Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.1.1. One-Pot Sol–Gel Auto-Combustion Method
2.1.2. Green Pulsed Laser Ablation in Liquid (PLAL) Method
3. Results and Discussion
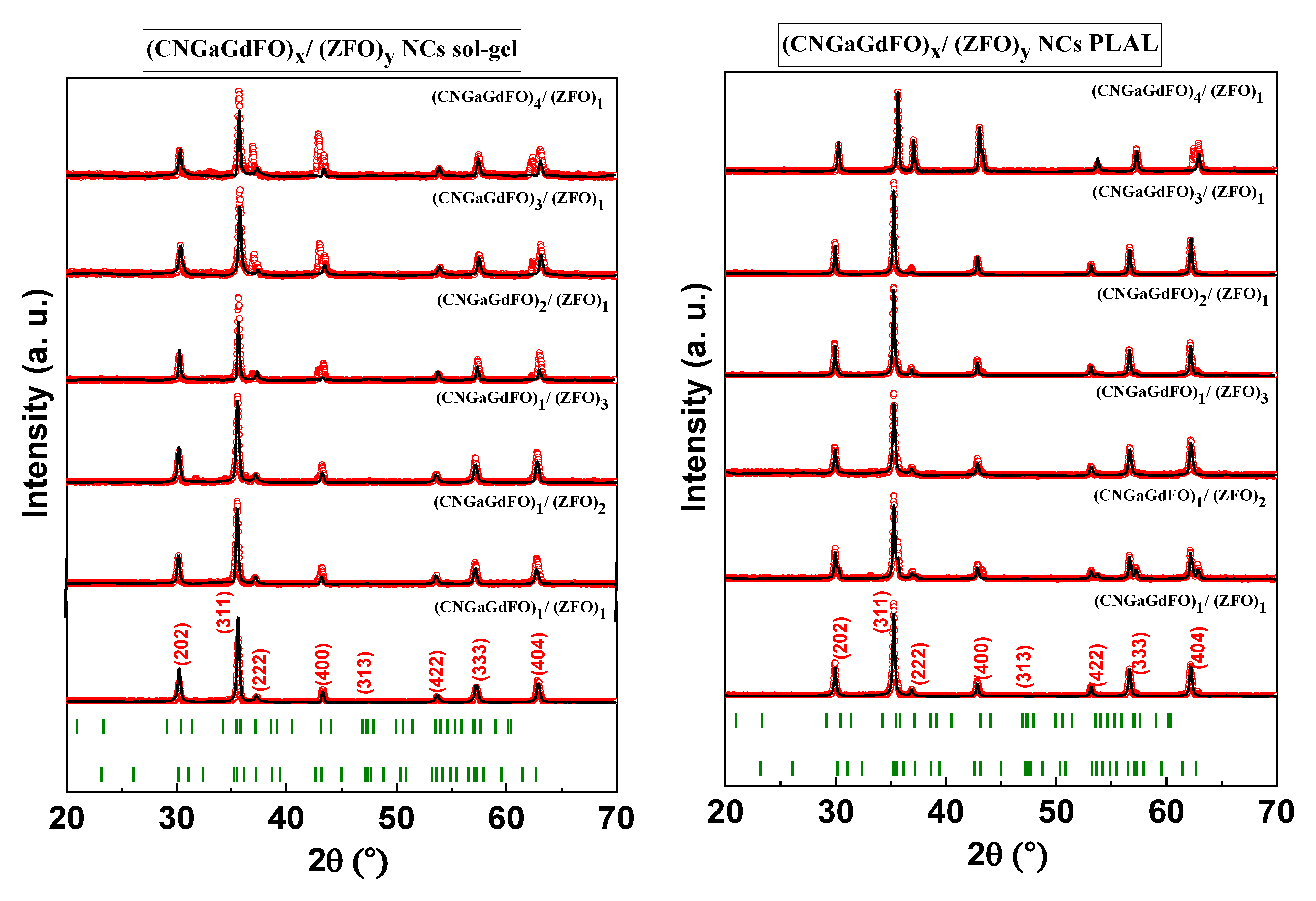
3.1. Phase Characterization
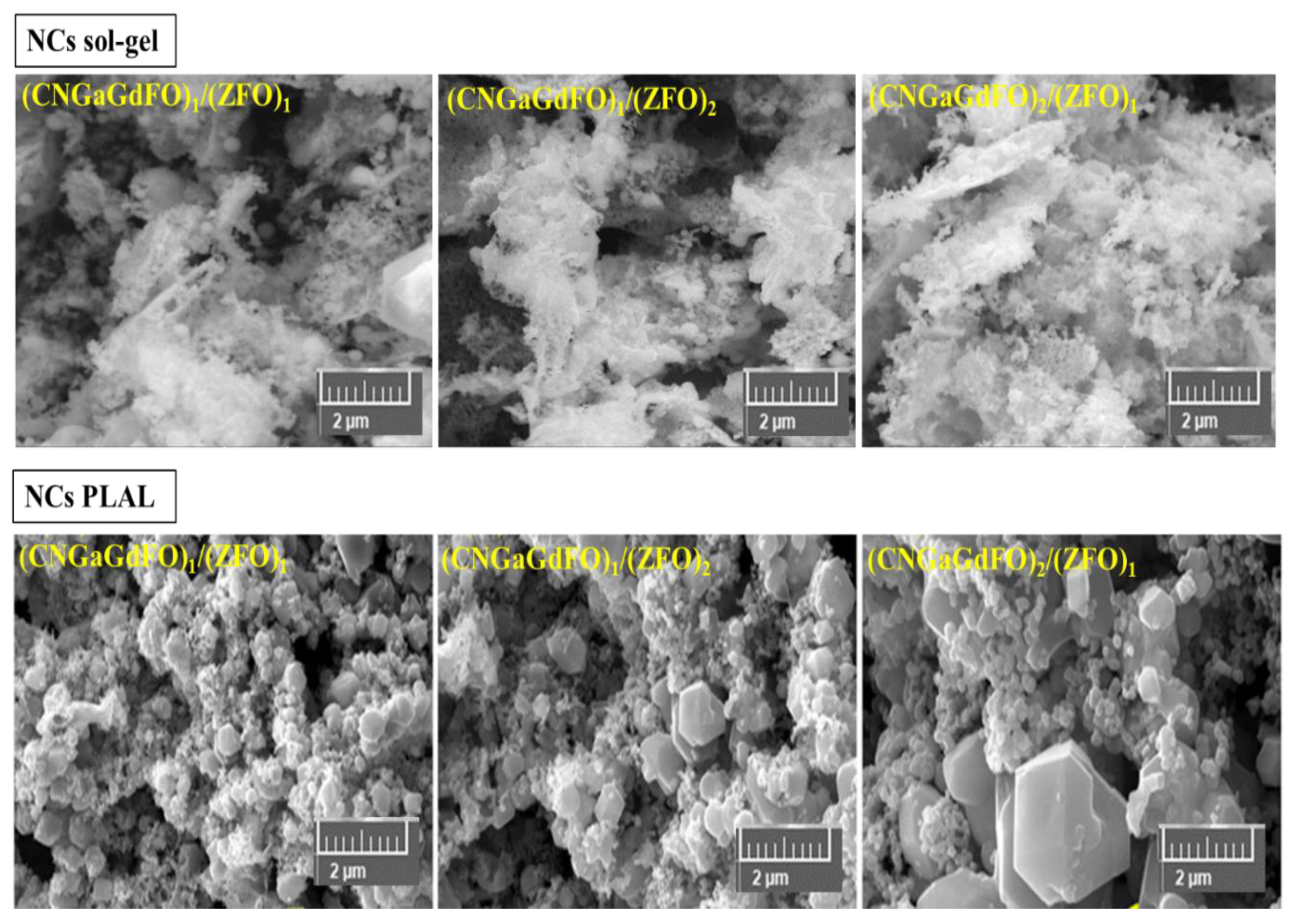
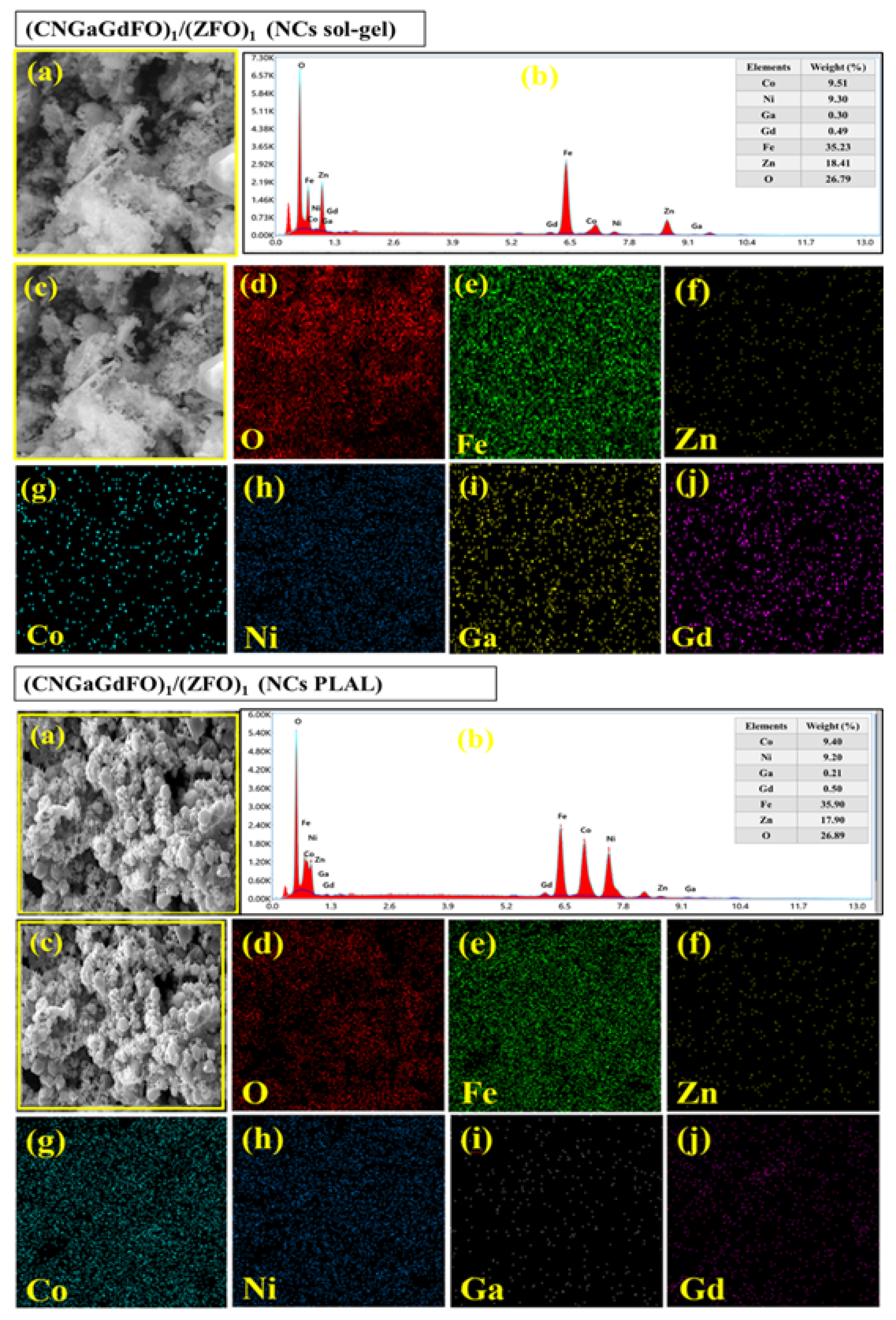
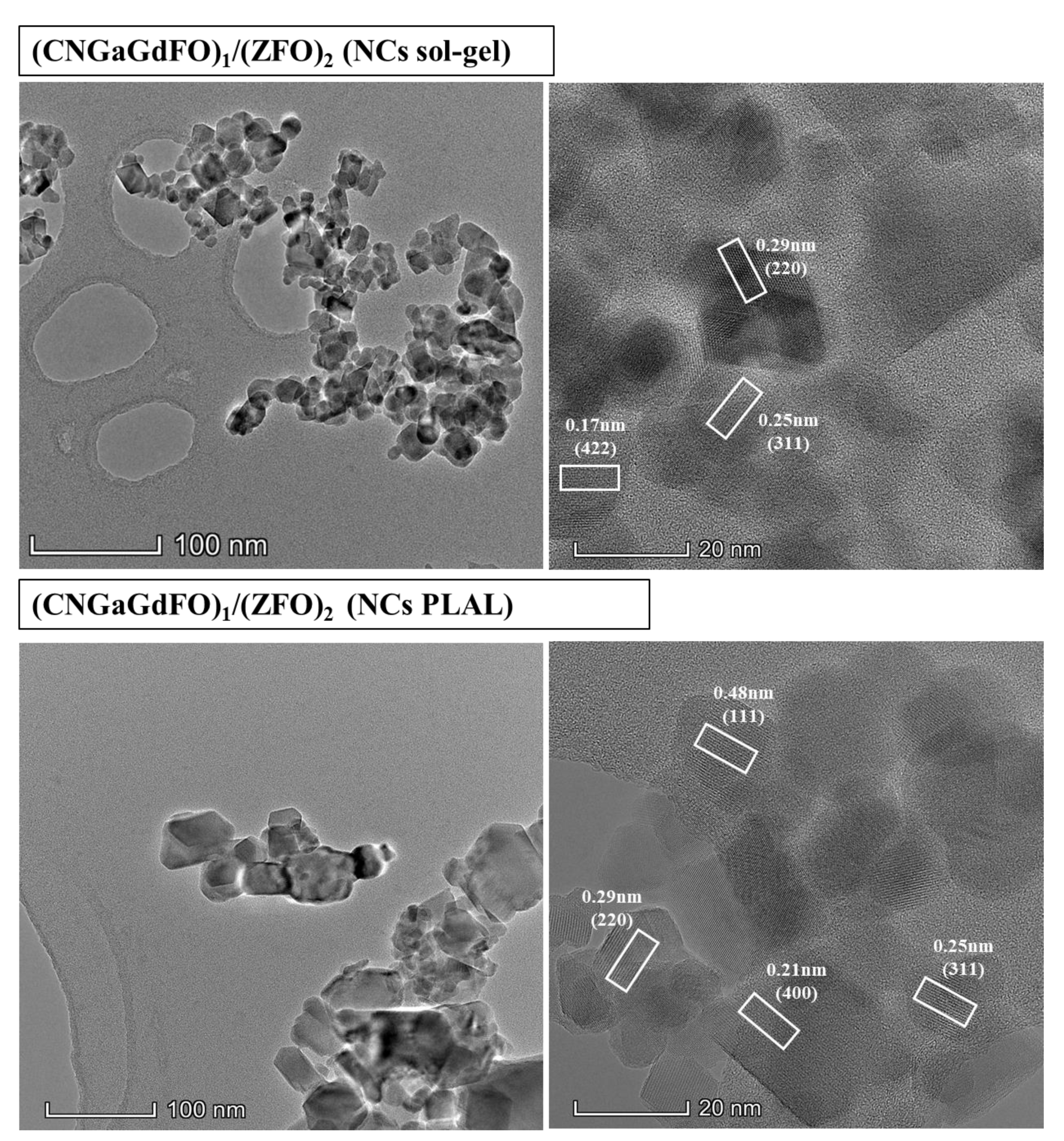
3.2. Microstructural Features
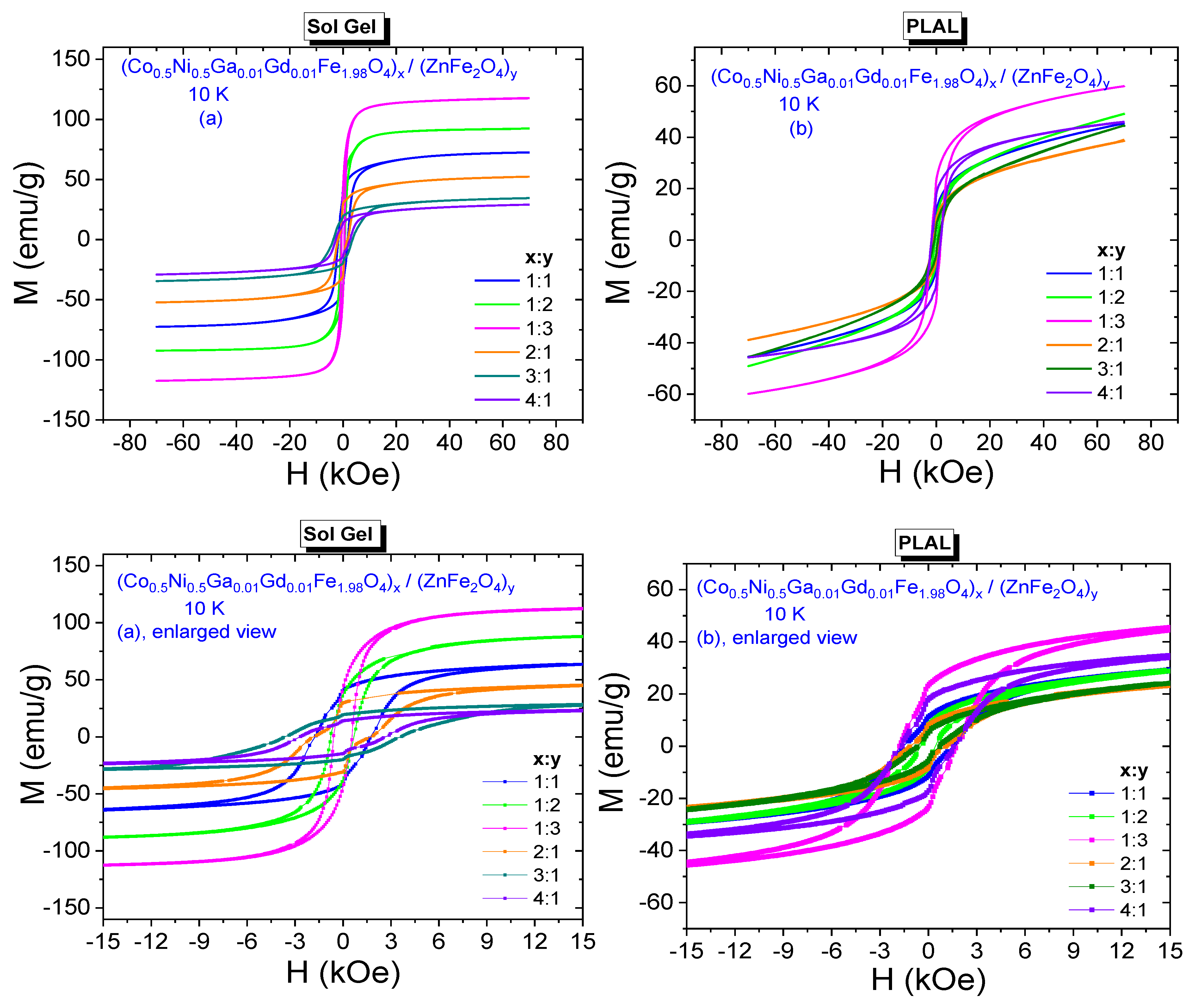
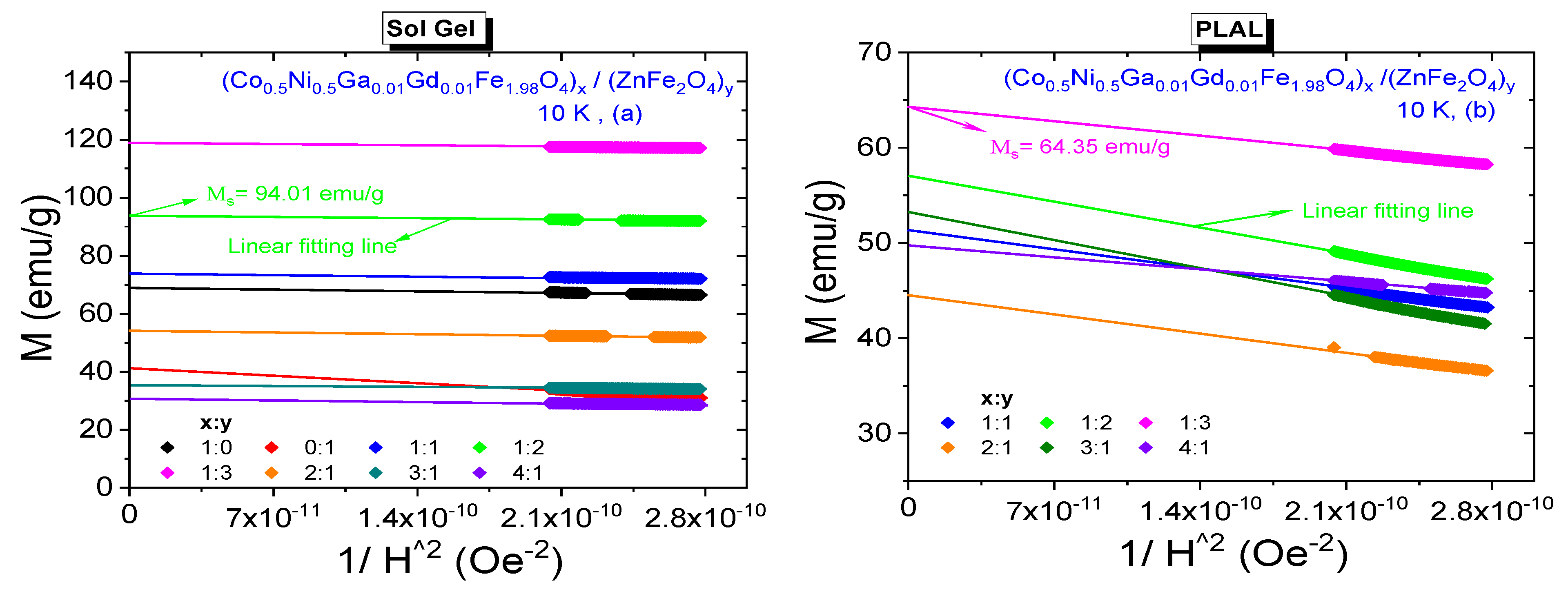
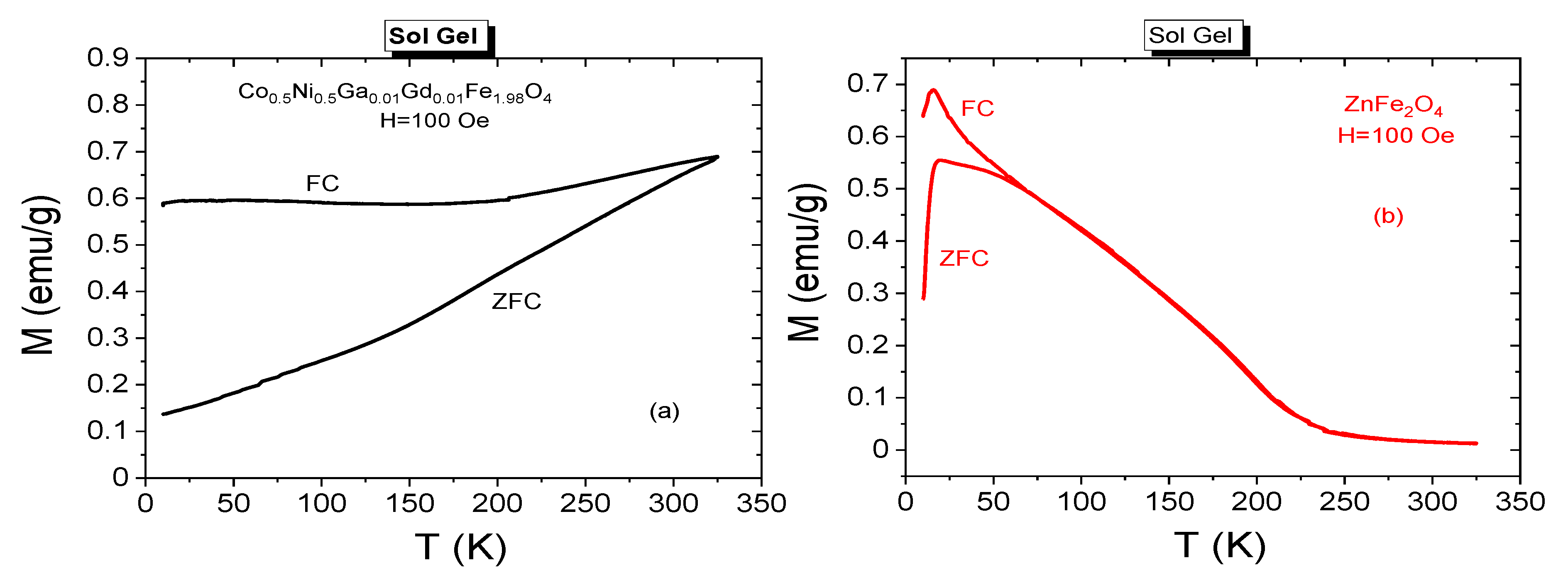
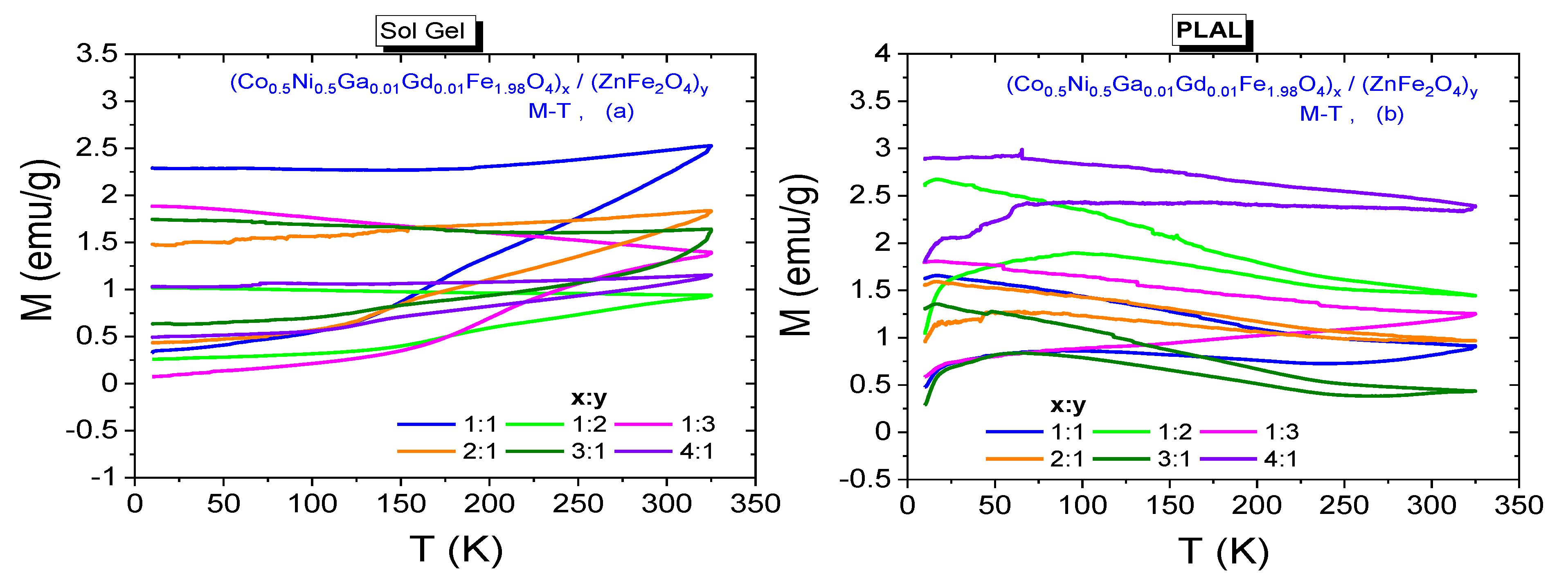
3.3. Magnetic Properties
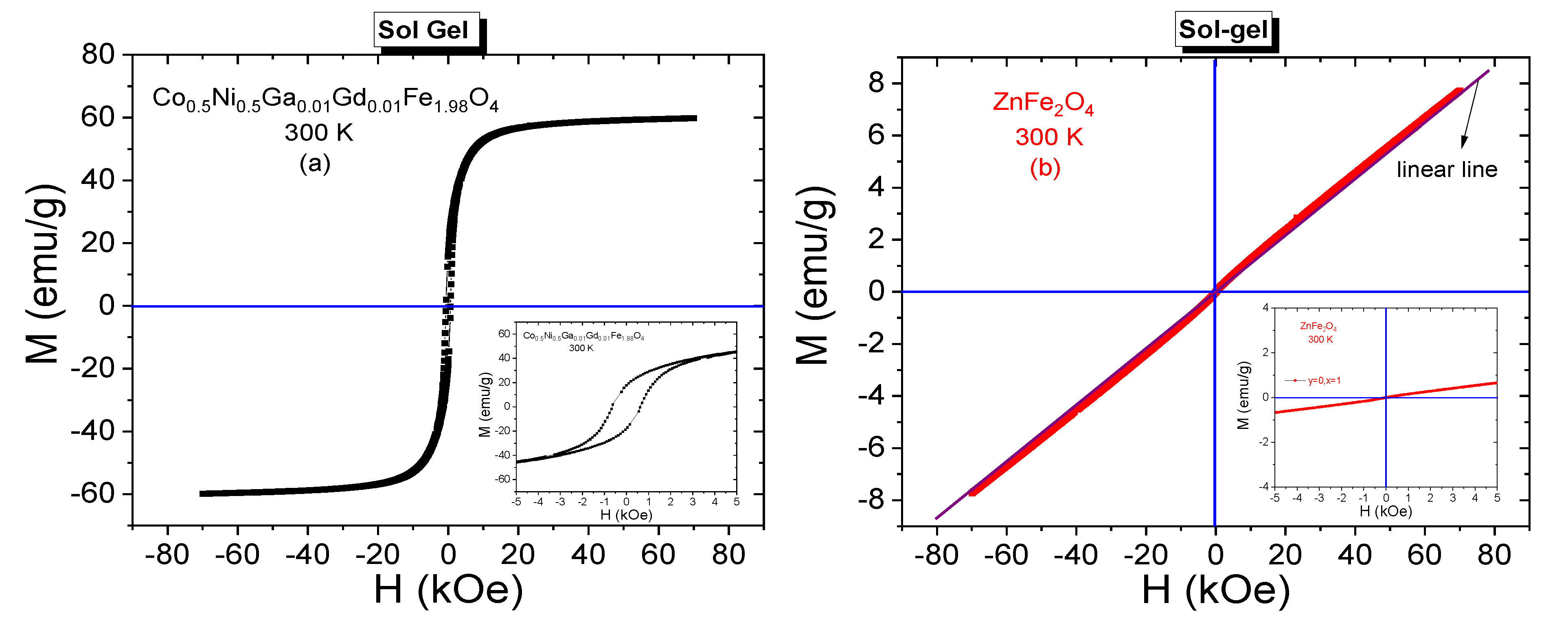
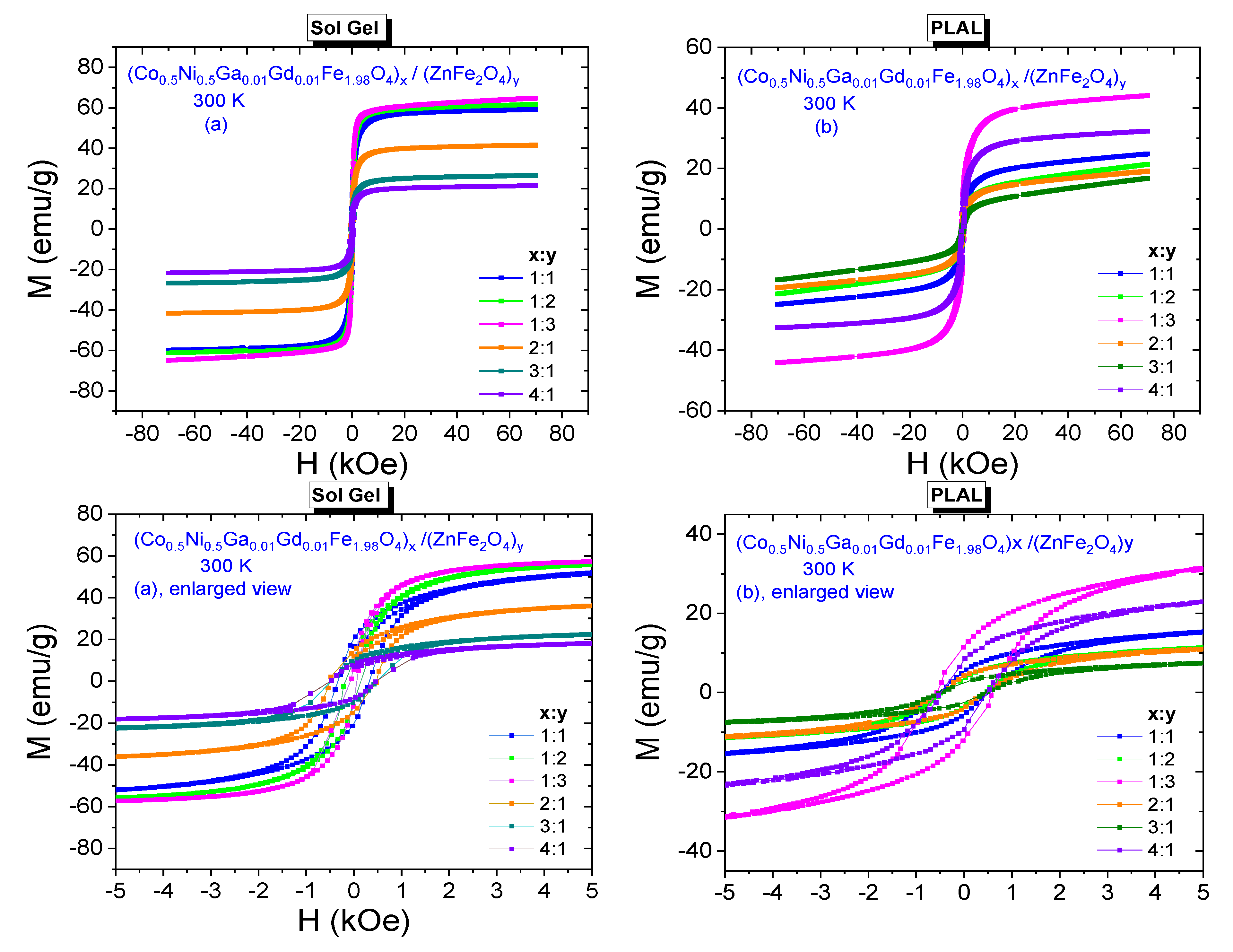
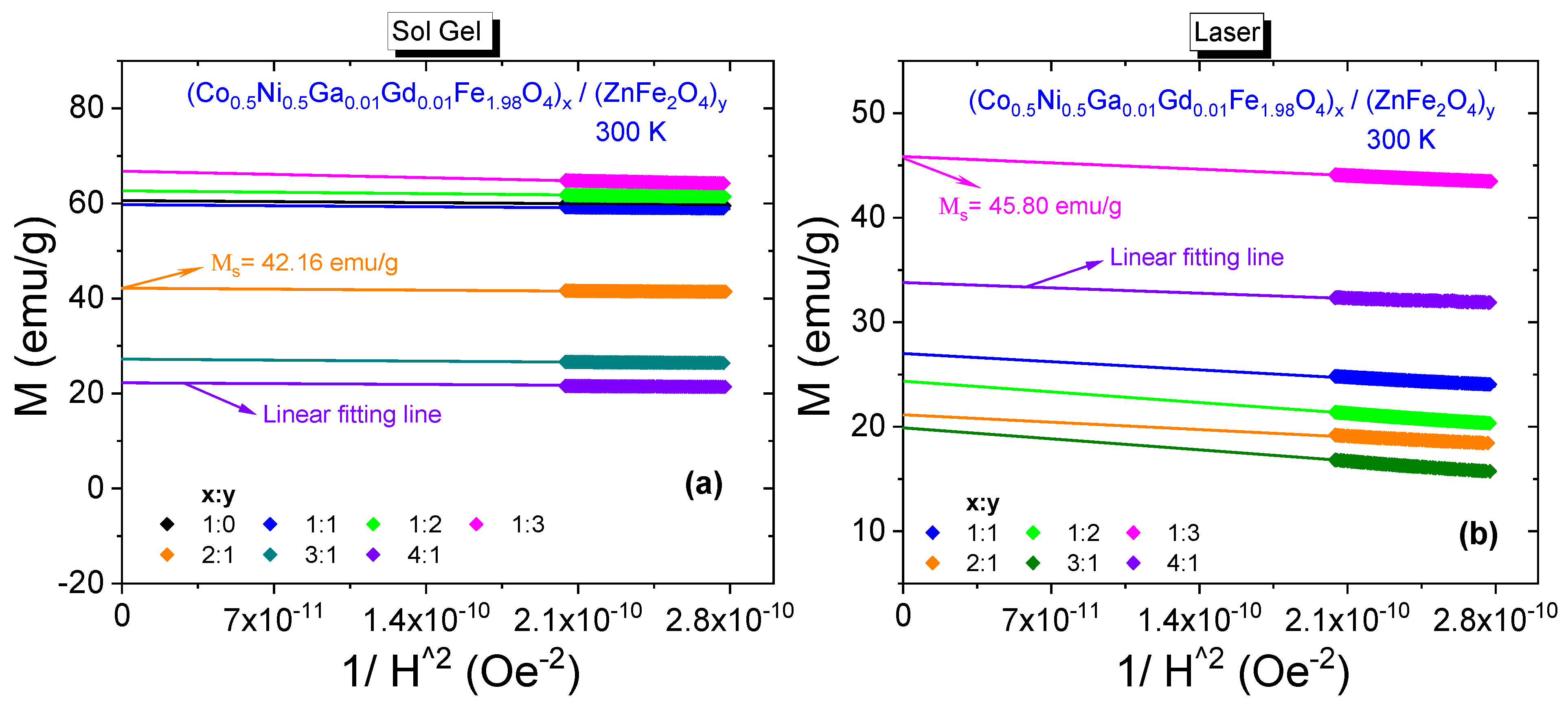
3.3.1. Room-Temperature Field Dependences
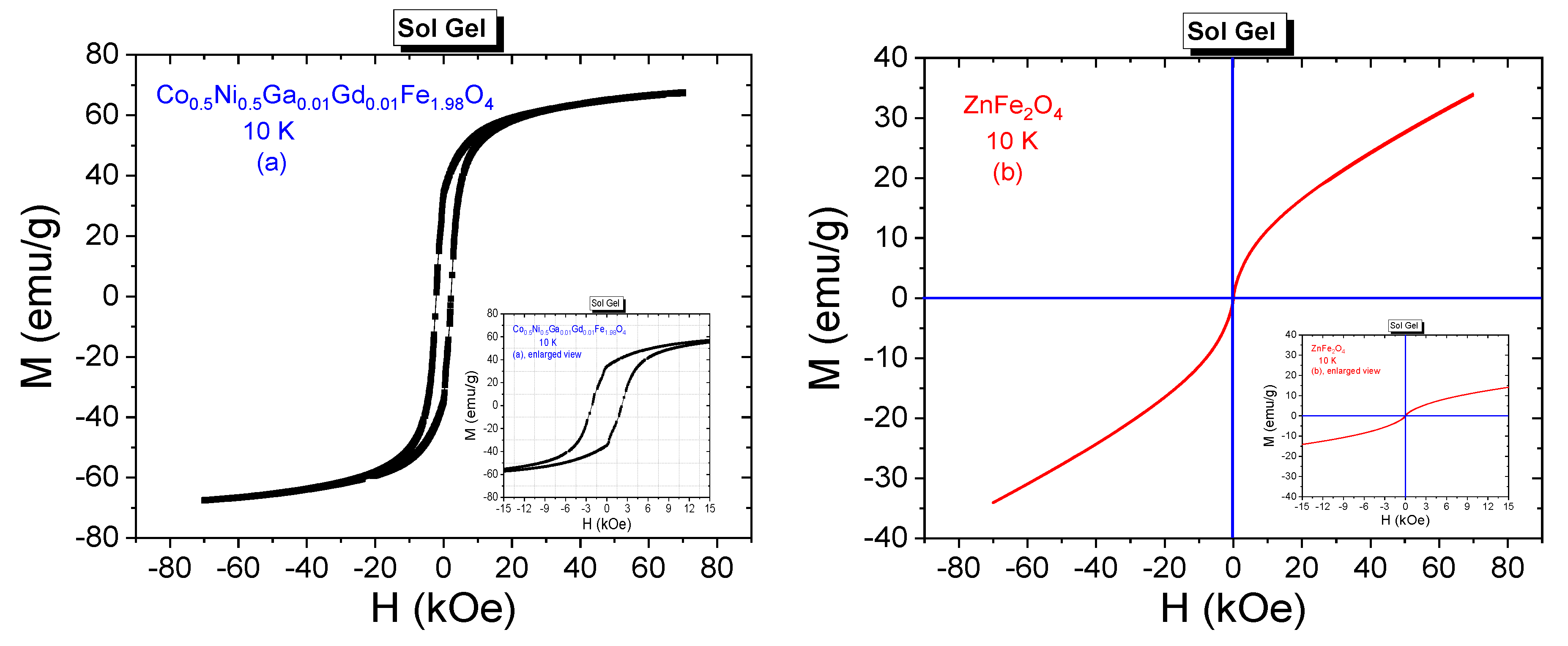
3.3.2. Low-Temperature Field Dependences
3.3.3. Temperature Dependences
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algarou, N.A.; Slimani, Y.; Almessiere, M.A.; Sadaqat, A.; Trukhanov, A.V.; Gondal, M.A.; Hakeem, A.S.; Trukhanov, S.V.; Vakhitov, M.G.; Klygach, D.S.; et al. Functional Sr0.5Ba0.5Sm0.02Fe11.98O4/x(Ni0.8Zn0.2Fe2O4) hard-soft ferrite nanocomposites: Structure, magnetic and microwave properties. Nanomaterials 2020, 10, 2134. [Google Scholar] [CrossRef]
