Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy
Abstract
1. Cancer
2. Photodynamic Therapy
3. Phthalocyanines as Therapeutic Agents in PDT
4. Polymeric Nanoparticle Delivery Systems
4.1. Polymeric Micelles
4.2. Polymersomes
4.3. Polymeric Nanoparticles
4.4. Dendrimers
5. Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy
5.1. Pc-PEGylated Delivery Systems in PDT
5.2. Pc-Polymeric Nanocarriers Based on Synthetic Polymers in PDT
5.3. Polymeric Nanocarriers Based on Natural Polymers
5.4. Polymeric Nanocarriers in PDT with Assistance of UCNPs
5.5. Pc-Polymeric Nanocarriers in Combination of PDT with Chemotherapy
5.6. Bioresponsive Pc-Polymeric Nanocarriers in PDT
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| λmax | maximum absorption |
| ΦΔ | singlet oxygen quantum yield |
| AlPc | aluminum-phthalocyanine chloride |
| AlPc-sulfo4 | tetrasulfonated aluminum phthalocyanine |
| CPT | camptothecin |
| DEGMA | di(ethylene glycol) methyl ether methacrylate |
| Dex-b-AcDex | dextran-block-acetalated dextran |
| DOX | doxorubicin |
| DTT | dithiothreitol |
| FDA | Food and Drug Administration |
| GaPc | gallium (III) phthalocyanine chloride |
| GSH | glutathione |
| HEMA | 2-hydroxyethyl methacrylate |
| InPc | indium(III) phthalocyanine chloride |
| MNP | magnetic nanoparticle |
| NP | nanoparticle |
| PEG-b-PLLA | poly(L-lactide)-b-poly(ethylene oxide) block copolymer |
| PEG-b-PNIPAAM | poly(ethylene glycol)-b-poly(N-isopropylacrylamide) |
| OEGMA | oligo(ethylene glycol) methyl ether methacrylate |
| PAH | polyalkylamine hydrochloride |
| PBLA | poly(β-benzyl-L-aspartate) |
| Pc | phthalocyanine |
| PCI | photochemical internalization |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PEG-b-PCL | poly(ethylene glycol)-b-poly(ε-caprolactone) diblock copolymer |
| PEG-PMAN | poly(ethylene glycol)-poly[2-(methylacryloyl)ethylnicotinate] |
| PEG-b-PLGA | poly(ethylene glycol)-b-poly(lactide-co-glycolide) |
| PMMA | poly(methyl methacrylate) |
| pNIPAM | poly(N-isopropylacrylamide) |
| P(R)-b-PPEGA | poly(N-substituted acrylamide)-b-poly(polyethylene glycol monomethyl ether acrylate) |
| PS | photosensitizer |
| PS-b-PAA | poly(styrene)-b-poly(acrylic acid) |
| PSt-b-PPEGA | polystyrene-b-poly(polyethylene glycol monomethyl ether acrylate) |
| PSS | poly(4-styrene sulfonate) |
| PTT | photothermal therapy |
| RuPc(4–12 PEG) | (ruthenium(II) phthalocyanines functionalized with 4–12 PEG chains |
| SiPc | silicon (IV) phthalocyanine dichloride |
| ZnPc | zinc (II) phthalocyanine |
| ZnPcBCH3 | 2(3), 9(10), 16(17), 23(24)-tetrakis-(4′-methyl-benzyloxy) phthalocyanine zinc(II) |
| ZnPcF16 | zinc 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro29H,31H-phthalocyanine |
| ZnPc-sulfo4 | Zinc(II) phthalocyanine tetrasulfonic acid |
| ZnTAPc | zinc(II) tetra-aminophthalocyanine |
References
- Available online: https://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 29 July 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Carbone, A. Cancer Classification at the Crossroads. Cancers 2020, 12, 980. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.S.; Guo, H.C.; Ho, P.C.-L.; Mahendran, R.; Zhang, Y. Mesoporous-Silica-Coated Up-Conversion Fluorescent Nanoparticles for Photodynamic Therapy. Small 2009, 5, 2285–2290. [Google Scholar] [CrossRef]
- Borzęcka, W.; Trindade, T.; Torres, T.; Tomé, J. Targeting Cancer Cells with Photoactive Silica Nanoparticles. Curr. Pharm. Des. 2016, 22, 6021–6038. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.; Freeman, H. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817. [Google Scholar] [CrossRef] [PubMed]
- Deda, D.K.; Araki, K. Nanotechnology, Light and Chemical Action: An Effective Combination to Kill Cancer Cells. J. Braz. Chem. Soc. 2015, 26, 2448–2470. [Google Scholar] [CrossRef]
- Allison, R.; Sibata, C. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef]
- Al-Omari, S. Toward a molecular understanding of the photosensitizer-copper interaction for tumor destruction. Biophys. Rev. 2013, 5, 305–311. [Google Scholar] [CrossRef][Green Version]
