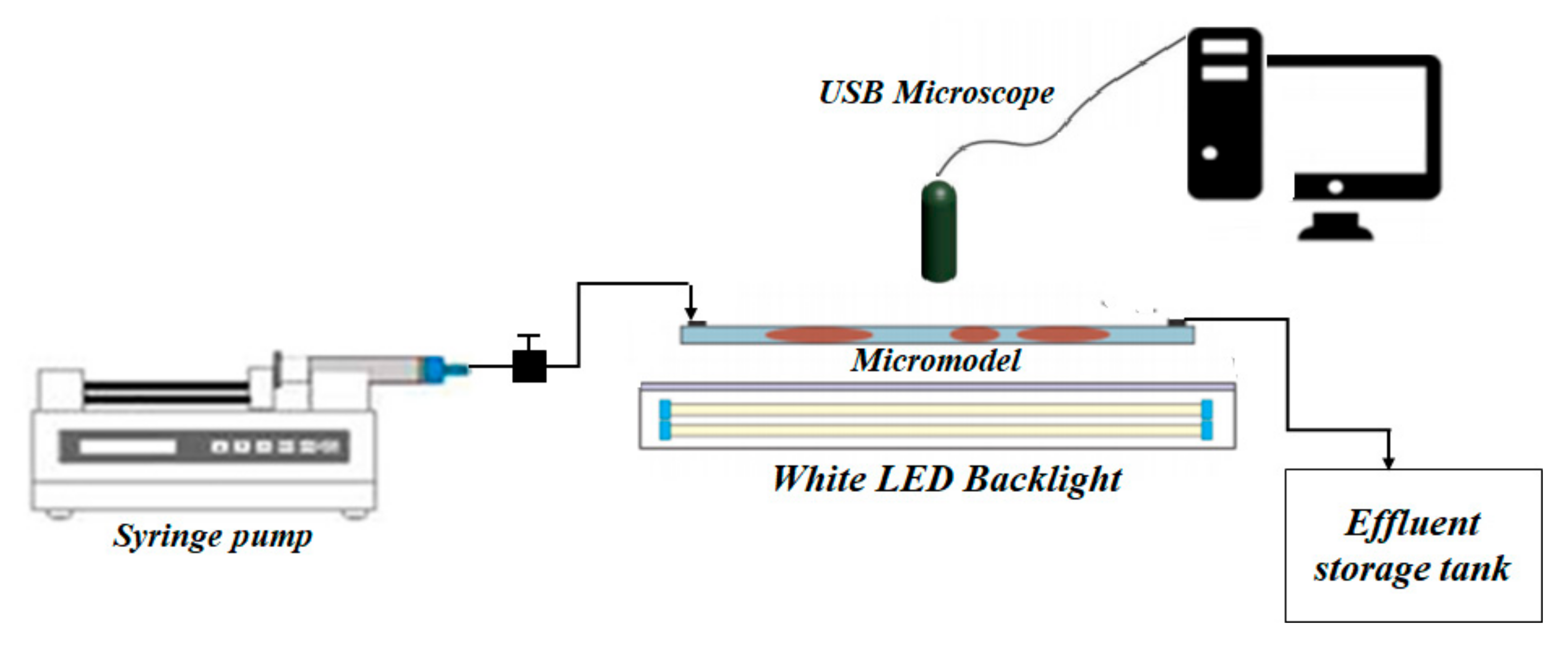
2.2.6. Displacement Experiments
3. Results and Discussion
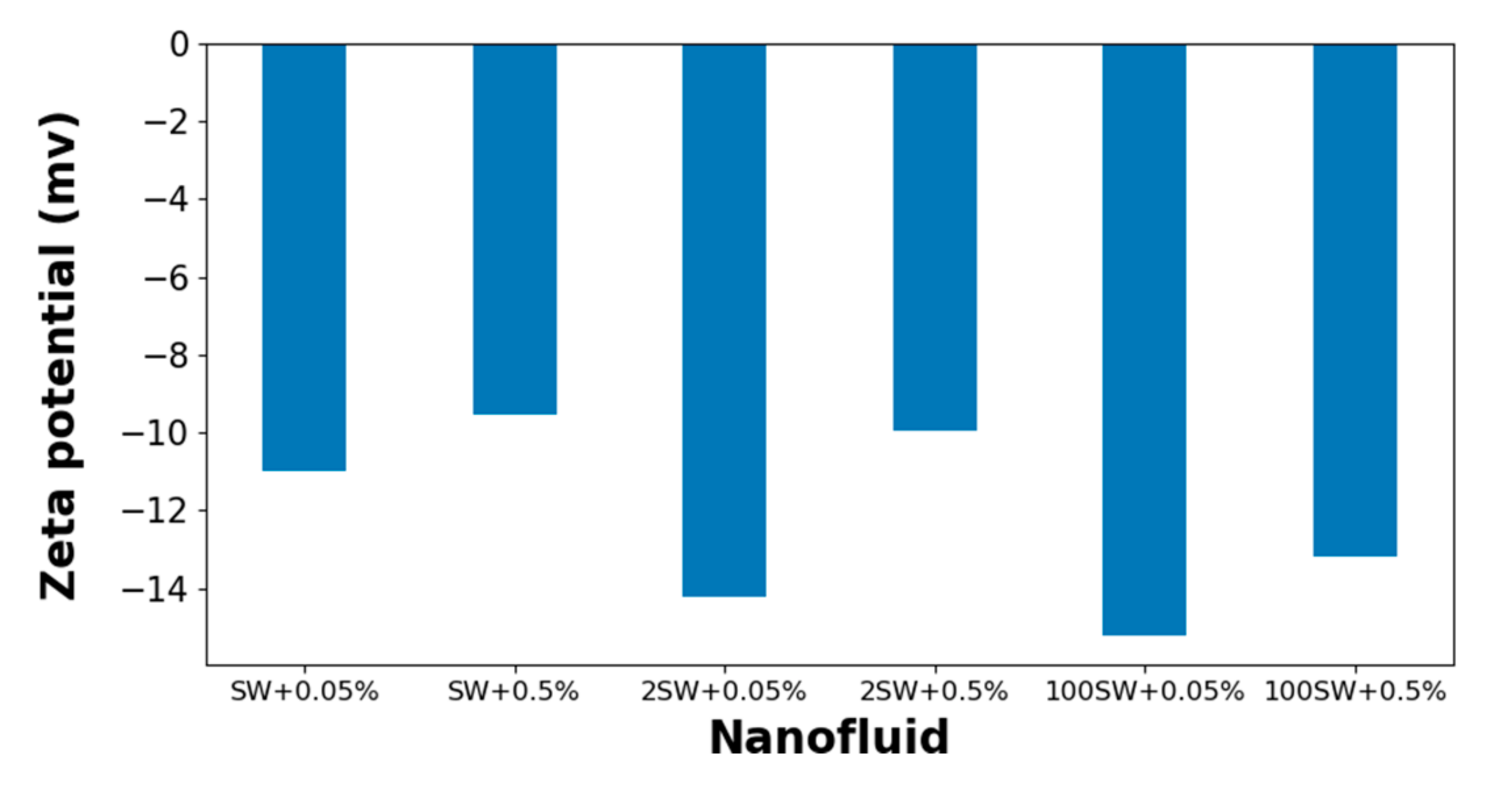
3.1. Colloidal Stability
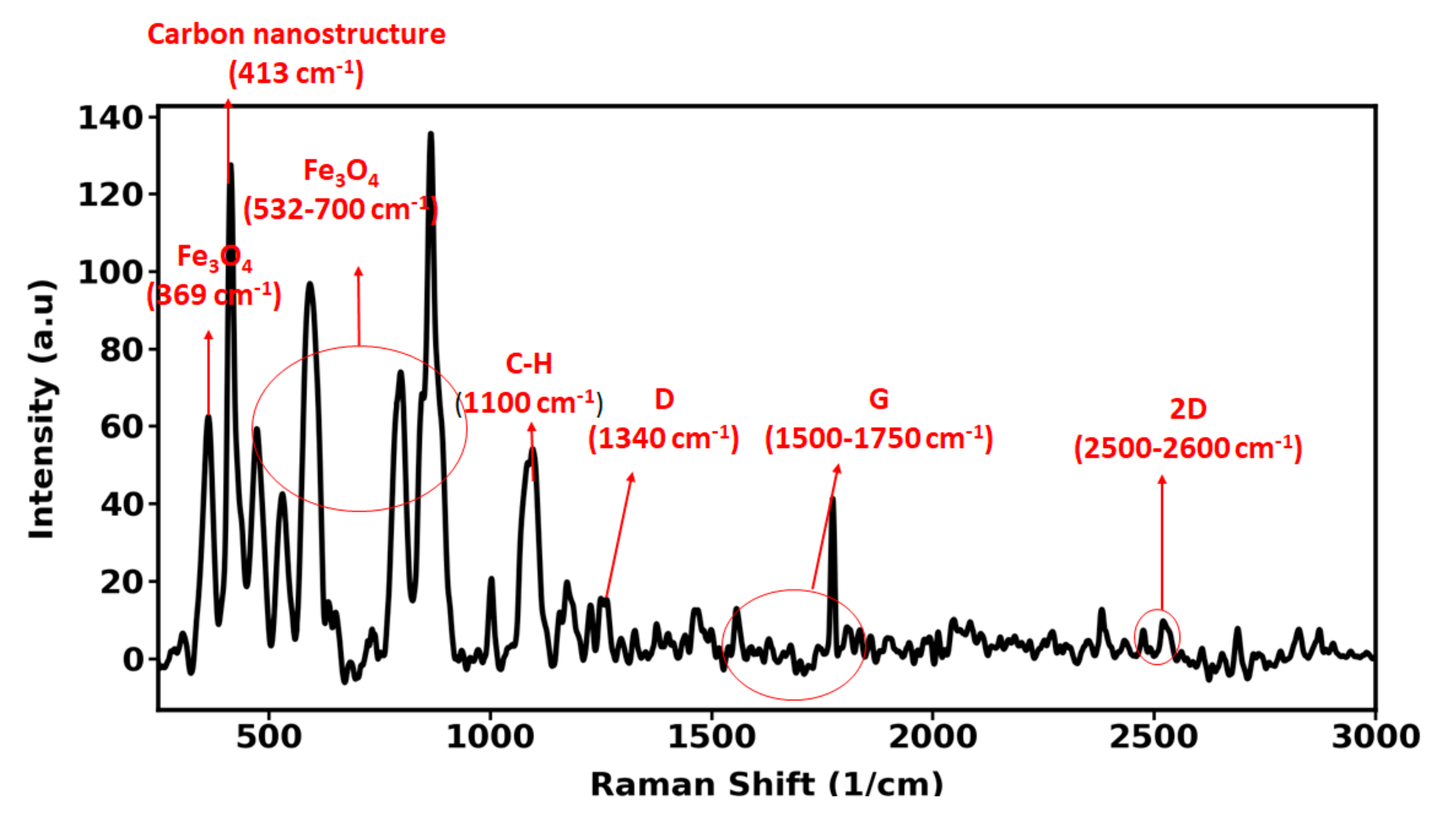
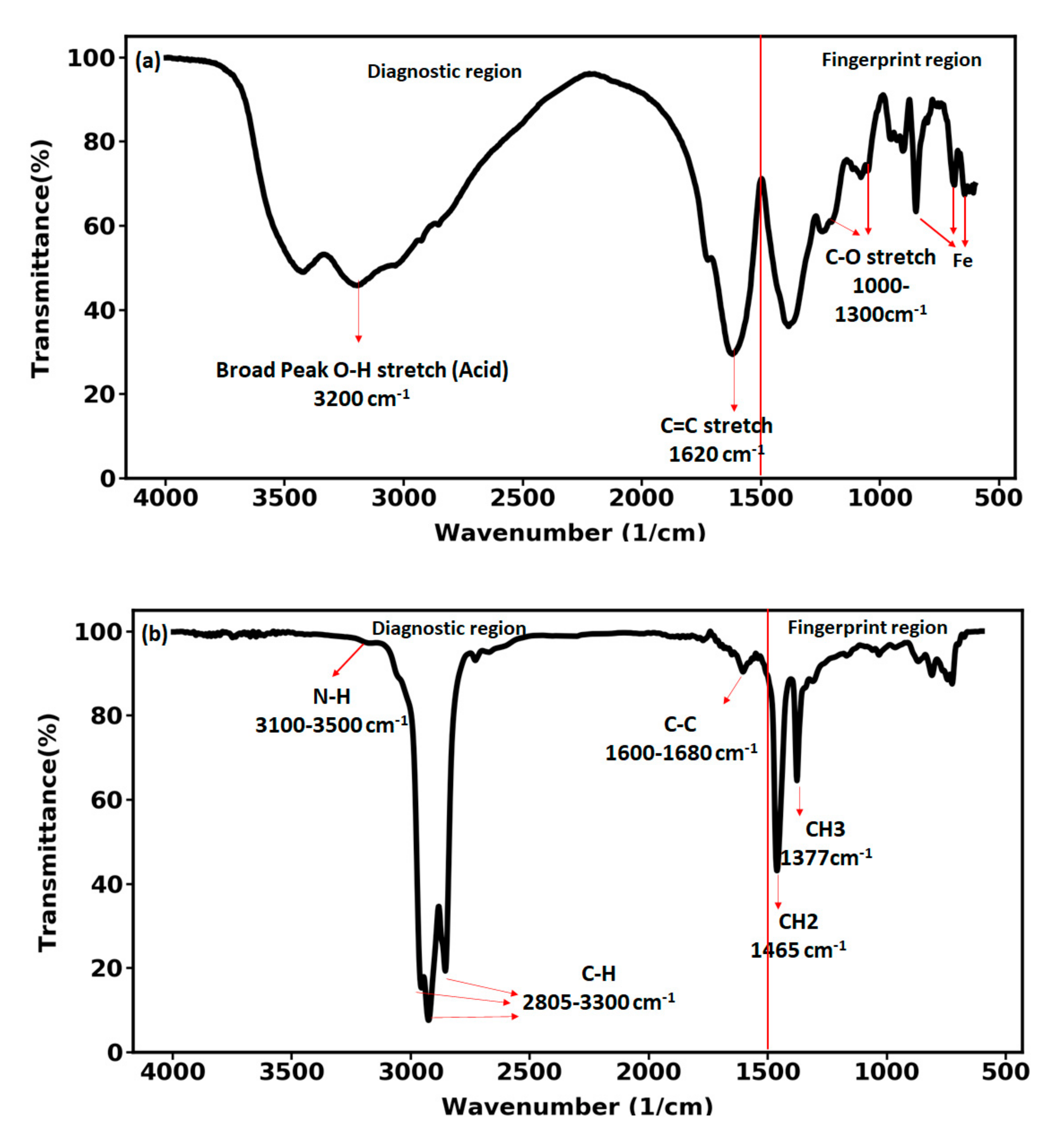
3.2. FTIR Measurements of the Carbon Nanohybrid
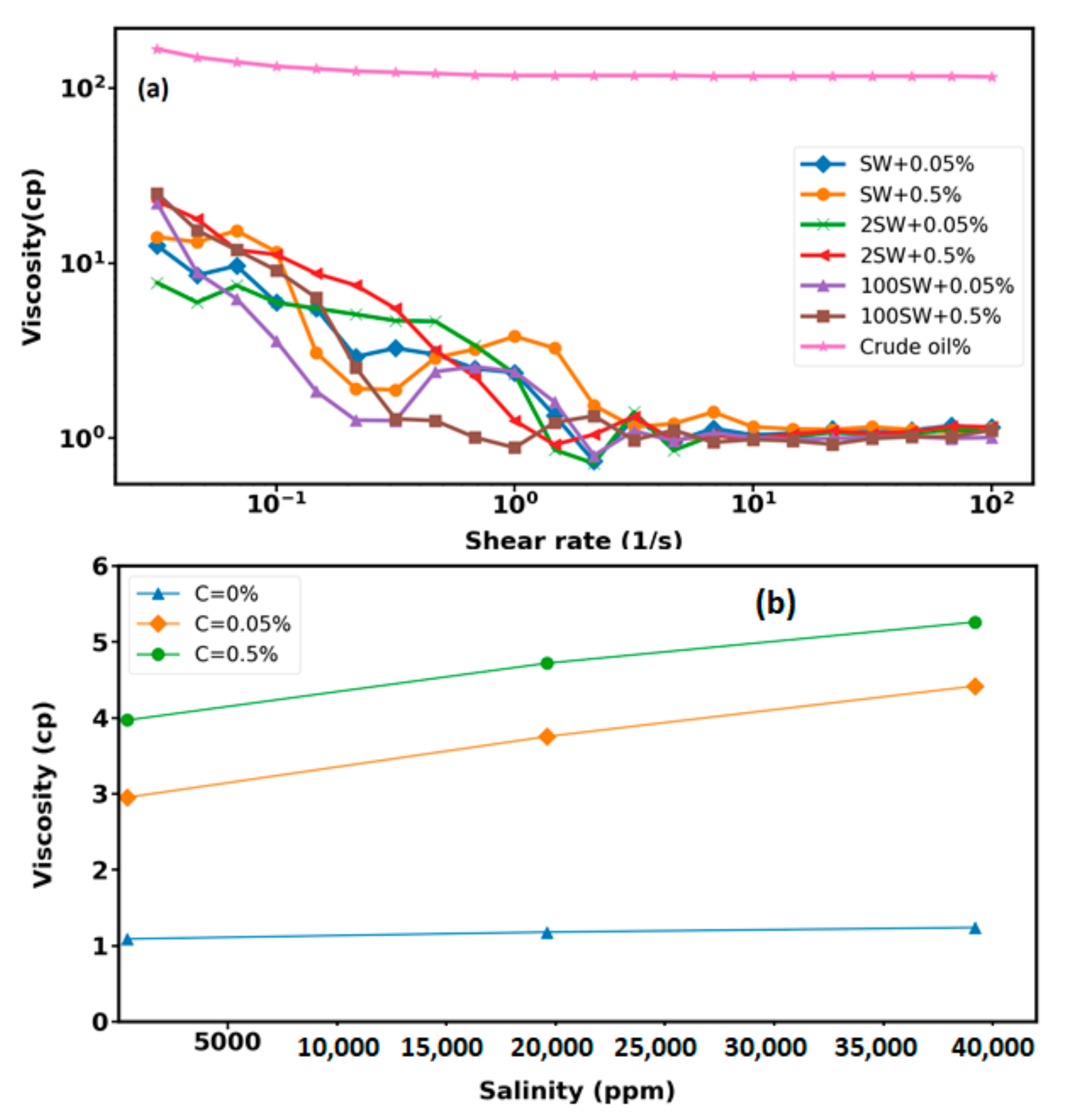
3.3. Viscosity Measurements
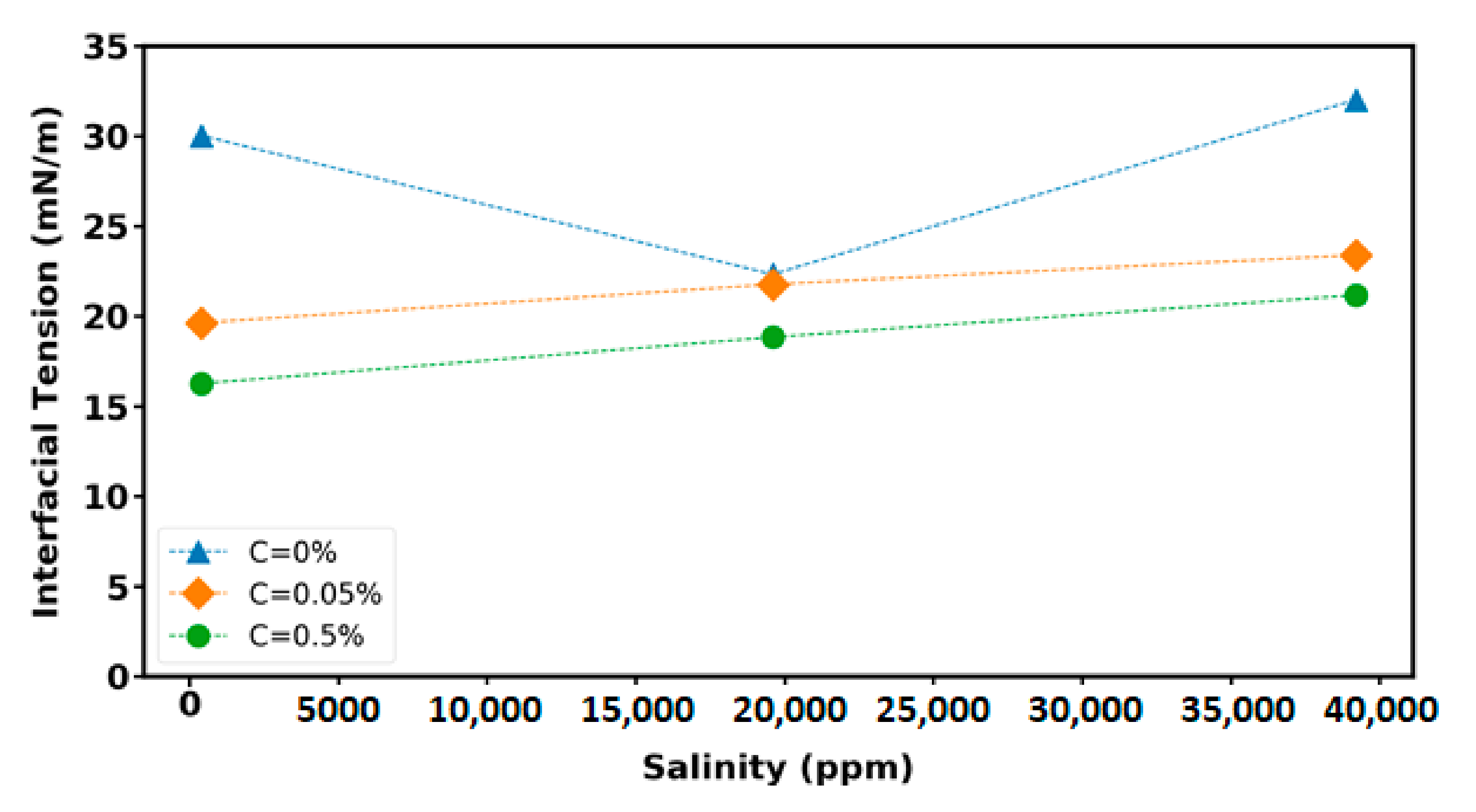
3.4. Interfacial Tension (IFT) Measurement
3.5. Contact Angle Measurement
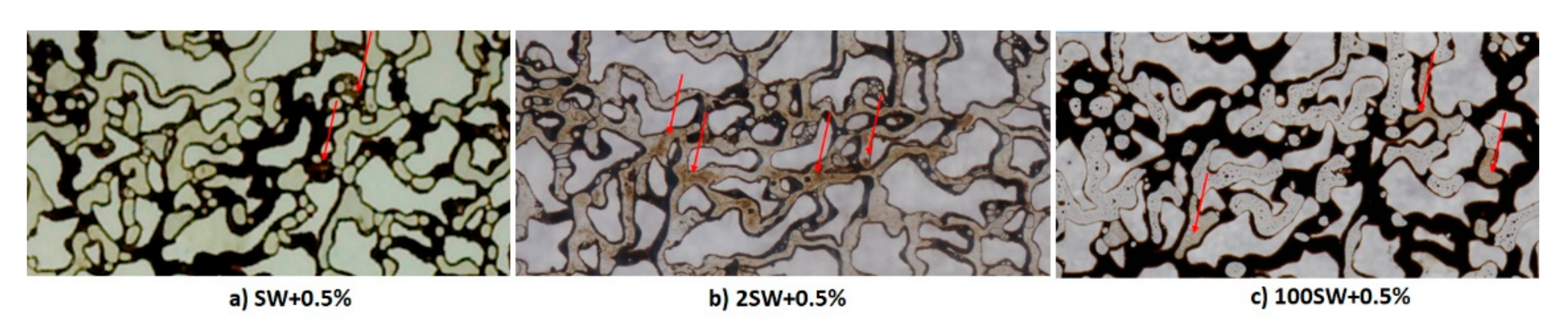
3.6. Quantitative and Qualitative Analyses of Micro-Dispersion
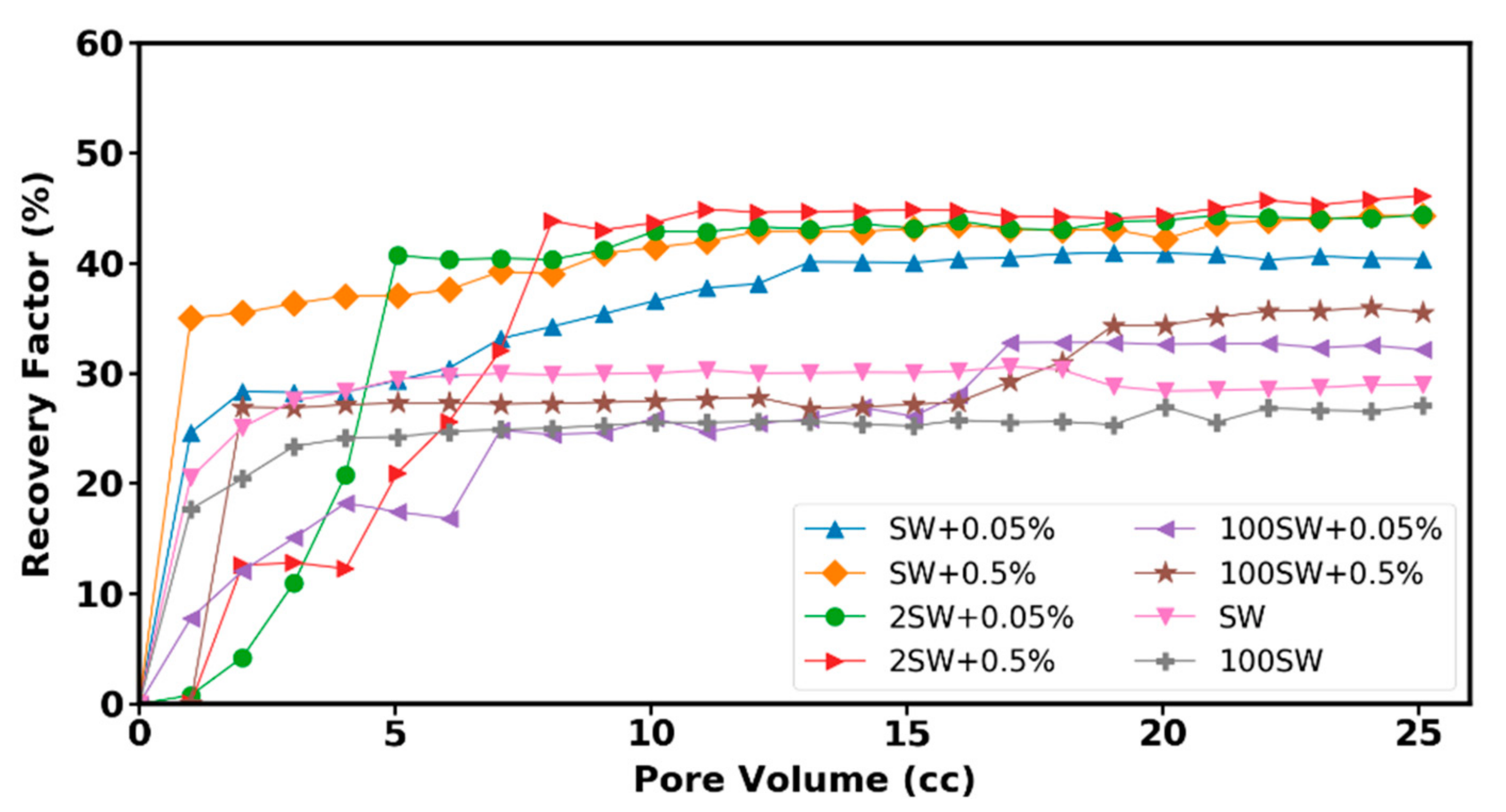
3.7. Displacement Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lau, H.C.; Yu, M.; Nguyen, Q.P. Nanotechnology for oilfield applications: Challenges and impact. J. Pet. Sci. Eng. 2017, 157, 1160–1169. [Google Scholar] [CrossRef]
- Sabet, M.; Hosseini, S.N.; Zamani, A.; Hosseini, Z.; Soleimani, H. Application of nanotechnology for enhanced oil recovery: A review. Defect Diffus. Forum 2016, 367, 149–156. [Google Scholar] [CrossRef]
- Cao, N.; Mohammed, M.A.; Babadagli, T. Wettability Alteration of heavy-oil-bitumen-containing carbonates by use of solvents, high-ph solutions, and nano/ionic liquids. SPE Reserv. Eval. Eng. 2016, 20, 9. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Society of Petroleum Engineers, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Huh, C.; Nizamidin, N.; Pope, G.A.; Milner, T.E.; Wang, B. Hydrophobic Paramagnetic Nanoparticles as Intelligent Crude Oil Tracers. WO Patent WO2014123672A1, 14 August 2014. [Google Scholar]
