ZnO Nanoparticles Induce Dyslipidemia and Atherosclerotic Lesions Leading to Changes in Vascular Contractility and Cannabinoid Receptors Expression as Well as Increased Blood Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. ZnONPs Preparation
2.4. Measurement of the Blood Pressure in Rats
2.5. Biochemical Determinations
2.6. Histological Analysis
2.7. CB1 and CB2 Receptors Expression in Aorta Rings by Immunofluorescence
2.8. Vascular Contractility
2.9. Statistical Analysis
3. Results
3.1. Effects of the ZnONPs on Lipid Profile and Atherogenic Risk
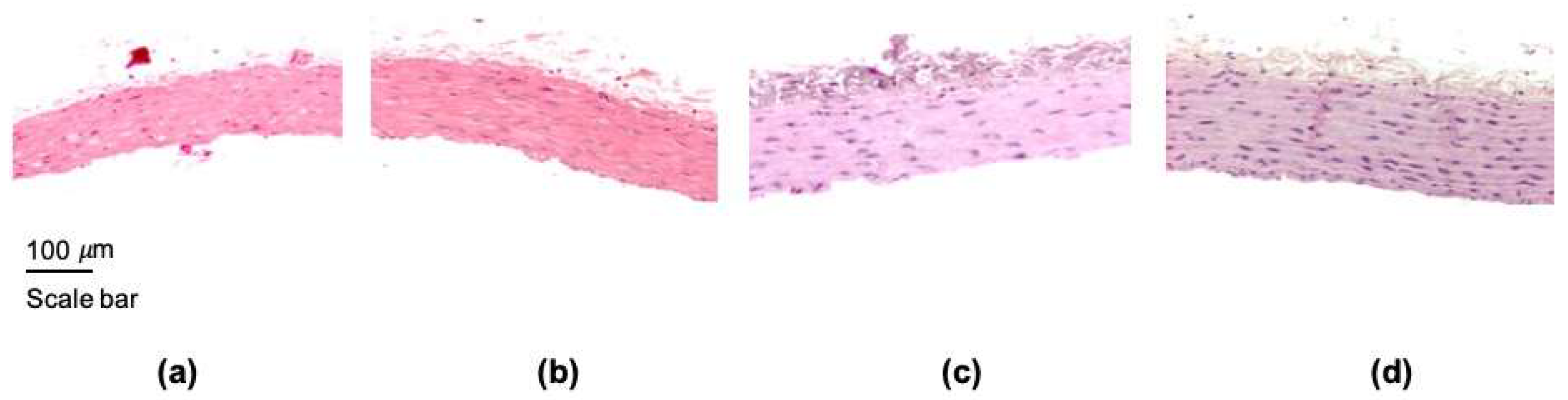
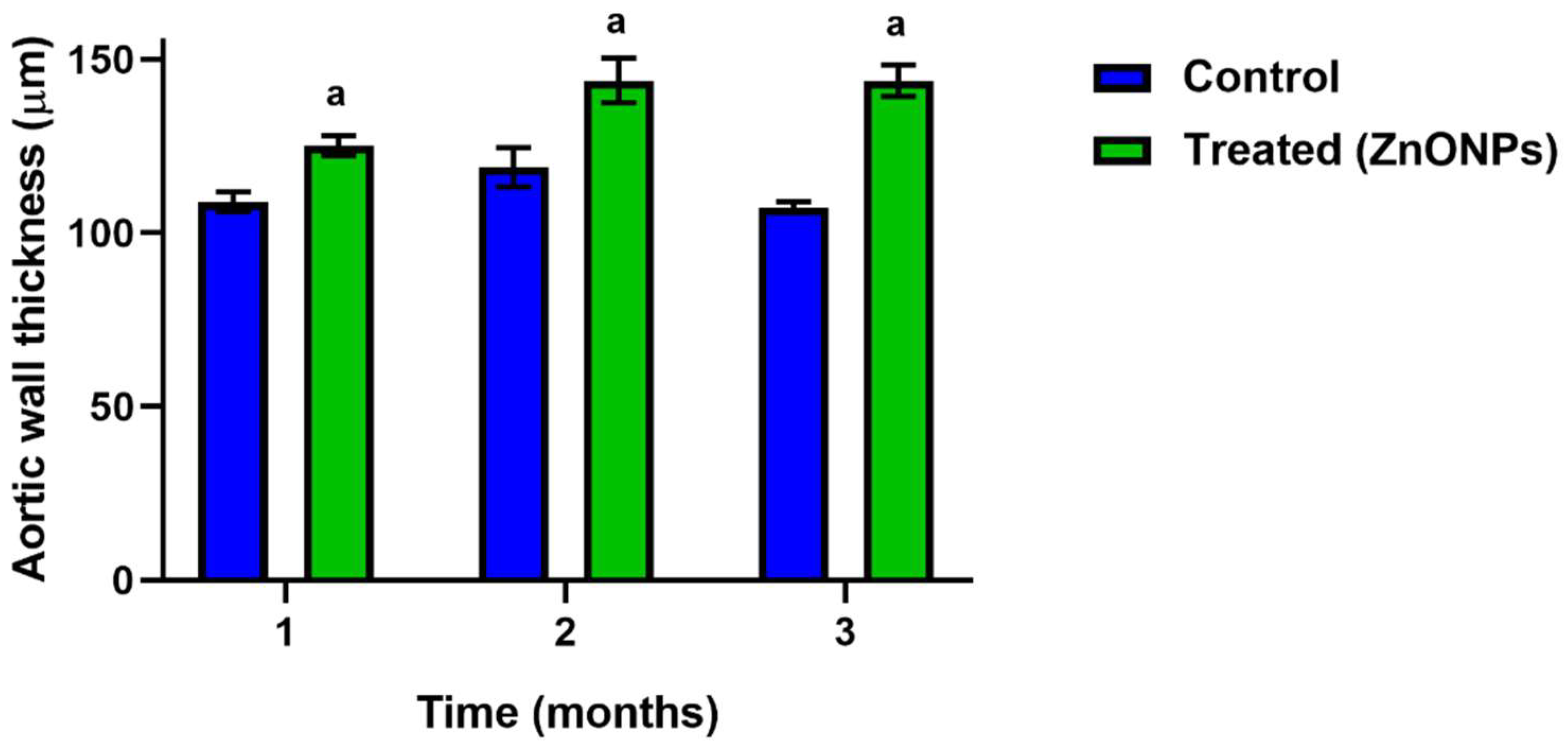
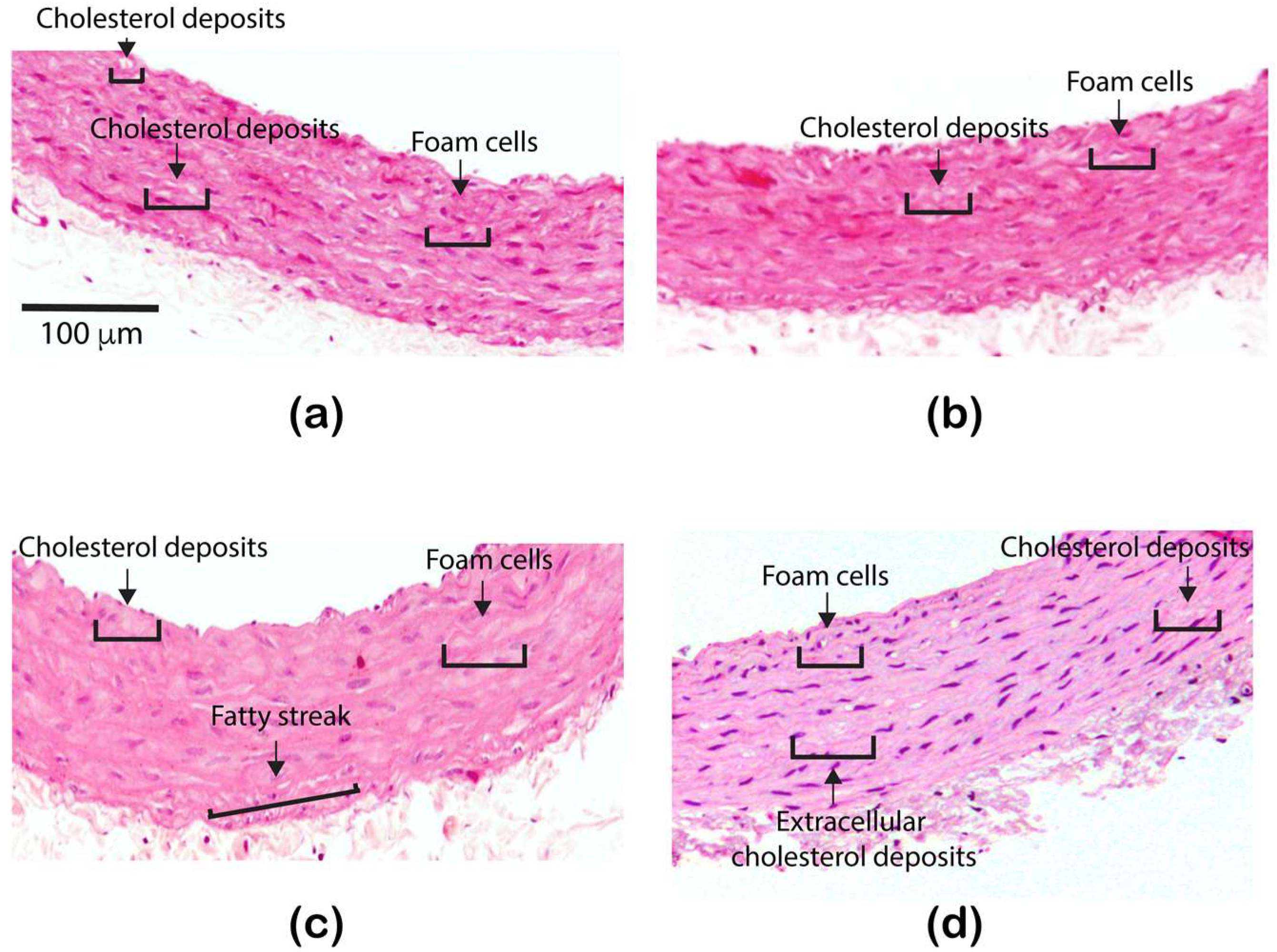
3.2. Effect of the ZnONPs Treatment on the Structure of the Aorta Wall
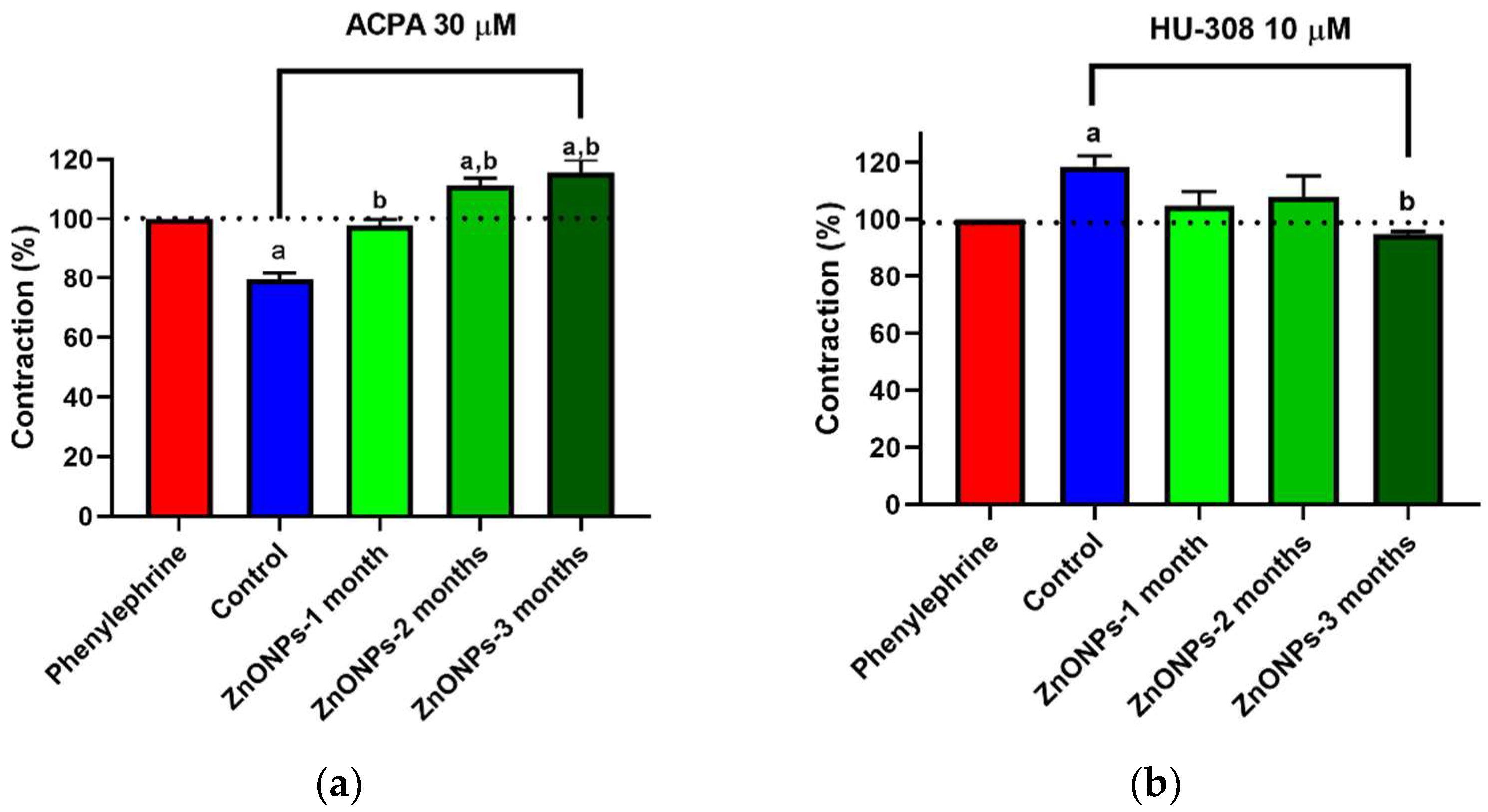
3.3. Alterations in ZnONPs-Induced Vascular Contractility
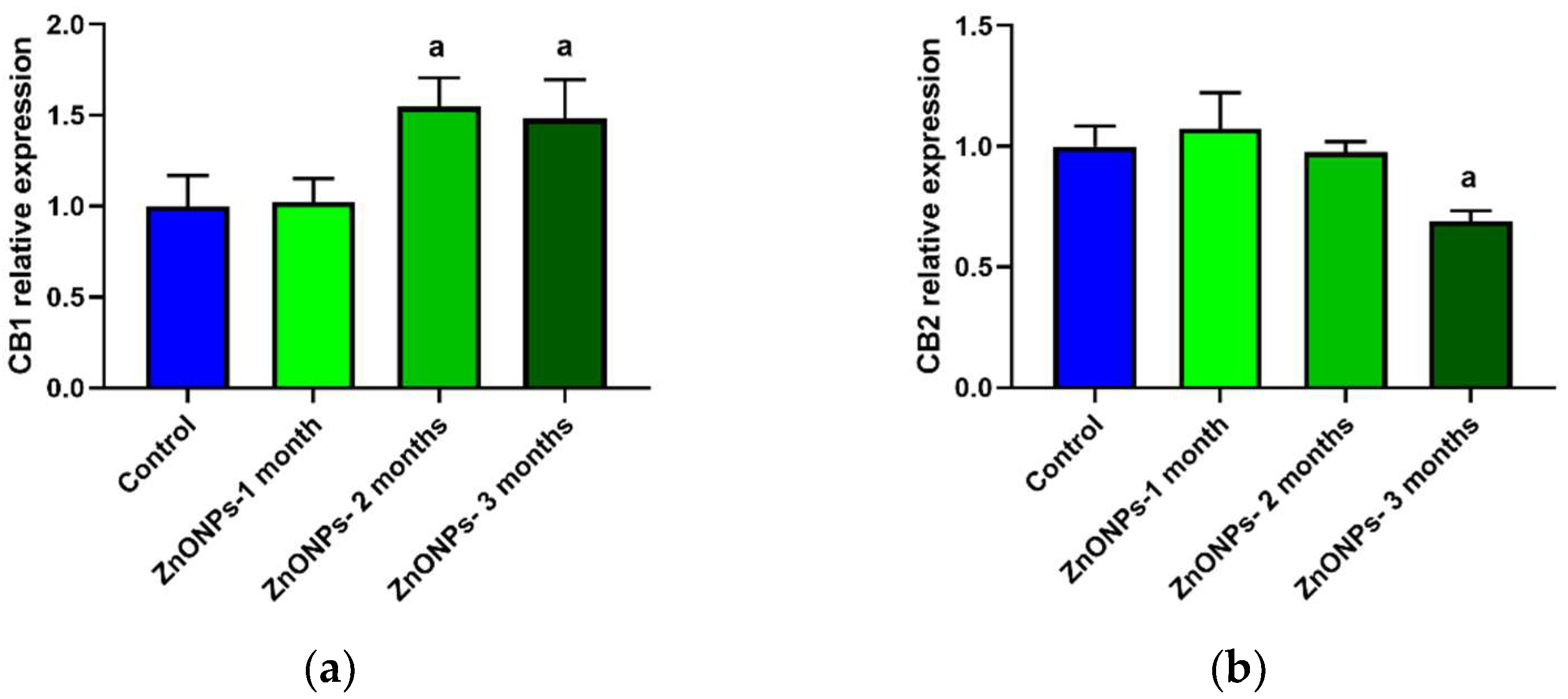
3.4. CB1 and CB2 Receptors Expression in Aorta Rings of ZnONPs-Treated Rats
3.5. ZnONPs on Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B.A. Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Asri-Rezaie, S. Comparative Study of Antidiabetic Activity and Oxidative Stress Induced by Zinc Oxide Nanoparticles and Zinc Sulfate in Diabetic Rats. AAPS PharmSciTech 2016, 17, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umrani, R.D.; Paknikar, K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar] [CrossRef]
