Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery
Abstract
1. Introduction
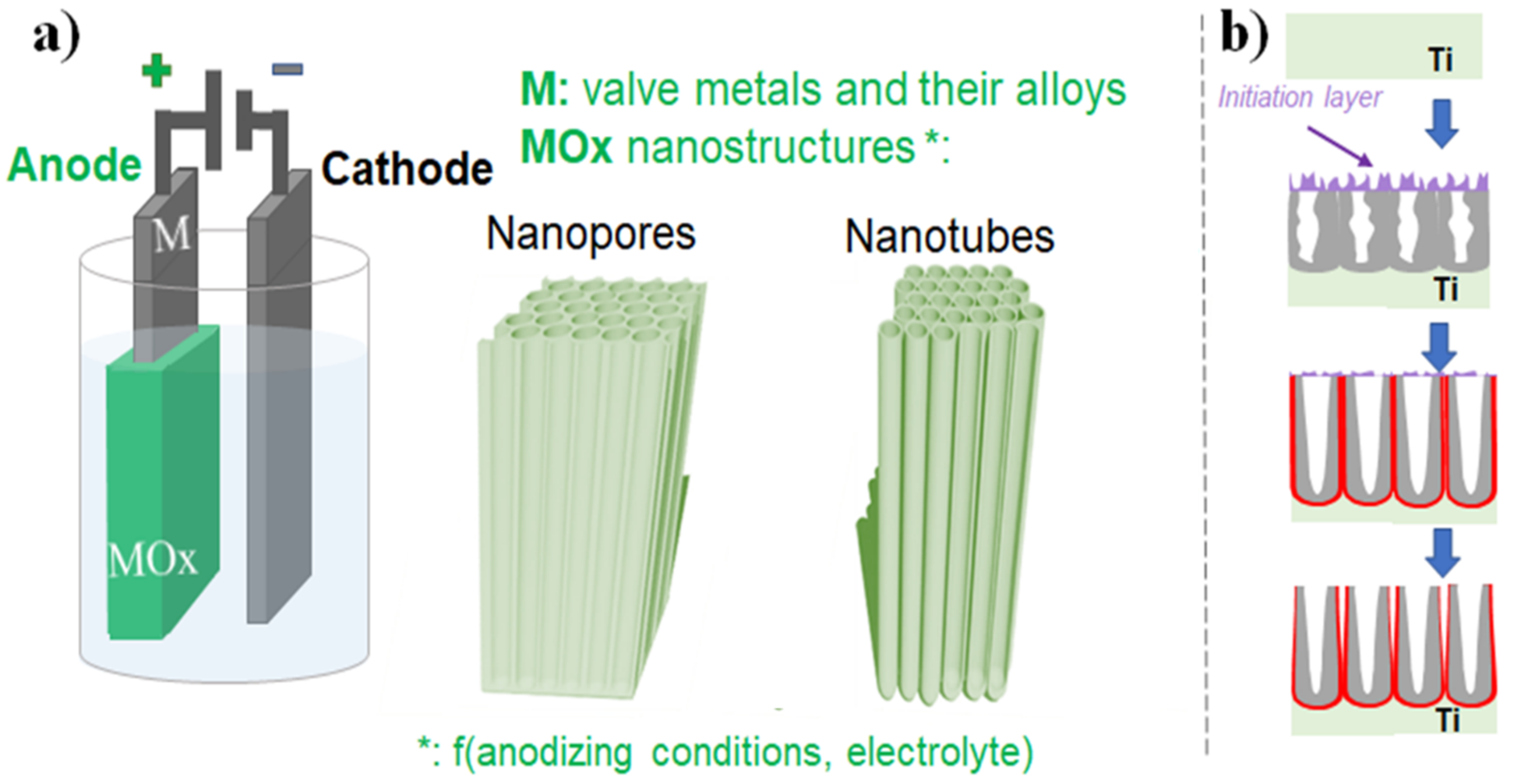
2. Anodic TiO2 Nanotubes
2.1. Nanomorphology and Critical Aspects of Anodic TiO2 Nanotubes
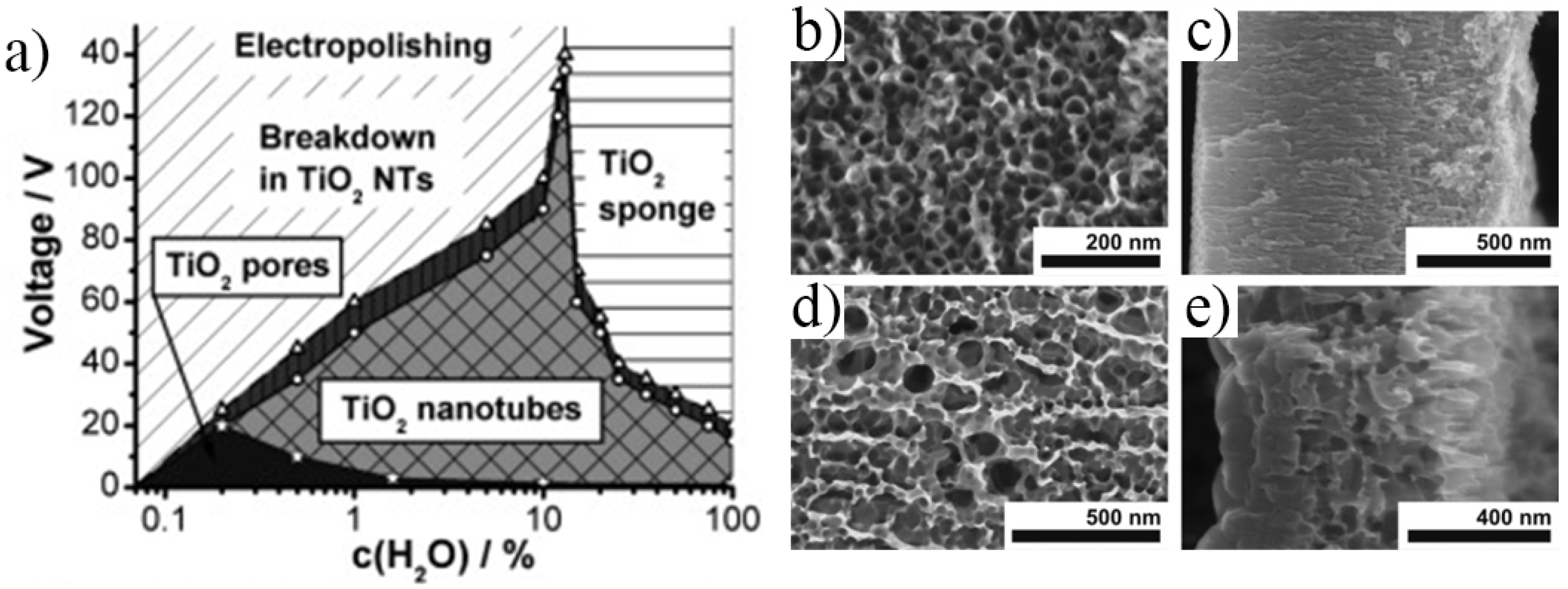
2.1.1. Electrochemical Anodization
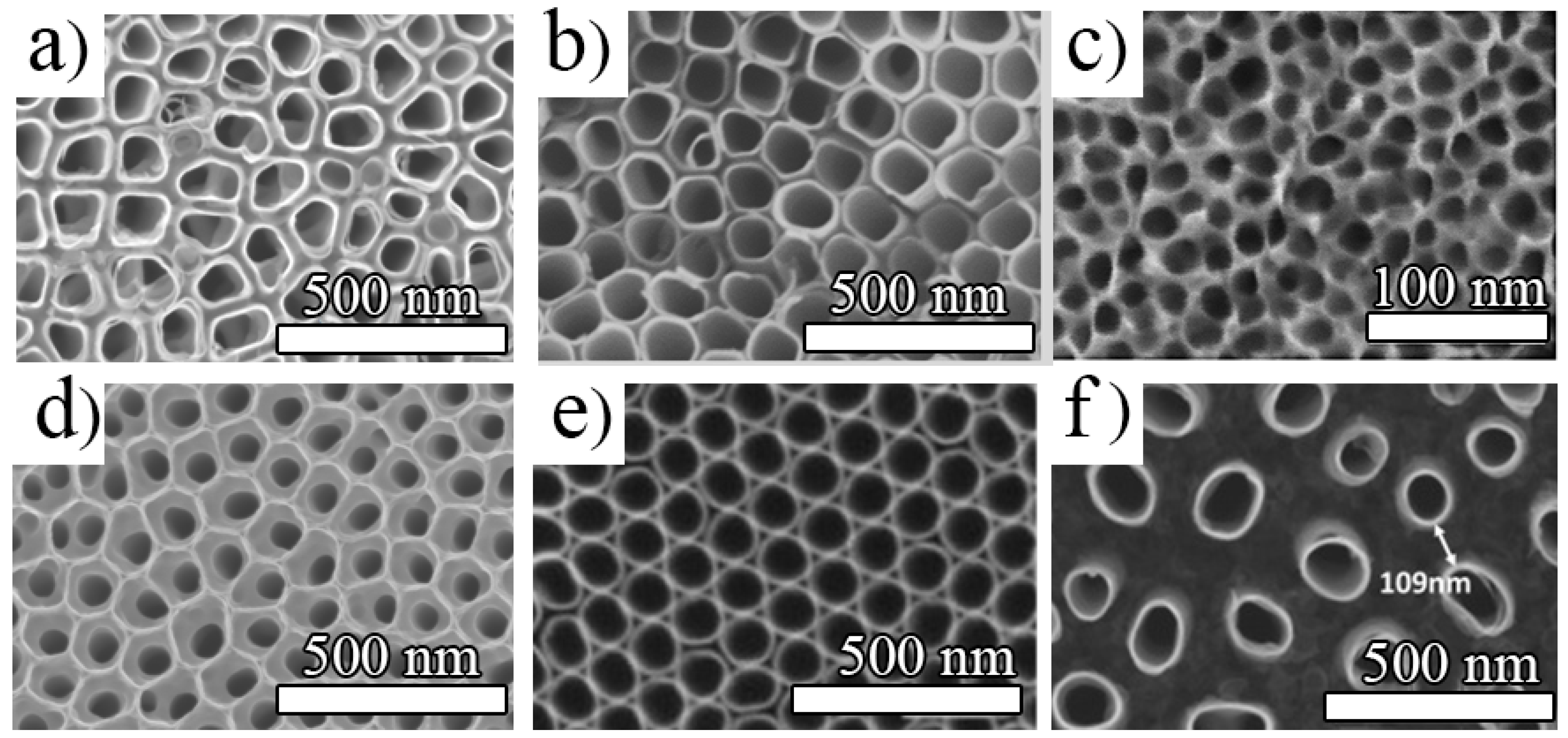
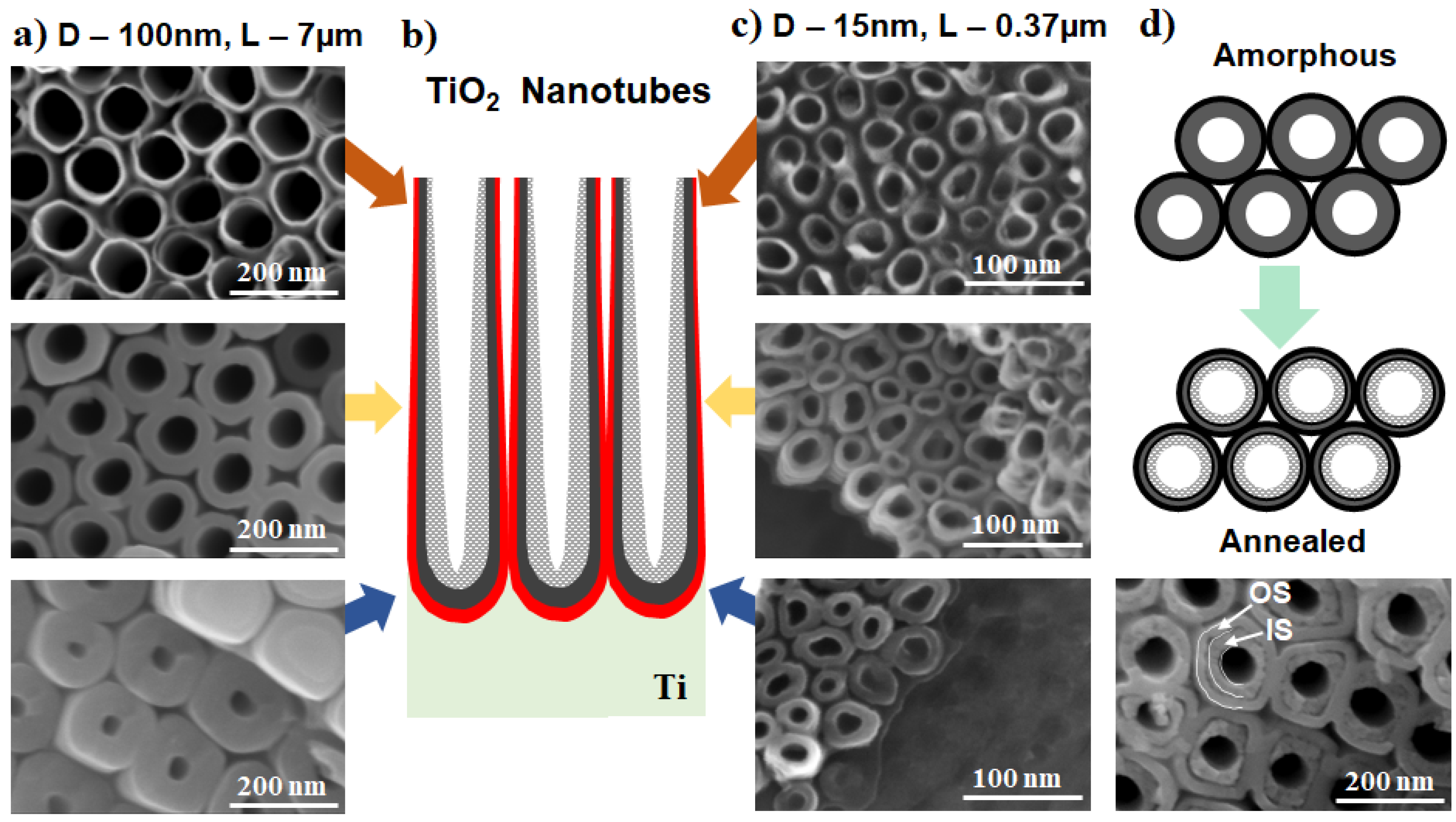
2.1.2. Morphology Aspects of Anodic TiO2 Nanotubes
2.2. Key Properties and Their Improvement for Biomedical Applications
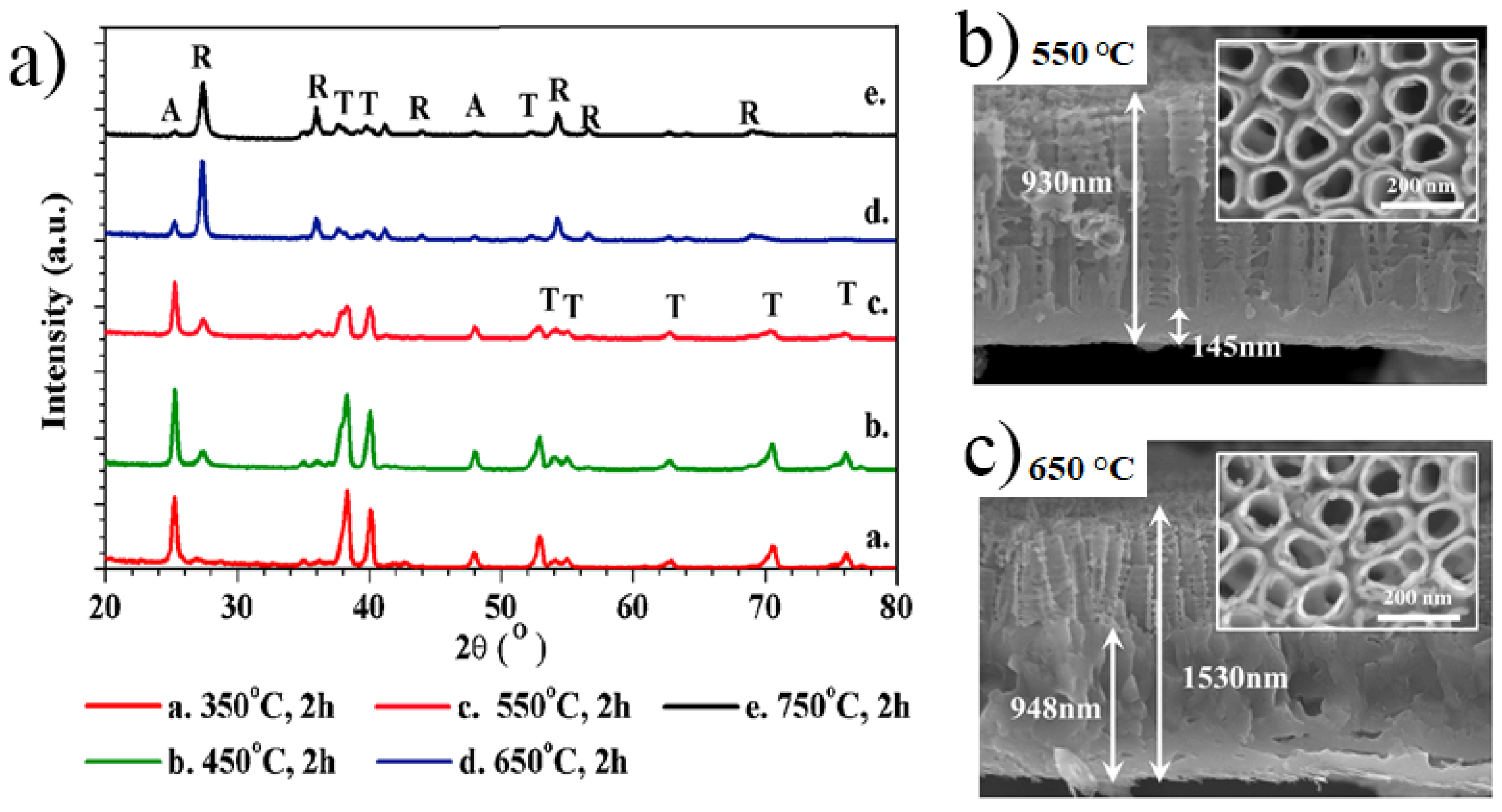
2.2.1. Crystallinity of Anodic TiO2 Nanotubes
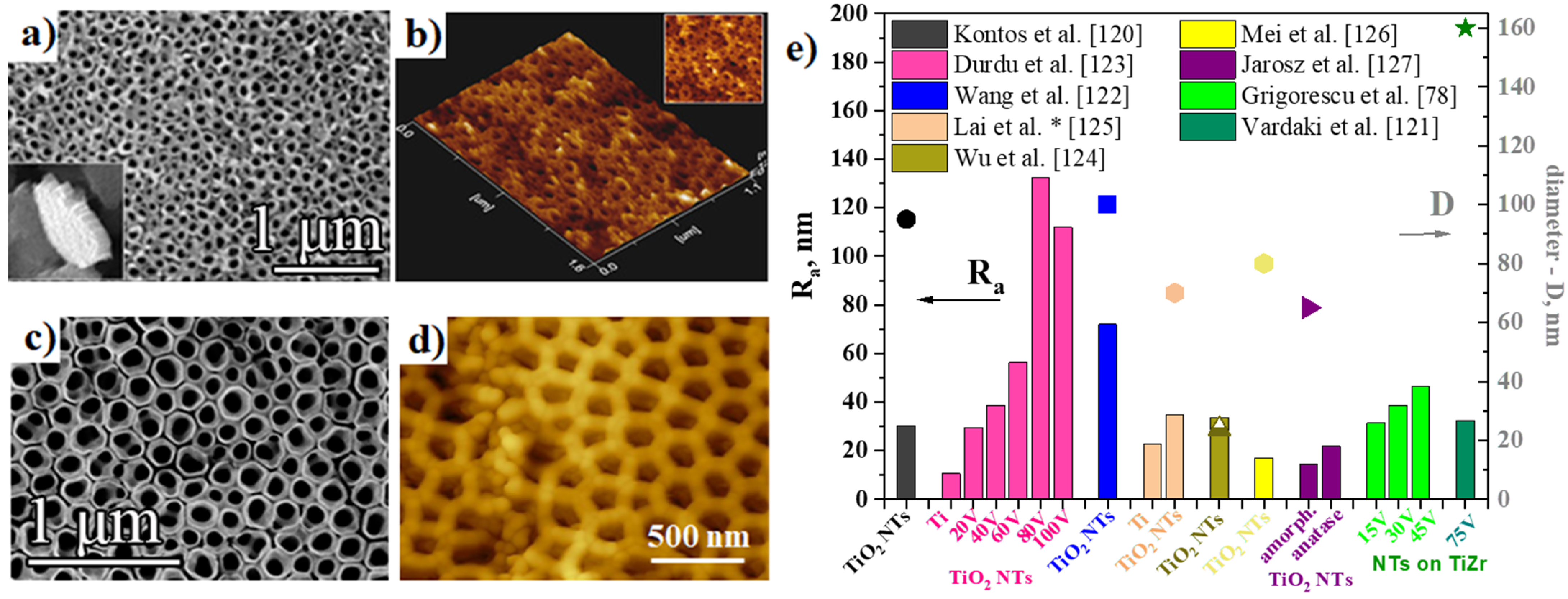
2.2.2. Surface Roughness of Anodic TiO2 Nanotubes
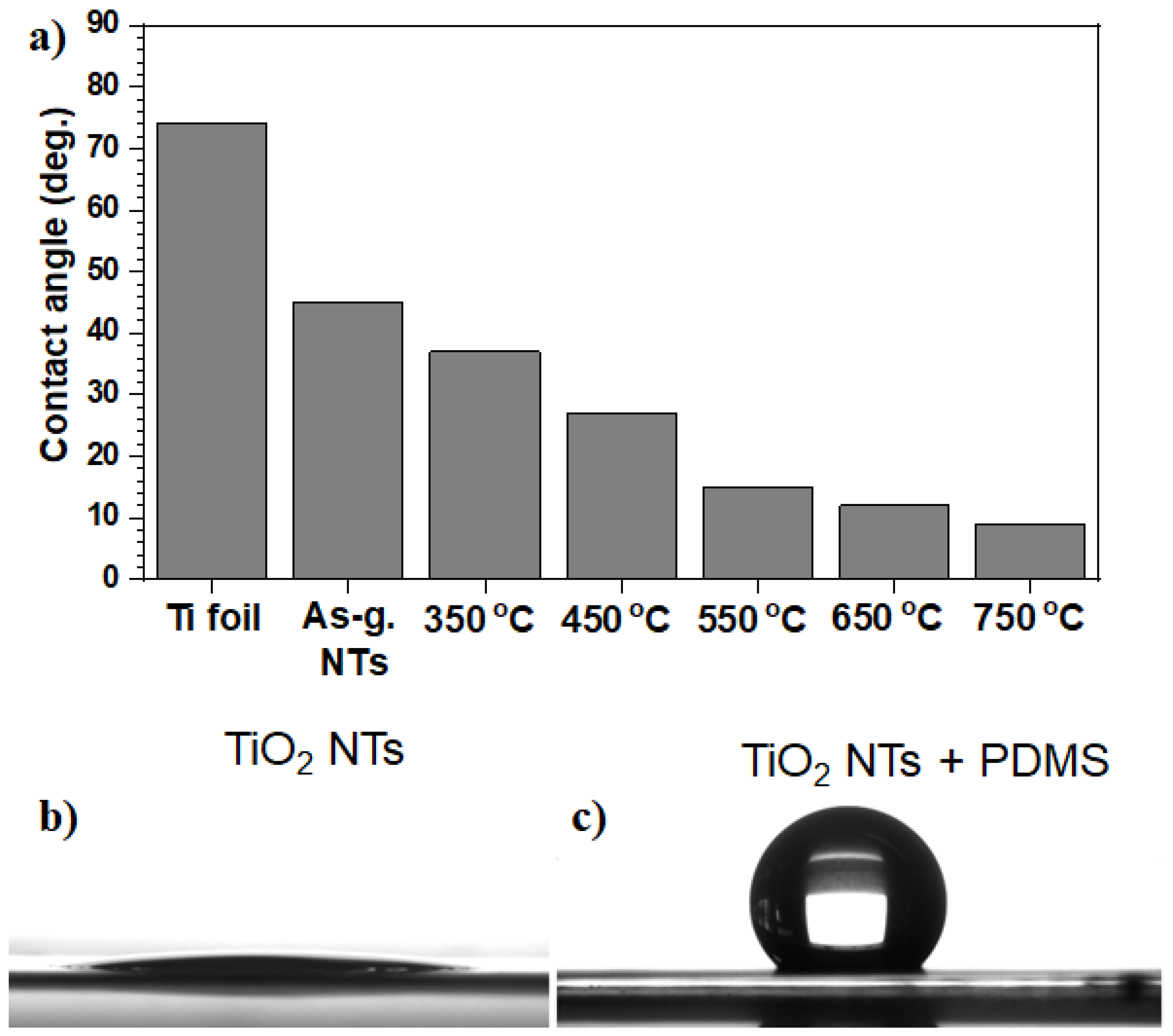
2.2.3. Wetting Characteristics of Anodic TiO2 Nanotubes
2.2.4. Corrosion Resistance of Anodic TiO2 Nanotubes
3. Tailoring Osteoinduction with Anodic TiO2 Nanotubes
3.1. Advantages of Anodic TiO2 Nanotubes for Osteoinduction
3.1.1. Nanotopographical Cues of Anodic TiO2 Nanotubes
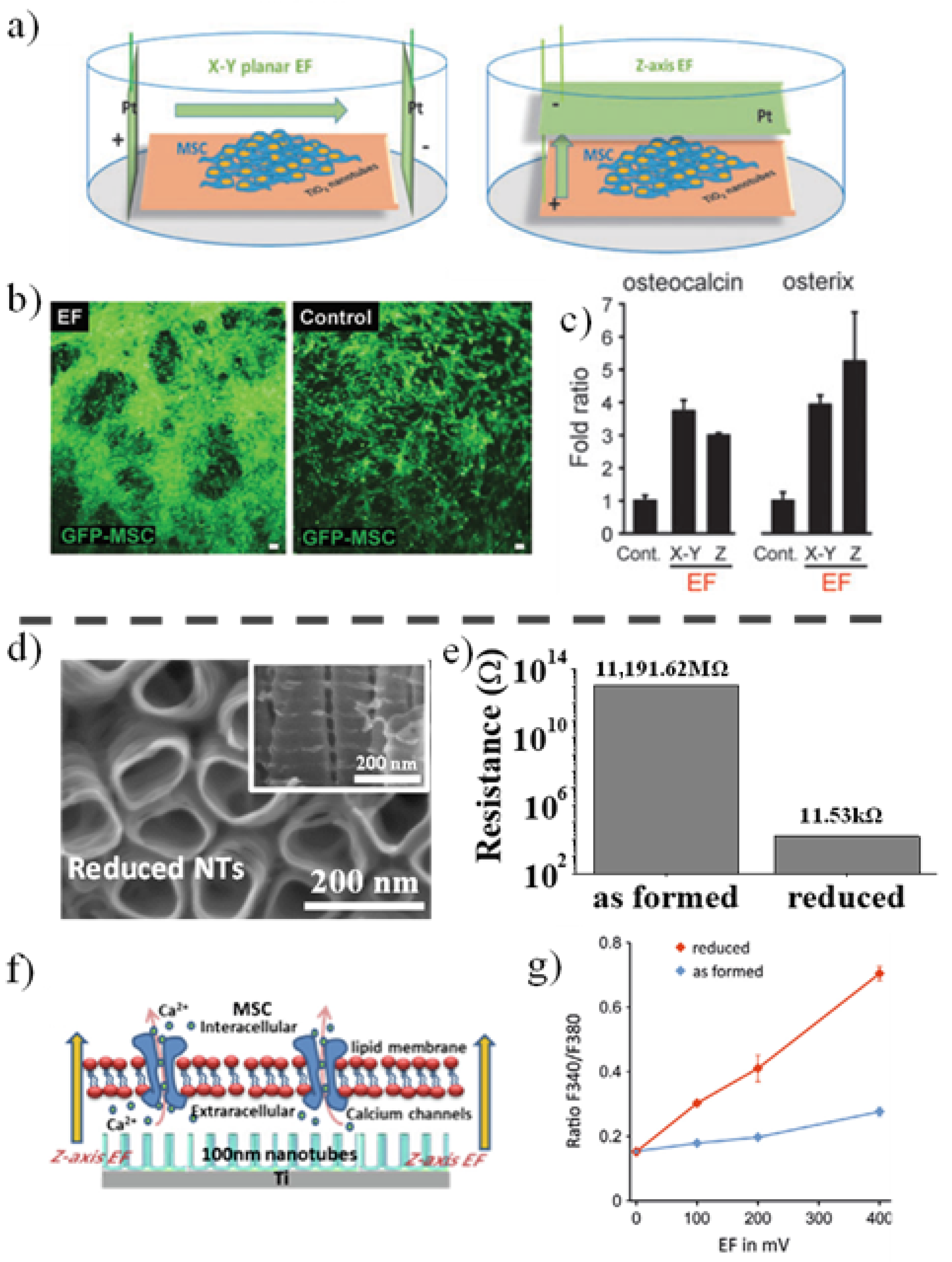
3.1.2. Electric Field Stimulation of Anodic TiO2 Nanotubes
3.2. Drug Delivery Applications Based on Anodic TiO2 Nanotubes
3.2.1. Release Rate from Anodic TiO2 Nanotubes
3.2.2. Drug Delivery for Antibacterial and Osteoinductive Activities
3.2.3. In Vivo Drug Delivery Approaches Using Anodic TiO2 Nanotube Implants
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. Roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng. Part A 2017, 23, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef]
- Cavalcanti-Adam, E.A.; Micoulet, A.; Blümmel, J.; Auernheimer, J.; Kessler, H.; Spatz, J.P. Lateral spacing of integrin ligands influences cell spreading and focal adhesion assembly. Eur. J. Cell Biol. 2006, 85, 219–224. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schmuki, P.; Mark, K. Von Der Narrow Window in Nanoscale Dependent Activation of Endothelial Cell Growth and Differentiation on TiO2 Nanotube Surfaces 2009. Nano Lett. 2009, 9, 3157–3164. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 62002. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2012, 58, 261–326. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Espanol, M.; Montufar, E.B.; Mestres, G.; Aparicio, C.; Gil, F.J.; Ginebra, M.P. Bioactive Ceramic and Metallic Surfaces for Bone Engineering. In Biomaterials Surface Science; Wiley-VCH Verlag GmbH & Co. KGaA: Singapore, 2013; pp. 337–374. ISBN 9783527649600. [Google Scholar]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: Thematic review. Front. Bioeng. Biotechnol. 2020, 8, 1261. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug Delivery Systems Based on Titania Nanotubes and Active Agents for Enhanced Osseointegration of Bone Implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Kulkarni, M.; Humpolíček, P.; Capáková, Z.; Burja, B.; Mazare, A.; Schmuki, P.; Mrak-Poljšak, K.; Flašker, A.; Žigon, P.; et al. Could Titanium Dioxide Nanotubes Represent a Viable Support System for Appropriate Cells in Vascular Implants? In Advances in Biomembranes and Lipid Self-Assembly; Academic Press: Cambridge, MA, USA, 2017; Volume 25, pp. 1–39. [Google Scholar]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Schmuki, P.; Cimpean, A. Attenuation of the macrophage inflammatory activity by TiO2 nanotubes via inhibition of MAPK and NF-κB pathways. Int. J. Nanomed. 2015, 10, 6455–6467. [Google Scholar] [CrossRef]
- Salamanna, F.; Gambardella, A.; Contartese, D.; Visani, A.; Fini, M. Nano-based biomaterials as drug delivery systems against osteoporosis: A systematic review of preclinical and clinical evidence. Nanomaterials 2021, 11, 530. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Cimpean, A. The state of the art and prospects for osteoimmunomodulatory biomaterials. Materials 2021, 14, 1357. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Chamberlain, L.M.; Brammer, K.S.; Johnston, G.W.; Chien, S.; Jin, S. Macrophage Inflammatory Response to TiO2 Nanotube Surfaces. J. Biomater. Nanobiotechnol. 2011, 2, 293–300. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Takeuchi, M.; Abe, Y.; Yoshida, Y.; Nakayama, Y.; Okazaki, M.; Akagawa, Y. Acid pretreatment of titanium implants. Biomaterials 2003, 24, 1821–1827. [Google Scholar] [CrossRef]
- John, A.A.; Jaganathan, S.K.; Supriyanto, E.; Manikandan, A. Surface modification of titanium and its alloys for the enhancement of osseointegration in orthopaedics. Curr. Sci. 2016, 111, 1003–1015. [Google Scholar] [CrossRef]
- Karthega, M.; Rajendran, N. Hydrogen peroxide treatment on Ti-6Al-4V alloy: A promising surface modification technique for orthopaedic application. Appl. Surf. Sci. 2010, 256, 2176–2183. [Google Scholar] [CrossRef]
- Janson, O.; Gururaj, S.; Pujari-Palmer, S.; Karlsson Ott, M.; Strømme, M.; Engqvist, H.; Welch, K. Titanium surface modification to enhance antibacterial and bioactive properties while retaining biocompatibility. Mater. Sci. Eng. C 2019, 96, 272–279. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Zhou, L.; Ma, J.; Zhang, X.; Li, H.; Liang, C.; Liu, S.; Wang, H. Influence of surface structures on biocompatibility of TiO2/HA coatings prepared by MAO. Mater. Chem. Phys. 2018, 215, 339–345. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Fu, S.; Wu, Y.; Lu, X.; Yang, L.; Liu, H.; Dong, Z. Synthesis, microstructure, anti-corrosion property and biological performances of Mn-incorporated Ca-P/TiO2 composite coating fabricated via micro-arc oxidation. Mater. Sci. Eng. C 2020, 117, 111321. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wei, D.; Cheng, S.; Wang, Y.; Li, B.; Jia, D.; Zhou, Y. Rapid structural evolution and bone inducing mechanism of the multilayer coating with silicon-doped hydroxyapatite crystals on the microwave water steaming-hydrothermally treated titania coating. Appl. Surf. Sci. 2021, 539, 148153. [Google Scholar] [CrossRef]
- Ansar, E.B.; Ravikumar, K.; Suresh Babu, S.; Fernandez, F.B.; Komath, M.; Basu, B.; Harikrishna Varma, P.R. Inducing apatite pre-layer on titanium surface through hydrothermal processing for osseointegration. Mater. Sci. Eng. C 2019, 105, 110019. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Cheon, K.H.; Jung, H.-D.; Song, J.; Kim, H.E.; Jang, T.S. Antibacterial and bioactive properties of stabilized silver on titanium with a nanostructured surface for dental applications. Appl. Surf. Sci. 2018, 451, 232–240. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Demetrescu, I.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Film. 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Astinchap, B.; Abdolghaderi, S.; Shafiekhani, A.; Morozov, I.A. Multifractal investigation of Ag/DLC nanocomposite thin films. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Choi, D.S.; Lee, J.K.; Kim, W.T.; Cha, B.K.; Choi, W.Y. Effect of drug-loaded TiO2 nanotube arrays on osseointegration in an orthodontic miniscrew: An in-vivo pilot study. Biomed. Microdevices 2017, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Choi, D.S.; Jang, I.; Choi, W.Y. Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with recombinant human bone morphogenetic protein-2: A pilot in vivo study. Int. J. Nanomed. 2015, 10, 1145–1154. [Google Scholar] [CrossRef]
- de Stefani, A.; Bruno, G.; Preo, G.; Gracco, A. Application of nanotechnology in orthodontic materials: A state-of-the-art review. Dent. J. 2020, 8, 126. [Google Scholar] [CrossRef]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, M.H.; Oh, N.; Park, J.A.; Leesungbok, R.; Ahn, S.J. Correlation between surface hydrophilicity and osteoblastic differentiation on microgrooved titanium substrata. J. Oral Implantol. 2012, 38, 11–19. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; Von Der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nguyen, N.T.; Ozkan, S.; Schmuki, P. Anodic TiO2 nanotube layers: Why does self-organized growth occur—A mini review. Electrochem. Commun. 2014, 46, 157–162. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Roy, P.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Formation of a non-thickness-limited titanium dioxide mesosponge and its use in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2009, 48, 9326–9329. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Roy, P.; Paramasivam, I.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Anodic formation of thick anatase TiO2mesosponge layers for high efficiency photocatalysis. Mater. Sci. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Ghicov, A.; Schmuki, P. Enhanced photochromism of Ag loaded self-organized TiO2 nanotube layers. Chem. Phys. Lett. 2007, 445, 233–237. [Google Scholar] [CrossRef]