- Roustaei, A.; Moghadasi, J.; Bagherzadeh, H.; Shahrabadi, A. An Experimental Investigation of Polysilicon Nanoparticles’ Recovery Efficiencies Through Changes in Interfacial Tension and Wettability Alteration. In Proceedings of the SPE Society of Petroleum Engineers, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Shah, R.D. Application of nanoparticle saturated injectant gases for EOR of heavy oils. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 8, 156–159. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Colloid. Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Zhang, H.; Nikolov, A.; Wasan, D.T. Enhanced oil recovery using nanoparticle dispersions: Underlying mechanism and imbibition experiments. Energy Fuels 2014, 28, 3002–3009. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereiraalmao, P.; Cortes, F.B. Nanoparticles for inhibition of asphaltenes damage: Adsorption study and displacement test on porous media. energy fuels. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Eshraghi, S.E.; Kazemi, K.; Sourani, S.; Mehrabi, M.; Ahmadi, Y. Behavior of asphaltene adsorption onto the metal oxide nanoparticle surface and its effect on heavy oil recovery. Ind. Eng. Chem. Res. 2015, 54, 233–239. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O. An Experimental Investigation of EOR Mechanisms for Nanoparticles Fluid in Glass Micromodel. In Proceedings of the International Symposium of the Society of Core Analysts, Avignon, France, 8–11 September 2014. [Google Scholar]