- Rio, Y.; Rodriguez-Morgade, M.S.; Torres, T. Modulating the electronic properties of porphyrinoids: A voyage from the violet to the infrared regions of the electromagnetic spectrum. Org. Biomol. Chem. 2008, 6, 1877–1894. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- De la Escosura, A.; Martínez-Díaz, M.V.; Thordarson, P.; Rowan, A.E.; Nolte, R.J.M.; Torres, T. Donor−Acceptor Phthalocyanine Nanoaggregates. J. Am. Chem. Soc. 2003, 125, 12300–12308. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Chen, J.; Xu, S.; Zhou, Y.; Zhu, L.; Xiang, Y.; Xia, D. The effect of a hydrogen bond on the supramolecular self-aggregation mode and the extent of metal-free benzoxazole-substituted phthalocyanines. New J. Chem. 2015, 39, 5750–5758. [Google Scholar] [CrossRef]
- Jing, C.; Wang, R.; Ou, H.; Li, A.; An, Y.; Guo, S.; Shi, L. Axial modification inhibited H-aggregation of phthalocyanines in polymeric micelles for enhanced PDT efficacy. Chem. Commun. 2018, 54, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zaidi, S.I.A.; Rodgers, M.A.J.; Mukhtar, H.; Kenney, M.E.; Oleinick, N.L.; He, J.; Larkin, H.E.; Rihter, B.D. The Synthesis, Photophysical and Photobiological Properties and in vitro Structure-Activity Relationships of a Set of Silicon Phthalocyanine PDT Photosensitizers. Photochem. Photobiol. 1997, 65, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Song, M.; Huang, J.; Chen, N.; Xue, J.; Huang, M. Photocyanine: A novel and effective phthalocyanine-based photosensitizer for cancer treatment. J. Innov. Opt. Health Sci. 2020, 13, 2030009. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmuş, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(ii) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar] [CrossRef]
- Sekkat, N.; Bergh, H.V.D.; Nyokong, T.; Lange, N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules 2012, 17, 98–144. [Google Scholar] [CrossRef]
- Nyokong, T.; Antunes, E. Influence of nanoparticle materials on the photophysical behavior of phthalocyanines. Coord. Chem. Rev. 2013, 257, 2401–2418. [Google Scholar] [CrossRef]
- Idowu, M.; Nyokong, T. Photophysical and photochemical properties of zinc and aluminum phthalocyanines in the presence of magnetic fluid. J. Photochem. Photobiol. A Chem. 2007, 188, 200–206. [Google Scholar] [CrossRef]
- Alonso, L.; Sampaio, R.N.; Souza, T.F.M.; Silva, R.C.; Neto, N.M.B.; Ribeiro, A.O.; Alonso, A.; Gonçalves, P.J. Photodynamic evaluation of tetracarboxy-phthalocyanines in model systems. J. Photochem. Photobiol. B Biol. 2016, 161, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pound-Lana, G.E.N.; Garcia, G.M.; Trindade, I.C.; Capelari-Oliveira, P.; Pontifice, T.G.; Vilela, J.M.C.; Andrade, M.S.; Nottelet, B.; Postacchini, B.B.; Mosqueira, V.C.F. Phthalocyanine photosensitizer in polyethylene glycol-block-poly(lactide-co-benzyl glycidyl ether) nanocarriers: Probing the contribution of aromatic donor-acceptor interactions in polymeric nanospheres. Mater. Sci. Eng. C 2019, 94, 220–233. [Google Scholar] [CrossRef]
- Teles Ferreira, J.; Pina, J.; Alberto Fontes Ribeiro, C.; Fernandes, R.; Tomé, J.P.C.; Rodríguez-Morgade, M.S.; Torres, T. PEG-containing ruthenium phthalocyanines as photosensitizers for photodynamic therapy: Synthesis, characterization and in vitro evaluation. J. Mater. Chem. B 2017, 5, 5862–5869. [Google Scholar] [CrossRef]
- Ongarora, B.G.; Hu, X.; Verberne-Sutton, S.D.; Garno, J.C.; Vicente, M.G.H. Syntheses and Photodynamic Activity of Pegylated Cationic Zn(II)-Phthalocyanines in HEp2 Cells. Theranostics 2012, 2, 850–870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, B.; Duan, W.; Lo, P.-C.; Duan, L.; Wu, C.; Ng, D.K.P. Mono-PEGylated Zinc(II) Phthalocyanines: Preparation, Nanoparticle Formation, and In Vitro Photodynamic Activity. Chem. Asian J. 2013, 8, 55–59. [Google Scholar] [CrossRef]
- Mehraban, N.; Musich, P.R.; Freeman, H.S. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Lv, H.; Zhang, H.; Ma, D.; Yang, H.; Xie, S.; Peng, Y. Triblock copolymers encapsulated poly (aryl benzyl ether) dendrimer zinc(II) phthalocyanine nanoparticles for enhancement in vitro photodynamic efficacy. Photodiagnosis Photodyn. Ther. 2016, 16, 124–131. [Google Scholar] [CrossRef]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; van Nostrum, C.F.; de la Escosura, A.; Torres, T. Amphiphilic phthalocyanines in polymeric micelles: A supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2020, 8, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, X.; Wang, Y.; Guo, Q.; Ye, Q.; Guo, R.; Xiao, S.; Ye, Q.; Huang, Y.; Peng, Y. Benzyl ester dendrimer silicon phthalocyanine based polymeric nanoparticle for in vitro photodynamic therapy of glioma. J. Lumin. 2019, 207, 597–601. [Google Scholar] [CrossRef]