- Mazare, A.; Paramasivam, I.; Schmidt-Stein, F.; Lee, K.; Demetrescu, I.; Schmuki, P. Flame annealing effects on self-organized TiO2 nanotubes. Electrochim. Acta 2012, 66, 12–21. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 μm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 65–67. [Google Scholar] [CrossRef]
- Albu, S.P.; Schmuki, P. Highly defined and ordered top-openings in TiO2 nanotube arrays. Phys. Status Solidi Rapid Res. Lett. 2010, 4, 151–153. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef]
- So, S.; Hwang, I.; Riboni, F.; Yoo, J.E.; Schmuki, P. Robust free standing flow-through TiO2 nanotube membranes of pure anatase. Electrochem. Commun. 2016, 71, 73–78. [Google Scholar] [CrossRef]
- Mohammadpour, F.; Behzadi, F.; Moradi, M. Fast anodically growth of long, small diameter TiO2 nanotubes by electropolishing of Ti foils in an ethanol-containing solution. Mater. Lett. 2015, 150, 81–83. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglič, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45. [Google Scholar] [CrossRef]
- Tesler, A.B.; Altomare, M.; Schmuki, P. Morphology and Optical Properties of Highly Ordered TiO2 Nanotubes Grown in NH4F/ o-H3PO4 Electrolytes in View of Light-Harvesting and Catalytic Applications. ACS Appl. Nano Mater. 2020, 3, 10646–10658. [Google Scholar] [CrossRef]
- Özkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Controlled spacing of self-organized anodic TiO2 nanotubes. Electrochem. Commun. 2016, 69, 76–79. [Google Scholar] [CrossRef]
- Kanta, A.F.; Poelman, M.; Decroly, A. Electrochemical characterisation of TiO2 nanotube array photoanodes for dye-sensitized solar cell application. Sol. Energy Mater. Sol. Cells 2015, 133, 76–81. [Google Scholar] [CrossRef]
- Tang, Y.X.; Tao, J.; Zhang, Y.Y.; Wu, T.; Tao, H.J.; Bao, Z.G. Preparation and characterization of TiO2 nanotube arrays via anodization of titanium films deposited on FTO conducting glass at room temperature. Wuli Huaxue Xuebao/Acta Phys. Chim. Sin. 2008, 24, 2191–2197. [Google Scholar] [CrossRef]
- Ali, G.; Chen, C.; Yoo, S.H.; Kum, J.M.; Cho, S.O. Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti. Nanoscale Res. Lett. 2011, 6, 332. [Google Scholar] [CrossRef]
- Wei, W.; Berger, S.; Hauser, C.; Meyer, K.; Yang, M.; Schmuki, P. Transition of TiO2 nanotubes to nanopores for electrolytes with very low water contents. Electrochem. Commun. 2010, 12, 1184–1186. [Google Scholar] [CrossRef]
- Berger, S.; Hahn, R.; Roy, P.; Schmuki, P. Self-organized TiO2 nanotubes: Factors affecting their morphology and properties. Phys. Status Solidi Basic Res. 2010, 247, 2424–2435. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglic, A. Influence of anodization parameters on morphology of TiO2 nanostructured surfaces. Adv. Mater. Lett. 2016, 7, 23–28. [Google Scholar] [CrossRef]
- Yoo, J.E.; Lee, K.; Altomare, M.; Selli, E.; Schmuki, P. Self-organized arrays of single-metal catalyst particles in TiO2 cavities: A highly efficient photocatalytic system. Angew. Chem. Int. Ed. 2013, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.E.; Lee, K.; Schmuki, P. Dewetted Au films form a highly active photocatalytic system on TiO2 nanotube-stumps. Electrochem. Commun. 2013, 34, 351–355. [Google Scholar] [CrossRef]
- Albu, S.P.; Schmuki, P. TiO2 nanotubes grown in different organic electrolytes: Two-size self-organization, single vs. double-walled tubes, and giant diameters. Phys. Status Solidi Rapid Res. Lett. 2010, 4, 215–217. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ozkan, S.; Hwang, I.; Mazare, A.; Schmuki, P. TiO2 nanotubes with laterally spaced ordering enable optimized hierarchical structures with significantly enhanced photocatalytic H2 generation. Nanoscale 2016, 8, 16868–16873. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Hahn, R.; Cerri, I.; Schmuki, P. Fast growth of TiO2 nanotube arrays with controlled tube spacing based on a self-ordering process at two different scales. Electrochem. Commun. 2017, 77, 1–142. [Google Scholar] [CrossRef]
- Albu, S.P.; Roy, P.; Virtanen, S.; Schmuki, P. Self-organized TiO2 nanotube arrays: Critical effects on morphology and growth. Isr. J. Chem. 2010, 50, 453–467. [Google Scholar] [CrossRef]
- So, S.; Hwang, I.; Schmuki, P. Hierarchical DSSC structures based on “single walled” TiO2 nanotube arrays reach a back-side illumination solar light conversion efficiency of 8%. Energy Environ. Sci. 2015, 8, 849–854. [Google Scholar] [CrossRef]
- Mirabolghasemi, H.; Liu, N.; Lee, K.; Schmuki, P. Formation of ‘single walled’ TiO2 nanotubes with significantly enhanced electronic properties for higher efficiency dye-sensitized solar cells. Chem. Commun. 2013, 49, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ghosh Pal, B. Metallic Biomaterials For Dental Implant Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081022054. [Google Scholar]
- Li, Q.; Ma, G.; Li, J.; Niinomi, M.; Nakai, M.; Koizumi, Y.; Wei, D.X.; Kakeshita, T.; Nakano, T.; Chiba, A.; et al. Development of low-Young’s modulus Ti–Nb-based alloys with Cr addition. J. Mater. Sci. 2019, 54, 8675–8683. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res. Part A 2005, 75, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Hu, T.; Gao, A.; Gao, N.; Starink, M.J.; Chen, Y.; Sun, W.; Liao, Q.; Tong, L.; Xu, X.; et al. Homogeneous Anodic TiO2 Nanotube Layers on Ti–6Al–4V Alloy with Improved Adhesion Strength and Corrosion Resistance. Adv. Mater. Interfaces 2019, 6, 1801964. [Google Scholar] [CrossRef]
- Mazare, A.; Dilea, M.; Ionita, D.; Demetrescu, I. Electrochemical behavior in simulated body fluid of TiO2 nanotubes on TiAlNb alloy elaborated in various anodizing electrolyte. Surf. Interface Anal. 2014, 46, 186–192. [Google Scholar] [CrossRef]
- Yasuda, K.; Schmuki, P. Control of morphology and composition of self-organized zirconium titanate nanotubes formed in (NH4)2SO4/NH4F electrolytes. Electrochim. Acta 2007, 52, 4053–4061. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef]
- López-Pavón, L.; Dagnino-Acosta, D.; López-Cuéllar, E.; Meléndez-Anzures, F.; Zárate-Triviño, D.; Barrón-González, M.; Moreno-Cortez, I.; Kim, H.Y.; Miyazaki, S. Synthesis of nanotubular oxide on Ti–24Zr–10Nb–2Sn as a drug-releasing system to prevent the growth of Staphylococcus aureus. Chem. Pap. 2021, 75, 2441–2450. [Google Scholar] [CrossRef]
- Ossowska, A.; Sobieszczyk, S.; Supernak, M.; Zielinski, A. Morphology and properties of nanotubular oxide layer on the “Ti-13Zr-13Nb” alloy. Surf. Coat. Technol. 2014, 258, 1239–1248. [Google Scholar] [CrossRef]
- Pérez, D.A.G.; Jorge Junior, A.M.; Asato, G.H.; Lepretre, J.C.; Roche, V.; Bolfarini, C.; Botta, W.J. Surface anodization of the biphasic Ti13Nb13Zr biocompatible alloy: Influence of phases on the formation of TiO2 nanostructures. J. Alloy. Compd. 2019, 796, 93–102. [Google Scholar] [CrossRef]
- Feng, X.J.; Macak, J.M.; Albu, S.P.; Schmuki, P. Electrochemical formation of self-organized anodic nanotube coating on Ti-28Zr-8Nb biomedical alloy surface. Acta Biomater. 2008, 4, 318–323. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Nah, Y.-C.; Tsuchiya, H.; Schmuki, P. Self-organized nano-tubes of TiO2-MoO3 with enhanced electrochromic properties. Chem. Commun. 2009, 2008–2010. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Verdério, J.F.; Bolfarini, C. Obtaining self-organized nanotubes on biomedical Ti-Mo alloys. Electrochem. Commun. 2013, 35, 139–141. [Google Scholar] [CrossRef]
- Feng, X.; Macak, J.M.; Schmuki, P. Flexible self-organization of two size-scales oxide nanotubes on Ti45Nb alloy. Electrochem. Commun. 2007, 9, 2403–2407. [Google Scholar] [CrossRef]