- Rehman, S.; Almessiere, M.A.; Al-Jameel, S.S.; Ali, U.; Slimani, Y.; Taskhandi, N.; Al-Saleh, N.S.; Manikandan, A.; Khan, F.A.; Al-Suhaimi, E. Designing of Co0.5Ni0.5GaxFe2-xO4 (0.0 ≤ x ≤ 1.0) microspheres via hydrothermal approach and their selective inhibition on the growth of cancerous and fungal cells. Pharmaceutics 2021, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikušová, Z.; Skládal, P. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Microchim. Acta 2019, 186, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Almessiere, M.; Slimani, Y.; Auwal, I.; Shirsath, S.; Gondal, M.; Sertkol, M.; Baykal, A. Biosynthesis effect of Moringa oleifera leaf extract on structural and magnetic properties of Zn doped Ca-Mg nano-spinel ferrites. Arab. J. Chem. 2021, 14, 103261. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef]
- Makovec, D.; Kodre, A.; Arčon, I.; Drofenik, M. The structure of compositionally constrained zinc-ferrite spinel nanoparticles. J. Nanoparticle Res. 2010, 13, 1781–1790. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.A.; Hassan, M.; Gondal, M.A.; Cevik, E.; Baykal, A. Investigation of hard/soft CoFe2O4/NiSc0.03Fe1.97O4 nanocomposite for energy storage applications. Int. J. Energy Res. 2021, 45, 16691–16708. [Google Scholar] [CrossRef]
- Ghasemi, A.; Kheirmand, M.; Heli, H. A study on the supercapacitive behavior of zinc substituted manganese ferrite nanoparticles. J. Iran. Chem. Soc. 2019, 16, 841–849. [Google Scholar] [CrossRef]