- Simioni, A.R.; Primo, F.L.; Tedesco, A. Silicon(IV) phthalocyanine-loaded-nanoparticles for application in photodynamic process. J. Laser Appl. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Cai, X.; Xie, Z.; Li, D.; Kassymova, M.; Zang, S.-Q.; Jiang, H.-L. Nano-sized metal-organic frameworks: Synthesis and applications. Coord. Chem. Rev. 2020, 417, 213366. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconj. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Chawla, J.S.; Amiji, M.M. Biodegradable poly(epsilon-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002, 249, 127–138. [Google Scholar] [CrossRef]
- Ricci-Júnior, E.; Marchetti, J.M. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 2006, 310, 187–195. [Google Scholar] [CrossRef]
- Souto, C.A.Z.; Madeira, K.P.; Rettori, D.; Baratti, M.O.; Rangel, L.B.A.; Razzo, D.; da Silva, A.R. Improved photodynamic action of nanoparticles loaded with indium (III) phthalocyanine on MCF-7 breast cancer cells. J. Nanoparticle Res. 2013, 15, 1879. [Google Scholar] [CrossRef]
- Lorenzoni, D.; Souto, C.A.Z.; Araujo, M.B.; de Souza Berger, C.; da Silva, L.C.D.; Baratti, M.O.; Ribeiro, J.N.; Endringer, D.C.; Guimarães, M.C.C.; da Silva, A.R. PLGA-PEG nanoparticles containing gallium phthalocyanine: Preparation, optimization and analysis of its photodynamic efficiency on red blood cell and Hepa-1C1C7. J. Photochem. Photobiol. B Biol. 2019, 198, 111582. [Google Scholar] [CrossRef]
- De Toledo, M.C.M.C.; Abreu, A.D.S.; Carvalho, J.A.; Ambrósio, J.A.R.; Godoy, D.d.S.; dos Santos Pinto, B.C.; Beltrame, M., Jr.; Simioni, A.R. Zinc phthalocyanine tetrasulfonate-loaded polyelectrolytic PLGA nanoparticles for photodynamic therapy applications. Photodiagn. Photodyn. Ther. 2020, 32, 101966. [Google Scholar] [CrossRef]
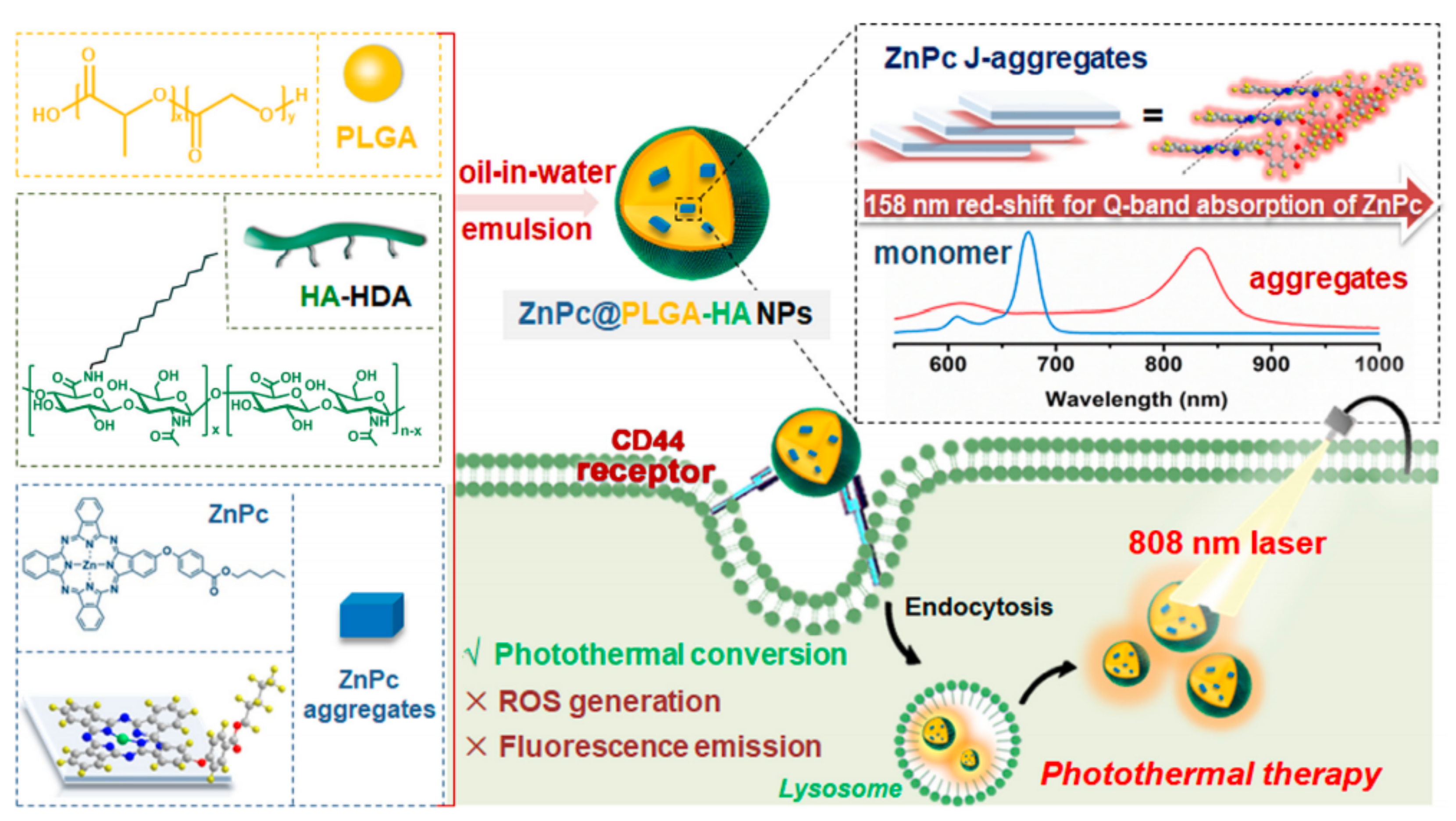
- Gao, D.; Wong, R.C.H.; Wang, Y.; Guo, X.; Yang, Z.; Lo, P.-C. Shifting the absorption to the near-infrared region and inducing a strong photothermal effect by encapsulating zinc(II) phthalocyanine in poly(lactic-co-glycolic acid)-hyaluronic acid nanoparticles. Acta Biomater. 2020, 116, 329–343. [Google Scholar] [CrossRef]
- Lamch, Ł.; Kulbacka, J.; Pietkiewicz, J.; Rossowska, J.; Dubińska-Magiera, M.; Choromańska, A.; Wilk, K.A. Preparation and characterization of new zinc(II) phthalocyanine—Containing poly(l-lactide)-b-poly(ethylene glycol) copolymer micelles for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2016, 160, 185–197. [Google Scholar] [CrossRef]
- Lamch, Ł.; Kulbacka, J.; Dubińska-Magiera, M.; Saczko, J.; Wilk, K.A. Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagn. Photodyn. Ther. 2019, 25, 480–491. [Google Scholar] [CrossRef]
- Lamch, Ł.; Tsirigotis-Maniecka, M.; Kulbacka, J.; Wilk, K.A. Synthesis of new zinc (II) phthalocyanine conjugates with block copolymers for cancer therapy. ARKIVOC 2016, 2017, 433–445. [Google Scholar] [CrossRef]
- Keyal, U.; Luo, Q.; Bhatta, A.K.; Luan, H.; Zhang, P.; Wu, Q.; Zhang, H.; Liu, P.; Zhang, L.; Wang, P.; et al. Zinc pthalocyanine-loaded chitosan/mPEG-PLA nanoparticles-mediated photodynamic therapy for the treatment of cutaneous squamous cell carcinoma. J. Biophotonics 2018, 11, e201800114. [Google Scholar] [CrossRef]
- Conte, C.; Costabile, G.; d’Angelo, I.; Pannico, M.; Musto, P.; Grassia, G.; Ialenti, A.; Tirino, P.; Miro, A.; Ungaro, F.; et al. Skin transport of PEGylated poly(ε-caprolactone) nanoparticles assisted by (2-hydroxypropyl)-β-cyclodextrin. J. Colloid Interface Sci. 2015, 454, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, X.; Ye, Q.; Chen, K.; Xiao, W.; Guan, X.; Huang, B.; Liu, G.; Wei, H.; Peng, Y. Prop-2-ynyloxybenzyloxy substituted phthalocyanine-based polymeric nanoparticles: Synthesis, photophysical properties and in vitro PDT efficacy. J. Coord. Chem. 2020, 73, 1232–1244. [Google Scholar] [CrossRef]
- Asem, H.; El-Fattah, A.A.; Nafee, N.; Zhao, Y.; Khalil, L.; Muhammed, M.; Hassan, M.; Kandil, S. Development and biodistribution of a theranostic aluminum phthalocyanine nanophotosensitizer. Photodiagn. Photodyn. Ther. 2016, 13, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Py-Daniel, K.R.; Namban, J.S.; de Andrade, L.R.; de Souza, P.E.N.; Paterno, L.G.; Azevedo, R.B.; Soler, M.A.G. Highly efficient photodynamic therapy colloidal system based on chloroaluminum phthalocyanine/pluronic micelles. Eur. J. Pharm. Biopharm. 2016, 103, 23–31. [Google Scholar] [CrossRef]