- Ghicov, A.; Aldabergenova, S.; Tsuchyia, H.; Schmuki, P. TiO2-Nb2O5 nanotubes with electrochemically tunable morphologies. Angew. Chem. Int. Ed. 2006, 45, 6993–6996. [Google Scholar] [CrossRef]
- Kim, J.J.; Byeon, I.S.; Brantley, W.A.; Choe, H.C. Highly ordered nanotubular film formation on Ti-25Nb-xZr and Ti-25Ta-xHf. Thin Solid Film. 2015, 596, 94–100. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, E.J.; Brantley, W.A.; Choe, H.C. Morphology of hydroxyapatite nanoparticles in coatings on nanotube-formed Ti-Nb-Zr alloys for dental implants. Vacuum 2014, 107, 297–303. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, W.G.; Choe, H.C.; Brantley, W.A. Control of nanotube shape and morphology on Ti-Nb(Ta)-Zr alloys by varying anodizing potential. Thin Solid Film. 2014, 572, 105–112. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Electrochemical and surface behavior of hydyroxyapatite/Ti film on nanotubular Ti-35Nb-xZr alloys. Appl. Surf. Sci. 2012, 258, 2129–2136. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C.; Brantley, W.A. An electrochemical study on self-ordered nanoporous and nanotubular oxide on Ti-35Nb-5Ta-7Zr alloy for biomedical applications. Acta Biomater. 2009, 5, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, S.; Han, X.; Hao, Y.; Ai, H. Effects of the surface characteristics of nanoporous titanium oxide films on Ti-24Nb-4Zr-8Sn alloy on the initial adhesion of osteoblast-like MG-63 cells. Exp. Ther. Med. 2013, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choe, H.C. Magnesium, silicon, and hydroxyapatite deposition on the Ti-xNb-2Ag-2Pt alloy by co-sputtering after nanotube formation. Surf. Coat. Technol. 2020, 404, 126487. [Google Scholar] [CrossRef]
- Jha, H.; Hahn, R.; Schmuki, P. Ultrafast oxide nanotube formation on TiNb, TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Hang, R.; Liu, Y.; Liu, S.; Bai, L.; Gao, A.; Zhang, X.; Huang, X.; Tang, B.; Chu, P.K. Size-dependent corrosion behavior and cytocompatibility of Ni-Ti-O nanotubes prepared by anodization of biomedical NiTi alloy. Corros. Sci. 2016, 103, 173–180. [Google Scholar] [CrossRef]
- Hang, R.; Huang, X.; Tian, L.; He, Z.; Tang, B. Preparation, characterization, corrosion behavior and bioactivity of Ni2O3-doped TiO2 nanotubes on NiTi alloy. Electrochim. Acta 2012, 70, 382–393. [Google Scholar] [CrossRef]
- Choe, H.C. Nanotubular surface and morphology of Ti-binary and Ti-ternary alloys for biocompatibility. Thin Solid Film. 2011, 519, 4652–4657. [Google Scholar] [CrossRef]
- Jarosz, M.; Grudzień, J.; Kapusta-Kołodziej, J.; Chudecka, A.; Sołtys, M.; Sulka, G.D. Anodization of Titanium Alloys for Biomedical Applications. In Nanostructured Anodic Metal Oxides: Synthesis and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 211–275. ISBN 9780128167069. [Google Scholar]
- Sarraf, M.; Nasiri-Tabrizi, B.; Yeong, C.H.; Madaah Hosseini, H.R.; Saber-Samandari, S.; Basirun, W.J.; Tsuzuki, T. Mixed oxide nanotubes in nanomedicine: A dead-end or a bridge to the future? Ceram. Int. 2021, 47, 2917–2948. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Domanska, M.; Łazinska, M.; Łukasiewicz, J.; Mol, J.M.C.; Durejko, T. Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes. Materials 2020, 13, 4743. [Google Scholar] [CrossRef]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [CrossRef]
- Zhang, Y.; Andrukhov, O.; Berner, S.; Matejka, M.; Wieland, M.; Rausch-Fan, X.; Schedle, A. Osteogenic properties of hydrophilic and hydrophobic titanium surfaces evaluated with osteoblast-like cells (MG63) in coculture with human umbilical vein endothelial cells (HUVEC). Dent. Mater. 2010, 26, 1043–1051. [Google Scholar] [CrossRef]
- Tighineanu, A.; Ruff, T.; Albu, S.; Hahn, R.; Schmuki, P. Conductivity of TiO2 nanotubes: Influence of annealing time and temperature. Chem. Phys. Lett. 2010, 494, 260–263. [Google Scholar] [CrossRef]
- Albu, S.P.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 nanotubes—Annealing effects on detailed morphology and structure. Eur. J. Inorg. Chem. 2010, 4351–4356. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar] [CrossRef]
- Regonini, D.; Jaroenworaluck, A.; Stevens, R.; Bowen, C.R. Effect of heat treatment on the properties and structure of TiO2 nanotubes: Phase composition and chemical composition. Surf. Interface Anal. 2010, 42, 139–144. [Google Scholar] [CrossRef]
- Mazare, A.; Paramasivam, I.; Lee, K.; Schmuki, P. Improved water-splitting behaviour of flame annealed TiO2 nanotubes. Electrochem. Commun. 2011, 13, 1030–1034. [Google Scholar] [CrossRef]
- Tighineanu, A.; Albu, S.P.; Schmuki, P. Conductivity of anodic TiO2 nanotubes: Influence of annealing conditions. Phys. Status Solidi Rapid Res. Lett. 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Małek, K.; Hnida, K.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Heat treatment effect on crystalline structure and photoelectrochemical properties of anodic TiO2 nanotube arrays formed in ethylene glycol and glycerol based electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Schenk, R.K.; Lussi, A.; Higginbottom, F.L.; Buser, D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: A histometric study in the canine mandible. J. Biomed. Mater. Res. 1998, 40, 1–11. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Hallgren, C.; Widmark, G.; Sennerby, L.; Wennerberg, A. Histologic evaluation of the bone integration of TiO2 blasted and turned titanium microimplants in humans. Clin. Oral Implant. Res. 2001, 12, 128–134. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.; Ferreira, J.A.; Rizzante, F.A.P.; Moura, G.F.; Mendonça, D.B.S.; de Magalhães, D.; Cimões, R.; Mendonça, G. Hydrophilic titanium surface modulates early stages of osseointegration in osteoporosis. J. Periodontal Res. 2021, 56, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Saruta, J.; Taniyama, T.; Kitajima, H.; Hirota, M.; Ikeda, T.; Ogawa, T. UV-pre-treated and protein-adsorbed titanium implants exhibit enhanced osteoconductivity. Int. J. Mol. Sci. 2020, 21, 4194. [Google Scholar] [CrossRef]
- Anitha, V.C.; Menon, D.; Nair, S.V.; Prasanth, R. Electrochemical tuning of titania nanotube morphology in inhibitor electrolytes. Electrochim. Acta 2010, 55, 3703–3713. [Google Scholar] [CrossRef]
- Kontos, A.G.; Kontos, A.I.; Tsoukleris, D.S.; Likodimos, V.; Kunze, J.; Schmuki, P.; Falaras, P. Photo-induced effects on self-organized TiO2 nanotube arrays: The influence of surface morphology. Nanotechnology 2009, 20, 045603. [Google Scholar] [CrossRef]
- Vardaki, M.; Mohajernia, S.; Pantazi, A.; Nica, I.C.; Enachescu, M.; Mazare, A.; Demetrescu, I.; Schmuki, P. Post treatments effect on TiZr nanostructures fabricated via anodizing. J. Mater. Res. Technol. 2019, 8, 5802–5812. [Google Scholar] [CrossRef]
- Wang, G.; Feng, H.; Jin, W.; Gao, A.; Peng, X.; Li, W.; Wu, H.; Li, Z.; Chu, P.K. Long-term antibacterial characteristics and cytocompatibility of titania nanotubes loaded with Au nanoparticles without photocatalytic effects. Appl. Surf. Sci. 2017, 414, 230–237. [Google Scholar] [CrossRef]
- Durdu, S.; Cihan, G.; Yalcin, E.; Altinkok, A. Characterization and mechanical properties of TiO2 nanotubes formed on titanium by anodic oxidation. Ceram. Int. 2021, 47, 10972–10979. [Google Scholar] [CrossRef]
- Wu, S.; Shen, X.; Chen, M.; Yie, K.H.R.; Zhou, Z.; Al-Baadani, M.A.; Fang, K.; Al-Bishari, A.M.; Deng, Z.; Liu, J.; et al. Multifunctional TaCu-Nanotubes Coated Titanium for Enhanced Bacteriostatic, Angiogenic and Osteogenic Properties; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 120, ISBN 8605778806. [Google Scholar]
- Lai, M.; Yan, X.; Shen, K.; Tang, Q.; Fang, X.; Zhang, C.; Zhu, Z.; Hou, Y. The effect of calcitonin gene-related peptide functionalized TiO2 nanotubes on osteoblast and osteoclast differentiation in vitro. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124899. [Google Scholar] [CrossRef]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf. B Biointerfaces 2016, 143, 447–454. [Google Scholar] [CrossRef]