- Bhujun, B.; Tan, M.T.T.; Shanmugam, A.S. Study of mixed ternary transition metal ferrites as potential electrodes for supercapacitor applications. Res. Phys. 2017, 7, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Islam, M.U.; Meydan, T.; Cuenca, J.A.; Melikhov, Y.; Mustafa, G.; Murtaza, G.; Jamil, Y. Microwave absorption properties of CoGd substituted ZnFe2O4 ferrites synthesized by co-precipitation technique. Ceram. Int. 2018, 44, 5909–5914. [Google Scholar] [CrossRef] [Green Version]
- Heiba, Z.K.; Mohamed, M.B.; Ahmed, M.; Moussa, M.; Hamdeh, H. Cation distribution and dielectric properties of nanocrystalline gallium substituted nickel ferrite. J. Alloy. Compd. 2014, 586, 773–781. [Google Scholar] [CrossRef]
- Hamdy, S.; Mohamed, M.B.; Ata-Allah, S.S. Effect of Zn, Ga and Gd Doping on Structural and Magnetic Properties of Nanonickel Ferrite Prepared by Two Different Methods. J. Supercond. Nov. Magn. 2019, 32, 115–125. [Google Scholar] [CrossRef]
- Venugopal Rao, S.; Krishna Podagatlapalli, G.; Hamad, S. Ultrafast Laser Ablation in Liquids for Nanomaterials and Applications. J. Nanosci. Nanotechnol. 2014, 14, 1364–1388. [Google Scholar]
- Yang, G. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Sasaki, K.; Takada, N. Liquid-phase laser ablation. Pure Appl. Chem. 2010, 82, 1317–1327. [Google Scholar] [CrossRef]
- Manikandan, A.; Kennedy, L.J.; Bououdina, M.; Vijaya, J.J. Synthesis, optical and magnetic properties of pure and Co-doped ZnFe2O4 nanoparticles by microwave combustion method. J. Magn. Magn. Mater. 2014, 349, 249–258. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Amir, M.; Baykal, A.; Güner, S.; Sertkol, M.; Sözeri, H.; Toprak, M. Synthesis and characterization of CoxZn1-xAlFeO4 nanoparticles. J. Inorg. Organomet. Polym. Mater. 2015, 25, 747–754. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Korkmaz, A.D.; Trukhanov, S.V.; Guner, S.; Alahmari, F.; Trukhanov, A.V.; Baykal, A. Correlation between chemical composition, electrical, magnetic and microwave properties in Dy-substituted Ni-Cu-Zn ferrites. Mater. Sci. Eng. B 2021, 270, 115202. [Google Scholar] [CrossRef]
- Baykal, A.; Güner, S.; Demir, A.; Esir, S.; Genç, F. Effect of zinc substitution on magneto-optical properties of Mn1−xZnxFe2O4/SiO2 nanocomposites. Ceram. Int. 2014, 40, 13401–13408. [Google Scholar] [CrossRef]
- Baykal, A.; Güner, S.; Demir, A. Synthesis and magneto-optical properties of triethylene glycol stabilized Mn1−xZnx Fe2O4 nanoparticles. J. Alloys Compd. 2015, 619, 5–11. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos. Trans. R. Soc. A 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Korkmaz, A.D.; Güner, S.; Baykal, A.; Shirsath, S.; Ercan, I.; Kögerler, P. Sonochemical synthesis of Dy3+ substituted Mn0.5Zn0.5Fe2−xO4 nanoparticles: Structural, magnetic and optical characterizations. Ultrason. Sonochemistry 2020, 61, 104836. [Google Scholar] [CrossRef] [PubMed]
- Slimani, Y.; Almessiere, M.A.; Güner, S.; Kurtan, U.; Shirsath, S.E.; Baykal, A.; Ercan, I. Magnetic and microstructural features of Dy3+ substituted NiFe2O4 nanoparticles derived by sol-gel approach. J. Sol-Gel Sci. Technol. 2020, 95, 202–210. [Google Scholar] [CrossRef]