- Mike Motloung, B.; Babu, B.; Prinsloo, E.; Nyokong, T. The photophysicochemical properties and photodynamic therapy activity of In and Zn phthalocyanines when incorporated into individual or mixed Pluronic® micelles. Polyhedron 2020, 188, 114683. [Google Scholar] [CrossRef]
- Chiarante, N.; García Vior, M.C.; Awruch, J.; Marino, J.; Roguin, L.P. Phototoxic action of a zinc(II) phthalocyanine encapsulated into poloxamine polymeric micelles in 2D and 3D colon carcinoma cell cultures. J. Photochem. Photobiol. B Biol. 2017, 170, 140–151. [Google Scholar] [CrossRef]
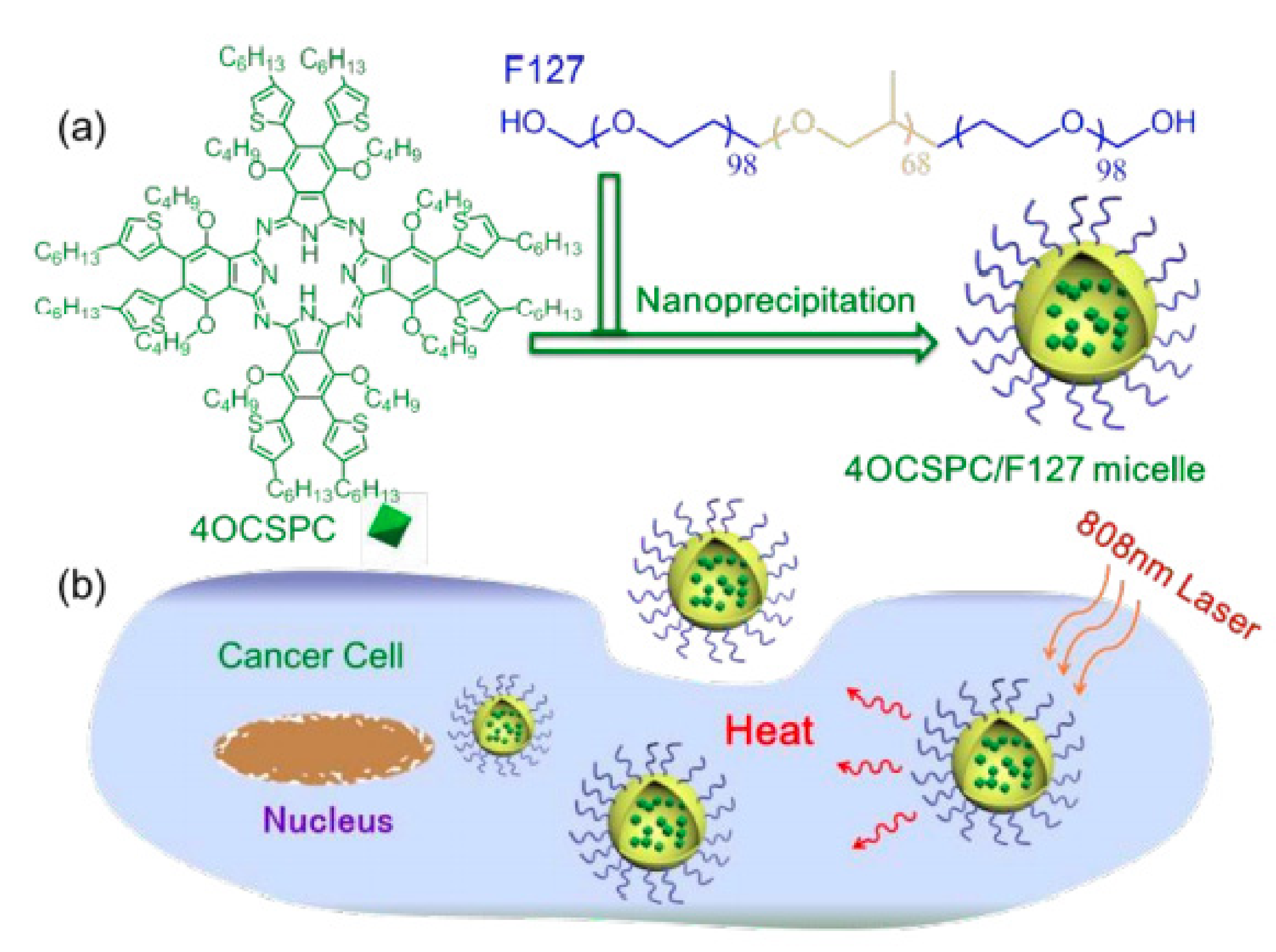
- Li, L.; Yang, Q.; Shi, L.; Zheng, N.; Li, Z.; Li, K.; Qiao, S.; Jia, T.; Sun, T.; Wang, Y. Novel phthalocyanine-based polymeric micelles with high near-infrared photothermal conversion efficiency under 808 nm laser irradiation for in vivo cancer therapy. J. Mater. Chem. B 2019, 7, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Qu, Z.; Shi, L.; Tan, S.; Ha, E.; Jia, T.; Sun, T. Novel phthalocyanine-based micelles/PNIPAM composite hydrogels: Spatially/temporally controlled drug release triggered by NIR laser irradiation. New J. Chem. 2020, 44, 8705–8709. [Google Scholar] [CrossRef]
- Feuser, P.E.; Gaspar, P.C.; Jacques, A.V.; Tedesco, A.C.; Santos Silva, M.C.D.; Ricci-Júnior, E.; Sayer, C.; de Araújo, P.H.H. Synthesis of ZnPc loaded poly(methyl methacrylate) nanoparticles via miniemulsion polymerization for photodynamic therapy in leukemic cells. Mater. Sci. Eng. C 2016, 60, 458–466. [Google Scholar] [CrossRef]
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. RAFT synthesis of polystyrene-block-poly(polyethylene glycol monomethyl ether acrylate) for zinc phthalocyanine-loaded polymeric micelles as photodynamic therapy photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 560–570. [Google Scholar] [CrossRef]
- Obata, M.; Masuda, S.; Takahashi, M.; Yazaki, K.; Hirohara, S. Effect of the hydrophobic segment of an amphiphilic block copolymer on micelle formation, zinc phthalocyanine loading, and photodynamic activity. Eur. Polym. J. 2021, 147, 110325. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Witt, M.A.; Barbosa, P.M.; Montanha, M.C.; Nunes, C.S.; Bellettini, I.C.; de Castro, L.V.; Sato, F.; Baesso, M.L.; Muniz, E.C.; et al. Formulation of chloroaluminum phthalocyanine incorporated into PS-b-PAA diblock copolymer nanomicelles. J. Mol. Liq. 2018, 271, 949–958. [Google Scholar] [CrossRef]
- Yu, W.; Ye, M.; Zhu, J.; Wang, Y.; Liang, C.; Tang, J.; Tao, H.; Shen, Y. Zinc phthalocyanine encapsulated in polymer micelles as a potent photosensitizer for the photodynamic therapy of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1099–1110. [Google Scholar] [CrossRef]
- Master, A.M.; Livingston, M.; Oleinick, N.L.; Sen Gupta, A. Optimization of a Nanomedicine-Based Silicon Phthalocyanine 4 Photodynamic Therapy (Pc 4-PDT) Strategy for Targeted Treatment of EGFR-Overexpressing Cancers. Mol. Pharm. 2012, 9, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Master, A.M.; Qi, Y.; Oleinick, N.L.; Gupta, A.S. EGFR-mediated intracellular delivery of Pc 4 nanoformulation for targeted photodynamic therapy of cancer: In vitro studies. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Gupta, A.S. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [CrossRef]
- Chen, K.; Pan, S.; Zhuang, X.; Lv, H.; Que, S.; Xie, S.; Yang, H.; Peng, Y. Effect of diblock copolymer properties on the photophysical properties of dendrimer silicon phthalocyanine nanoconjugates. J. Nanoparticle Res. 2016, 18, 197. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, D.; Pan, S.; Lin, P.; Lin, Y.; Yang, H.; Peng, Y. Comparative study of aluminum phthalocyanine incorporating into two types of block copolymer: Photo-physical property, size, and in vitro photodynamic therapy efficacy. J. Nanoparticle Res. 2015, 17, 41. [Google Scholar] [CrossRef]
- Conte, C.; Ungaro, F.; Maglio, G.; Tirino, P.; Siracusano, G.; Sciortino, M.T.; Leone, N.; Palma, G.; Barbieri, A.; Arra, C.; et al. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J. Control. Release 2013, 167, 40–52. [Google Scholar] [CrossRef]
