Structural, Magnetic, and AC Measurements of Nanoferrites/Graphene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ferrite Nanoparticles Synthesis
2.2. Structure Investigation
2.3. Preparation of the Composites
2.4. Magnetic and Dielectric Properties
3. Results and Discussion
3.1. Characterization
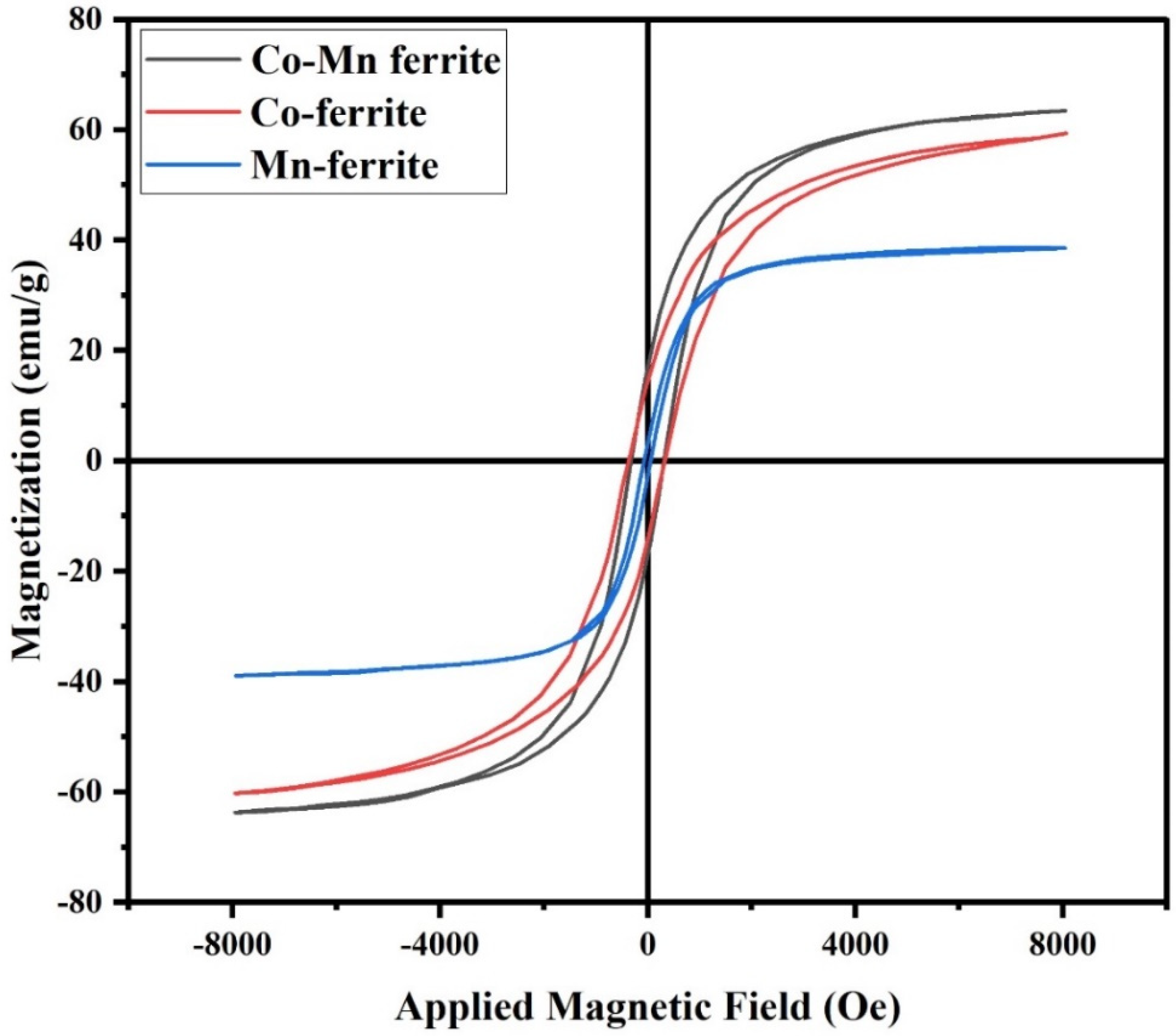
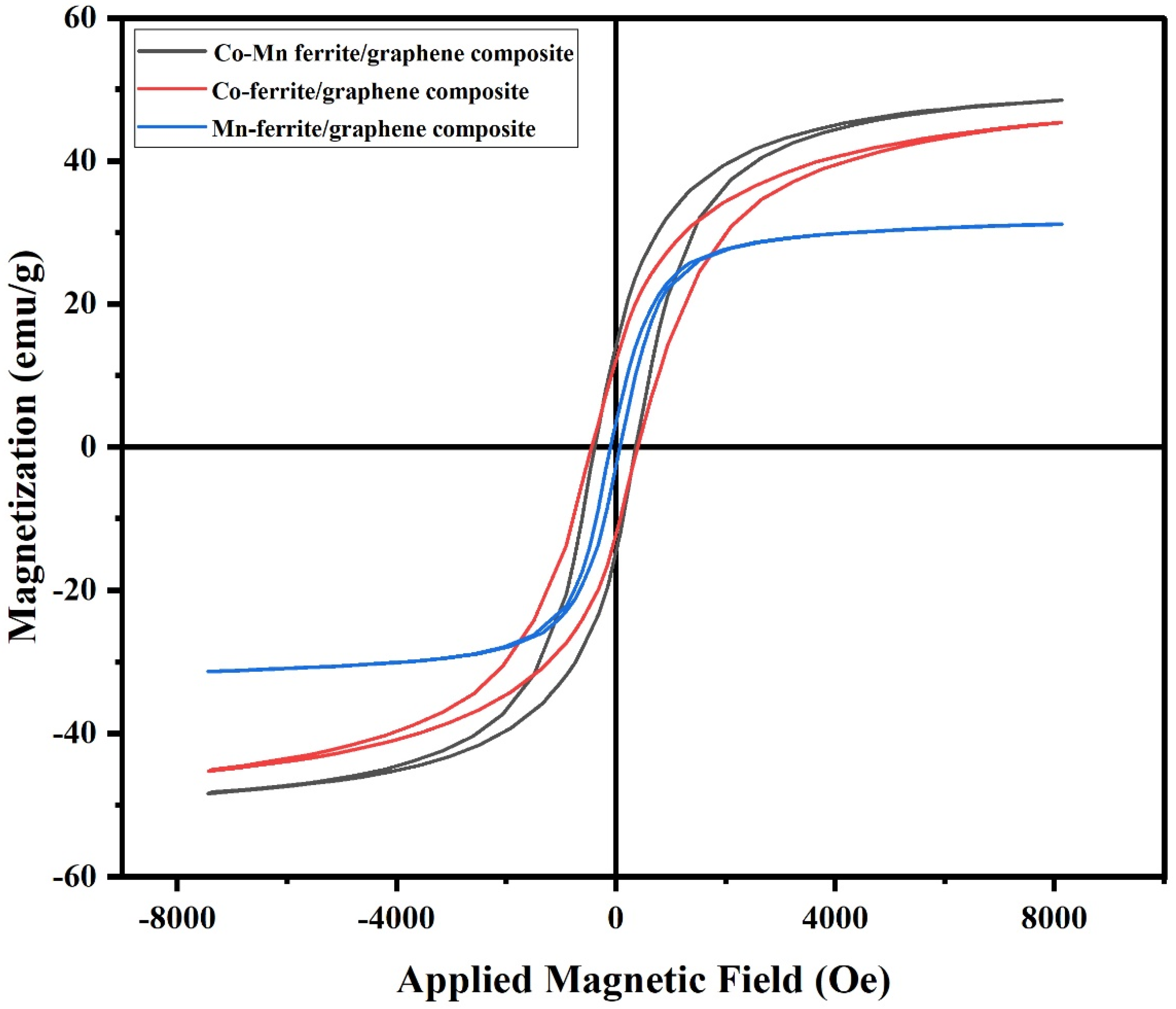
3.2. VSM Measurements
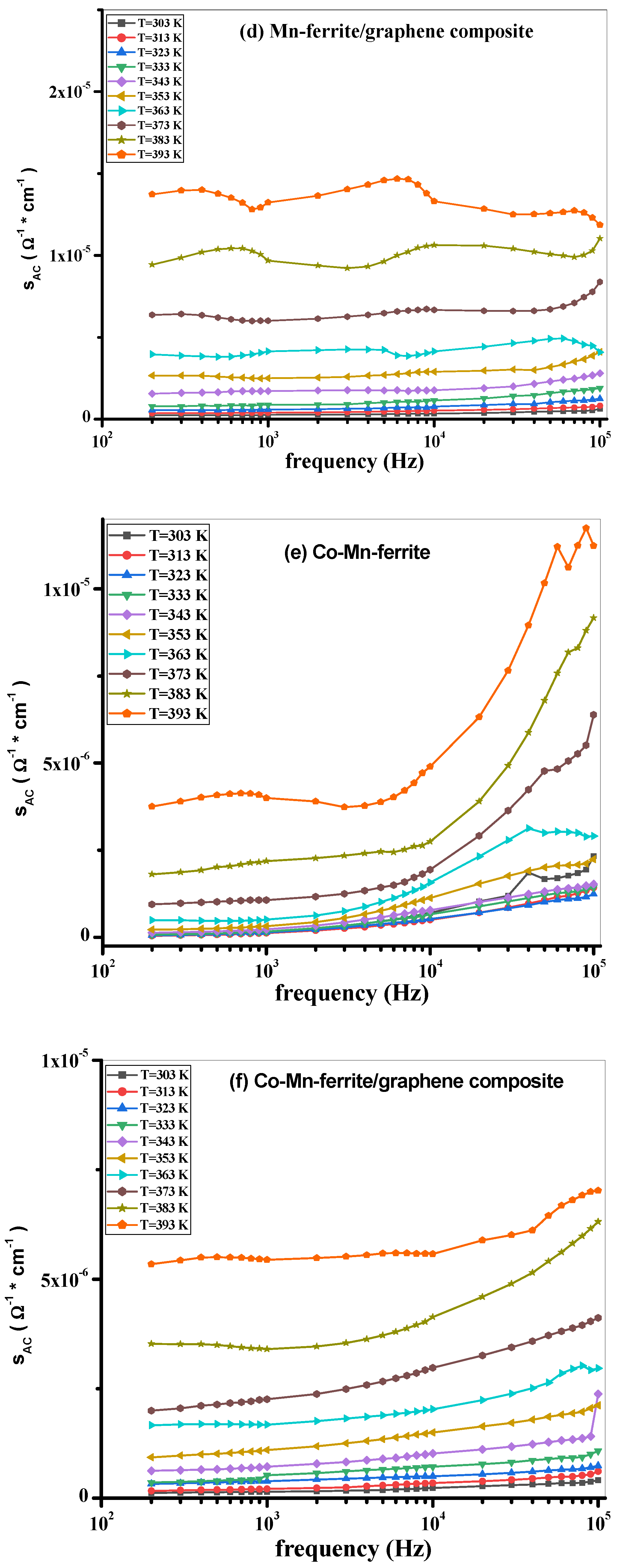
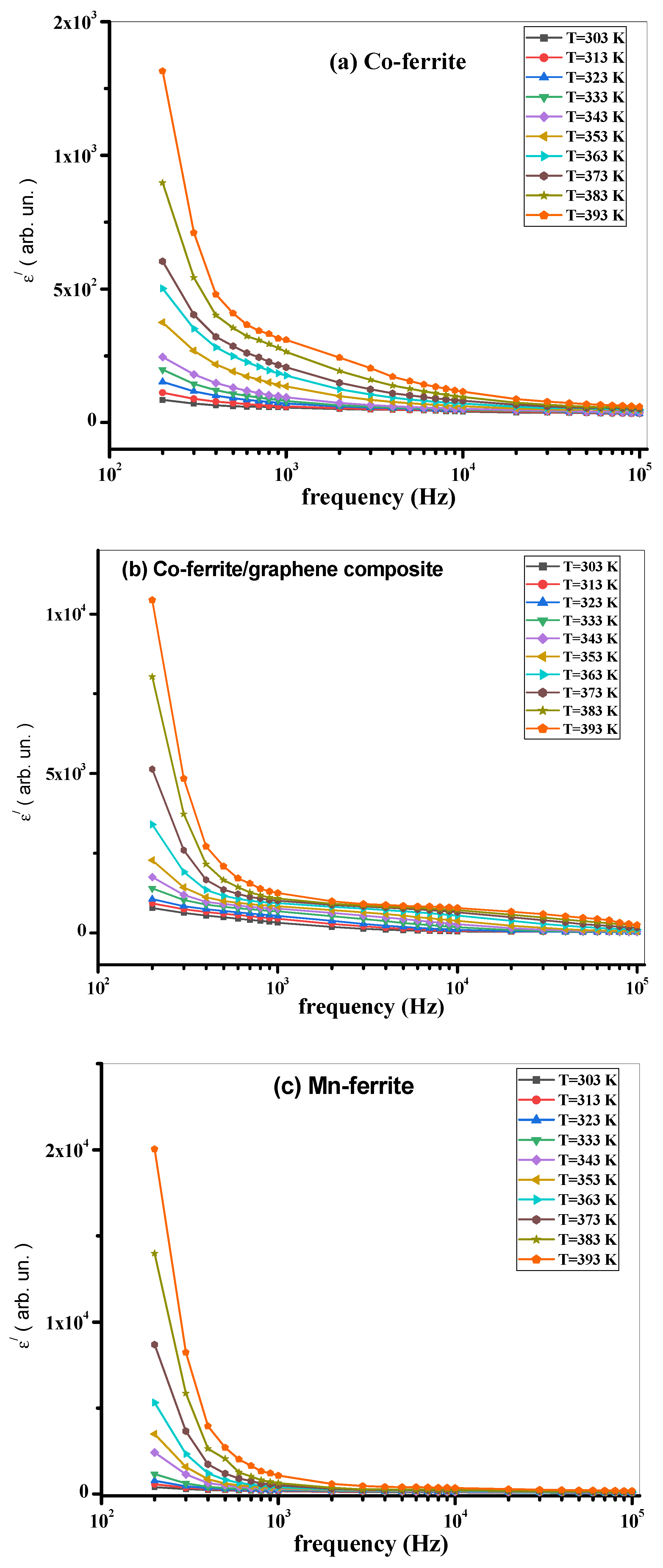
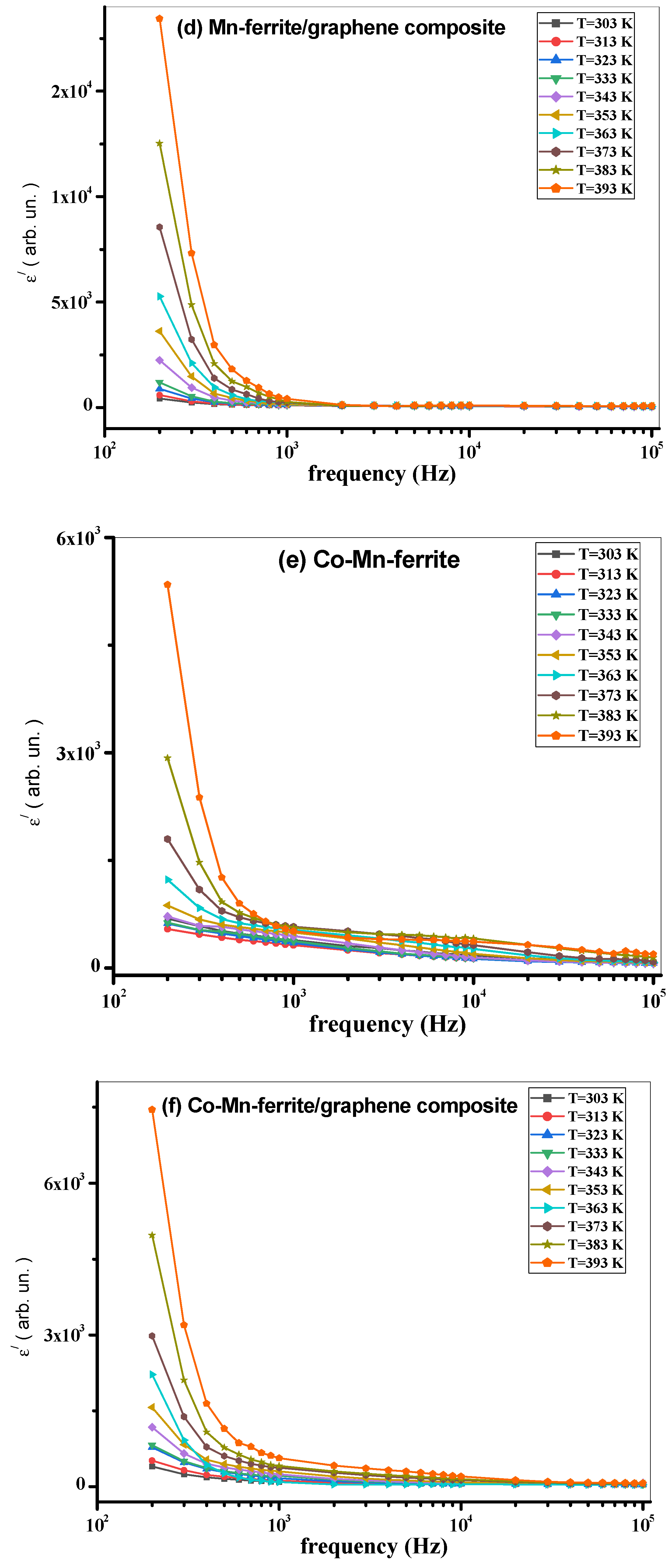
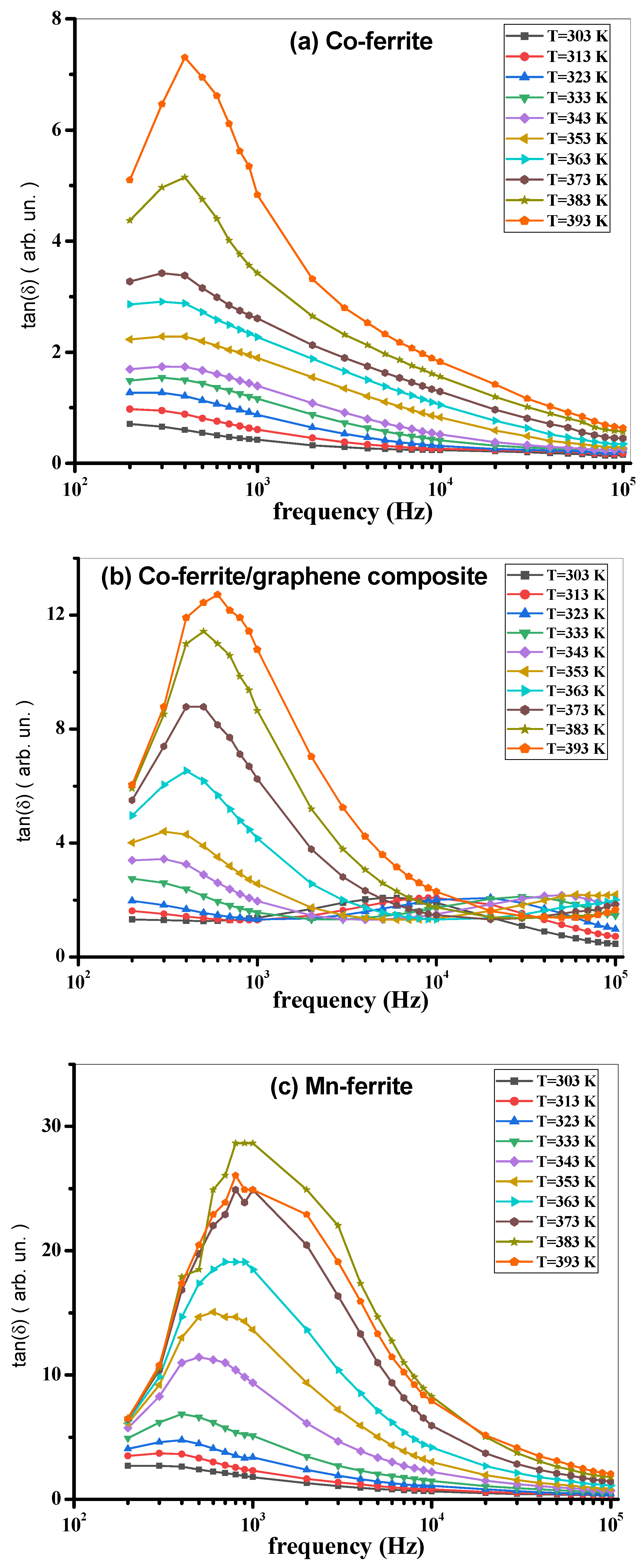
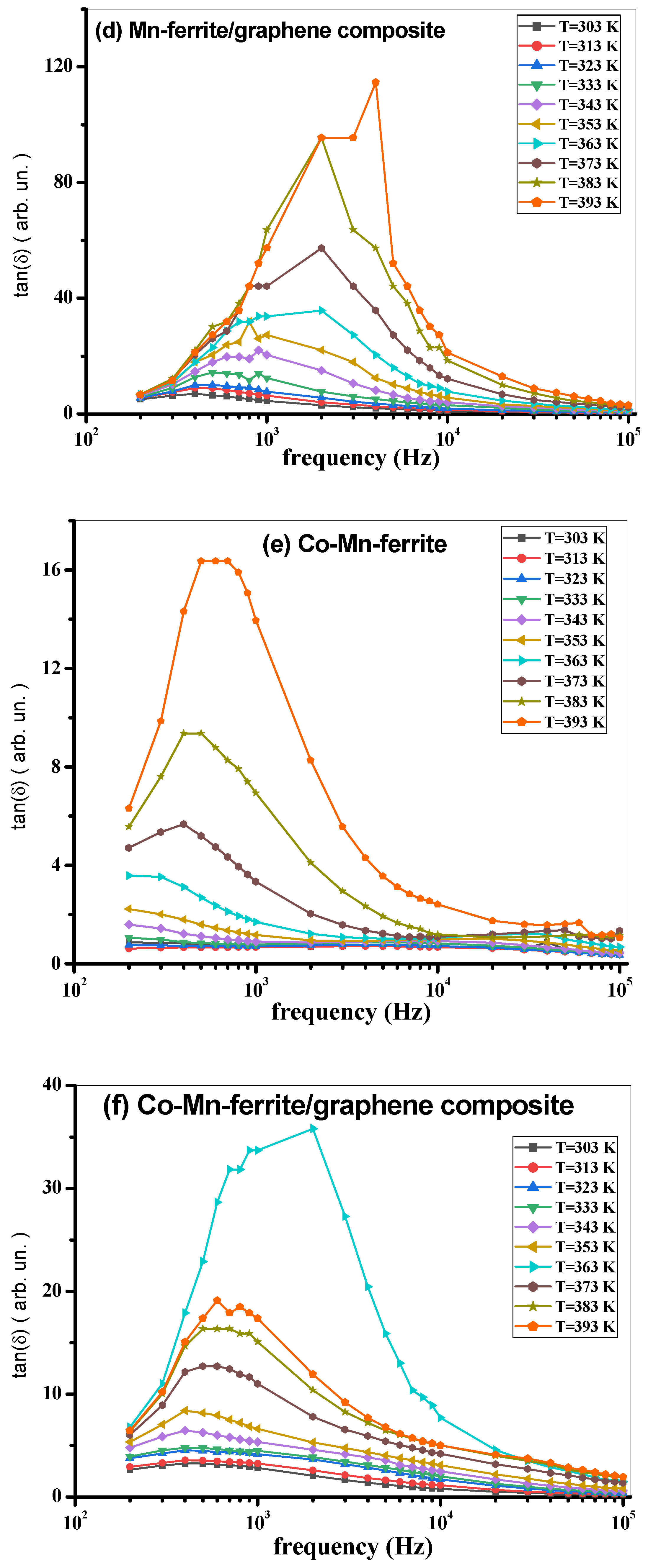
3.3. AC Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Xiang, K.; Wang, Y.; Zhou, W.; Zhu, Y.; Chen, W.; Chen, X.; Chen, H.; Cheng, H.; Lu, Z. Selective preparation of graphene- and rope-like NanoCarbons from camellia wastes as high performance electrode materials for energy storage. J. Alloys Compd. 2019, 811, 151616. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Talaie, E.; Bonnick, P.; Sun, X.; Pang, Q.; Liang, X.; Nazar, L.F. Methods and protocols for electrochemical energy storage materials research. Chem. Mater. 2017, 29, 90–105. [Google Scholar] [CrossRef]
- Nivetha, R.; Grace, A.N. Manganese and zinc ferrite based graphene nanocomposites for electrochemical hydrogen evolution reaction. J. Alloys Compd. 2019, 796, 185–195. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Ye, T.; Yang, H.; Wang, F.; Liu, C. Synthesis and characterization of graphene/0.8BaFe12O19/0.2Y3Fe5O12 nanocomposite. J. Alloys Compd. 2016, 683, 559–566. [Google Scholar] [CrossRef]
- Yang, H.; Ye, T.; Lin, Y.; Zhu, J.; Wang, F. Microwave absorbing properties of the ferrite composites based on graphene. J. Alloys Compd. 2016, 683, 567–574. [Google Scholar] [CrossRef]