- Wang, D.; Tian, B.; Cao, M.; Sun, Y.; Li, S.; Lu, T.; Wang, J. Mechanism Discussion of Nanofluid for Enhanced Oil Recovery: Adhesion Work Evaluation and Direct Force Measurements between Nanoparticles and Surfaces. Energy Fuels 2018, 32, 11390–11397. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O.A. Stabilizer that Enhances the oil recovery process using silica-based nanofluids. Transp. Porous Media 2015, 108, 679–696. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Effect of Hydrophobic and hydrophilic metal oxide nanoparticles on the performance of xanthan gum solutions for heavy oil recovery. Nanomaterials 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Mohammadalinejad, P.; Hosseinpour, N.; Rahmati, N.; Rasaei, M.R. Formation damage during oil displacement by aqueous SiO2 nanofluids in water-wet/oil-wet glass micromodel porous media. J. Pet. Sci. 2019, 182, 106297. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and charging of colloidal silica particles: Effect of particle size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K. Toxicity of metal oxides nanoparticles. Adv. Mol. Toxicol. 2011, 5, 145–178. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability literature survey-part 1: Rock/oil/brine interactions and the effects of core handling on wettability. J. Pet. Technol. 1986, 38, 1125–1144. [Google Scholar] [CrossRef]
- Strand, S.; Puntervold, T.; Austad, T. Water based EOR from clastic oil reservoirs by wettability alteration: A review of chemical aspects. J. Pet. Sci. Eng. 2016, 146, 1079–1091. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M. Review of low salinity waterflooding mechanisms: Wettability alteration and its impact on oil recovery. Fuel 2020, 267, 117112. [Google Scholar] [CrossRef]