- Qiu, J.; Gu, M.; Shen, H. Microwave absorption properties of Al- and Cr-substituted M-type barium hexaferrite. J. Magn. Magn. Mater. 2005, 295, 263–268. [Google Scholar] [CrossRef]
- Algarou, N.; Slimani, Y.; Almessiere, M.; Baykal, A.; Guner, S.; Manikandan, A.; Ercan, I. Enhancement on the exchange coupling behavior of SrCo0.02Zr0.02Fe11.96O19/MFe2O4 (M = Co, Ni, Cu, Mn and Zn) as hard/soft magnetic nanocomposites. J. Magn. Magn. Mater. 2019, 499, 166308. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Güner, S.; Kurtan, U.; Baykal, A. Impacts of sol-gel auto-combustion and ultrasonication approaches on structural, magnetic, and Optical properties of Sm-Tm co-substituted Sr0.5Ba0.5Fe12O19 nanohexaferrites: Comparative study. Nanomaterials 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almessiere, M.; Slimani, Y.; Guner, S.; Aldakhil, S.; Korkmaz, A.; Sertkol, M.; Gungunes, H.; Yasin, G.; Baykal, A. Ultrasonic synthesis, magnetic and optical characterization of Tm3+ and Tb3+ ions co-doped barium nanohexaferrites. J. Solid State Chem. 2020, 286, 121310. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Rehman, S.; Khan, F.A.; Güngüneş, Ç.D.; Güner, S.; Shirsath, S.E.; Baykal, A. Magnetic properties, anticancer and antibacterial effectiveness of sonochemically produced Ce3+/Dy3+ co-activated Mn-Zn nanospinel ferrites. Arab. J. Chem. 2020, 13, 7403–7417. [Google Scholar] [CrossRef]