- Ambikeswari, N.; Manivannan, S. Superior magnetodielectric properties of room temperature synthesized superparamagnetic cobalt ferrite—Graphene oxide composite. J. Alloys Compd. 2018, 763, 711–718. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, G.; Zhang, J.; Wang, X.; Wang, M.; Gan, Y.; Shi, J.; He, J. Fabrication of reduced graphene oxide/multi-walled carbon nanotubes/zinc ferrite hybrid composites as high-performance microwave absorbers. J. Alloys Compd. 2018, 736, 1–11. [Google Scholar] [CrossRef]
- Bashir, B.; Shaheen, W.; Asghar, M.; Warsi, M.F.; Khan, M.A.; Haider, S.; Shakir, I.; Shahid, M. Copper doped manganese ferrites nanoparticles anchored on graphene nano-sheets for high performance energy storage applications. J. Alloys Compd. 2017, 695, 881–887. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Lei, W.; Xia, X.; Wang, X. Ternary nitrogen-doped graphene/nickel ferrite/polyaniline nanocomposites for high-performance supercapacitors. J. Power Sources 2014, 269, 250–259. [Google Scholar] [CrossRef]
- Zheng, L.; Guan, L.; Yang, G.; Chen, S.; Zheng, H. One-pot synthesis of CoFe2O4/rGO hybrid hydrogels with 3D networks for high capacity electrochemical energy storage devices. RSC Adv. 2018, 8, 8607–8614. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, Q.; Zhang, W.; Wang, Y.; Chen, W. Nano nickel oxide coated graphene/polyaniline composite film with high electrochemical performance for flexible supercapacitor. Electrochim. Acta 2016, 211, 1066–1075. [Google Scholar] [CrossRef]
- Meng, F.; Yang, M.; Zhao, L.; Zhang, Y.; Shang, X.; Jin, P.; Zhang, W. A comparative study of the structural, magnetic and electrochemical properties of Al3+ and Cu2+ substituted NiZn ferrite/reduced graphene oxide nanocomposites. Ceram. Int. 2017, 43, 15959–15964. [Google Scholar] [CrossRef]