- Peng, Z.; Ni, J. Surface properties and bioactivity of TiO2 nanotube array prepared by two-step anodic oxidation for biomedical applications. R. Soc. Open Sci. 2019, 6, 181948. [Google Scholar] [CrossRef] [PubMed]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Song, W.; Mano, J.F. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter 2013, 9, 2985–2999. [Google Scholar] [CrossRef]
- De Jonge, L.T.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Organic-inorganic surface modifications for titanium implant surfaces. Pharm. Res. 2008, 25, 2357–2369. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Tsuchiya, H.; Schmuki, P. Wetting behaviour of layers of TiO2 nanotubes with different diameters. J. Mater. Chem. 2005, 15, 4488–4491. [Google Scholar] [CrossRef]
- Tesler, A.B.; Prado, L.H.; Khusniyarov, M.M.; Thievessen, I.; Mazare, A.; Fischer, L.; Virtanen, S.; Goldmann, W.H.; Schmuki, P. A One-Pot Universal Approach to Fabricate Lubricant-Infused Slippery Surfaces on Solid Substrates. Adv. Funct. Mater. 2021, 30, 2101090. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Xia, H.; Kim, E.; Sun, H.B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Wang, M.; Huang, W.; Chu, P.K. Electrochemical stability of TiO2 nanotubes with different diameters in artificial saliva. Surf. Coat. Technol. 2011, 206, 63–67. [Google Scholar] [CrossRef]
- Man, I.; Pirvu, C.; Demetrescu, I. Enhancing titanium stability in Fusayama saliva using electrochemical elaboration of TiO2 nanotubes. Rev. Chim. 2008, 59, 615–617. [Google Scholar] [CrossRef]
- Yu, W.Q.; Qiu, J.; Xu, L.; Zhang, F.Q. Corrosion behaviors of TiO2 nanotube layers on titanium in Hank’s solution. Biomed. Mater. 2009, 4, 065012. [Google Scholar] [CrossRef]
- Ali Yahia, S.A.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Sutter, E.M.M. Effect of Anodizing Potential on the Formation and EIS Characteristics of TiO2 Nanotube Arrays. J. Electrochem. Soc. 2012, 159, K83–K92. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 nanotubes on Ti dental implant. Part 2: EIS characterization in Hank’s solution. Metals 2017, 7, 220. [Google Scholar] [CrossRef]
- Acquesta, A.; Carangelo, A.; Monetta, T. TiO2 Nanotubes on Ti dental implant. Part 3: Electrochemical behavior in hank’s solution of titania nanotubes formed in ethylene glycol. Metals 2018, 8, 489. [Google Scholar] [CrossRef]
- Al-Saady, F.A.A.; Rushdi, S.A.; Abbar, A.H. Improvement the corrosion Behavior of Titanium by Nanotubular Oxide in a simulated saliva solution. IOP Conf. Ser. Mater. Sci. Eng. 2020, 870, 012060. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Rajendran, N. In-vitro biocompatibility and corrosion resistance of strontium incorporated TiO2 nanotube arrays for orthopaedic applications. J. Biomater. Appl. 2014, 29, 113–129. [Google Scholar] [CrossRef]
- Mazare, A.; Ionita, D.; Totea, G.; Demetrescu, I. Calcination condition effect on microstructure, electrochemical and hemolytic behavior of amorphous nanotubes on Ti6Al7Nb alloy. Surf. Coat. Technol. 2014, 252, 87–92. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Rajendran, N. Electrochemical behavior and effect of heat treatment on morphology, crystalline structure of self-organized TiO2 nanotube arrays on Ti–6Al–7Nb for biomedical applications. Mater. Sci. Eng. C 2015, 50, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Choe, H.C. Nanostructure and corrosion behaviors of nanotube formed Ti-Zr alloy. Trans. Nonferrous Met. Soc. China 2009, 19, 1005–1008. [Google Scholar] [CrossRef]
- Indira, K.; Kamachi Mudali, U.; Rajendran, N. Corrosion behavior of electrochemically assembled nanoporous titania for biomedical applications. Ceram. Int. 2013, 39, 959–967. [Google Scholar] [CrossRef]
- Mazǎre, A.; Voicu, G.; Truscǎ, R.; Ioniţǎ, D. Heat treatment of TiO2 nanotubes, a way to significantly change their behaviour. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2011, 73, 97–108. [Google Scholar]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; Taylor & Francins Inc.: Abingdon, UK, 2002; ISBN 0824799178. [Google Scholar]
- Kear, G.; Walsh, F.C. The characteristics of a true tafel slope. Corros. Mater. 2005, 30, 51–55. [Google Scholar]
- Burstein, G.T. A hundred years of Tafel’s Equation: 1905-2005. Corros. Sci. 2005, 47, 2858–2870. [Google Scholar] [CrossRef]
- Buchanan, R.A.; Stansbury, E.E. Electrochemical Corrosion. In Handbook of Environmental Degradation of Materials, 2nd ed.; Elesevier: Amsterdam, The Netherlands, 2013; pp. 87–125. ISBN 978-1-4377-3455-3. [Google Scholar]
- Al-Mobarak, N.A.; Al-Swayih, A.A. Development of titanium surgery implants for improving osseointegration through formation of a titanium nanotube layer. Int. J. Electrochem. Sci. 2014, 9, 32–45. [Google Scholar]
- Sun, Y.; Rong, Y.; Zhao, Y.; Zhao, Y.; Hang, R.; Yao, X.; Chu, P.K. Electrochemical stability, corrosion behavior, and biological properties of Ni–Ti–O nanoporous layers anodically on NiTi alloy. Corros. Sci. 2021, 179, 109104. [Google Scholar] [CrossRef]
- Barjaktarević, D.R.; Djokić, V.R.; Bajat, J.B.; Dimić, I.D.; Cvijović-Alagić, I.L.; Rakin, M.P. The influence of the surface nanostructured modification on the corrosion resistance of the ultrafine-grained Ti–13Nb–13Zr alloy in artificial saliva. Theor. Appl. Fract. Mech. 2019, 103, 102307. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Von Der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef]
- von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part II: Cellular recognition of biomaterial surfaces: Lessons from cell–matrix interactions. Prog. Mater. Sci. 2013, 58, 327–381. [Google Scholar] [CrossRef]
- Huang, S.; Chen, C.S.; Ingber, D.E. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell 1998, 9, 3179–3193. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Yarwood, S.J.; Wilkinson, C.D.W.; Curtis, A.S.G. Nucleus alignment and cell signaling in fibroblasts: Response to a micro-grooved topography. Exp. Cell Res. 2003, 284, 272–280. [Google Scholar] [CrossRef]
- Spatz, J.P. Cell-Nanostructure Interactions. In Nanobiotechnology: Concepts, Applications and Perspectives; Niemeyer, C.M., Mirkin, C.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 3-527-30658-7. [Google Scholar]
- Seifert, G.; Eschrig, E.; Bieger, W.; Porezag, D.; Seifert, G.; Widany, J.; Weich, F.; Porezag, D.; Bilek, M.M.M.; Mckenzie, D.R.; et al. Oxidation-Resistant Gold-55 Clusters. Science 2002, 297, 1533–1537. [Google Scholar]
- Liu, H.; Webster, T.J. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Pittrof, A.; Song, Y.-Y.; von der Mark, K.; Schmuki, P. Covalent functionalization of TiO2 nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cells. Integr. Biol. 2011, 3, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef]
- Khaw, J.S.; Bowen, C.R.; Cartmell, S.H. Effect of TiO2 nanotube pore diameter on human mesenchymal stem cells and human osteoblasts. Nanomaterials 2020, 10, 2117. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Shao, Y.; Liu, Y.; Xia, R.; Tong, Z.; Zhang, J.; Zhai, Z.; Cheng, W.; Li, H. Mechanical strain promotes osteogenic differentiation of mesenchymal stem cells on TiO2 nanotubes substrate. Biochem. Biophys. Res. Commun. 2019, 511, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Liu, Y.; Xia, R.; Chang, Y.; Hu, Y.; Liu, P.; Zhai, Z.; Zhang, J.; Li, H. F-actin Regulates Osteoblastic Differentiation of Mesenchymal Stem Cells on TiO2 Nanotubes Through MKL1 and YAP/TAZ. Nanoscale Res. Lett. 2020, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-induced osteogenic differentiation of stem cells—A systematic review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef]