- Alomari, G.; Al-Trad, B.; Hamdan, S.; Aljabali, A.A.A.; Al Zoubi, M.S.; Al-Batanyeh, K.; Qar, J.; Eaton, G.J.; Alkaraki, A.K.; Alshaer, W.; et al. Alleviation of diabetic nephropathy by zinc oxide nanoparticles in streptozotocin-induced type 1 diabetes in rats. IET Nanobiotechnol. 2021, 15, 473–483. [Google Scholar] [CrossRef]
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative Effect of Zinc Oxide Nanoparticles on Antioxidants and Sperm Characteristics in Streptozotocin-Induced Diabetic Rat Testes. BioMed Res. Int. 2015, 2015, 153573. [Google Scholar] [CrossRef]
- Shanker, K.; Naradala, J.; Mohan, G.K.; Kumar, G.S.; Pravallika, P.L. A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Adv. 2017, 7, 37158–37167. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.; Zhang, S.H.; Zhang, J.L.; Hao, X.Q.; Yang, F.; Zhang, C.; Yang, Z.J.; Zhang, M.Y.; Wang, J. Acute and Cumulative Effects of Unmodified 50-nm Nano-ZnO on Mice. Biol. Trace Elem. Res. 2018, 185, 124–134. [Google Scholar] [CrossRef]
- Hong, T.K.; Tripathy, N.; Son, H.J.; Ha, K.T.; Jeong, H.S.; Hahn, Y.B. A comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J. Mater. Chem. B 2013, 1, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.J.; Lee, T.J.; Kim, G.Y.; Meang, E.; Hong, J.S.; Kim, S.H.; Koh, S.B.; Hong, S.G.; Sun, Y.S.; et al. A 90-day study of sub-chronic oral toxicity of 20 nm positively charged zinc oxide nanoparticles in Sprague Dawley rats. Int. J. Nanomed. 2014, 9, 93–107. [Google Scholar]
- Liu, H.; Yang, H.; Fang, Y.; Li, K.; Tian, L.; Liu, X.; Zhang, W.; Tan, Y.; Lai, W.; Bian, L.; et al. Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci. Total Environ. 2020, 705, 135809, Erratum in: Sci. Total Environ. 2021, 765, 145120. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, P.; Luo, Y.; Xie, H.Q.; Chakraborty, S.; Monikh, F.A.; Lynch, I. Intranasal exposure to ZnO nanoparticles induces alterations in cholinergic neurotransmission in rat brain. Nano Today 2020, 35, 100977. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, W.; Wu, Y.; Wang, W.; Li, B.; Liang, N.; Wu, W. Zinc oxide nanoparticle-induced atherosclerotic alterations in vitro and in vivo. Int. J. Nanomed. 2017, 12, 4433–4442. [Google Scholar] [CrossRef] [Green Version]
- Asri-Rezaei, S.; Dalir-Naghadeh, B.; Nazarizadeh, A.; Noori-Sabzikar, Z. Comparative study of cardio-protective effects of zinc oxide nanoparticles and zinc sulfate in streptozotocin-induced diabetic rats. J. Trace Elem. Med. Biol. 2017, 42, 129–141. [Google Scholar] [CrossRef]
