Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process of PSBMA Nanogels
2.3. Drug Loading
2.4. Hydrogel Preparation
2.5. Characterization
2.5.1. Structure Characterization
2.5.2. Particle Size Measurement
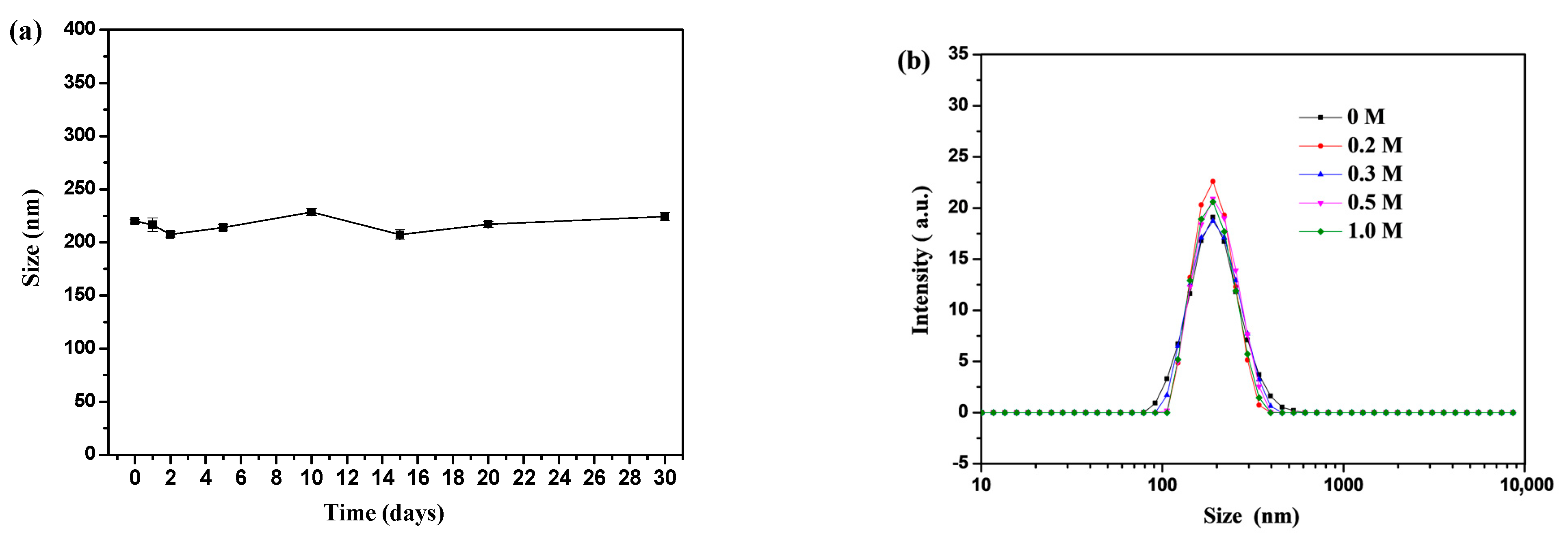
2.5.3. Stability Characterization
2.5.4. Morphological Observation
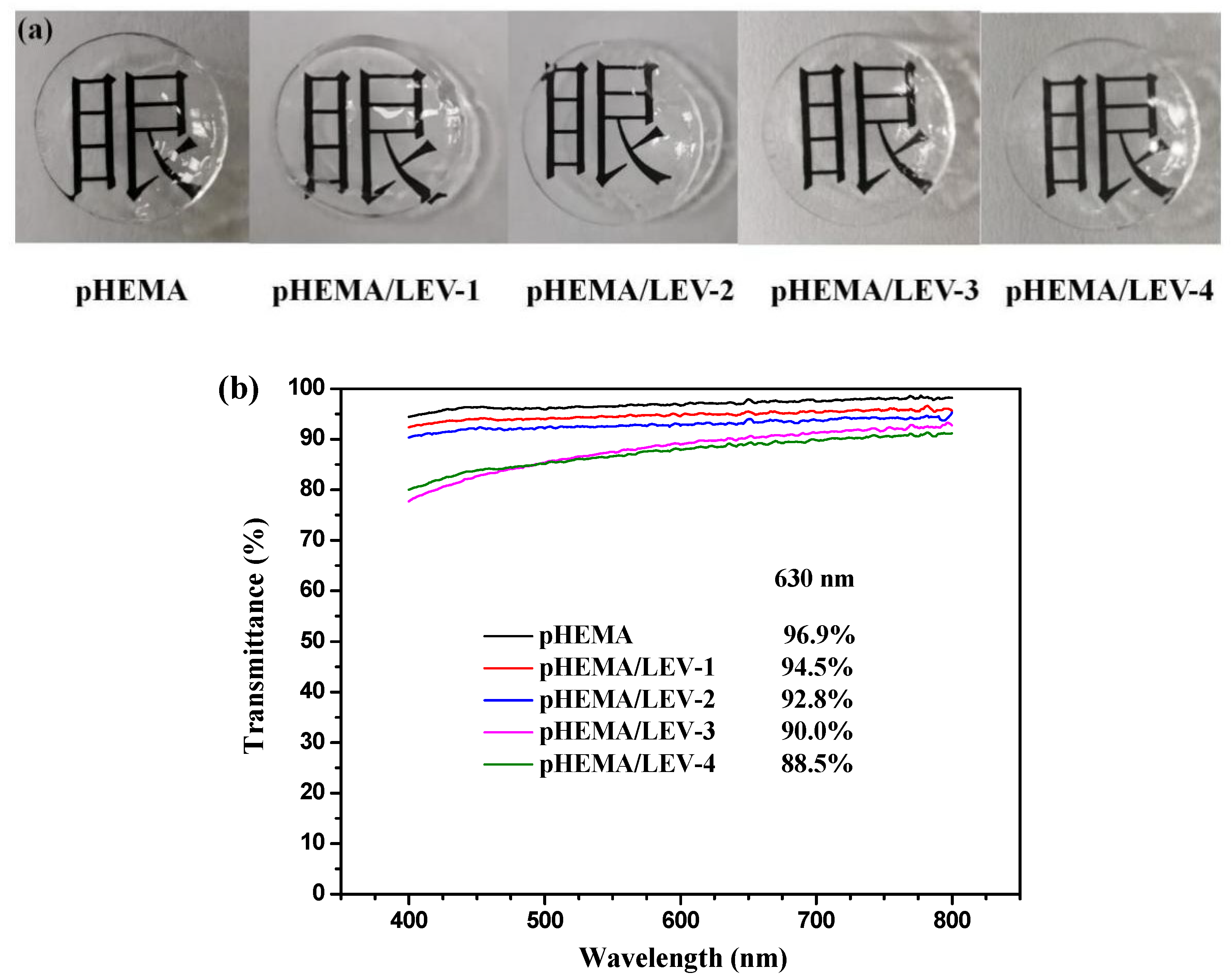
2.5.5. Transmittance
2.5.6. Water Contact Angle
2.5.7. Equilibrium Water Content
2.5.8. Swelling Properties
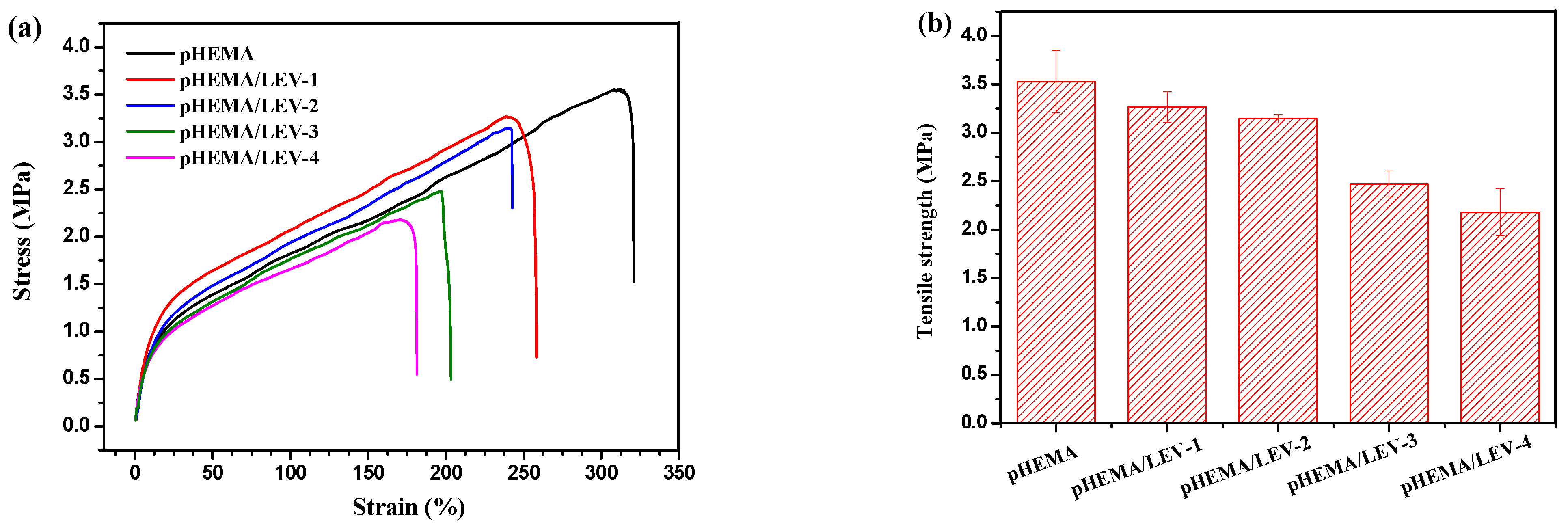
2.5.9. Mechanical Properties
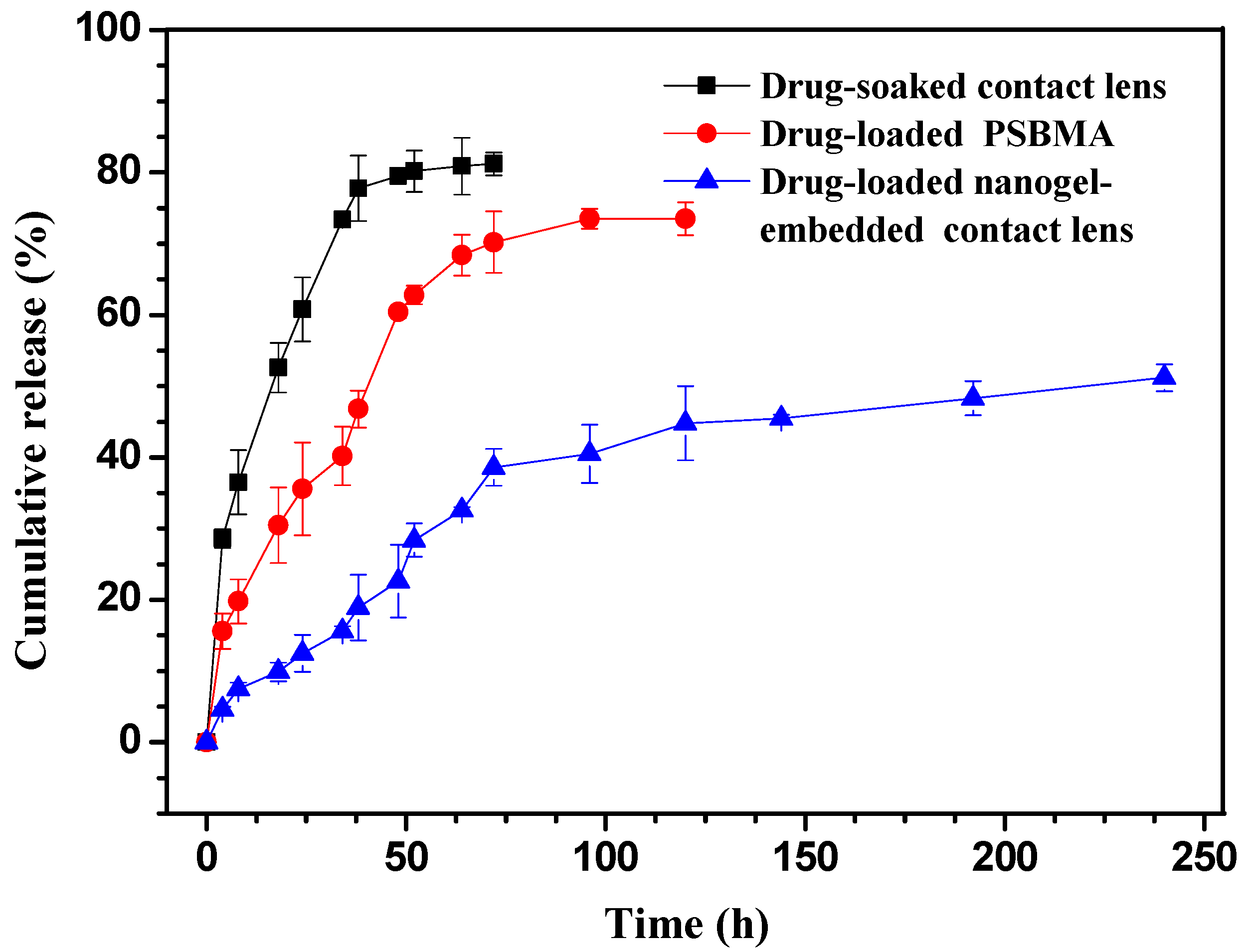
2.6. In Vitro Drug Release
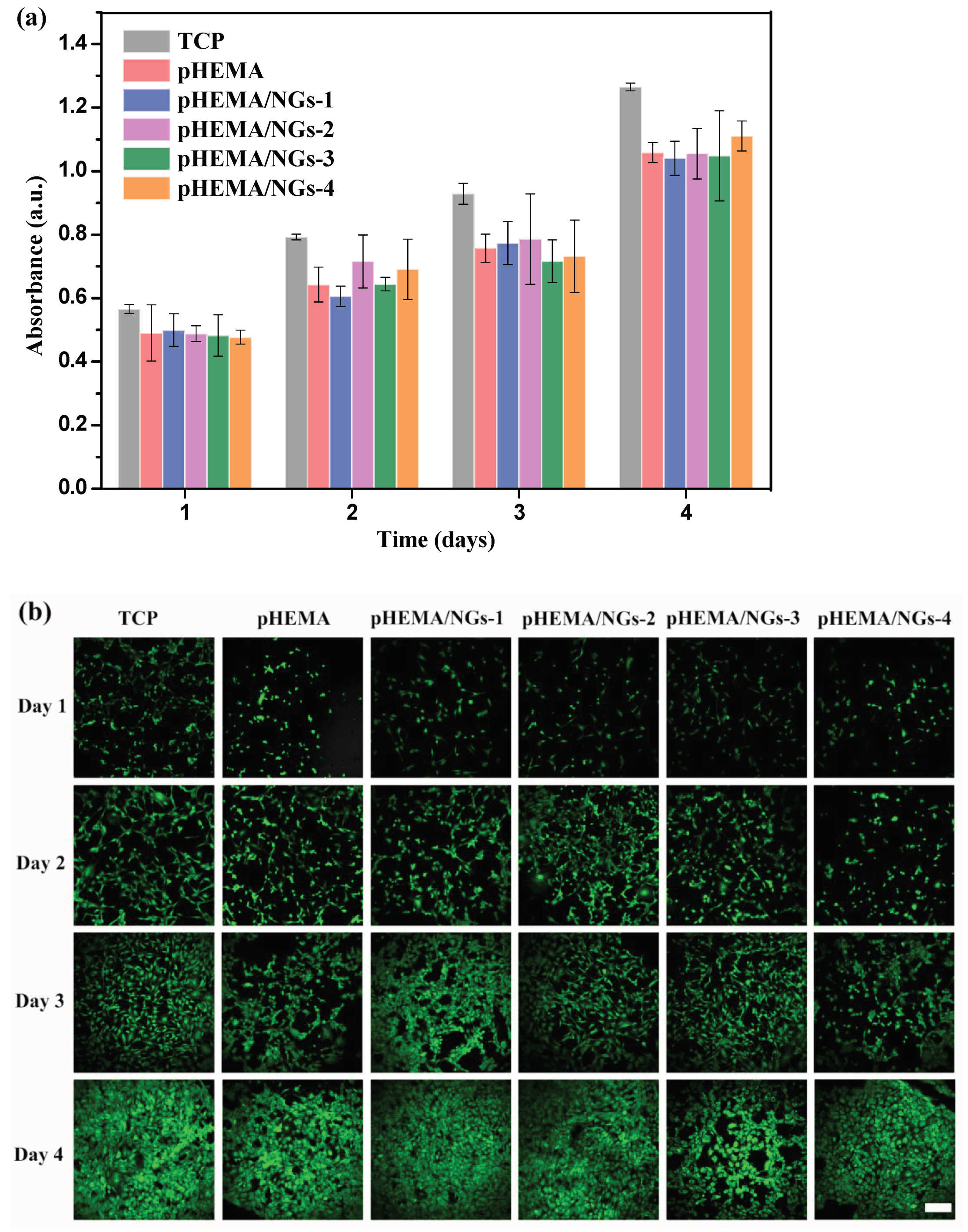
2.7. Cell Culture and Toxicity Test
3. Results
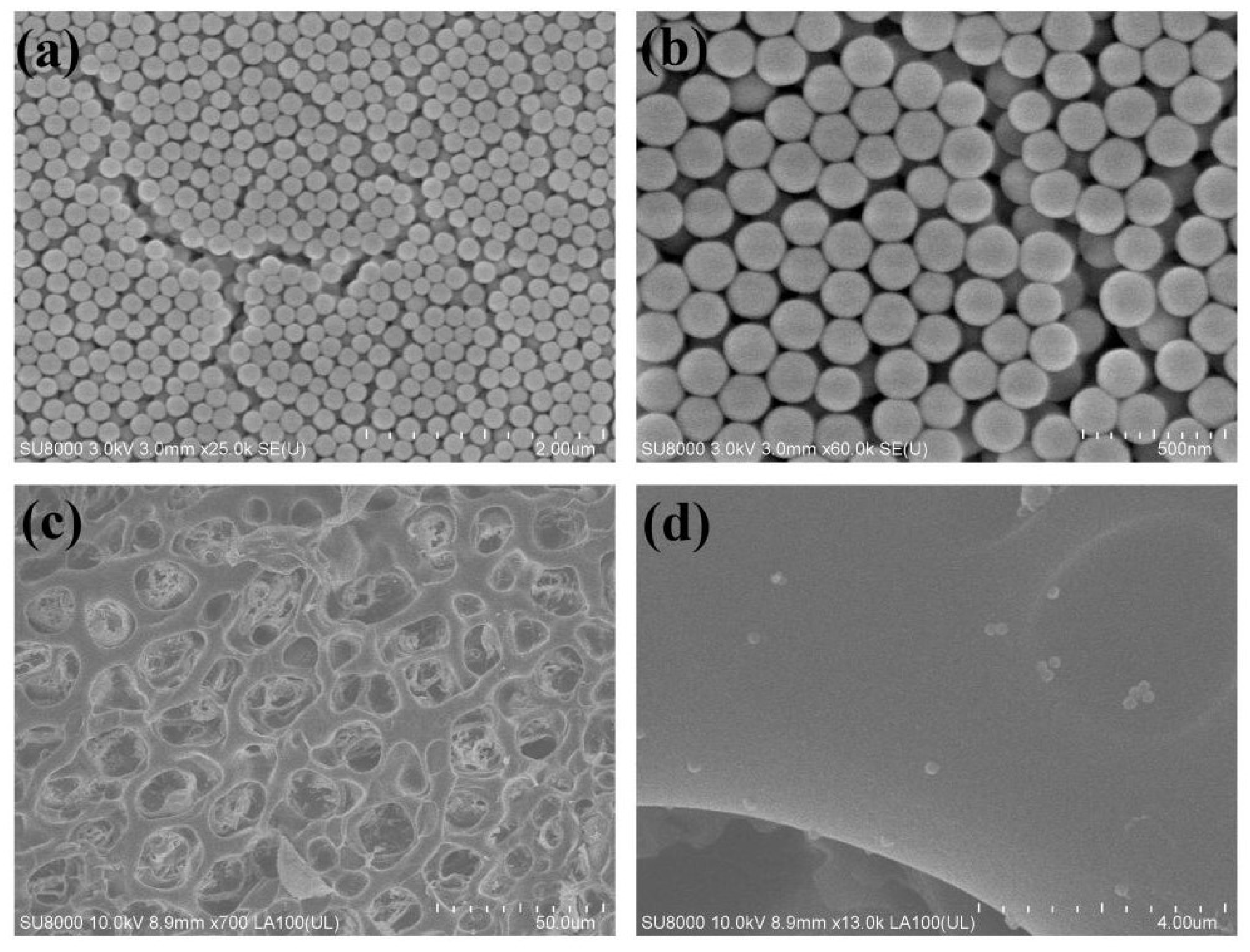
3.1. Preparation and Characterization of PSBMA Nanogels
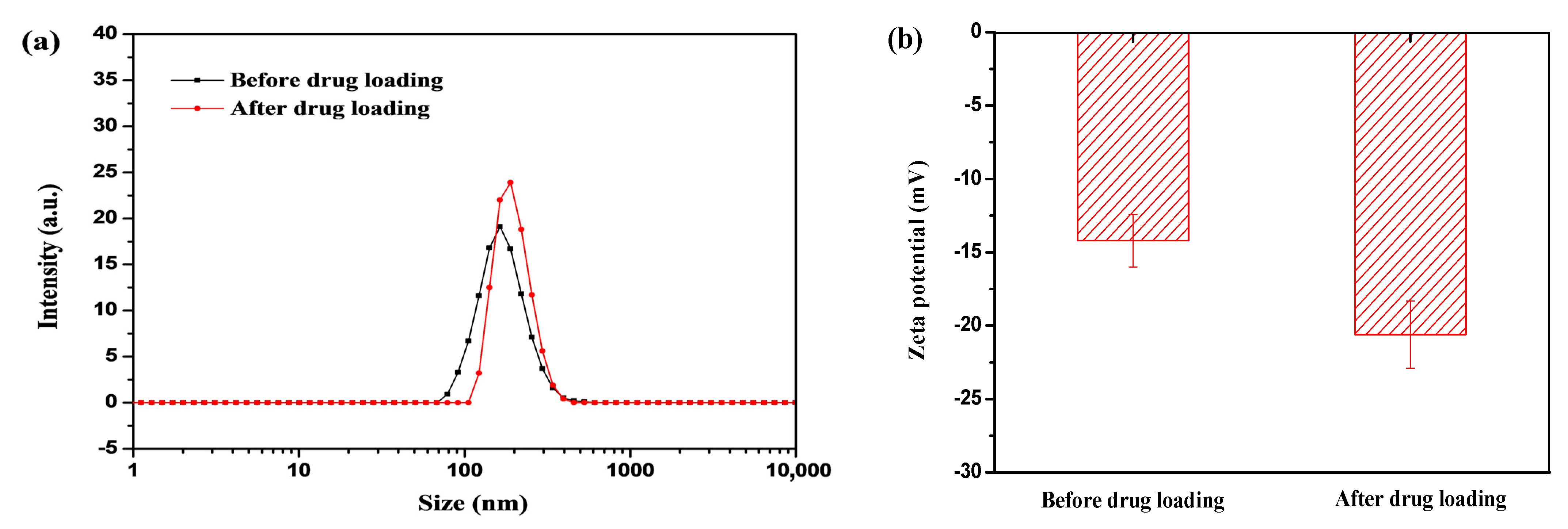
3.2. Drug Encapsulation in Nanogels
3.3. Synthesis and Characterization of Nanogel-Embedded Contact Lenses
3.4. In Vitro Drug Release of Nanogels and Nanogel-Embedded Contact Lens
3.5. In Vitro Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of Commercial Soft Contact Lenses for Ocular Drug Delivery: A Review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Wang, Z.; Chen, X.; Dana, R.; Annabi, N. Advanced Nanodelivery Platforms for Topical Ophthalmic Drug Delivery. Drug Discov. Today 2021, 26, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Mangiacotte, N.; Prosperi-Porta, G.; Liu, L.; Dodd, M.; Sheardown, H. Mucoadhesive Nanoparticles for Drug Delivery to the Anterior Eye. Nanomaterials 2020, 10, 1400. [Google Scholar] [CrossRef]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From Fundamentals to Applications. Adv. Healthc. Mater. 