- Carta, D.; Casula, M.F.; Falqui, A.; Loche, D.; Mountjoy, G.; Sangregorio, C.; Corrias, A. A Structural and Magnetic Investigation of the Inversion Degree in Ferrite Nanocrystals MFe2O4(M = Mn, Co, Ni). J. Phys. Chem. C 2009, 113, 8606–8615. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Ali, S.; Manikandan, A.; Ercan, I.; Baykal, A.; Trukhanov, A. Magnetic Attributes of NiFe2O4 Nanoparticles: Influence of Dysprosium Ions (Dy3+) Substitution. Nanomaterials 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Hakim, M.; Haque, M.M.; Huq, M.; Nordblad, P. Spin-glass-like ordering in the spinel ZnFe2O4 ferrite. Phys. B 2011, 406, 48–51. [Google Scholar] [CrossRef]
| Product | Sol–Gel | PLAL | ||||
|---|---|---|---|---|---|---|
| DXRD (nm) | a (Å) | V (Å3) | DXRD (nm) | a (Å) | V(Å3) | |
| (CNGaGdFeO)1/(ZFO)1 NCs | 38.9 | 8.3603 | 584.3400 | 70.1 | 8.4366 | 600.4810 |
| (CNGaGdFeO)1/(ZFO)2 NCs | 48.4 | 8.3628 | 585.7145 | 75.9 | 8.4375 | 600.6861 |
| (CNGaGdFeO)1/(ZFO)3 NCs | 42.1 | 8.3667 | 585.6830 | 88.6 | 8.4379 | 600.7714 |
| (CNGaGdFeO)2/(ZFO)1 NCs | 59.1 | 8.3470 | 581.5556 | 86.3 | 8.4423 | 601.6946 |
| (CNGaGdFeO)3/(ZFO)1 NCs | 59.9 | 8.3406 | 580.2148 | 85.2 | 8.4585 | 605.1651 |
| (CNGaGdFeO)4/(ZFO)1 NCs | 52.4 | 8.3400 | 576.7385 | 55.8 | 8.4654 | 683.3110 |
| x:y | MW g/mol | Sol-Gel | |||||
| Mr emu/g | HC Oe | MS emu/g | SQR Mr/Ms | Keff × 104 Erg/g | Ha Oe | ||
| 1:0 | 235.65 | 17.75 | 625 | 60.63 | 0.294 | 5.92 | 1953 |
| 1:1 | 476.73 | 20.0 | 340 | 59.93 | 0.334 | 3.18 | 1062 |