- Supriya, S.; Kumar, S.; Kar, M. CFO-graphene nano composite for high performance electrode material. Mater. Today Proc. 2017, 4, 5651–5656. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, S.R.; Fan, S.C.; Hu, W.Q.; Zheng, M.S.; Dong, Q.F. Reduced graphene oxide anchoring CoFe2O4 nanoparticles as an effective catalyst for non-aqueous lithium-oxygen batteries. Faraday Discuss. 2014, 172, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Radich, J.G.; McGinn, P.J.; Kamat, P.V. Graphene-based composites for electrochemical energy storage. Interface Mag. 2011, 20, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Zhang, X.; Xia, S.; Sun, T.; Ling, Y.; Zhou, S.; Guang, H.; Chen, Y.; Xu, Z.; Liang, M.; et al. Magnetic graphene oxide/carbon fiber composites with improved interfacial properties and electromagnetic interference shielding performance. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106811. [Google Scholar] [CrossRef]
- Ullah, T.; Gul, K.; Khan, H.; Ara, B.; Zia, T.U.H. Efficient removal of selected fluoroquinolones from the aqueous environment using reduced magnetic graphene oxide/polyaniline composite. Chemosphere 2022, 293, 133452. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, H.; Lu, M.; Xie, J.; Guan, S.; Wang, X.; Liu, X.; Lu, J. Preparation and adsorbability of magnetic composites based on cellulose nanofiber/graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128373. [Google Scholar] [CrossRef]
- Masrour, R.; Jabar, A. Magnetic properties of bilayer graphene armchair nanoribbons: A Monte Carlo study. J. Magn. Magn. Mater. 2017, 426, 225–229. [Google Scholar] [CrossRef]
- Masrour, R.; Jabar, A. Size and diluted magnetic properties of diamond shaped graphene quantum dots: Monte Carlo study. Phys. A Stat. Mech. Appl. 2018, 497, 211–217. [Google Scholar] [CrossRef]