- Zhang, P.; Austad, T. Wettability and oil recovery from carbonates: Effects of temperature and potential determining ions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 179–187. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. Smart water as a wettability modifier in chalk: The effect of salinity and ionic composition. Energy Fuel 2010, 24, 2514–2519. [Google Scholar] [CrossRef] [Green Version]
- Strand, S.; Høgnesen, E.J.; Austad, T. Wettability alteration of carbonates—Effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 1–10. [Google Scholar] [CrossRef]
- Karoussi, O.; Hamouda, A.A. Imbibition of sulfate and magnesium ions into carbonate rocks at elevated temperatures and their influence on wettability alteration and oil recovery. Energy Fuel 2007, 21, 2138–2146. [Google Scholar] [CrossRef]
- Shariatpanahi, S.F.; Hopkins, P.; Aksulu, H.; Strand, S.; Puntervold, T.; Austad, T. Water based EOR by wettability alteration in dolomite. Energy Fuel 2016, 30, 180–187. [Google Scholar] [CrossRef]
- Prabhakar, S.; Melnik, R. Wettability alteration of calcite oil wells: Influence of smart water ions. Sci. Rep. 2017, 7, 17365. [Google Scholar] [CrossRef] [Green Version]
- Romero-Zeron, L.B. The Role of Porous Media Wettability on Foamed Gel Propagation and Fluid Diverting Performance. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2004. [Google Scholar]