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Hui, A.; Phan, C.M.; Read, M.L.; Azar, D.; Buch, J.; Ciolino, J.B.; Naroo, S.A.; Pall, B.; Romond, K.; et al. CLEAR—Contact Lens Technologies of the Future. Contact Lens Anterior Eye 2021, 44, 398–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic Contact Lenses for Ophthalmic Drug Delivery: Major Challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-Generation Contact Lenses: Towards Bioresponsive Drug Delivery and Smart Technologies in Ocular Therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Shayani Rad, M.; Mohajeri, S.A. Extended Ciprofloxacin Release Using Vitamin E Diffusion Barrier From Commercial Silicone-Based Soft Contact Lenses. Eye Contact Lens Sci. Clin. Pract. 2017, 43, 103–109. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Chen, J.; Ding, H.; Deng, D.; Xie, Z. Self-Reporting Colorimetric Analysis of Drug Release by Molecular Imprinted Structural Color Contact Lens. ACS Appl. Mater. Interfaces 2018, 10, 34611–34617. [Google Scholar] [CrossRef]
- Li, R.; Guan, X.; Lin, X.; Guan, P.; Zhang, X.; Rao, Z.; Du, L.; Zhao, J.; Rong, J.; Zhao, J. Poly(2-Hydroxyethyl Methacrylate)/β-Cyclodextrin-Hyaluronan Contact Lens with Tear Protein Adsorption Resistance and Sustained Drug Delivery for Ophthalmic Diseases. Acta Biomater. 