- Dag, A.; Cakilkaya, E.; Omurtag Ozgen, P.S.; Atasoy, S.; Yigit Erdem, G.; Cetin, B.; Çavuş Kokuroǧlu, A.; Gürek, A.G. Phthalocyanine-Conjugated Glyconanoparticles for Chemo-photodynamic Combination Therapy. Biomacromolecules 2021, 22, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Sun, X.; Zhang, B.; Kang, H.; Zhang, F.; Jin, Y. Doxorubicin-Loaded Photosensitizer-Core pH-Responsive Copolymer Nanocarriers for Combining Photodynamic Therapy and Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 1008–1016. [Google Scholar] [CrossRef]
- De Souza, T.D.; Ziembowicz, F.I.; Müller, D.F.; Lauermann, S.C.; Kloster, C.L.; Santos, R.C.V.; Lopes, L.Q.S.; Ourique, A.F.; Machado, G.; Villetti, M.A. Evaluation of photodynamic activity, photostability and in vitro drug release of zinc phthalocyanine-loaded nanocapsules. Eur. J. Pharm. Sci. 2016, 83, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, P.; Chen, Y.; Dong, E.; Feng, Z.; He, Z.; Zhou, C.; Wang, C.; Liu, Y.; Feng, C. Preparation of zinc phthalocyanine-loaded amphiphilic phosphonium chitosan nanomicelles for enhancement of photodynamic therapy efficacy. Colloids Surf. B Biointerfaces 2021, 202, 111693. [Google Scholar] [CrossRef] [PubMed]
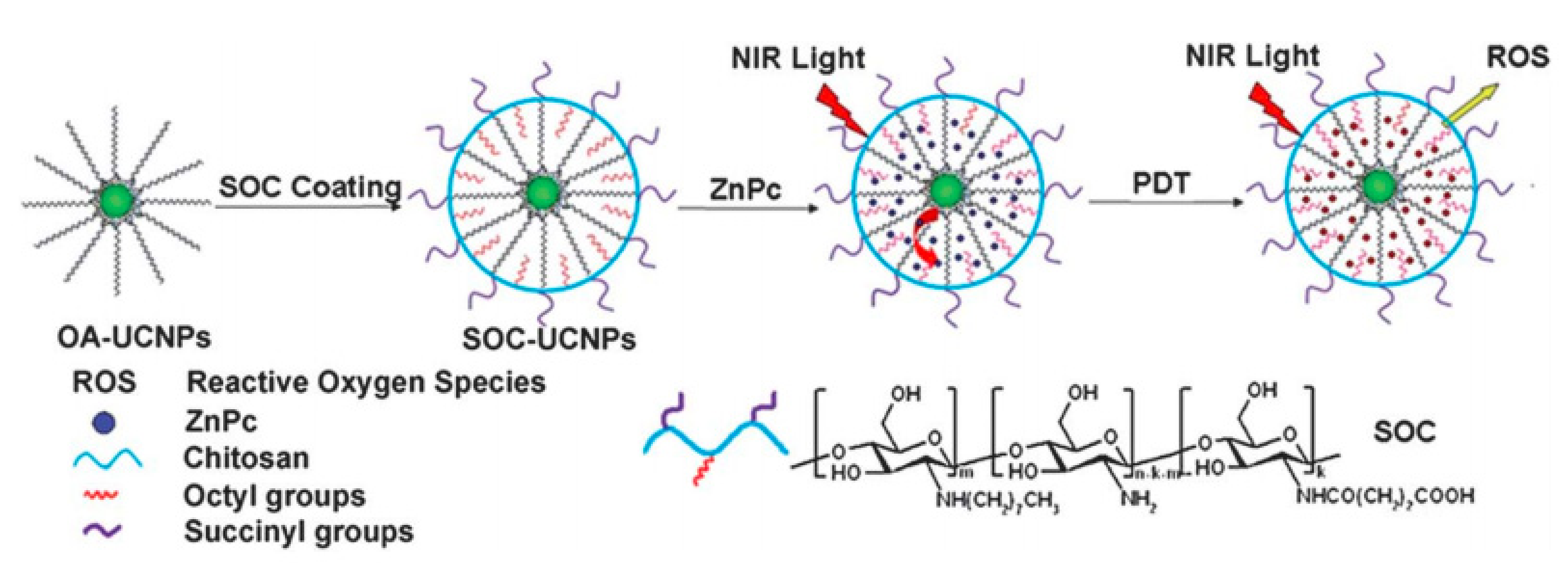
- Cui, S.; Chen, H.; Zhu, H.; Tian, J.; Chi, X.; Qian, Z.; Achilefu, S.; Gu, Y. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In Vivo Targeted Deep-Tissue Photodynamic Therapy Based on Near-Infrared Light Triggered Upconversion Nanoconstruct. ACS Nano 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z.; Lv, L.; Yin, D.; Chen, D.; Han, Z.; Ma, Y.; Zhang, M.; Yang, M.; Gu, Y. Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics 2016, 6, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, Y.; Zhang, C.; Alfranca, G.; Cheng, S.; Ma, L.; Liu, Y.; Zhi, X.; Ni, J.; Jiang, W.; et al. ROS-Responsive Mitochondria-Targeting Blended Nanoparticles: Chemo- and Photodynamic Synergistic Therapy for Lung Cancer with On-Demand Drug Release upon Irradiation with a Single Light Source. Theranostics 2016, 6, 2352–2366. [Google Scholar] [CrossRef] [PubMed]
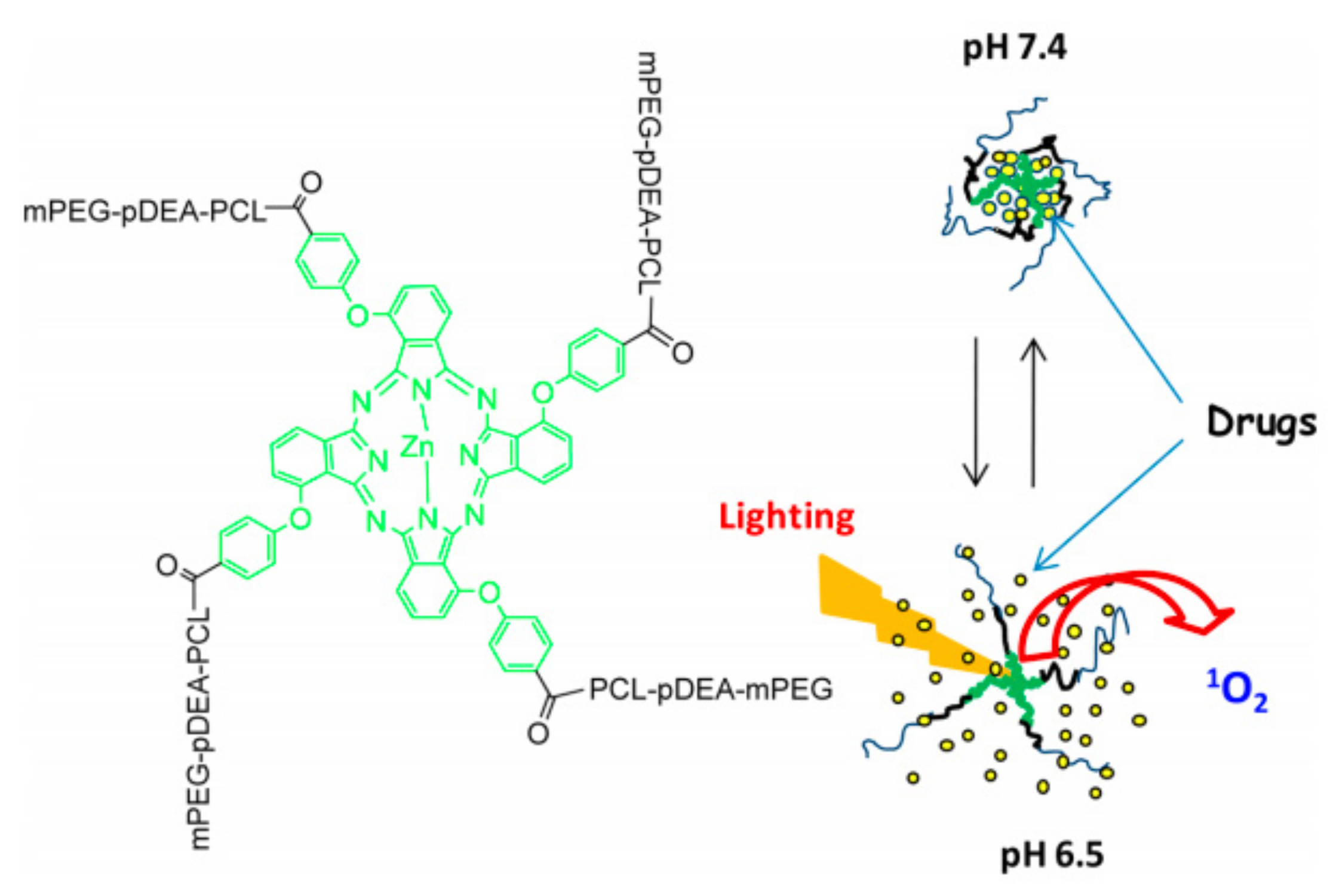
- Gao, D.; Lo, P.-C. Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy. J. Control. Release 2018, 282, 46–61. [Google Scholar] [CrossRef]
- Breitenbach, B.B.; Steiert, E.; Konhäuser, M.; Vogt, L.-M.; Wang, Y.; Parekh, S.H.; Wich, P.R. Double stimuli-responsive polysaccharide block copolymers as green macrosurfactants for near-infrared photodynamic therapy. Soft Matter 2019, 15, 1423–1434. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Hu, Z.; Jiang, X.-J.; Ngai, T.; Lo, P.-C.; Zhang, W.; Chen, G. Novel phthalocyanine and PEG-methacrylates based temperature-responsive polymers for targeted photodynamic therapy. Polym. Chem. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Zhang, Z.; Weng, Y.; Chen, G.; Yuan, B.; Yang, K.; Ma, Y. Encapsulation of Hydrophobic Phthalocyanine with Poly(N-isopropylacrylamide)/Lipid Composite Microspheres for Thermo-Responsive Release and Photodynamic Therapy. Materials 2014, 7, 3481–3493. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Li, Y.; Liu, Y.; Duan, Q.; Kakuchi, T. Synthesis of water-soluble and thermoresponsive phthalocyanine ended block copolymers as potential photosensitizer. Dye. Pigment. 2017, 142, 88–99. [Google Scholar] [CrossRef]
- Feuser, P.E.; Fernandes, A.C.; Nele, M.; Viegas, A.D.C.; Ricci-Junior, E.; Tedesco, A.C.; Sayer, C.; de Araújo, P.H.H. Simultaneous encapsulation of magnetic nanoparticles and zinc phthalocyanine in poly(methyl methacrylate) nanoparticles by miniemulsion polymerization and in vitro studies. Colloids Surfaces B Biointerfaces 2015, 135, 357–364. [Google Scholar] [CrossRef]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of near-infrared photoactivable phthalocyanine-loaded nanoparticles to kill tumor cells: An improved tool for photodynamic therapy of solid cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, 1902604. [Google Scholar] [CrossRef]
- Alibolandi, M.; Ramezani, M.; Abnous, K.; Sadeghi, F.; Hadizadeh, F. Comparative evaluation of polymersome versus micelle structures as vehicles for the controlled release of drugs. J. Nanoparticle Res. 2015, 17, 76. [Google Scholar] [CrossRef]