- Masrour, R.; Jabar, A. Study of magnetic order of domain walls based on zigzag graphene nanoribbons under size effect. Synth. Met. 2021, 273, 116694. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharya, S.; Sharma, P. Novel insights into adsorption of heavy metal ions using magnetic graphene composites. J. Environ. Chem. Eng. 2021, 9, 106212. [Google Scholar] [CrossRef]
- El-Ghazzawy, E.H.; Amer, M.A. Structural, elastic and magnetic studies of the as-synthesized Co1−xSrxFe2O4 nanoparticles. J. Alloys Compd. 2017, 690, 293–303. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Q.; He, M.; Wan, Y.; Sun, X.; Xia, H.; Wang, X. Copper ferrite-graphene hybrid: A multifunctional heteroarchitecture for photocatalysis and energy storage. Ind. Eng. Chem. Res. 2012, 51, 11700–11709. [Google Scholar] [CrossRef]
- Rehman, M.A.; Yusoff, I.; Alias, Y. Structural, morphological and magnetic investigations of CuCe0.2Fe1.8O4 graphene-supported nanocomposites. Ceram. Int. 2016, 42, 1399–1407. [Google Scholar] [CrossRef]
- El Nimr, M.K.; Moharram, B.M.; Saafan, S.A.; Assar, S.T. Particle size distribution, magnetic permeability and dc conductivity of nano-structured and bulk LiNiZn–ferrite samples. J. Magn. Magn. Mater. 2010, 322, 2108–2112. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Israr, M.; Iqbal, J.; Arshad, A.; Gómez-Romero, P. Sheet-on-sheet like calcium ferrite and graphene nanoplatelets nanocomposite: A multifunctional nanocomposite for high-performance supercapacitor and visible light driven photocatalysis. J. Solid State Chem. 2021, 293, 121646. [Google Scholar] [CrossRef]
- Hosseini Mohammadabadi, F.; Masoudpanah, S.M.; Alamolhoda, S.; Koohdar, H.R. Electromagnetic microwave absorption properties of high entropy spinel ferrite ((MnNiCuZn)1−xCoxFe2O4)/graphene nanocomposites. J. Mater. Res. Technol. 2021, 14, 1099–1111. [Google Scholar] [CrossRef]