2020, 110, 105–118. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, C.; Sun, Z.; Zhang, X.; Liang, N.; Mao, S. Inner Layer-Embedded Contact Lenses for PH-Triggered Controlled Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 128, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hui, A. Contact Lenses for Ophthalmic Drug Delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired Hydrogels for Drug-Eluting Contact Lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A Comprehensive Review on Contact Lens for Ophthalmic Drug Delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Shan, C.L.; Yu, L.Y. Advances in Contact Lenses as Ocular Drug Delivery System. Chin. Pharm. J. 2018, 53, 574–578. [Google Scholar]
- Hu, X.; Hao, L.; Wang, H.; Yang, X.; Zhang, G.; Wang, G.; Zhang, X. Hydrogel Contact Lens for Extended Delivery of Ophthalmic Drugs. Int. J. Polym. Sci. 2011, 2011, 814163. [Google Scholar] [CrossRef]
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yu, Y.; Sekar, P.; Chauhan, A. Commercialization Challenges for Drug Eluting Contact Lenses. Expert Opin. Drug Deliv. 2020, 17, 1133–1149. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, T.; Cao, A.; Sun, J.; Jia, L.; Sheng, R. Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials 2018, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Longo, R.; Gorrasi, G.; Guadagno, L. Electromagnetically Stimuli-Responsive Nanoparticles-Based Systems for Biomedical Applications: Recent Advances and Future Perspectives. Nanomaterials 2021, 11, 848. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S.; Dehghan, M.M.; Chaleshtori, S.S.; Shafiee, A. Preparation and Evaluation of Contact Lenses Embedded with Polycaprolactone-Based Nanoparticles for Ocular Drug Delivery. Biomacromolecules 2016, 17, 485–495. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-Delivery of Latanoprost and Timolol from Micelles-Laden Contact Lenses for the Treatment of Glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Heidari keshel, S. Preparation of Nanoparticle-Containing Ring-Implanted Poly(Vinyl Alcohol) Contact Lens for Sustained Release of Hyaluronic Acid. Macromol. Biosci. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Sun, J.; Lei, Y.; Dai, Z.; Liu, X.; Huang, T.; Wu, J.; Xu, Z.P.; Sun, X. Sustained Release of Brimonidine from a New Composite Drug Delivery System for Treatment of Glaucoma. ACS Appl. Mater. Interfaces 2017, 9, 7990–7999. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Choksi, H.H.; Desai, A.R.; Patel, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. PH Triggered Controlled Drug Delivery from Contact Lenses: Addressing the Challenges of Drug Leaching during Sterilization and Storage. Colloids Surf. B Biointerfaces 2017, 157, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Aggarwal, R.; Chauhan, M.K. Extended Levobunolol Release from Eudragit Nanoparticle-Laden Contact Lenses for Glaucoma Therapy. Futur. J. Pharm. Sci. 2020, 6, 109. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials 2021, 11, 780. [Google Scholar] [CrossRef]
