BiVO4 Ceramic Photoanode with Enhanced Photoelectrochemical Stability
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Materials
2.2. Preparation of BiVO4 and Mo doped BiVO4 Powders
2.3. Preparation of BiVO4 and Mo Doped BiVO4 Ceramics
2.4. Fabrication of BiVO4 and Mo Doped BiVO4 Films on FTO Substrate
2.5. Preparation of BiVO4 and Mo-BiVO4 Ceramic Photoanode
2.6. Photoelectrochemical Characterizations
2.7. Characterizations
3. Results and Discussion
3.1. Morphologies and Structures of As-Prepared Samples
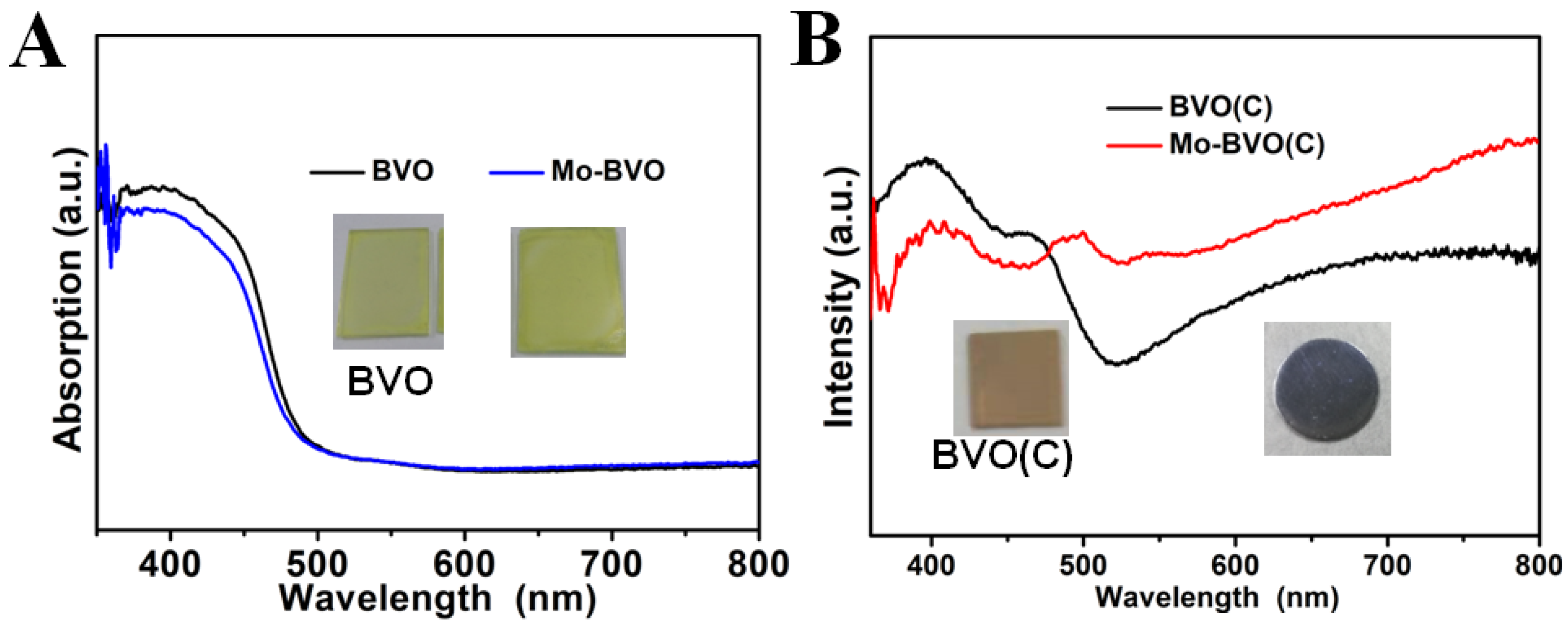
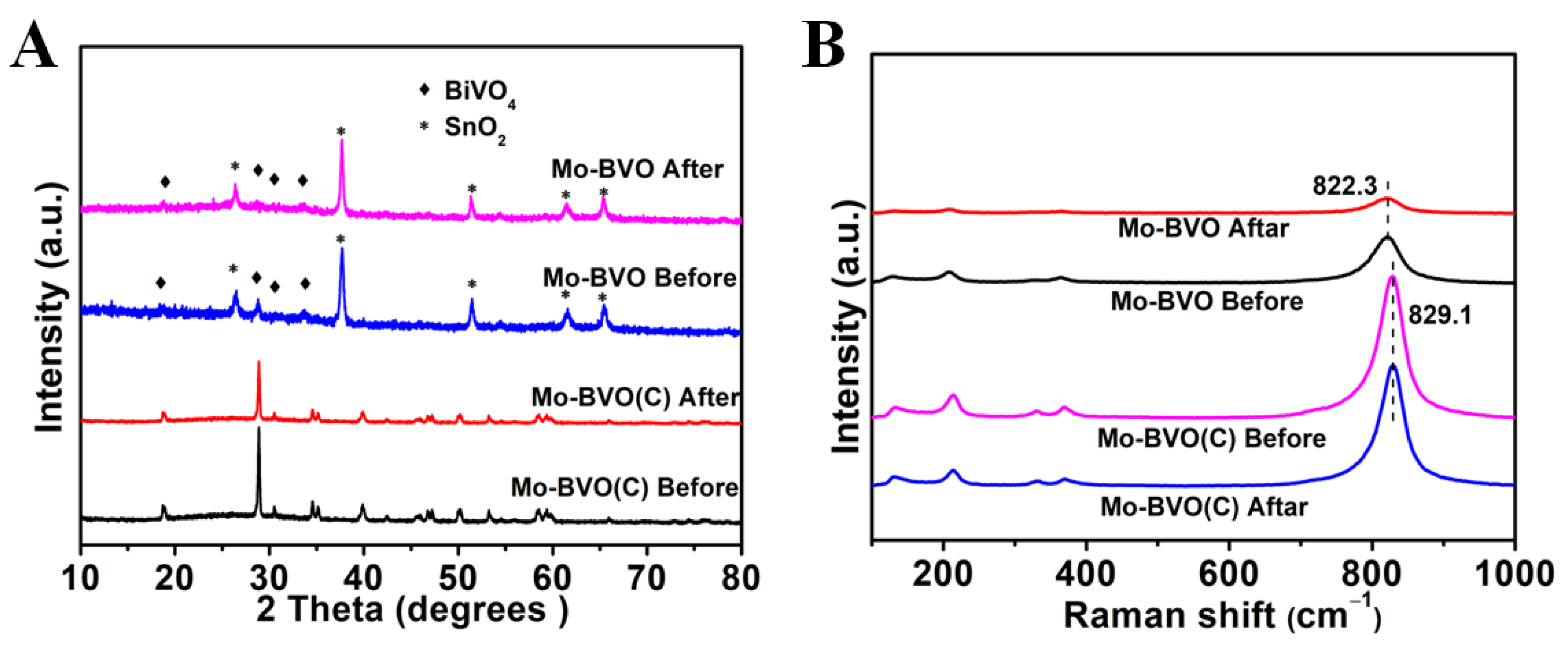
3.2. X-ray Diffraction and UV-Vis DRS Measurements
3.3. Photoelectrochemical Properties of BVO, BVO(C), Mo-BVO(C), and Mo-BVO
3.4. Photoelectrochemical Properties, SEM, XRD, Raman, XPS, and EDS of the Mo-BVO and Mo-BVO (C) Electrodes after the Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.Y.; Huang, B.B.; Zhang, X.Y.; Qin, X.Y.; Li, H.L.; Dai, Y.; Li, Y.J. Anisotropic Photoelectrochemical (PEC) Performances of ZnO Single Crystalline Photoanode: Effect of Internal Electrostatic Fields on the Separation of Photogenerated Charge Carriers during PEC Water Splitting. Chem. Mater. 2016, 28, 6613–6620. [Google Scholar] [CrossRef]
- Qiu, Y.C.; Liu, W.; Chen, W.; Chen, W.; Zhou, G.M.; Hsu, P.-C.; Zhang, R.F.; Liang, Z.; Fan, S.S.; Zhang, Y.G.; et al. Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells. Sci. Adv. 2016, 2, e1501764. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.E.; Wang, F.Y.; Wang, Q. Nanostructure-based WO3 photoanodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2012, 4, 7894–7911. [Google Scholar] [CrossRef]
- Cooper, J.K.; Gul, S.; Toma, F.M.; Chen, L.; Glans, P.-A.; Guo, J.H.; Ager, J.W.; Yano, J.; Sharp, I.D. Electronic structure of monoclinic BiVO4. Chem. Mater. 2014, 26, 5365–5373. [Google Scholar] [CrossRef]