- Meybodi, E.H.; Kharrat, R.; Araghi, N.M. Experimental studying of pore morphology and wettability effects on microscopic and macroscopic displacement efficiency of polymer flooding. J. Pet. Sci. Eng. 2011, 78, 347–363. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Zarko, V.I.; Leboda, R.; Chibowski, E. Aqueous suspension of fumed oxides: Particle size distribution and zeta potential. Adv. Colloid. Interface Sci. 2001, 91, 1–112. [Google Scholar] [CrossRef]
- Tajik, S.; Nasernejad, B.; Rashidi, A. Preparation of silica-graphene nanohybrid as a stabilizer of emulsions. J. Mol. Liq. 2016, 222, 788–795. [Google Scholar] [CrossRef]
- Jagdale, S.C.; Deore, G.K.; Chabukswar, A.R. Development of microemulsion based nabumetone transdermal delivery for treatment of arthritis. Recent Pat. Drug Deliv. Formul. 2018, 12, 130–149. [Google Scholar] [CrossRef]
- Mahzari, P.; Sohrabi, M.; Cooke, A.J.; Carnegie, A. Direct pore-scale visualization of interactions between different crude oils and low salinity brine. J. Pet. Sci. 2018, 166, 73–84. [Google Scholar] [CrossRef]
- Akhlaq, M.S. Characterization of the isolated wetting crude oil components with infrared spectroscopy. J. Pet. Sci. 1999, 22, 229–235. [Google Scholar] [CrossRef]
- Abbas, S.; Yadav, I.; Kumar, S.; Aswal, V.K.; Kohlbrecher, J. Structure and interaction in pathway of charged nanoparticles aggregation in saline water as probed by scattering techniques. Chem. Phys. Lett. 2017, 675, 124–130. [Google Scholar] [CrossRef]
- Rostami, P.; Fattahi Mehraban, M.; Sharifi, M.; Dejam, M.; Ayatollahi, S. Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: A mechanistic study. Petroleum 2019, 5, 367–374. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S. Effect of asphaltene and resin on interfacial tension of acidic crude oil/sulfate aqueous solution: Experimental study. Fluid Phase Equilib. 2016, 414, 149–155. [Google Scholar] [CrossRef]