- Fulmer, M.L.; Thewke, D.P. The Endocannabinoid System and Heart Disease: The Role of Cannabinoid Receptor Type 2. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Sachdeva, R.; Mehta, J.L. Cannabinoids and Atherosclerotic Coronary Heart Disease. Clin Cardiol 2012, 35, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Rami, M.; Herzig, S.; Steffens, S. Endocannabinoid Signalling in Atherosclerosis and Related Metabolic Complications. Thromb. Haemost. 2019, 119, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB22 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgen-Ortiz, A.; Apolinar-Iribe, A.; Díaz-Reval, I.; Parra-Delgado, H.; Limón-Miranda, S.; Sánchez-Pastor, E.A.; Castro-Sánchez, L.; Jesús Castillo, S.; Dagnino-Acosta, A.; Bonales-Alatorre, E.; et al. Zinc Oxide Nanoparticles Induce an Adverse Effect on Blood Glucose Levels Depending on the Dose and Route of Administration in Healthy and Diabetic Rats. Nanomaterials 2020, 10, 2005. [Google Scholar] [CrossRef]
- Pour-Mohammad, P.; Alipanah-Moghadam, R.; Amani, F.; Nemati, A.; Malekzadeh, V. Effect of Zinc Oxide Nanoparticles on Blood Lipid Profile in Wistar Male Rats. J. Ardabil. Univ. Med. Sci. 2018, 18, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.E.; Ahmed-Farid, O.A.H.; Abdelmottaleb-Moussa, S.; Omara, E.A.; Abdel Jaleel, G.A.; Ibrahim, F.A.A. Efficacy of zinc oxide nanoparticles on hepatocellular carcinomainduced biochemical and trace element alterations in rats. J. Appl. Pharm. Sci 2021, 11, 108–117. [Google Scholar]