- Toma, F.M.; Cooper, J.K.; Kunzelmann, V.M.; McDowell, M.T.; Yu, J.; Larson, D.M.; Borys, N.J.; Abelyan, C.; Beeman, J.W.; Yu, K.M.; et al. Mechanistic insights into chemical and photochemical transformations of bismuth vanadate photoanodes. Nat. Commun. 2016, 7, 12012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabold, J.A.; Choi, K.S. Efficient and stable photooxidation of water by a bismuth vanadate photoanode couples with an iron oxyhydroxide oxygen evolution catalyst. J. Am. Chem. Soc. 2012, 134, 2186–2192. [Google Scholar] [CrossRef]
- Nair, V.; Perkins, C.L.; Lin, Q.Y.; Law, M. Textured nanoporous Mo: BiVO4 photoanodes with high charge transport and charge transfer quantum efficiencies for oxygen evolution. Energy Environ. Sci. 2016, 9, 412–1429. [Google Scholar] [CrossRef] [Green Version]
- Tayebi, M.; Lee, B.-K. Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting. Renew. Sust. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Choi, K.S.; Shin, H.-M.; Kim, T.L.; Song, J.; Yoon, S.; Jang, H.W.; Yoon, M.-H.; Jeon, C.; Lee, J.; et al. Enhanced photocatalytic performance depending on morphology of bismuth vanadate thin film synthesized by pulsed laser deposition. ACS Appl. Mater. Interfaces 2017, 9, 505–512. [Google Scholar] [CrossRef]
- Zhang, L.W.; Lin, C.-Y.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic Enhancement in BiVO4 Photonic Crystals for Effi cient Water Splitting. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Bao, J.; Xu, Y.; Zhang, J.J.; Xie, J.F.; Guan, M.L.; Wang, C.L.; Wen, L.Y.; Lei, Y.; Xie, Y. Photoelectrodes Based upon Mo: BiVO4 Inverse Opals for Photoelectrochemical Water Splitting. ACS Nano 2014, 8, 7088–7098. [Google Scholar] [CrossRef] [PubMed]
- Talasila, G.; Sachdev, S.; Srivastva, U.; Saxena, D.; Ramakumar, S.S.V. Modified synthesis of BiVO4 and effect of doping (Mo or W) on its photoelectrochemical performance for water splitting. Energy Rep. 2020, 6, 1963–1972. [Google Scholar] [CrossRef]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co-Pi” Catalyst-Modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef]