- Seo, C.H.; Jeong, H.; Feng, Y.; Montagne, K.; Ushida, T.; Suzuki, Y.; Furukawa, K.S. Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials 2014, 35, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, S.; Xu, L.; Li, Y.; Fu, Y.; Zhang, H.; Song, J. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and β-catenin. Acta Biomater. 2019, 96, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hou, Y.; Li, Z.; Hu, J.; Huo, D.; Zheng, H.; Zhang, J.; Yao, X.; Gao, R.; Wu, X.; et al. mTORC2 regulates hierarchical micro/nano topography-induced osteogenic differentiation via promoting cell adhesion and cytoskeletal polymerization. J. Cell. Mol. Med. 2021, 25, 6695–6708. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.; Ma, Q.; Wang, Q.; Chu, P.K.; Zhang, Y. The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials 2012, 33, 7993–8002. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Zhang, P.; Bai, X.; Ma, X.; Wang, Y.; Li, H.; Wang, L.; Zhou, Y. The epigenetic mechanisms of nanotopography-guided osteogenic differentiation of mesenchymal stem cells via high-throughput transcriptome sequencing. Int. J. Nanomed. 2018, 13, 5605–5623. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography Modulates Mechanotransduction of Stem Cells and Induces Differentiation through Focal Adhesion Kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Necula, M.G.; Mazare, A.; Ion, R.N.; Ozkan, S.; Park, J.; Schmuki, P.; Cimpean, A. Lateral spacing of TiO2 nanotubes modulates osteoblast behavior. Materials 2019, 12, 2956. [Google Scholar] [CrossRef]
- Smith, B.S.; Capellato, P.; Kelley, S.; Gonzalez-Juarrero, M.; Popat, K.C. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater. Sci. 2013, 1, 322–332. [Google Scholar] [CrossRef]
- Yao, S.; Feng, X.; Li, W.; Wang, L.N.; Wang, X. Regulation of RAW 264.7 macrophages behavior on anodic TiO2 nanotubular arrays. Front. Mater. Sci. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Chen, P.; Zhang, X.; Huang, X.; Du, Z.; Crawford, R.; Yao, X.; Tang, B.; Hang, R.; et al. Targeting Early Healing Phase with Titania Nanotube Arrays on Tunable Diameters to Accelerate Bone Regeneration and Osseointegration. Small 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Zheng, D.; Song, R.; Zhang, Y.; Jiang, P.; Vogler, E.A.; Lin, C. Effect of construction of TiO2 nanotubes on platelet behaviors: Structure-property relationships. Acta Biomater. 2017, 51, 505–512. [Google Scholar] [CrossRef]
- Gong, Z.; Hu, Y.; Gao, F.; Quan, L.; Liu, T.; Gong, T.; Pan, C. Effects of diameters and crystals of titanium dioxide nanotube arrays on blood compatibility and endothelial cell behaviors. Colloids Surf. B Biointerfaces 2019, 184, 110521. [Google Scholar] [CrossRef]
- Bai, L.; Yang, Y.; Mendhi, J.; Du, Z.; Hao, R.; Hang, R.; Yao, X.; Huang, N.; Tang, B.; Xiao, Y. The effects of TiO2 nanotube arrays with different diameters on macrophage/endothelial cell response and ex vivo hemocompatibility. J. Mater. Chem. B 2018, 6, 6322–6333. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In Vivo Evaluation of Anodic TiO2 Nanotubes; An Experimental Study in the Pig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Alves-Rezende, M.C.R.; Capalbo, L.C.; De Oliveira Limírio, J.P.J.; Capalbo, B.C.; Limírio, P.H.J.O.; Rosa, J.L. The role of TiO2 nanotube surface on osseointegration of titanium implants: Biomechanical and histological study in rats. Microsc. Res. Tech. 2020, 83, 817–823. [Google Scholar] [CrossRef]
- Baker, E.A.; Vara, A.D.; Salisbury, M.R.; Fleischer, M.M.; Baker, K.C.; Fortin, P.T.; Roberts, R.V.; Friedrich, C.R. Titania nanotube morphologies for osseointegration via models of in vitro osseointegrative potential and in vivo intramedullary fixation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Role of TiO2 Nanotubes on the Surface of Implants in Osseointegration in Animal Models: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Yao, C.; Webster, T.J. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J. Biomed. Mater. Res. Part A 2008, 84, 447–453. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef]
- Tao, B.; Deng, Y.; Song, L.; Ma, W.; Qian, Y.; Lin, C.; Yuan, Z.; Lu, L.; Chen, M.; Yang, X.; et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surf. B Biointerfaces 2019, 177, 242–252. [Google Scholar] [CrossRef]
- Keceli, H.G.; Bayram, C.; Celik, E.; Ercan, N.; Demirbilek, M.; Nohutcu, R.M. Dual delivery of platelet-derived growth factor and bone morphogenetic factor-6 on titanium surface to enhance the early period of implant osseointegration. J. Periodontal Res. 2020, 55, 694–704. [Google Scholar] [CrossRef]
- Lai, M.; Jin, Z.; Su, Z. Surface modification of TiO2 nanotubes with osteogenic growth peptide to enhance osteoblast differentiation. Mater. Sci. Eng. C 2017, 73, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Markx, G.H. The use of electric fields in tissue engineering: A review. Organogenesis 2008, 4, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Bayat, A. Electrical stimulation in bone healing: Critical analysis by evaluating levels of evidence. Eplasty 2011, 11, e34. [Google Scholar] [PubMed]
- Hart, F.X.; Palisano, J.R. The Application of Electric Fields in Biology and Medicine. In Electric Field; IntechOpen: London, UK, 2018; pp. 161–186. ISBN 9781789231861. [Google Scholar]
- Iwasa, S.N.; Babona-Pilipos, R.; Morshead, C.M. Environmental Factors That Influence Stem Cell Migration: An “electric Field”. Stem Cells Int. 2017, 2017, 4276927. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Mycielska, M.E.; Djamgoz, M.B.A. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J. Cell Sci. 2004, 117, 1631–1639. [Google Scholar] [CrossRef]
- Srirussamee, K.; Xue, R.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Changes in the extracellular microenvironment and osteogenic responses of mesenchymal stem/stromal cells induced by in vitro direct electrical stimulation. J. Tissue Eng. 2021, 12, 2041731420974147. [Google Scholar] [CrossRef]
- Stephan, M.; Zimmermann, J.; Klinder, A.; Sahm, F.; van Rienen, U.; Kämmerer, P.W.; Bader, R.; Jonitz-Heincke, A. Establishment and Evaluation of an In Vitro System for Biophysical Stimulation of Human Osteoblasts. Cells 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Liu, N.; Pritchard-Oh, A.; Mok, A.; Badawi, D.; Popovic, M.R.; Morshead, C.M. Calcium influx differentially regulates migration velocity and directedness in response to electric field application. Exp. Cell Res. 2018, 368, 202–214. [Google Scholar] [CrossRef]
- Naskar, S.; Kumaran, V.; Markandeya, Y.S.; Mehta, B.; Basu, B. Neurogenesis-on-Chip: Electric field modulated transdifferentiation of human mesenchymal stem cell and mouse muscle precursor cell coculture. Biomaterials 2020, 226, 119522. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chen, C.H.; Kuo, H.W.; Yen, T.L.; Mao, Y.Y.; Hu, W.W. Electrical stimulation through conductive substrate to enhance osteo-differentiation of human dental pulp-derived stem cells. Appl. Sci. 2019, 9, 3938. [Google Scholar] [CrossRef]
- Jaatinen, C. The Effect of an Applied Electric Current on Cell Proliferation, Viability, Morphology, Adhesion, and Stem Cell Differentiation. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2017. publication 1462. [Google Scholar]
- Gabi, M.; Sannomiya, T.; Larmagnac, A.; Puttaswamy, M.; Vörös, J. Influence of applied currents on the viability of cells close to microelectrodes. Integr. Biol. 2009, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Grunert, P.C.; Jonitz-Heincke, A.; Su, Y.; Souffrant, R.; Hansmann, D.; Ewald, H.; Krüger, A.; Mittelmeier, W.; Bader, R. Establishment of a Novel In Vitro Test Setup for Electric and Magnetic Stimulation of Human Osteoblasts. Cell Biochem. Biophys. 2014, 70, 805–817. [Google Scholar] [CrossRef]
- Hiemer, B.; Ziebart, J.; Jonitz-Heincke, A.; Grunert, P.C.; Su, Y.; Hansmann, D.; Bader, R. Magnetically induced electrostimulation of human osteoblasts results in enhanced cell viability and osteogenic differentiation. Int. J. Mol. Med. 2016, 38, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Kim, G. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/β-TCP based bio-composites on cellular activities for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Kang, E.T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef]
- Park, J.; Mazare, A.; Schneider, H.; von der Mark, K.; Fischer, M.J.M.; Schmuki, P. Electric Field-Induced Osteogenic Differentiation on TiO 2 Nanotubular Layer. Tissue Eng. Part C Methods 2016, 22, 809–821. [Google Scholar] [CrossRef]