- Israr, M.; Iqbal, J.; Arshad, A.; Gómez-Romero, P.; Benages, R. Multifunctional MgFe2O4/GNPs nanocomposite: Graphene-promoted visible light driven photocatalytic activity and electrochemical performance of MgFe2O4 nanoparticles. Solid State Sci. 2020, 110, 106363. [Google Scholar] [CrossRef]
- El-Ghazzawy, E.H. Conductivity and dielectric relaxation properties of annealed Cr-substituted Ni-ferrite nanoparticles. J. Electron. Mater. 2017, 46, 5599–5607. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Synthesis and characterization of magnetically separable and recyclable crumbled MgFe2O4/reduced graphene oxide nanoparticles for removal of methylene blue dye from aqueous solutions. J. Phys. Chem. Solids 2021, 149, 109760. [Google Scholar] [CrossRef]
- Heidari, P.; Masoudpanah, S.M. Structural, magnetic and optical properties and photocatalytic activity of magnesium-calcium ferrite powders. J. Phys. Chem. Solids 2021, 148, 109681. [Google Scholar] [CrossRef]
- Amulya, M.A.S.; Nagaswarupa, H.P.; Kumar, M.R.A.; Ravikumar, C.R.; Kusuma, K.B. Sonochemical synthesis of MnFe2O4 nanoparticles and their electrochemical and photocatalytic properties. J. Phys. Chem. Solids 2021, 148, 109661. [Google Scholar] [CrossRef]
- El Shater, R.E.; El-Ghazzawy, E.H.; El-Nimr, M.K. Study of the sintering temperature and the sintering time period effects on the structural and magnetic properties of M-type hexaferrite BaFe12O19. J. Alloys Compd. 2018, 739, 327–334. [Google Scholar] [CrossRef]
- El-Alaily, T.M.; El-Nimr, M.K.; Saafan, S.A.; Kamel, M.M.; Meaz, T.M.; Assar, S.T. Construction and calibration of a low cost and fully automated vibrating sample magnetometer. J. Magn. Magn. Mater. 2015, 386, 25–30. [Google Scholar] [CrossRef]
- Saafan, S.A.; Assar, S.T.; Moharram, B.M.; El Nimr, M.K. Comparison study of some structural and magnetic properties of nano-structured and bulk Li–Ni–Zn ferrite samples. J. Magn. Magn. Mater. 2010, 322, 628–632. [Google Scholar] [CrossRef]