- Li, P.; Zeng, L.P.; Guo, H.L.; Guo, H.; Li, W.H. Research Progress in Zwitterionic Hydrogels. Acta Polym. Sin. 2020, 51, 1307–1320. [Google Scholar]
- Xu, L.; Ma, P.; Yuan, B.; Chen, Q.; Lin, S.; Chen, X.; Hua, Z.; Shen, J. Anti-Biofouling Contact Lenses Bearing Surface-Immobilized Layers of Zwitterionic Polymer by One-Step Modification. RSC Adv. 2014, 4, 15030–15035. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Lin, Y.; Wang, L.; Wu, S. Preparation and Characterization of Protein-Resistant Hydrogels for Soft Contact Lens Applications via Radical Copolymerization Involving a Zwitterionic Sulfobetaine Comonomer. J. Biomater. Sci. Polym. Ed. 2017, 28, 1935–1949. [Google Scholar] [CrossRef]
- Ogawa, H.; Nakaji-Hirabayashi, T.; Matsumura, K.; Yoshikawa, C.; Kitano, H.; Saruwatari, Y. Novel Anti-Biofouling and Drug Releasing Materials for Contact Lenses. Colloids Surf. B Biointerfaces 2020, 189, 110859. [Google Scholar] [CrossRef]
- Chen, J.S.; Ting, Y.S.; Tsou, H.M.; Liu, T.Y. Highly Hydrophilic and Antibiofouling Surface of Zwitterionic Polymer Immobilized on Polydimethylsiloxane by Initiator-Free Atmospheric Plasma-Induced Polymerization. Surf. Coat. Technol. 2018, 344, 621–625. [Google Scholar] [CrossRef]
- Fan, M.; Wang, F.; Wang, C. Reflux Precipitation Polymerization: A New Platform for the Preparation of Uniform Polymeric Nanogels for Biomedical Applications. Macromol. Biosci. 2018, 18, 1800077. [Google Scholar] [CrossRef]