- Chidanguro, T.; Simon, Y.C. Bent out of shape: Towards non-spherical polymersome morphologies. Polym. Int. 2021, 70, 951–957. [Google Scholar] [CrossRef]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-polymer Platforms Into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.V.; Perez, L.R.; Battaglia, G. Block copolymer nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Choucair, A.; Eisenberg, A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur. Phys. J. E 2003, 10, 37–44. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Eisenberg, A. Morphogenic Effect of Solvent on Crew-Cut Aggregates of Apmphiphilic Diblock Copolymers. Macromolecules 1998, 31, 1144–1154. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Eisenberg, A. Multiple pH-Induced Morphological Changes in Aggregates of Polystyrene-block-poly(4-vinylpyridine) in DMF/H2O Mixtures. J. Am. Chem. Soc. 1999, 121, 2728–2740. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Patravale, V.; Joshi, M. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar]
- Riley, T.; Stolnik, S.; Heald, C.R.; Xiong, C.D.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Physicochemical Evaluation of Nanoparticles Assembled from Poly(lactic acid)−Poly(ethylene glycol) (PLA−PEG) Block Copolymers as Drug Delivery Vehicles. Langmuir 2001, 17, 3168–3174. [Google Scholar] [CrossRef]
- Heald, C.R.; Stolnik, S.; Kujawinski, K.S.; De Matteis, C.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Poly(lactic acid)−Poly(ethylene oxide) (PLA−PEG) Nanoparticles: NMR Studies of the Central Solidlike PLA Core and the Liquid PEG Corona. Langmuir 2002, 18, 3669–3675. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Chitkara, D.; Kumar, N.; Pawar, R.; Domb, A.; Corn, B. Polymeric Carriers for Regional Drug Therapy. In Smart Polymers: Applications in Biotechnology and Biomedicine; CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef]
- Li, T.; Yan, L. Functional Polymer Nanocarriers for Photodynamic Therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized Micellar Systems for Cancer Targeted Drug Delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Li, L.; Huh, K.M. Polymeric nanocarrier systems for photodynamic therapy. Biomater. Res. 2014, 18, 19. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, C.Y.; Lee, S.; Lee, D.; Chung, H.-M.; Kim, G.; Heo, S.-H.; Kim, C.; Hong, K.-S.; Yoon, J. Nanostructured Phthalocyanine Assemblies with Protein-Driven Switchable Photoactivities for Biophotonic Imaging and Therapy. J. Am. Chem. Soc. 2017, 139, 10880–10886. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Targeting the Oncofetal Thomsen–Friedenreich Disaccharide Using Jacalin-PEG Phthalocyanine Gold Nanoparticles for Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2012, 51, 6158–6162. [Google Scholar] [CrossRef]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef]
- García Calavia, P.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.M.; Bayoumi, N.A.; El-Shershaby, H.M.; Allahloubi, N.M. Targeted photoimmunotherapy based on photosensitizer-antibody conjugates for multiple myeloma treatment. J. Photochem. Photobiol. B Biol. 2020, 203, 111777. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.M.; Al-Ashkar, E.A. Synthesis, photochemical and photophysical properties of a sulfo-pegylated zinc-phthalocyanine star polymer potential for biomedical applications. Adv. Appl. Sci. 2015, 9, 126–134. [Google Scholar]
- Domiński, A.; Konieczny, T.; Duale, K.; Krawczyk, M.; Pastuch-Gawołek, G.; Kurcok, P. Stimuli-Responsive Aliphatic Polycarbonate Nanocarriers for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 2890. [Google Scholar] [CrossRef] [PubMed]
- Lamch, Ł.; Tylus, W.; Jewgiński, M.; Latajka, R.; Wilk, K.A. Location of Varying Hydrophobicity Zinc(II) Phthalocyanine-Type Photosensitizers in Methoxy Poly(ethylene oxide) and Poly(l-lactide) Block Copolymer Micelles Using 1H NMR and XPS Techniques. J. Phys. Chem. B 2016, 120, 12768–12780. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, W.; Chen, G.; Zhang, W.; Zhu, X. A novel approach to synthesize polymers for potential photodynamic therapy: From benzenedinitrile to phthalocyanine. Polym. Chem. 2014, 5, 2872–2879. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Li, C.; Li, Y.; Liu, J.; Tu, Y.; Zhang, W.; Zhou, N.; Zhu, X. Preparation and characterization of solution processable phthalocyanine-containing polymers via a combination of RAFT polymerization and post-polymerization modification techniques. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 691–698. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, H.; Wu, H.; Huang, B.; Gan, L.; Chen, Z. The synthesis and photophysical properties of zinc (II) phthalocyanine bearing poly(aryl benzyl ether) dendritic substituents. Dye. Pigment. 2010, 87, 10–16. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.R.; Vilsinski, B.H.; Nunes, C.S.; Bonkovoski, L.C.; Garcia, F.; Nakamura, C.V.; Caetano, W.; Valente, A.J.M.; Martins, A.F.; Muniz, E.C. Application of a polyelectrolyte complex based on biocompatible polysaccharides for colorectal cancer inhibition. Carbohydr. Res. 2021, 499, 108194. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef]
- Qin, Y.L.; Huang, X.; Chen, J.Y. Conjugation of sulfonated aluminum phthalocyanine to doxorubicin can improve the efficacy of photodynamic cancer therapy. Anti Cancer Drugs 2012, 23, 1047–1053. [Google Scholar] [CrossRef]
- Thapa, P.; Li, M.; Bio, M.; Rajaputra, P.; Nkepang, G.; Sun, Y.; Woo, S.; You, Y. Far-Red Light-Activatable Prodrug of Paclitaxel for the Combined Effects of Photodynamic Therapy and Site-Specific Paclitaxel Chemotherapy. J. Med. Chem. 2016, 59, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Gao, D.; Lo, P.-C. Combined pH-responsive chemotherapy and glutathione-triggered photosensitization to overcome drug-resistant hepatocellular carcinoma—A SPP/JPP Young Investigator Award paper. J. Porphyr. Phthalocyanines 2020, 24, 1387–1401. [Google Scholar] [CrossRef]
- Guo, X.; Jin, H.; Lo, P.-C. Encapsulating an acid-activatable phthalocyanine–doxorubicin conjugate and the hypoxia-sensitive tirapazamine in polymeric micelles for multimodal cancer therapy. Biomater. Sci. 2021, 9, 4936–4951. [Google Scholar] [CrossRef]
- Rijcken, C.J.F.; Hofman, J.-W.; van Zeeland, F.; Hennink, W.E.; van Nostrum, C.F. Photosensitiser-loaded biodegradable polymeric micelles: Preparation, characterisation and in vitro PDT efficacy. J. Control. Release 2007, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef] [PubMed]
- Kiew, L.V.; Cheah, H.Y.; Voon, S.H.; Gallon, E.; Movellan, J.; Ng, K.H.; Alpugan, S.; Lee, H.B.; Dumoulin, F.; Vicent, M.J.; et al. Near-infrared activatable phthalocyanine-poly-L-glutamic acid conjugate: Increased cellular uptake and light-dark toxicity ratio toward an effective photodynamic cancer therapy. Nanomedicine 2017, 13, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Cheah, H.Y.; Gallon, E.; Dumoulin, F.; Hoe, S.Z.; Japundžić-Žigon, N.; Glumac, S.; Lee, H.B.; Anand, P.; Chung, L.Y.; Vicent, M.J.; et al. Near-Infrared Activatable Phthalocyanine–Poly-L-Glutamic Acid Conjugate: Enhanced in Vivo Safety and Antitumor Efficacy toward an Effective Photodynamic Cancer Therapy. Mol. Pharm. 2018, 15, 2594–2605. [Google Scholar] [CrossRef]
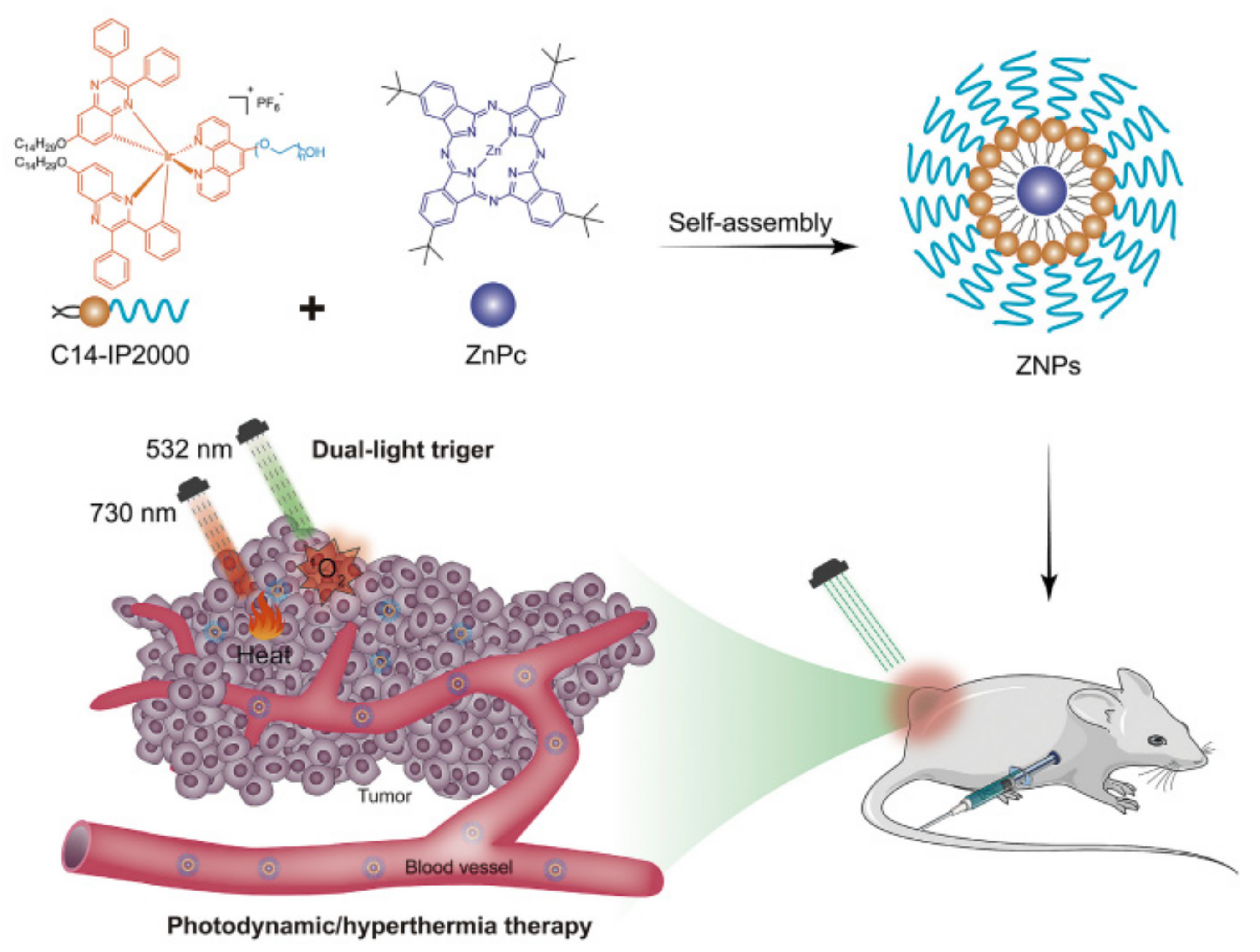
- Deng, Y.; Wang, X.; Liu, Y.; Xu, Y.; Zhang, J.; Huang, F.; Li, B.; Miao, Y.; Sun, Y.; Li, Y. Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy. Acta Biomater. 2021, 119, 323–336. [Google Scholar] [CrossRef]
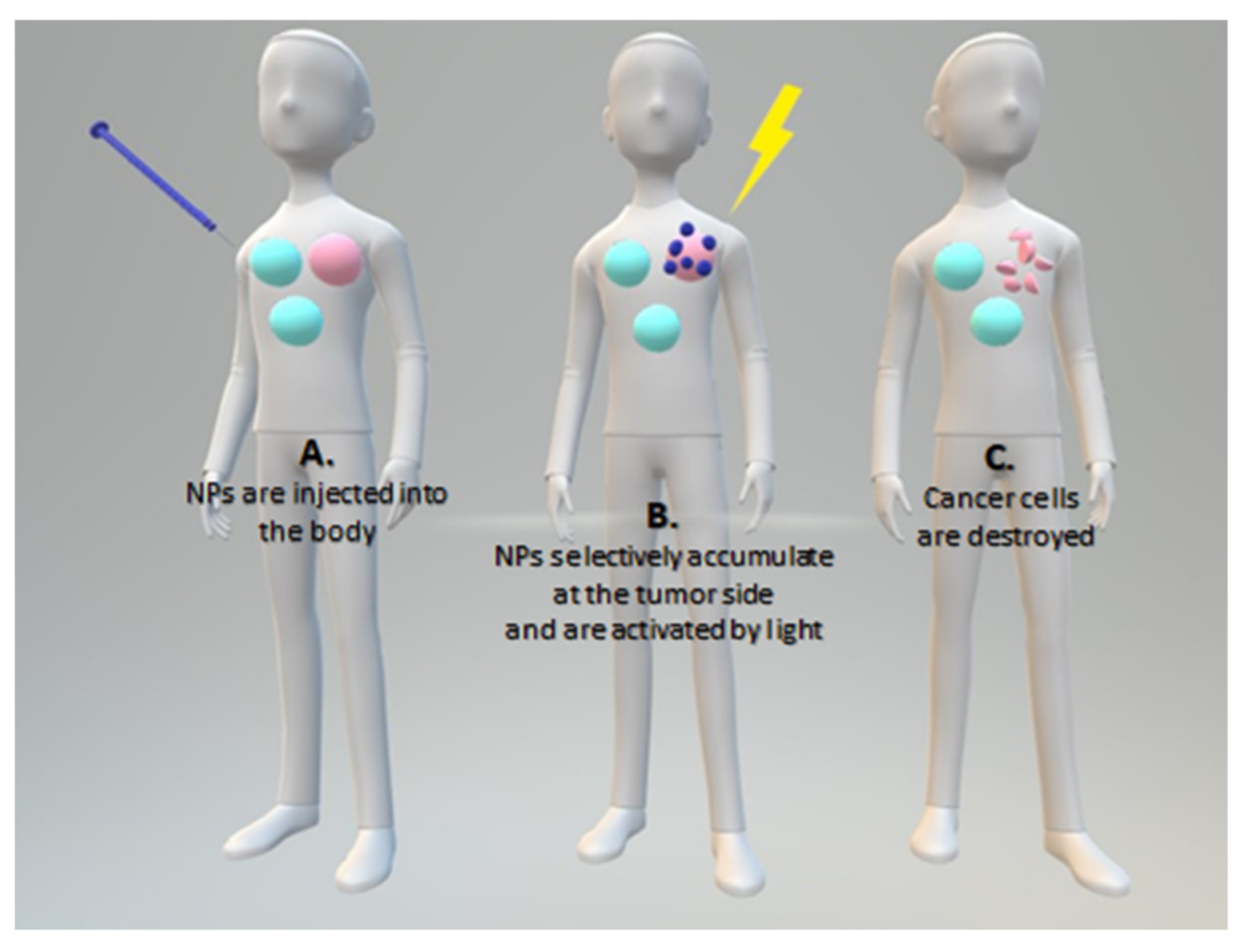

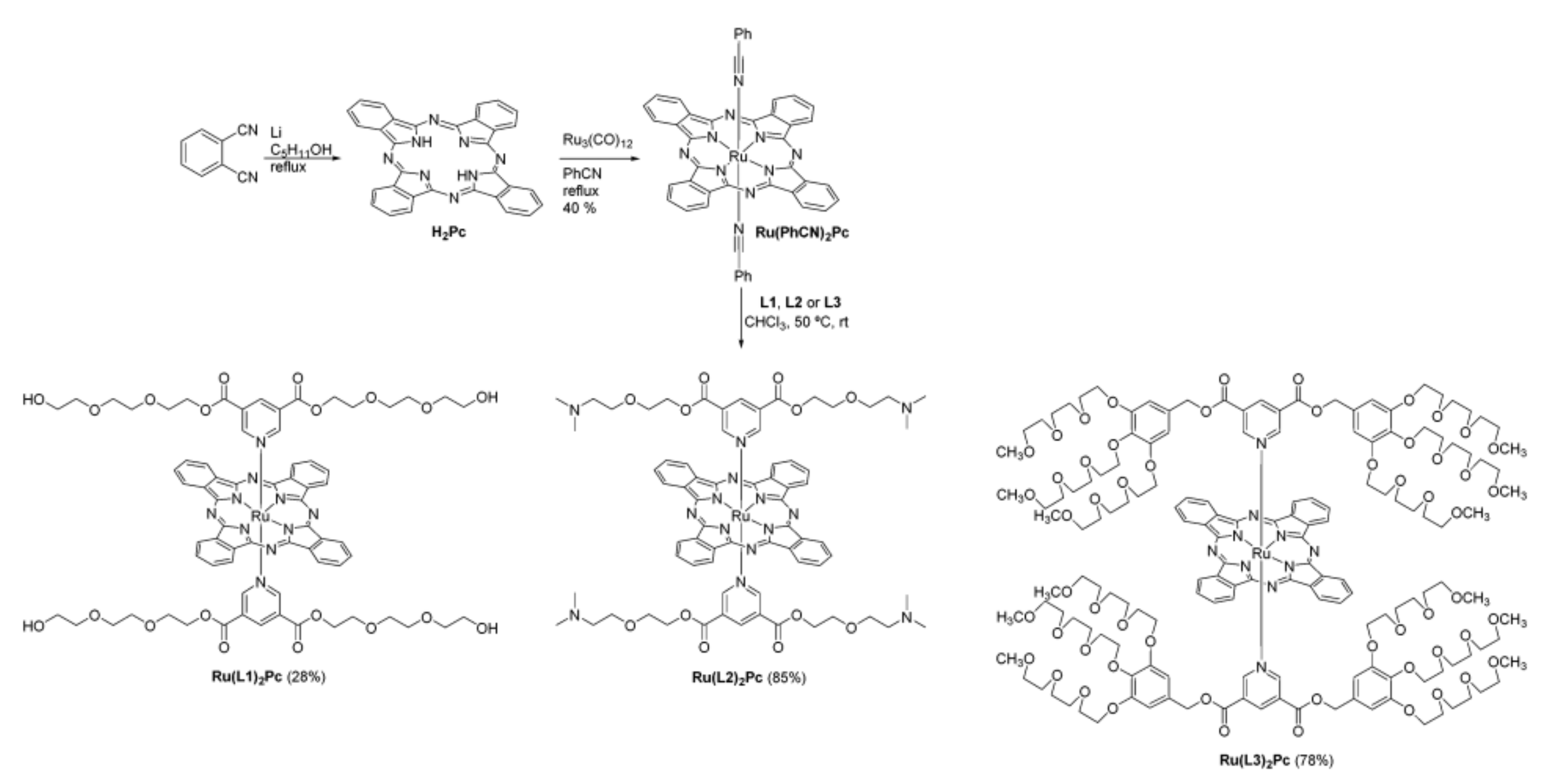
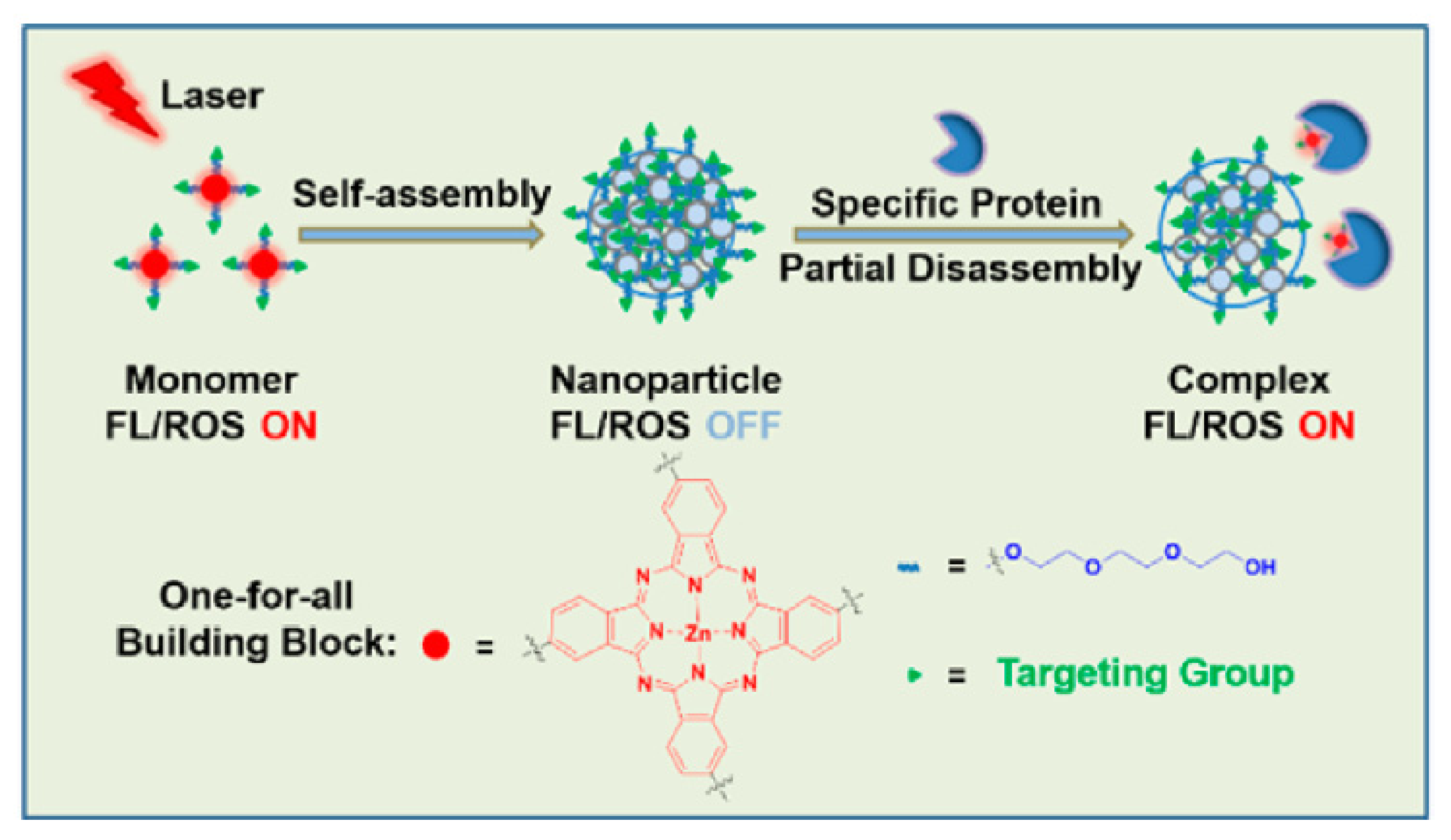

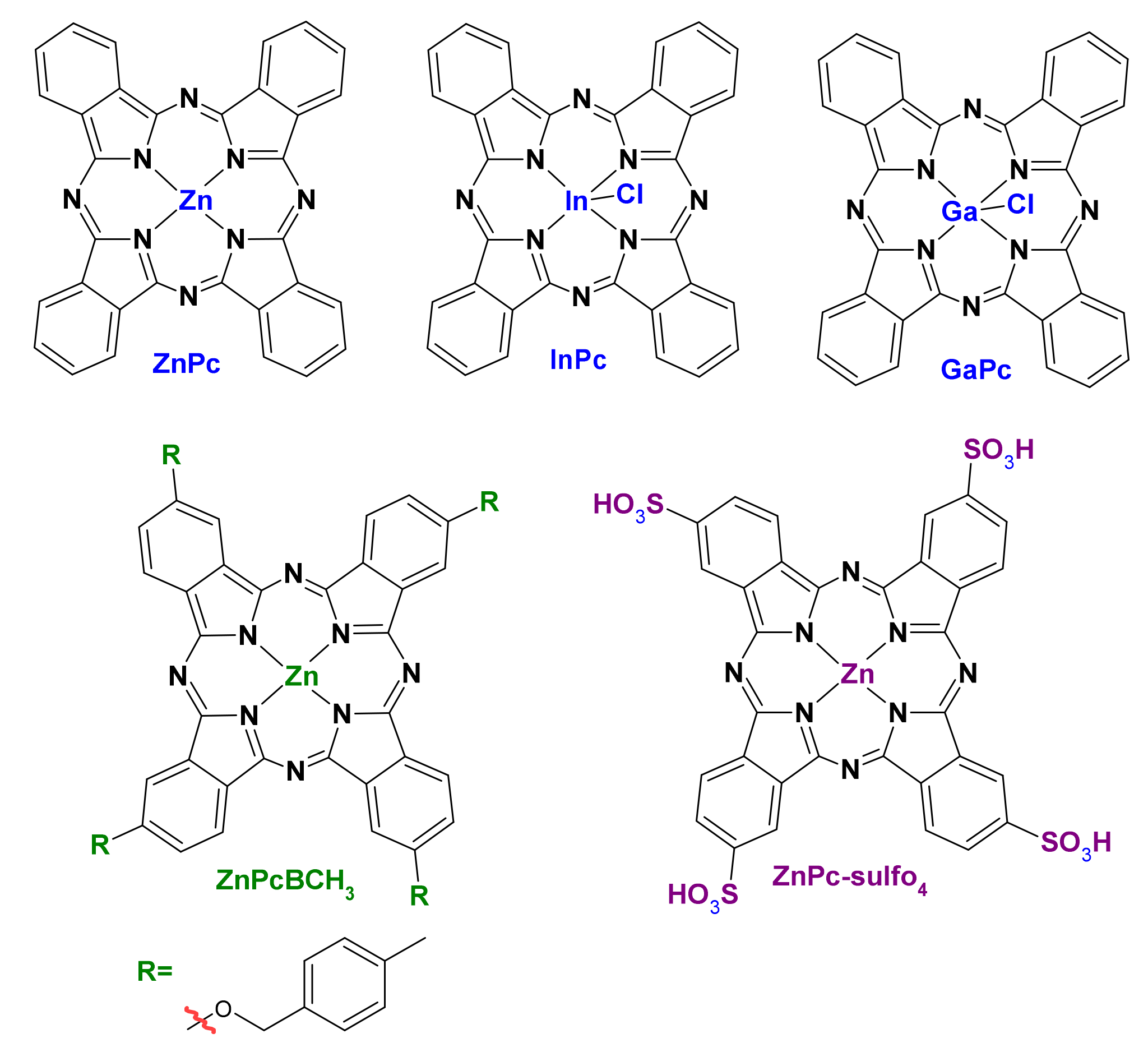




| Name | λmax (nm) | ΦΔ | Cancer Type/Cell Line/Animal Model |
|---|---|---|---|
| Photosens (sulfonated AlPcs) [14,26] | 676 (DMF) | 0.38 (DMF) | skin, stomach, lip, breast, and oral cancer (2) |
| Pc 4 (silicon phthalocyanine 4) [14,26] | 675 (CH3CN) | 0.43 [27] (CH3CN) | actinic keratosis, Bowen’s disease, skin cancer, mycosis fungoides (1) |
| Photocyanine [22,28] | 675 (DMSO) | 0.53 (DMSO) | HepG2 (1) |
| ZnPc [29,30,31] | 672 (DMSO) | 0.67 (DMSO) | cutaneous and subcutaneous lesions from diverse solid tumor origins (1,3) |
| AlPc [32,33] | 680 (DMSO) | 0.29 (DMSO) | J774A.1, Vero cells [34] (3) |
| RuPc-(4–12 PEG) [35] | 638–642 (H2O/DMSO 99: 1) | 0.76 (DMSO) | HT-1376 (3) |
| PEG-ZnPc [36] | 679–686 (DMF) | - | HEp2 (3) |
| Tetraethyleneglycol-substituted ZnPc [29] | 676–702 (DMSO) | 0.34–0.72 (DMSO) | HT-29 (3) |
| Mono-PEGylated ZnPc [37] | 672 (DMF0 | 0.53–0.56 (DMF) | HepG2 (3) |
| ZnPcBCH3 [38] | 681 (DMF) | 0.51 (DMSO) | A549 (3) |
| Poly(aryl benzyl ether)dendrimer ZnPc [39] | 620–630 (PBS) | 0.43–0.56 (DMSO) | HeLa (3) |