- Grigioni, I.; Abdellah, M.; Corti, A.; Dozzi, M.V.; Hammarström, L.; Selli, E. Photoinduced Charge-Transfer Dynamics in WO3/BiVO4 Photoanodes Probed through Midinfrared Transient Absorption Spectroscopy. J. Am. Chem. Soc. 2018, 140, 14042–14045. [Google Scholar] [CrossRef] [Green Version]
- Bielinski, A.R.; Lee, S.; Brancho, J.J.; Esarey, S.L.; Gayle, A.J.; Kazyak, E.; Sun, K.; Bartlett, B.M.; Dasgupta, N.P. Atomic Layer Deposition of Bismuth Vanadate Core-Shell Nanowire Photoanodes. Chem. Mater. 2019, 31, 3221–3227. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lichterman, M.F.; Spurgeon, J.M.; Hu, S.; Sharp, I.D.; Brunschwig, B.S.; Lewis, N.S. Improved Stability of Polycrystalline Bismuth Vanadate Photoanodes by Use of Dual-Layer Thin TiO2/Ni Coatings. J. Phys. Chem. C 2014, 118, 19618–19624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Huang, X.J.; Zhang, Y.; Lu, G.X.; Chou, L.J.; Bi, Y.P. Unveiling the Activity and Stability Origin of BiVO4 Photoanodes with FeNi Oxyhydroxides for Oxygen Evolution. Angew. Chem. Int. Ed. 2020, 132, 19152–19157. [Google Scholar] [CrossRef]
- Lee, D.K.; Cho, K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Qiu, W.T.; Xiao, S.; Ke, J.W.; Wang, Z.; Tang, S.Y.; Zhang, K.; Qian, W.; Huang, Y.C.; Huang, D.; Tong, Y.X.; et al. Freeing the polarons to facilitate charge transport in BiVO4 from oxygen vacancies with an oxidative 2D precursor. Angew. Chem. Int. Ed. 2019, 58, 19087–19095. [Google Scholar] [CrossRef]
- Meng, Q.J.; Zhang, B.B.; Fan, L.Z.; Liu, H.D.; Valvo, M.; Edström, K.; Coartero, M.; Marco, R.D.; Crespo, G.A.; Sun, L.C. Efficient BiVO4 photoanodes by postsynthetic treatment: Remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. Int. Ed. 2019, 58, 19027–19033. [Google Scholar] [CrossRef] [Green Version]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/ Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Wang, M.; Che, Y.S.; Niu, C.; Dang, M.Y.; Dong, D. Effective visible light-active boron and europium co-doped BiVO4 synthesized by sol-gel method for photodegradion of methyl orange. J. Hazard. Mater. 2013, 262, 447–455. [Google Scholar] [CrossRef]