- Sanchez-Marcos, J.; Mazario, E.; Rodriguez-Velamazan, J.A.; Salas, E.; Herrasti, P.; Menendez, N. Cation distribution of cobalt ferrite electrosynthesized nanoparticles. A methodological comparison. J. Alloys Compd. 2018, 739, 909–917. [Google Scholar] [CrossRef] [Green Version]
- El-Ghazzawy, E.H. Effect of heat treatment on structural, magnetic, elastic and optical properties of the co-precipitated Co0.4Sr0.6Fe2O4. J. Magn. Magn. Mater. 2020, 497, 166017. [Google Scholar] [CrossRef]
- Makovec, D.; Kodre, A.; Arčon, I.; Drofenik, M. Structure of manganese zinc ferrite spinel nanoparticles prepared with co-precipitation in reversed microemulsions. J. Nanopart. Res. 2009, 11, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Darwish, M.A.; Saafan, S.A.; El-Kony, D.; Salahuddin, N.A. Preparation and investigation of dc conductivity and relative permeability of epoxy/Li-Ni-Zn ferrite composites. J. Magn. Magn. Mater. 2015, 385, 99–106. [Google Scholar] [CrossRef]
- Saafan, S.A.; Meaz, T.M.; El-Ghazzawy, E.H.; El Nimr, M.K.; Ayad, M.M.; Bakr, M. AC and DC conductivity of NiZn ferrite nanoparticles in wet and dry conditions. J. Magn. Magn. Mater. 2010, 322, 2369–2374. [Google Scholar] [CrossRef]
- Aen, F.; Ahmad, M.; Rana, M.U. The role of Ga substitution on magnetic and electromagnetic properties of nano-sized W-type hexagonal ferrites. Curr. Appl. Phys. 2013, 13, 41–46. [Google Scholar] [CrossRef]
- Darwish, M.A.; Trukhanov, A.V.; Senatov, O.S.; Morchenko, A.T.; Saafan, S.A.; Astapovich, K.A.; Trukhanov, S.V.; Trukhanova, E.L.; Pilyushkin, A.A.; Sombra, A.S.B.; et al. Investigation of AC-Measurements of Epoxy/Ferrite Composites. Nanomaterials 2020, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Koops, C.G. On the Dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 1951, 83, 121–124. [Google Scholar] [CrossRef]