- Men, Y.; Peng, S.; Yang, P.; Jiang, Q.; Zhang, Y.; Shen, B.; Dong, P.; Pang, Z.; Yang, W. Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 23509–23521. [Google Scholar] [CrossRef]
- Peng, S.; Men, Y.; Xie, R.; Tian, Y.; Yang, W. Biodegradable Phosphorylcholine-Based Zwitterionic Polymer Nanogels with Smart Charge-Conversion Ability for Efficient Inhibition of Tumor Cells. J. Colloid Interface Sci. 2019, 539, 19–29. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, J.; Tang, X.; Ren, Q.; Wang, Y.; Yang, W. Near-Infrared Light-Responsive Nanogels with Diselenide-Cross-Linkers for on-Demand Degradation and Triggered Drug Release. Part. Part. Syst. Charact. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Tranoudis, I.; Efron, N. Water Properties of Soft Contact Lens Materials. Contact Lens Anterior Eye 2004, 27, 193–208. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, W.; Li, S.; Tao, X.; Mu, G. Bimatoprost Loaded Microemulsion Laden Contact Lens to Treat Glaucoma. J. Drug Deliv. Sci. Technol. 2019, 54, 101330. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Opdahl, A.; Kim, S.H.; Koffas, T.S.; Marmo, C.; Somorjai, G.A. Surface Mechanical Properties of PHEMA Contact Lenses: Viscoelastic and Adhesive Property Changes on Exposure to Controlled Humidity. J. Biomed. Mater. Res. 2003, 67A, 350–356. [Google Scholar] [CrossRef]