- Gromboni, M.F.; Coelho, D.; Mascaro, L.H.; Pockett, A.; Marken, F. Enhancing activity in a nanostructured BiVO4 photoanode with a coating of microporous Al2O3. Appl. Catal. B Environ. 2017, 200, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Lamers, M.; Fiechter, S.; Friedrich, D.; Abdi, F.F.; Van de Krol, R. Formation and suppression of defects during heat treatment of BiVO4 photoanodes for solar water splitting. J. Mater. Chem. A 2018, 6, 18694–18700. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.R.; Wang, Z.Y.; Zhang, B.; Jiang, W.Y.; Bao, X.L.; Cheng, H.F.; Zheng, Z.K.; Wang, P.; Liu, Y.Y.; Whangbo, M.-H.; et al. Enhancing the Photoelectrochemical Water Oxidation Reaction of BiVO4 Photoanode by Employing Carbon Spheres as Electron Reservoirs. ACS Catal. 2020, 10, 13031–13039. [Google Scholar] [CrossRef]
- Zhang, H.P.; Li, H.L.; Wang, Z.Y.; Zheng, Z.K.; Wang, P.; Liu, Y.Y.; Zhang, X.Y.; Qin, X.Y.; Dai, Y.; Huang, B.B. Fabrication of BiVO4 Photoanode Consisted of Mesoporous Nanoparticles with Improved Bulk Charge Separation Efficiency. Appl. Catal. B Environ. 2018, 238, 586–591. [Google Scholar] [CrossRef]
- Hirota, K.; Komatsu, G.; Yamashita, M.; Takemura, H.; Yamaquchi, O. Formation, Characterization and singtering of Alkoxy-derived Bismuth Vanadate. Mat. Res. Bull. 1992, 27, 823–830. [Google Scholar] [CrossRef]
- Rettie, A.J.E.; Lee, H.C.; Marshall, L.G.; Lin, J.-F.; Capan, C.; Lindemuth, J.; McCloy, J.S.; Zhou, J.S.; Bard, A.J.; Buddie Mullins, C. Combined Charge Carrier Transport and Photoelectrochemical Characterization of BiVO4 Single Crystals: Intrinsic Behavior of a Complex Metal Oxide. J. Am. Chem. Soc. 2013, 135, 11389–11396. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.S.; Park, H.L.; Kim, J.N.; Ro, J.H.; Park, Y.H. Raman Spectrum in Monoclinic BiVO4. Jpn. J. Appl. Phys. 1985, 24, 506–507. [Google Scholar] [CrossRef]
- Frost, R.L.; Henry, D.A.; Weier, M.L.; Martens, W. Raman spectroscopy of three polymorphs of BiVO4: Clinobisvanite, dreyerite and pucherite, with comparisons to (VO4)3-bearing minerals: Namibite, pottsite and schumacherite. J. Raman Spectrosc. 2006, 37, 722–732. [Google Scholar] [CrossRef] [Green Version]