- Mokhtari, R.; Ayatollahi, S.; Fatemi, M. Experimental investigation of the influence of fluid-fluid interactions on oil recovery during low salinity water flooding. J. Pet. Sci. 2019, 182, 106194. [Google Scholar] [CrossRef]
- Mehraban, M.F.; Afzali, S.; Ahmadi, Z.; Mokhtari, R.; Ayatollahi, S.; Sharifi, M.; Kazemi, A.; Nasiri, M.; Fathollahi, S. Smartwater flooding in a carbonate asphaltenic fractured oil reservoir—Comprehensive fluidfluid-rock mechanistic study. In Proceedings of the 19th European Symposium on Improved Oil Recovery: Sustainable 539 IOR in a Low Oil Price World, Stavanger, Norway, 24–27 April 2017. [Google Scholar] [CrossRef]
- Ghorbanizadeh, S.; Rostami, B. Surface and interfacial tension behavior of salt water containing dissolved amphiphilic compounds of crude oil: The role of single-salt ionic composition. Energy Fuels 2017, 31, 9117–9124. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Manteghian, M. Investigation of synergistic effects between silica nanoparticles, biosurfactant and salinity in simultaneous flooding for enhanced oil recovery. RSC Adv. 2019, 9, 20281. [Google Scholar] [CrossRef] [Green Version]
- Glaser, N.; Adams, D.J.; B¨oker, A.; Krausch, G. Janus particle at liquid-liquid interfaces. Langmuir 2006, 22, 5227–5229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Mejia, A.F.; Diaz, A.; Clearfield, A.; Mannan, M.S.; Chang, Y.W. Amphiphilic Nanosheets and Methods of Making the Same. U.S. Patent 9686983, 7 March 2017. [Google Scholar]