- Lu, D.R.; Lee, S.J.; Park, K. Calculation of Solvation Interaction Energies for Protein Adsorption on Polymer Surfaces. J. Biomater. Sci. Polym. Ed. 1992, 3, 127–147. [Google Scholar] [CrossRef]
- Chien, H.W.; Kuo, C.J. Preparation, Material Properties and Antimicrobial Efficacy of Silicone Hydrogel by Modulating Silicone and Hydrophilic Monomer. J. Biomater. Sci. Polym. Ed. 2019, 30, 1050–1067. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, A.R.; Choksi, H.H.; Patil, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Effect of Surfactant Chain Length on Drug Release Kinetics from Microemulsion-Laden Contact Lenses. Int. J. Pharm. 2017, 524, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.M.; Cho, P.; Boost, M.V. Cytotoxicity and Effects on Metabolism of Contact Lens Care Solutions on Human Corneal Epithelium Cells. Clin. Exp. Optom. 2012, 95, 198–206. [Google Scholar] [CrossRef]
- Liu, P.; Xie, Z.; Zheng, F.; Zhao, Y.; Gu, Z. Surfactant-Free HEMA Crystal Colloidal Paint for Structural Color Contact Lens. J. Mater. Chem. B 2016, 4, 5222–5227. [Google Scholar] [CrossRef]
- Shen, X.; Du, J.; Sun, J.; Guo, J.; Hu, X.; Wang, C. Transparent and UV Blocking Structural Colored Hydrogel for Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 39639–39648. [Google Scholar] [CrossRef]
- Xie, Z.; Li, L.; Liu, P.; Zheng, F.; Guo, L.; Zhao, Y.; Jin, L.; Li, T.; Gu, Z. Self-Assembled Coffee-Ring Colloidal Crystals for Structurally Colored Contact Lenses. Small 2015, 11, 926–936. [Google Scholar] [CrossRef]