- Beatrice, C.; Dobák, S.; Tsakaloudi, V.; Fiorillo, F.; Maniοudaki, A.; Zaspalis, V. Magnetic aging in TiO2-doped Mn-Zn ferrites. J. Magn. Magn. Mater. 2020, 502, 166576. [Google Scholar] [CrossRef]
- Karmakar, S.; Varma, S.; Behera, D. Investigation of structural and electrical transport properties of nano-flower shaped NiCo2O4 supercapacitor electrode materials. J. Alloys Compd. 2018, 757, 49–59. [Google Scholar] [CrossRef]
- Craciun, F.; Dimitriu, E.; Vasile, B.S.; Negrila, C.C.; Trusca, R.; Birjega, R.; Cernea, M. The enhancement mechanism of dielectric properties of Pb(Zr,Ti)O3 via (Mg2+,Sb3+) incorporation for supercapacitors. Mater. Today Chem. 2020, 18, 100350. [Google Scholar] [CrossRef]
- Yadav, R.S.; Anju; Jamatia, T.; Kuřitka, I.; Vilčáková, J.; Škoda, D.; Urbánek, P.; Machovský, M.; Masař, M.; Urbánek, M.; et al. Excellent, lightweight and flexible electromagnetic interference shielding nanocomposites based on polypropylene with MnFe2O4 spinel ferrite nanoparticles and reduced graphene oxide. Nanomaterials 2020, 10, 2481. [Google Scholar] [CrossRef]
- Nawaz, M.; Islam, M.U.; Nazir, M.A.; Bano, I.; Gul, I.H.; Ajmal, M. Transport properties in spinel ferrite/graphene oxide nanocomposites for electromagnetic shielding. Ceram. Int. 2021, 47, 25505–25513. [Google Scholar] [CrossRef]
- Ravinder, D.; Latha, K. Dielectric behaviour of mixed Mg-Zn ferrites at low frequencies. Mater. Lett. 1999, 41, 247–253. [Google Scholar] [CrossRef]
- Saafan, S.A.; El-Nimr, M.K.; Hussein, M.M.; Omar, M.K. FTIR, DC, and AC electrical measurements of Mg Zn Nano-ferrites and their composites with Polybenzoxazine. Appl. Phys. A Mater. Sci. Process. 2021, 127, 800. [Google Scholar] [CrossRef]

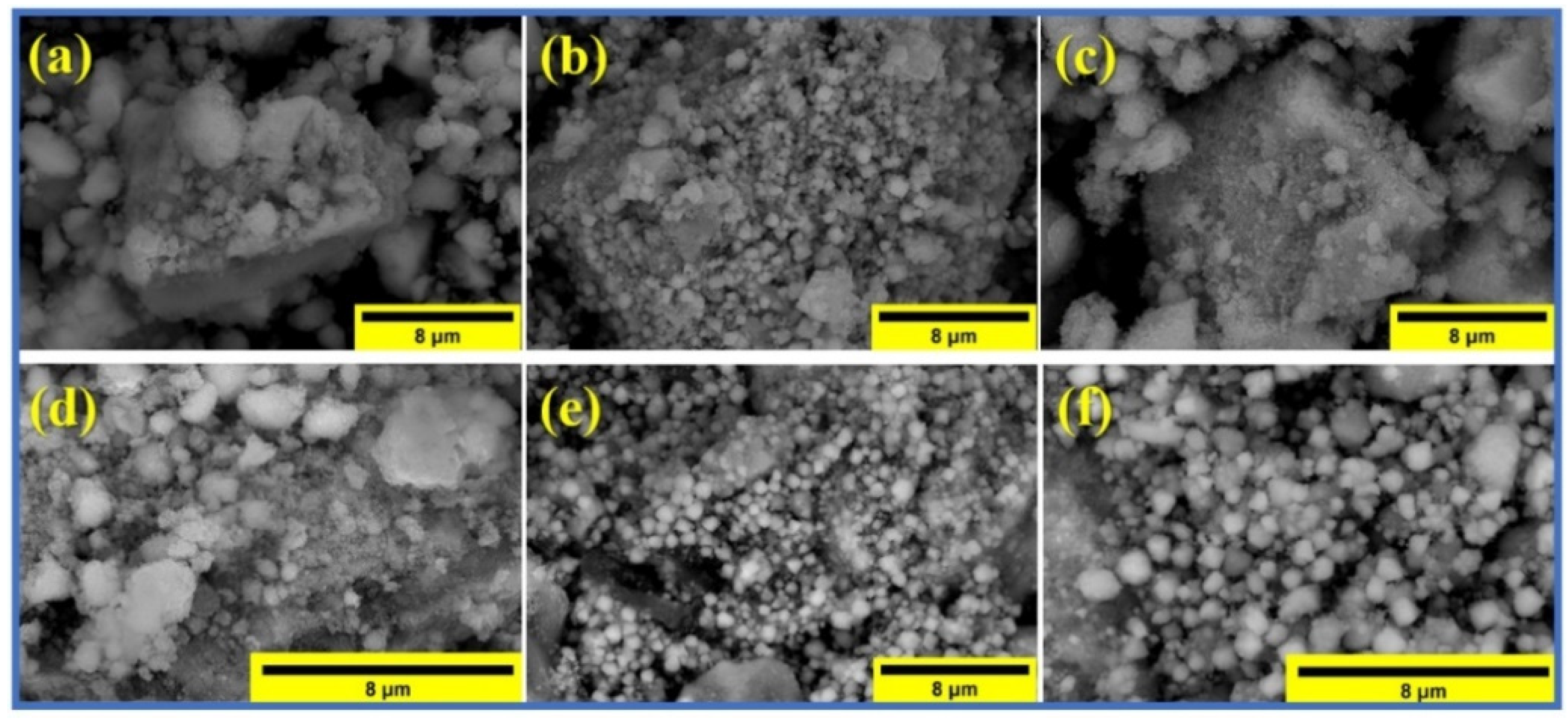