| 1:2 | 717.81 | 10.0 | 150 | 62.69 | 0.160 | 1.47 | 469 |
| 1:3 | 958.89 | 8.7 | 67 | 66.53 | 0.131 | 0.7 | 210 |
| 2:1 | 712.39 | 14.3 | 472 | 42.16 | 0.339 | 3.11 | 1475 |
| 3:1 | 948.04 | 10.0 | 480 | 27.19 | 0.368 | 2.04 | 1500 |
| 4:1 | 1183.7 | 8.2 | 500 | 22.13 | 0.371 | 1.73 | 1562 |
| x:y | MW g/mol | PLAL | |||||
| Mr emu/g | HC Oe | MS emu/g | SQR Mr/Ms | Keff × 104 Erg/g | Ha Oe | ||
| 1:1 | 476.73 | 5.4 | 531 | 26.97 | 0.200 | 2.24 | 1659 |
| 1:2 | 717.81 | 3.5 | 437 | 24.29 | 0.144 | 1.66 | 1366 |
| 1:3 | 958.89 | 11.6 | 570 | 45.80 | 0.253 | 4.08 | 1781 |
| 2:1 | 712.39 | 3.9 | 492 | 21.32 | 0.183 | 1.64 | 1537 |
| 3:1 | 948.04 | 2.4 | 467 | 19.85 | 0.121 | 1.45 | 1459 |
| 4:1 | 1183.7 | 8.6 | 480 | 33.61 | 0.256 | 2.52 | 1500 |
| x:y | MW g/mol | Sol-Gel | |||||
| Mr emu/g | HC Oe | MS emu/g | SQR Mr/Ms | Keff × 105 Erg/g | Ha Oe | ||
| 1:0 | 235.65 | 34.54 | 2207 | 69.84 | 0.495 | 2.40 | 6897 |
| 0:1 | 241.08 | - | - | 42.50 | - | - | - |
| 1:1 | 476.73 | 41.15 | 1750 | 73.87 | 0.557 | 2.02 | 5469 |
| 1:2 | 717.81 | 36.75 | 702 | 94.01 | 0.391 | 1.03 | 2194 |
| 1:3 | 958.89 | 42.27 | 571 | 118.7 | 0.356 | 1.06 | 1784 |
| 2:1 | 712.39 | 30.00 | 1709 | 54.06 | 0.555 | 1.44 | 5341 |
| 3:1 | 948.04 | 19.90 | 3564 | 35.78 | 0.556 | 1.99 | 11138 |
| 4:1 | 1183.7 | 14.55 | 2663 | 30.59 | 0.476 | 1.27 | 8321 |
| x:y | MW g/mol | PLAL | |||||
| Mr emu/g | HC Oe | MS emu/g | SQR Mr/Ms | Keff × 105 Erg/g | Ha Oe | ||