- Xiang, W.; Zhao, S.; Song, X.; Fang, S.; Wang, F.; Zhong, C.; Luo, Z. Amphiphilic nanosheet self-assembly at the water/oil interface: Computer simulations. Phys. Chem. Chem. Phys. 2017, 19, 7576–7586. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Emadi, A.; Sohrabi, M. Visual Investigation of Oil Recovery by Low Salinity Water Injection: Formation of Water Micro-Dispersions and Wettability Alteration. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Stiller, S.; Gers-Barlag, H.; Lergenmueller, M.; Pflucker, F.; Schulz, J.; Wittern, K.P.; Daniels, R. Investigation of the stability in emulsions stabilized with different surface modified titanium dioxides. Colloids. Surf. A 2004, 232, 261–267. [Google Scholar] [CrossRef]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering emulsions: Interfacial tension, colloidal layer morphology, and trapped-particle motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]



| Crude Name | Saturates (%wt) | Aromatics (%wt) | Resins (%wt) | Asphaltenes (%wt) | TAN (mg KOH/g) | TBN (mg KOH/g) | Density (gr/cc) | Viscosity (cp) | |
|---|---|---|---|---|---|---|---|---|---|
| BG | 19.2 | 51 | 31 | 8.3 | 9.7 | 0.09 | 2.94 | 0.939 | 251.39 |
| Ion Compositions | SW (mglit−1) | 2 SW (mglit−1) | 100 W (mglit−1) |
|---|---|---|---|
| Na+ | 12,355 | 6177.5 | 123.5 |
| Cl− | 21,981 | 10,990.5 | 219.81 |
| Ca2+ | 440 | 220 | 4.4 |
| Mg2+ | 1360 | 680 | 13.60 |
| K+ | 375 | 187.5 | 3.75 |
| SO42− | 2740 | 1370 | 27.4 |
| HCO3− | 200 | 100 | 2 |
| Sr2+ | 7.5 | 3.75 | 0.075 |
| Fe2+ | 1 | 0.5 | 0.01 |
| Al3+ | <1 | <0.5 | <0.01 |
| Li+ | <1 | <0.5 | <0.01 |
| TDS (ppm) | 39,182 | 19,591 | 391.82 |