- Mazare, A.; Park, J.; Simons, S.; Mohajernia, S.; Hwang, I.; Yoo, J.E.; Schneider, H.; Fischer, M.J.; Schmuki, P. Black TiO2 nanotubes: Efficient electrodes for triggering electric field-induced stimulation of stem cell growth. Acta Biomater. 2019, 97, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Portan, D.V.; Deligianni, D.D.; Papanicolaou, G.C.; Kostopoulos, V.; Psarras, G.C.; Tyllianakis, M. Combined Optimized Effect of a Highly Self-Organized Nanosubstrate and an Electric Field on Osteoblast Bone Cells Activity. Biomed Res. Int. 2019, 2019, 7574635. [Google Scholar] [CrossRef]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating electric fields modify the function of human osteoblasts growing on and in the surroundings of titanium electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania nanotubes for local drug delivery from implant surfaces. In Electrochemically Engineered Nanoporous Materials. Springer Series in Materials Science; Losic, D., Santos, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 220, pp. 307–355. ISBN 978-3-319-20346-1. [Google Scholar]
- Maher, S.; Losic, D. Nanoengineered titania nanotube arrays for localized drug delivery and enhanced osseointegration. In Nanotechnologies in Preventive and Regenerative Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–206. ISBN 9780323480635. [Google Scholar]
- De Santo, I.; Sanguigno, L.; Causa, F.; Monetta, T.; Netti, P.A. Exploring doxorubicin localization in eluting TiO2 nanotube arrays through fluorescence correlation spectroscopy analysis. Analyst 2012, 137, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Moseke, C.; Hage, F.; Vorndran, E.; Gbureck, U. TiO2 nanotube arrays deposited on Ti substrate by anodic oxidation and their potential as a long-term drug delivery system for antimicrobial agents. Appl. Surf. Sci. 2012, 258, 5399–5404. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Wang, Z.; Chen, S. Preparation, characterization and long-term antibacterial activity of Ag-poly(dopamine)-TiO2 nanotube composites. RSC Adv. 2016, 6, 14097–14104. [Google Scholar] [CrossRef]
- Nemati, S.H.; Hadjizadeh, A. Gentamicin-Eluting Titanium Dioxide Nanotubes Grown on the Ultrafine-Grained Titanium. AAPS PharmSciTech 2017, 18, 2180–2187. [Google Scholar] [CrossRef]
- Shirazi-Fard, S.; Mohammadpour, F.; Zolghadr, A.R.; Klein, A. Encapsulation and Release of Doxorubicin from TiO2 Nanotubes: Experiment, Density Functional Theory Calculations, and Molecular Dynamics Simulation. J. Phys. Chem. B 2021, 125, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Gulati, K.; Losic, D. Controlling Drug Release from Titania Nanotube Arrays Using Polymer Nanocarriers and Biopolymer Coating. J. Biomater. Nanobiotechnol. 2011, 2, 477–484. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Sinha-Ray, S.; Sukotjo, C.; Yarin, A.L. Intercalation of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2013, 3, 17380–17386. [Google Scholar] [CrossRef]
- Pawlik, A.; Jarosz, M.; Syrek, K.; Sulka, G.D. Co-delivery of ibuprofen and gentamicin from nanoporous anodic titanium dioxide layers. Colloids Surf. B Biointerfaces 2017, 152, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.; Anandan, C.; Rajendran, N. Drug release characteristics of quercetin-loaded TiO2 nanotubes coated with chitosan. Int. J. Biol. Macromol. 2016, 93, 1633–1638. [Google Scholar] [CrossRef]
- Shidfar, S.; Tavangarian, F.; Nemati, N.H.; Fahami, A. Drug delivery behavior of titania nanotube arrays coated with chitosan polymer. Mater. Discov. 2017, 8, 9–17. [Google Scholar] [CrossRef]
- Chen, X.; Cai, K.; Fang, J.; Lai, M.; Hou, Y.; Li, J.; Luo, Z.; Hu, Y.; Tang, L. Fabrication of selenium-deposited and chitosan-coated titania nanotubes with anticancer and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 103, 149–157. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, K.; Luo, Z.; Xu, D.; Xie, D.; Huang, Y.; Yang, W.; Liu, P. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012, 8, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Taipina, M.O.; de Mello, M.G.; Tamborlin, L.; Pereira, K.D.; Luchessi, A.D.; Cremasco, A.; Caram, R. A novel Ag doping Ti alloys route: Formation and antibacterial effect of the TiO2 nanotubes. Mater. Chem. Phys. 2021, 261, 124192. [Google Scholar] [CrossRef]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Dabbagh, A.; Abdul Razak, B.; Mahmoodian, R.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Abdullah, H.; Sukiman, N.L. Highly-ordered TiO2 nanotubes decorated with Ag2O nanoparticles for improved biofunctionality of Ti6Al4V. Surf. Coat. Technol. 2018, 349, 1008–1017. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Belcarz, A.; Pisarek, M.; Ginalska, G.; Lewandowska, M. TiO2 nanotube composite layers as delivery system for ZnO and Ag nanoparticles—An unexpected overdose effect decreasing their antibacterial efficacy. Mater. Sci. Eng. C 2015, 51, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, J.; Yuan, F.; Hang, R.; Huang, X.; Tang, B. In situ growth of self-organized Cu-containing nano-tubes and nano-pores on Ti90-xCu10Alx (x = 0, 45) alloys by one-pot anodization and evaluation of their antimicrobial activity and cytotoxicity. Surf. Coat. Technol. 2014, 240, 167–178. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, J.; Huo, K.; Hu, T.; Chu, P.K. Bioactive SrTiO3 Nanotube Arrays: Osteoporotic Bone Implants. ASC Nano 2009, 3, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, T.; Yang, Y.; Liu, T.; Gong, Z.; Wei, Y.; Quan, L.; Yang, Z.; Liu, S. Incorporation of Sr2+ and Ag nanoparticles into TiO2 nanotubes to synergistically enhance osteogenic and antibacterial activities for bone repair. Mater. Des. 2020, 196, 109086. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Li, B.; Hao, J.; Min, Y.; Xin, S.; Guo, L.; He, F.; Liang, C.; Wang, H.; Li, H. Biological properties of nanostructured Ti incorporated with Ca, P and Ag by electrochemical method. Mater. Sci. Eng. C 2015, 51, 80–86. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Belcarz, A.; Marcon, L.; Holdynski, M.; Andrzejczuk, M.; Janik-Czachor, M. Improvement of the bio-functional properties of TiO2 nanotubes. Appl. Surf. Sci. 2016, 388, 775–785. [Google Scholar] [CrossRef]
- Draghi, L.; Preda, V.; Moscatelli, M.; Santin, M.; Chiesa, R. Gentamicin-Loaded TiO2 Nanotubes as Improved Antimicrobial Surfaces for Orthopedic Implants. Front. Mater. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Mohammadnejad, J.; Houshmand, B.; Faghihi, S. Chitosan coating of tio2 nanotube arrays for improved metformin release and osteoblast differentiation. Int. J. Nanomed. 2020, 15, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ma, P.; Hu, Y.; Xu, G.; Xu, K.; Chen, W.; Ran, Q.; Dai, L.; Yu, Y.; Mu, C.; et al. Alendronate-loaded hydroxyapatite-TiO2 nanotubes for improved bone formation in osteoporotic rabbits. J. Mater. Chem. B 2016, 4, 1423–1436. [Google Scholar] [CrossRef]
- Lai, M.; Jin, Z.; Yang, X.; Wang, H.; Xu, K. The controlled release of simvastatin from TiO2 nanotubes to promote osteoblast differentiation and inhibit osteoclast resorption. Appl. Surf. Sci. 2017, 396, 1741–1751. [Google Scholar] [CrossRef]
- Perumal, A.; Kanumuri, R.; Rayala, S.K.; Nallaiyan, R. Fabrication of bioactive corrosion-resistant polyaniline/TiO2 nanotubes nanocomposite and their application in orthopedics. J. Mater. Sci. 2020, 55, 15602–15620. [Google Scholar] [CrossRef]