| Amphiphilic SiPc deriv [40]. | 686 (CHCl3) | 0.27 (DMSO) | RAW 264.7 (3) |
| Benzyl ester dendrimer SiPc [41] | 678 (DMF) | 0.31 (DMF) | - |
| NzPc [42] | 682 (EtOH) | 0.63 (EtOH) | - |
| Material Description | Nanocarrier Type | Pc-Type | NP Size [nm] | Cell Line/Animal Model | Active Targeting |
|---|---|---|---|---|---|
| PLGA [49] | NP | ZnPc | 285 ± 5.1 | P388-D1 | - |
| PLGA-PEG [50] | NP | InPc | 61–243 | MCF-7 | - |
| PLGA-PEG [51] | NP | GaPc | >200 | Hepa-1C1C7, blood red cell | - |
| PEG-b-PLGA [38] | NP | ZnPcBCH3 | 90.02 ± 0.07 | A549 | - |
| PEG-PLA-BGE [34] | M | AlPc | 60–130 | J774A.1, Vero cells | - |
| PLGA [52] | M | ZnPc-sulfo4 | 384.7 ± 138.6 | B16-F10 | - |
| PLGA-HA [53] | NP | ZnPc- | 259 | HT29, A549, LO2/HT29 tumor-bearing nude mice | HA |
| PEG-b-PLLA [54] | M | ZnPc | 32–35 | Me45, HaCaT, P388/D1, HUV-EC-C | - |
| FA-PEG-b-PLLA [55] | M | ZnPc | <150 | SKOV3, Me45 | FA |
| ZnPc-PEGylated Pluronic P123/PLLA [56] | M | ZnPc | 15–89 | MeWo | - |
| chitosan/mPEG-PLA [57] | NP | ZnPc | 189.7–3.5 | SCC, A431/SKH-1 hairless mice | - |
| PEG-b-PCL [58] | NP | ZnPc | 60 | SC | HPβCD |
| PEG-b-PCL [40] | M | SiPc deriv. | - | RAW 264.7 | - |
| PEG-b-PCL [59] | M | SiPc/ZnPc deriv. | 111/77 | MCF-7 | - |
| PEG-b-PCL [60] | M | AlPc | 66.5–99.1 | female Balb/c mice | |
| Pluronic F127 [61] | M | AlPc | 6 | A549 | - |
| Pluronic F127 [62] | M | InPc/ZnPc deriv. | 27.1–37.8 | MCF-7 | - |
| Tetronic 1107 [63] | M | ZnPc deriv. | 10–100 | CT26 | - |
| Pluronic F127 [64] | M | 4OCSPC | 193.2 | HeLa/mice bearing 4T1 tumor | - |
| Pluronic F127, pNIPAM [65] | M | 4OCSPC | 193.2 | HeLa | - |
| PMMA [66] | NP | ZnPc | 97 ± 2.5 | L929, HPBL, K562, Jurkat | - |
| PSt-b-PPEGA [67] | M | ZnPc | 190–210 | HeLa | - |
| P(R)-b-PPEGA [68] | M | ZnPc | 167–230 | RGK-1 | - |
| PS-b-PAA [69] | M | AlPc | 139.9 ± 0.8 | Caco-2 | - |
| PEG-PMAN [70] | M | ZnPc | 30 | MNNG/Hos, U2OS, Saos-2, MG-63/subcutaneous mouse | - |
| PEG-PCL [71,72,73] | M | Pc 4 | 80–100 | A431, MCF-7c3 | EGFR |
| PEG-b-PCL [25] | M | BtPc | 95–110 | HeLa cells | - |
| PEG-b-PCL [74] | M | SiPc deriv. | 45–70 | - | - |
| PLL-b-PEG-b-PLL, PEG-b-PLL [75] | M | S-AlPc | 10–70 | HUVECs | - |
| PEG5000-b-PLA3000 [41] | M | D-SiPc | 100 | U251 | - |
| PLL-b-PEG-b-PLL [39] | M | ZnPc-dendrimers | 80–150 | HeLa | - |
| PLGA [42] | NP | NzPc | 435 | WS-21 | - |
| PEO2000–b-PCL4300 PEO2000–PCL6800–b-PEO2000 [76] | NP | ZnPc/DTX | 60–100 | HeLa | - |
| P(MMA-b-MAEBA-b-FrucMA)-ZnPc/Dox [77] | NP | ZnPc/DTX | 30 | 3T3, MCF7, MDA-MB-231 | GLUT5 |
| mPEG-pDEA-PCL)4-ZnPc4 (PDCZP) [78] | M | PDCZP | 51–342 | MCF-7, SW480, HepG2/H22 tumor-bearing mice | pH |
| SOC, PCL [79] | NP | ZnPc | 100 | - | |
| phosphonium chitosan [80] | M | ZnPc | 103 ± 5 | Panc-1 | |
| SOC with UPNPs [81] | NP | ZnPc | 45 | HELF, MCF-7/S180 tumor-bearing mice | |
| folate-modified SOC UPNPs [82] | NP | ZnPc | 50 | HELF, MDA-MB-231/S180 tumor-bearing mice, Bel-7402 tumor bearing mice | FA |
| c(RGDyK) modified SOC with UPNPs [83] | NP | ZnPc | 52 | PC-3, WPMY-1/PC-3 tumor-bearing mice | αvβ3 |
| TL-CPT-PEG1K-TPP [84] | N | ZnPc/CPT | 77.1–149. | NCI-H460/Female BALB/c athymic nude mice | n |
| PEG-b-PBLA [85] | M | ZnPc/Dox | 160–180 | HepG2/HepG2 tumor-bearing nude mice | GSH |
| Dex-b-AcDex [86] | M | ZnPc | 120 | HeLa | pH |
| poly(OEGMA-co-DEGMA-co-HEMA) [87] | M | SiPc | 70 | - | temp. |
| pNIPAM/lipid [88] | microgelparticles | SiPc | 1000 | HeLa | temp. |
| PEG-b-PNIPAAM [89] | M | ZnTAPc | 45 | HeLa | temp. |
| PMMA, MNP [90] | NP | ZnPc | 104 ± 2.5 | U87MG | magnetic field |
| PMMA [91] | NP | AlPc-sulfo4 | 80 | MSC, PC3, SCID mice withPC3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzęcka, W.; Domiński, A.; Kowalczuk, M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials 2021, 11, 2426. https://doi.org/10.3390/nano11092426
Borzęcka W, Domiński A, Kowalczuk M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials. 2021; 11(9):2426. https://doi.org/10.3390/nano11092426
Chicago/Turabian StyleBorzęcka, Wioleta, Adrian Domiński, and Marek Kowalczuk. 2021. "Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy" Nanomaterials 11, no. 9: 2426. https://doi.org/10.3390/nano11092426
APA StyleBorzęcka, W., Domiński, A., & Kowalczuk, M. (2021). Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials, 11(9), 2426. https://doi.org/10.3390/nano11092426