| Sample | SBMA (mg) | DVB (mg) | AIBN (mg) | AN (mL) | Size (nm) a | PDI b | ZP (mV) c |
|---|---|---|---|---|---|---|---|
| PSBMA-1 | 180 | 20 | 4 | 40 | 294.7 ± 2.6 | 0.183 | −4.3 ± 0.7 |
| PSBMA-2 | 160 | 40 | 4 | 40 | 270.4 ± 3.6 | 0.124 | −9.2 ± 1.3 |
| PSBMA-3 | 140 | 60 | 4 | 40 | 248.6 ± 2.2 | 0.164 | −11.4 ± 1.5 |
| PSBMA-4 | 120 | 80 | 4 | 40 | 219.9 ± 1.7 | 0.104 | −14.2 ± 1.8 |
| Sample | NVP (mg) | HEMA (mg) | EGDMA (mg) | AIBN (mg) | Drug-Loaded Nanogels (mg) | Loaded LEV (mg) a |
|---|---|---|---|---|---|---|
| pHEMA | 150 | 815 | 15 | 20 | 0 | 0 |
| pHEMA/LEV-1 | 150 | 815 | 15 | 20 | 50 | 6.7 |
| pHEMA/LEV-2 | 150 | 815 | 15 | 20 | 80 | 10.6 |
| pHEMA/LEV-3 | 150 | 815 | 15 | 20 | 110 | 14.6 |
| pHEMA/LEV-4 | 150 | 815 | 15 | 20 | 130 | 17.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. https://doi.org/10.3390/nano11092328
Wang Z, Li X, Zhang X, Sheng R, Lin Q, Song W, Hao L. Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials. 2021; 11(9):2328. https://doi.org/10.3390/nano11092328
Chicago/Turabian StyleWang, Zhao, Xinhua Li, Xiaojuan Zhang, Ruilong Sheng, Qing Lin, Wenli Song, and Lingyun Hao. 2021. "Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery" Nanomaterials 11, no. 9: 2328. https://doi.org/10.3390/nano11092328
APA StyleWang, Z., Li, X., Zhang, X., Sheng, R., Lin, Q., Song, W., & Hao, L. (2021). Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials, 11(9), 2328. https://doi.org/10.3390/nano11092328