- Coman, A.N.; Mare, A.; Tanase, C.; Bud, E.; Rusu, A. Silver-Deposited Nanoparticles on the Titanium Nanotubes Surface as a Promising Antibacterial Material into Implants. Metals 2021, 11, 92. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qiao, Y.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. aureus. Biomaterials 2015, 65, 22–31. [Google Scholar] [CrossRef]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO2 Nanotube Surface Improves Osteogenesis Ability Through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a therapeutic agent in bone regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; et al. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, Z.; Kolawole, S.K.; Zeng, L.; Zhao, Y.; Ren, L.; Yang, K. In vitro study on cytocompatibility and osteogenesis ability of Ti–Cu alloy. J. Mater. Sci. Mater. Med. 2019, 30, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Song, Y.; Ma, A.; Li, C.; Zhang, X.; Li, H.; Zhang, Q.; Zhang, K. Improved osteoblast adhesion and osseointegration on TiO2 nanotubes surface with hydroxyapatite coating. Dent. Mater. J. 2019, 38, 278–286. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Peng, Q.; Lin, J.; Wen, C. Biocompatibility of nanoscale hydroxyapatite coating on TiO2 nanotubes. Materials 2019, 12, 1979. [Google Scholar] [CrossRef] [PubMed]
- Çalişkan, N.; Bayram, C.; Erdal, E.; Karahaliloǧlu, Z.; Denkbaş, E.B. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater. Sci. Eng. C 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Sutrisno, L.; Hu, Y.; Shen, X.; Li, M.; Luo, Z.; Dai, L.; Wang, S.; Zhong, J.L.; Cai, K. Fabrication of hyaluronidase-responsive biocompatible multilayers on BMP2 loaded titanium nanotube for the bacterial infection prevention. Mater. Sci. Eng. C 2018, 89, 95–105. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shen, G.; Zhao, J. Enhanced osteogenic activity and anti-inflammatory properties of Lenti-BMP-2-loaded TiO2 nanotube layers fabricated by lyophilization following trehalose addition. Int. J. Nanomed. 2016, 11, 429–439. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Yu, Q.; Wang, Y.A.; Zhao, J. Dose reduction of bone morphogenetic protein-2 for bone regeneration using a delivery system based on lyophilization with trehalose. Int. J. Nanomed. 2018, 13, 403–414. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Y.; Qian, S.; Li, J.; Chang, Q.; Ye, D.; Pan, H.; Zhang, M.; Cao, H.; Liu, X.; et al. Vacuum extraction enhances rhPDGF-BB immobilization on nanotubes to improve implant osseointegration in ovariectomized rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1809–1818. [Google Scholar] [CrossRef]
- Ma, A.; Shang, H.; Song, Y.; Chen, B.; You, Y.; Han, W.; Zhang, X.; Zhang, W.; Li, Y.; Li, C. Icariin-Functionalized Coating on TiO2 Nanotubes Surface to Improve Osteoblast Activity In Vitro and Osteogenesis Ability In Vivo. Coatings 2019, 9, 327. [Google Scholar] [CrossRef]
- Zhao, X.; You, L.; Wang, T.; Zhang, X.; Li, Z.; Ding, L.; Li, J.; Xiao, C.; Han, F.; Li, B. Enhanced osseointegration of titanium implants by surface modification with silicon-doped titania nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar] [CrossRef]
- Wu, F.; Wu, F.; Xu, J.; Yan, R.; Yan, R.; Hu, B.; Hu, B.; Li, G.; Li, G.; Jin, M.; et al. In vitro and in vivo evaluation of antibacterial activity of polyhexamethylene guanidine (PHMG)-loaded TiO2 nanotubes. Biomed. Mater. 2020, 15, 045016. [Google Scholar] [CrossRef]
- Somsanith, N.; Kim, Y.K.; Jang, Y.S.; Lee, Y.H.; Yi, H.K.; Jang, J.H.; Kim, K.A.; Bae, T.S.; Lee, M.H. Enhancing of osseointegration with propolis-loaded TiO2 nanotubes in rat mandible for dental implants. Materials 2018, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Termaat, M.F.; Den Boer, F.; Bakker, F.; Patka, P.; Haarman, H. Bone Morphogenetic Proteins. Development and Clinical Efficacy in the Treatment of Fractures and Bone Defects. J. Bone Jt. Surg. 2005, 87, 1367. [Google Scholar] [CrossRef]
- Kanakaris, N.K.; Giannoudis, P. V Clinical applications of bone morphogenetic proteins: Current evidence. J. Surg. Orthop. Adv. 2008, 17, 133–146. [Google Scholar] [PubMed]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, P.J.J.; Hosking, D.J. Alendronate sodium in the management of osteoporosis. Ther. Clin. Risk Manag. 2006, 2, 235–249. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, T.; Wen, L.M.; Li, R.; Zhang, Y.B.; Bi, W.J.; Feng, X.J.; Qi, M.C. Osteogenic capability of strontium and icariin-loaded TiO2 nanotube coatings in vitro and in osteoporotic rats. J. Biomater. Appl. 2021, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; You, Y.; Chen, B.; Wang, W.; Liu, J.; Qi, H.; Liang, Y.; Li, Y. Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect. Coatings 2020, 10, 427. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Meimandi-Parizi, A.; Oryan, A.; Sayahi, E.; Bigham-Sadegh, A. Propolis extract a new reinforcement material in improving bone healing: An in vivo study. Int. J. Surg. 2018, 56, 94–101. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Xie, L.; Liu, N.; Xiao, Y.; Liu, Y.; Yan, C.; Wang, G.; Jing, X. In Vitro and In Vivo Osteogenesis Induced by Icariin and Bone Morphogenetic Protein-2: A Dynamic Observation. Front. Pharmacol. 2020, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Zhang, X.; Wang, W.; Zhang, Y.; Wu, Z.; Chu, P.K. The osteogenic activity of strontium loaded titania nanotube arrays on titanium substrates. Biomaterials 2013, 34, 19–29. [Google Scholar] [CrossRef]
- Alenezi, A.; Galli, S.; Atefyekta, S.; Andersson, M.; Wennerberg, A. Osseointegration effects of local release of strontium ranelate from implant surfaces in rats. J. Mater. Sci. Mater. Med. 2019, 30, 116. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Xing, M.; Wang, X.; Wang, E.; Gao, L.; Chang, J. Bone tissue engineering strategy based on the synergistic effects of silicon and strontium ions. Acta Biomater. 2018, 72, 381–395. [Google Scholar] [CrossRef]
- Mladenović, Ž.; Johansson, A.; Willman, B.; Shahabi, K.; Björn, E.; Ransjö, M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014, 10, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Aw, M.S.; Santos, A.; Gulati, K.; Bariana, M. Titania nanotube arrays for local drug delivery: Recent advances and perspectives. Expert Opin. Drug Deliv. 2015, 12, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Losic, D. Advancing of titanium medical implants by surface engineering: Recent progress and challenges. Expert Opin. Drug Deliv. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Vitt, A.; Sofrata, A.; Slizen, V.; Sugars, R.V.; Gustafsson, A.; Gudkova, E.I.; Kazeko, L.A.; Ramberg, P.; Buhlin, K. Antimicrobial activity of polyhexamethylene guanidine phosphate in comparison to chlorhexidine using the quantitative suspension method. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Swiontek Brzezinska, M.; Walczak, M.; Richert, A.; Kalwasinska, A.; Pejchalová, M. The influence of polyhexamethylene guanidine derivatives introduced into polyhydroxybutyrate on biofilm formation and the activity of bacterial enzymes. Appl. Biochem. Microbiol. 2016, 52, 298–303. [Google Scholar] [CrossRef]



| Drug/ Compound | Nanostructures | Drug/Agent Deposition | Release Rate | Reference | |
| Type | D, L | ||||
| Sr2+ | TiO2 NTs (mainly anatase) | D: 110 nm L: 2.1 µm | Sr2+—dip coating | A viable alternative in orthopedics to provide improved corrosion resistance and enhanced biocompatibility | [149] |
| Ag or Vancomycin (VAN) | TiO2 NTs (anatase, rutile) | Aqueous: (a) D 70 nm, L 0.87 µm (b) D 100 nm, L 1.45 µm. Organic: L 6.5 µm | vacuum impregnation technique for both VAN or Ag, from 10% VAN or 10% silver nitrate (AgNO3) solutions, respectively | VAN release was significantly retarded from NTs in organic electrolytes (compared to aqueous). Ag release was retarded from aqueous nanotubes compared to Ti surfaces. | [230] |
| Gentamicin (GEN) | TiO2 NTs on coarse or ultrafine-grained Ti | D: not specified L: 8 µm or 15 µm | immersion of samples in phosphate-buffered saline solution containing GEN | Partly delayed release of gentamicin, for targeting bacterial inflammation around the implant. | [232] |
| Ibuprofen (IBU) or Gentamicin (GEN) | TiO2 NTs Amorphous Anatase Anatase and rutile | D: 65 nm L: 2.1 µm | 10 wt.% solution of IBU in methanol, and 10 wt.% GEN in water. 5× of 1 mL pipetting and drying (drying in air, room temperature or 75 °C) | The release process is governed by the desorption of the drug from the top surface, followed by a combination of desorption and slow diffusion of the drug from the inside of the nanostructure. | [127] |