- Du, J.Y.; Zhong, X.H.; He, H.C.; Huang, J.; Yang, M.J.; Ke, G.L.; Wang, J.; Zhou, Y.; Dong, F.Q.; Ren, Q.; et al. Enhanced Photoelectrochemical Water Oxidation Performance on BiVO4 by Coupling of CoMoO4 as a Hole-Transfer and Conversion Cocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 42207–42216. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; He, J.F.; Pena, B.; Berlinguette, C.P. Curing BiVO4 Photoanodes with Ultraviolet Light Enhances Photoelectrocatalysis. Angew. Chem. Int. Ed. 2016, 55, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Trześniewski, B.J.; Digdaya, I.A.; Nagaki, T.; Ravishankar, S.; Herraiz-Cardona, I.; Vermaas, D.A.; Longo, A.; Gimenez, S.; Smith, W.A. Near-complete suppression of surface losses and total internal quantum efficiency in BiVO4 photoanodes. Energy Environ. Sci. 2017, 10, 1517–1529. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Wang, M.; Li, Y.; Ma, F.; Li, J.; Jiang, W.; Liu, M.; Cheng, H.; Wang, Z.; Zheng, Z.; et al. BiVO4 Ceramic Photoanode with Enhanced Photoelectrochemical Stability. Nanomaterials 2021, 11, 2404. https://doi.org/10.3390/nano11092404
Zheng L, Wang M, Li Y, Ma F, Li J, Jiang W, Liu M, Cheng H, Wang Z, Zheng Z, et al. BiVO4 Ceramic Photoanode with Enhanced Photoelectrochemical Stability. Nanomaterials. 2021; 11(9):2404. https://doi.org/10.3390/nano11092404
Chicago/Turabian StyleZheng, Liren, Minrui Wang, Yujie Li, Fahao Ma, Jiyu Li, Weiyi Jiang, Mu Liu, Hefeng Cheng, Zeyan Wang, Zhaoke Zheng, and et al. 2021. "BiVO4 Ceramic Photoanode with Enhanced Photoelectrochemical Stability" Nanomaterials 11, no. 9: 2404. https://doi.org/10.3390/nano11092404
APA StyleZheng, L., Wang, M., Li, Y., Ma, F., Li, J., Jiang, W., Liu, M., Cheng, H., Wang, Z., Zheng, Z., Wang, P., Liu, Y., Dai, Y., & Huang, B. (2021). BiVO4 Ceramic Photoanode with Enhanced Photoelectrochemical Stability. Nanomaterials, 11(9), 2404. https://doi.org/10.3390/nano11092404