| 1:1 | 476.73 | 11.2 | 1490 | 51.43 | 0.218 | 1.20 | 4656 |
| 1:2 | 717.81 | 7.8 | 485 | 57.10 | 0.137 | 0.43 | 1516 |
| 1:3 | 958.89 | 23.5 | 1785 | 64.35 | 0.365 | 1.79 | 5578 |
| 2:1 | 712.39 | 8.0 | 1070 | 44.59 | 0.179 | 0.74 | 3344 |
| 3:1 | 948.04 | 5.5 | 685 | 53.04 | 0.104 | 0.57 | 2140 |
| 4:1 | 1183.7 | 18.0 | 1682 | 49.62 | 0.363 | 1.30 | 5256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almessiere, M.A.; Güner, S.; Slimani, Y.; Hassan, M.; Baykal, A.; Gondal, M.A.; Baig, U.; Trukhanov, S.V.; Trukhanov, A.V. Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches. Nanomaterials 2021, 11, 2461. https://doi.org/10.3390/nano11092461
Almessiere MA, Güner S, Slimani Y, Hassan M, Baykal A, Gondal MA, Baig U, Trukhanov SV, Trukhanov AV. Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches. Nanomaterials. 2021; 11(9):2461. https://doi.org/10.3390/nano11092461
Chicago/Turabian StyleAlmessiere, Munirah A., Sadik Güner, Yassine Slimani, Mohammed Hassan, Abdulhadi Baykal, Mohammed Ashraf Gondal, Umair Baig, Sergei V. Trukhanov, and Alex V. Trukhanov. 2021. "Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches" Nanomaterials 11, no. 9: 2461. https://doi.org/10.3390/nano11092461
APA StyleAlmessiere, M. A., Güner, S., Slimani, Y., Hassan, M., Baykal, A., Gondal, M. A., Baig, U., Trukhanov, S. V., & Trukhanov, A. V. (2021). Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches. Nanomaterials, 11(9), 2461. https://doi.org/10.3390/nano11092461