| Doxorubicin (DOX) | TiO2 NTs (amorphous) | D: 110 nm L: 0.80 µm | (a) immersion of samples in DOX solution (soaking) or (b) vacuum impregnation (drying under vacuum, several times) | DOX loaded by soaking, the elution time is around 7 days, while for wet vacuum impregnation it reached 30 days. | [229] |
| TiO2 NTs (amorphous) | D: 170—220 nm L: 0.93 µm | 15 cycles of deposition and drying under vacuum in DOX solution. Polyethylene glycol (PEG) layer for capping (1 to 50% PEG) | Release of doxorubicin can be controlled (slowed down) only during the first 3 h by the PEG layer. TiO2 NTs are competitive for drug release of low polarity drugs compared to other boron or carbon-based materials. | [233] | |
| Ibuprofen (IBU) and Gentamicin (GEN) | TiO2 NTs (anatase) | D: 49 nm L: 0.5, 0.8 or 1.8 µm | 10 wt.% solution of IBU in methanol, or GEN in water were prepared. 1 mL of each solution: a) IBU and GEN at the same time (de-noted IG), b) GEN and then IBU (GI), c) IBU and then GEN (I&G) | The length, crystallinity, and loading procedure of NTs influence the drug loading and release processes. Drug release can be modified by the loading procedure (GI approach led to the longest period or release time for GEN as the initial burst release was inhibited). | [236] |
| Drug/ Compound | Nanostructure | Drug/Compound Deposition | Biological Effects | Reference | |
| Type | D, L | ||||
| Sr2+, Ag | TiO2 NTs (anatase) | D: 70 nm L: not specified | Sr2+—hydrothermal treatment; Ag+ by photodeposition to Ag NPs | Enhancement of the osteobonding capability of the nanotubes, as well as of their antibacterial activities by combining the pro-osteogenic effects of Sr2+ and strong antibacterial effect of Ag NPs. | [250] |
| Ag2O | TiO2 NTs (amorphous) | D: 80 nm L: 6 µm to 2 µm, decreasing with Ag % | Ag2O nanoparticles are embedded into the nanotubes. Substrates are TiAg layers (magnetron sputtering) | Sustained antibacterial activity due to the controlled low dose Ag+ release, improved cell attachment and spreading, no deleterious effects on pre-osteoblast cell viability, proliferation, and differentiation. | [244] |
| Zn | TiO2 NTs (30 nm: anatase, rutile; 80 nm: anatase) | D: 30 nm, 80 nm L: not specified | Zn—deposition onto the NTs by hydrothermal treatment | Antibacterial effects depending on the amount of loaded and released Zn in NTs. 80 nm NTs (3 h Zn deposition) enhance MSC osteogenic differentiation (enhanced protein deposition, enabling cell functionalities and Zn release). | [246] |
| Polyaniline (PANI) | TiO2 NTs (anatase/rutile) | D: 85 nm L: not specified | PANI deposition by electropolymerization | PANI/TiO2 NTs supported the viability/proliferation of MG-63 osteoblasts and showed good anti-biofilm activity. | [258] |
| Se-Chitosan | TiO2 NTs (amorphous) | D: 110 nm L: 0.90 µm | Se is deposited by electrodeposition and Chitosan by spin coating | NTs-Se-Chi samples showed excellent antibacterial activity and promoted the proliferation and biological functions of healthy osteoblasts while inhibiting the growth of cancerous osteoblasts. | [239] |
| Metformin (MET)-Chitosan | TiO2 NTs | D: 160 nm L: ≈9–10 µm | 5 cycles of deposition-drying in air of MET solution in fetal bovine serum. Chitosan was deposited by spin coating | A 15-layer chitosan deposition could prolong the metformin release up to 21 days (with a significant decrease in the burst release), while the chitosan coating of the MET-loaded TiO2 NTs increased MSCs attachment, proliferation, and differentiation. | [255] |
| Bioactive Compound | Implant Characteristics | Drug Loading Method | Animal in Vivo Model/Biological Effects | Reference |
|---|---|---|---|---|
| rhBMP2 | TiO2 NTs D: ~70 nm, ~110 nm; Implant: D 3.5 mm; L 8.5 mm | Dip-coating in 1.5 mg rhBMP-2/mL (in a vacuum chamber) | Pilot in vivo study: New Zealand white rabbits, 4 types of implants (proximal tibia); rhBMP2-loaded implants: the highest BIC and enhanced bone remodeling. | [38] |
| rhBMP2/ Lenti-BMP2 | TiO2 NTs: D ~100; L: 400 nm; Ti rods (D: 2 mm; L: 8 mm) | Lyophilization of Lenti-BMP2 in the presence of trehalose | Femur defect model in Fisher 344 rats; TiO2-Lyo-Tre-BMP2 implant: most effective in terms of BMP2 stability, sustained release, bioactivity, bone regeneration. | [273,274] |
| rhBMP2 and Ibuprofen (IBU) | TiO2 NTs: D ~70 nm; L: 5 µm; Ti rods (D: 2 mm; L: 8 mm) | IBU (1.5 mg/mL) and rhBMP-2 (10 mg/mL) loading by dip coating (3×), lyophilized, freeze and vacuum dried. | IBU-NTs behaved as an anti-inflammatory drug and improved the osseointegration of orthodontic miniscrews in vivo. However, the effect of rhBMP2-loaded NTs on the osseointegration was slightly lower. | [37] |
| rhPDGF-BB | Ti rods (D: 2 mm; L: 8 mm) NTs: D—70 nm | Immersion in 100 μg/mL rhPDGF-BB at RT (PDGF group) or put in the vacuum pump (PDGF + Vacuum group) for 10 min | OVX rats with bilateral femurs were used for the implantation; the newly designed coating contributed to the new bone formation surrounding the implant and enhanced bone fixation in OVX rats showing great promise for clinical applications in osteoporotic patients. | [275] |
| Alendronate (ALN) | Ti rods (D: 3 mm; L: 13 mm); NTs D: 70 nm; L: 0.7–1.0 µm (anodic oxidation), HA layers: alternate immersion method on TiO2 NTs surface. | NTs-HA-ALN implant: immersion into ALN 20 mg/mL solution at RT (12 h). NT-ALN implant: physical absorption of ALN on TiO2 NTs | Implants into the femoral epiphysis of OVX female New Zealand white rabbits. NTs-HA-ALN implants showed great potential for increasing osseointegration as compared to Ti, NTs, and NTs-HA implants, with the highest anti-osteoporosis potential | [256] |
| Icariin (ICA) | Cylindrical implants (D: 1.5 mm, L: 2 mm); Anodic TiO2 NT (D: 80 ± 10 nm) | Immersion in ICA solution (2 days), drying at 37 °C (1 day): PLGA coating (twice in a dropwise manner) | Sprague Dawley rats received implants in the femora’s mid-diaphysis; TiO2 NT structure and ICA synergistically promote osteoblasts’ function and PLGA coating endowed the implant surface with better osteogenic/osseointegration ability. | [276] |
| Silicon (Si) | Ti screws, inner/ outer D: 1.7/2 mm, L: 10 mm. Anodic NTs: inner/ outer D 60/80 nm. | Si plasma immersion ion implantation (PIII) method | Sprague Dawley rats received implants in the distal femur in the horizontal direction; Si-TiO2-NTs induced enhanced early osseointegration positive effect on implant osseointegration and trabecular microarchitecture formation. | [277] |
| Polyhexa-methylene guanidine (PHMG) | cp-Ti rods (D: 3.175 mm; L: 1.5 ± 0.1 cm; Anodic TiO2 NTs: D—46.4 ± 5.9 nm, L—650–800 nm. | Addition of 100 μL of 25% PHMG aqueous solution onto rods dropwise and drying by a vacuum oven at RT for 1 h (×10 times). | Rabbits implanted with S. aureus-contaminated rods in the femoral medullary cavity; PHMG-NTs showed an excellent capacity to prevent bacterial infections, as well as to promote osteogenic differentiation by increased expression of osteogenic-related genes in the femur tissues around the implants. | [278] |
| Propolis (PL) | Ti rods (D: 0.85 mm; L: 4.5 mm) screw-processed at a thread angle of 20 degrees; anodic TiO2 NT (D: 60–90 nm). | Immersion in propolis solution for 24 h at 25 °C followed by vacuum-drying at 25 °C for 24 h | Sprague Dawley rat mandibular model; increased new bone formation and mineral density around the PL-NT-Ti implant; enhanced osteogenic differentiation and increased expression of collagen fibers while pro-inflammatory markers decreased | [279] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. https://doi.org/10.3390/nano11092359
Park J, Cimpean A, Tesler AB, Mazare A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials. 2021; 11(9):2359. https://doi.org/10.3390/nano11092359
Chicago/Turabian StylePark, Jung, Anisoara Cimpean, Alexander B. Tesler, and Anca Mazare. 2021. "Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery" Nanomaterials 11, no. 9: 2359. https://doi.org/10.3390/nano11092359
APA StylePark, J., Cimpean, A., Tesler, A. B., & Mazare, A. (2021). Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials, 11(9), 2359. https://doi.org/10.3390/nano11092359