| Dimension (cm) | Pore Diameter (µm) | Etched Depth (µm) | Pore Volume (mL) | Porosity (%) | Absolute Permeability (D) |
|---|---|---|---|---|---|
| 3.5 × 3.5 | 16–270 | 166 | 0.08 | 39 | 1.1 |
| Test No. | Injected Nanofluid (Base Fluid + Nanoparticle Concentration wt %) | Flow Rate | Capillary Number |
|---|---|---|---|
| 1 | SW + 0.05% | 0.00108 | 1 × 10−6 |
| 2 | SW + 0.5% | 0.0017 | 1 × 10−6 |
| 3 | 2SW + 0.05% | 0.00106 | 1 × 10−6 |
| 4 | 2SW + 0.5% | 0.0017 | 1 × 10−6 |
| 5 | 100SW + 0.05% | 0.0018 | 1 × 10−6 |
| 6 | 100SW + 0.5% | 0.0015 | 1 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razavirad, F.; Shahrabadi, A.; Babakhani Dehkordi, P.; Rashidi, A. Experimental Pore-Scale Study of a Novel Functionalized Iron-Carbon Nanohybrid for Enhanced Oil Recovery (EOR). Nanomaterials 2022, 12, 103. https://doi.org/10.3390/nano12010103
Razavirad F, Shahrabadi A, Babakhani Dehkordi P, Rashidi A. Experimental Pore-Scale Study of a Novel Functionalized Iron-Carbon Nanohybrid for Enhanced Oil Recovery (EOR). Nanomaterials. 2022; 12(1):103. https://doi.org/10.3390/nano12010103
Chicago/Turabian StyleRazavirad, Fatemeh, Abbas Shahrabadi, Parham Babakhani Dehkordi, and Alimorad Rashidi. 2022. "Experimental Pore-Scale Study of a Novel Functionalized Iron-Carbon Nanohybrid for Enhanced Oil Recovery (EOR)" Nanomaterials 12, no. 1: 103. https://doi.org/10.3390/nano12010103
APA StyleRazavirad, F., Shahrabadi, A., Babakhani Dehkordi, P., & Rashidi, A. (2022). Experimental Pore-Scale Study of a Novel Functionalized Iron-Carbon Nanohybrid for Enhanced Oil Recovery (EOR). Nanomaterials, 12(1), 103. https://doi.org/10.3390/nano12010103