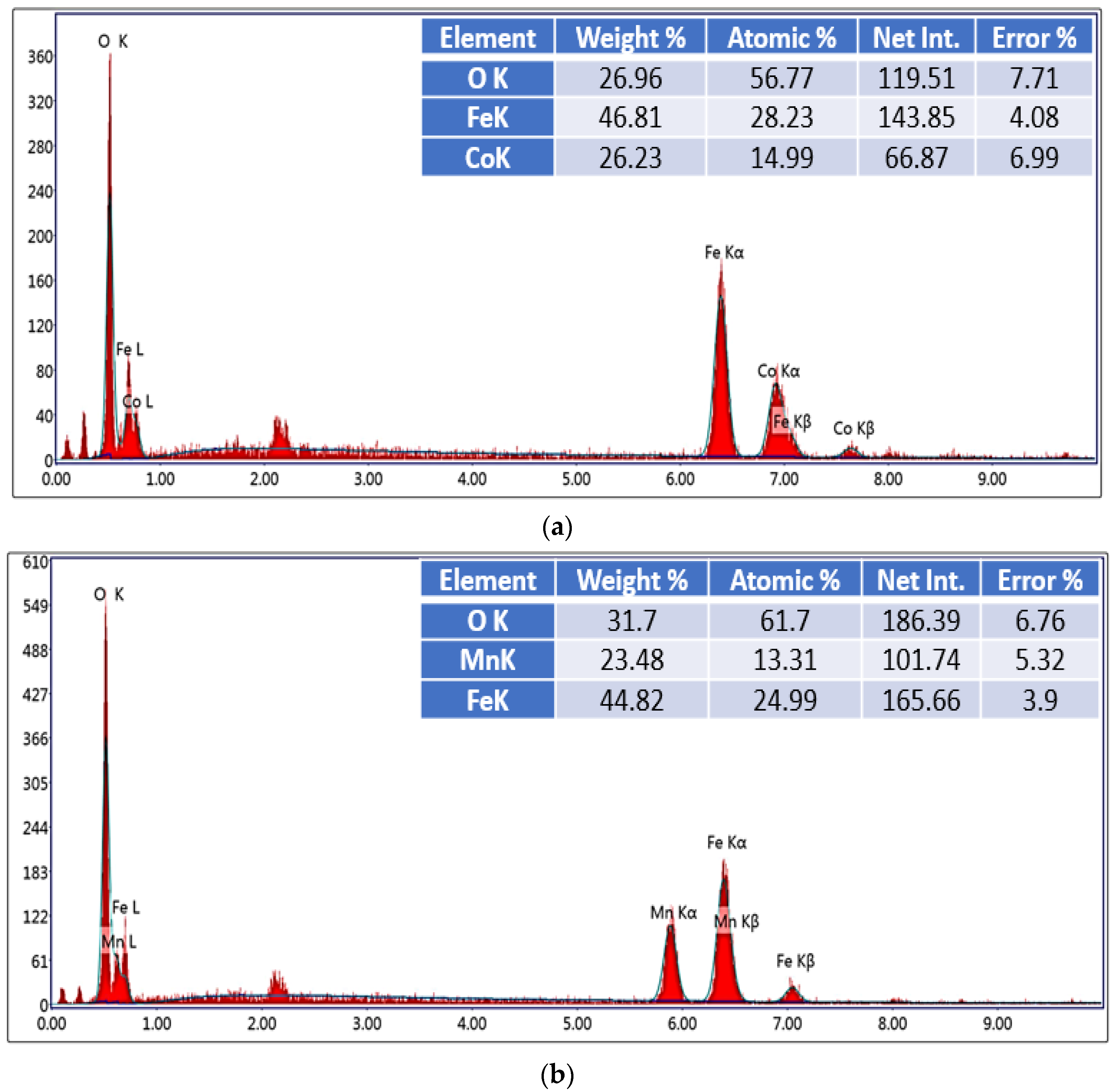



| Composition | Calculated from All Peaks, (nm) | Calculated from the Maximum Peak, (nm) | Calculated from SEM Images, (nm) |
|---|---|---|---|
| CoFe2O4 | 20 | 13 | 19.2 |
| CoMnFe2O4 | 25 | 16 | 18.8 |
| MnFe2O4 | 75 | 64 | 55.5 |
| Composition | Mr (emu/g) | Hc (Oe) | Ms (emu/g) |
|---|---|---|---|
| CoMnFe2O4 | 17.0 | 325 | 63 |
| CoFe2O4 | 14.5 | 350 | 60 |
| MnFe2O4 | 3.5 | 70 | 39 |
| CoMnFe2O4/graphene | 14.0 | 390 | 48 |
| CoFe2O4/graphene | 12.5 | 450 | 45 |
| MnFe2O4/graphene | 3.5 | 100 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, S.A.; Saafan, S.A.; Meaz, T.M.; Darwish, M.A.; Zhou, D.; Khandaker, M.U.; Islam, M.A.; Mohafez, H.; Trukhanov, A.V.; Trukhanov, S.V.; et al. Structural, Magnetic, and AC Measurements of Nanoferrites/Graphene Composites. Nanomaterials 2022, 12, 931. https://doi.org/10.3390/nano12060931
Habib SA, Saafan SA, Meaz TM, Darwish MA, Zhou D, Khandaker MU, Islam MA, Mohafez H, Trukhanov AV, Trukhanov SV, et al. Structural, Magnetic, and AC Measurements of Nanoferrites/Graphene Composites. Nanomaterials. 2022; 12(6):931. https://doi.org/10.3390/nano12060931
Chicago/Turabian StyleHabib, Shaimaa A., Samia A. Saafan, Talaat M. Meaz, Moustafa A. Darwish, Di Zhou, Mayeen U. Khandaker, Mohammad A. Islam, Hamidreza Mohafez, Alex V. Trukhanov, Sergei V. Trukhanov, and et al. 2022. "Structural, Magnetic, and AC Measurements of Nanoferrites/Graphene Composites" Nanomaterials 12, no. 6: 931. https://doi.org/10.3390/nano12060931
APA StyleHabib, S. A., Saafan, S. A., Meaz, T. M., Darwish, M. A., Zhou, D., Khandaker, M. U., Islam, M. A., Mohafez, H., Trukhanov, A. V., Trukhanov, S. V., & Omar, M. K. (2022). Structural, Magnetic, and AC Measurements of Nanoferrites/Graphene Composites. Nanomaterials, 12(6), 931. https://doi.org/10.3390/nano12060931