- Gordon, S.M.; Li, H.; Zhu, X.; Shah, A.S.; Lu, L.J.; Davidson, W.S. A comparison of the mouse and human lipoproteome: Suitability of the mouse model for studies of human lipoproteins. J. Proteome Res. 2015, 14, 2686–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Paigen, B. Genetics of variation in HDL cholesterol in humans and mice. Circ. Res. 2005, 96, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. BioMed Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [Green Version]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition. Environ. Health Perspect. 2007, 115, 403–409. [Google Scholar] [CrossRef]
- Li, C.H.; Liao, P.L.; Shyu, M.K.; Liu, C.W.; Kao, C.C.; Huang, S.H.; Kang, J.J. Zinc Oxide Nanoparticles–Induced Intercellular Adhesion Molecule 1 Expression Requires Rac1/Cdc42, Mixed Lineage Kinase 3, and c-Jun N-Terminal Kinase Activation in Endothelial Cells. Toxicol. Sci. 2012, 126, 162–172. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Zhao, D.; Hun, F.H.; Weng, L.; Liu, H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells. Cell Biol. Toxicol. 2011, 27, 333–342. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Gadolinium Oxide Nanoparticles Induce Toxicity in Human Endothelial HUVECs via Lipid Peroxidation, Mitochondrial Dysfunction and Autophagy Modulation. Nanomaterials 2020, 10, 1675. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 1, 20. [Google Scholar]
- Yeh, S.C.; Tsai, F.Y.; Chao, H.R.; Tsou, T.C. Zinc Ions Induce Inflammatory Responses in Vascular Endothelial Cells. Bull. Environ. Contam. Toxicol. 2011, 87, 113–116. [Google Scholar] [CrossRef]
- White, R.; Ho, W.S.; Bottrill, F.E.; Ford, W.R.; Hiley, C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001, 134, 921–929. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Chapnick, B.M.; Howlett, A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2046–H2054. [Google Scholar] [CrossRef] [PubMed]
- Lake, K.D.; Martin, B.R.; Kunos, G.; Varga, K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 1997, 29, 1204–1210. [Google Scholar] [CrossRef]
- Varga, K.; Lake, K.D.; Huangfu, D.; Guyenet, P.G.; Kunos, G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension 1996, 28, 682–686. [Google Scholar] [CrossRef]
- Mach, F.; Steffens, S. The Role of the Endocannabinoid System in Atherosclerosis. J. Neuroendocrinol. 2008, 20, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Karpov, R.S. Prospects for the use of cannabinoid receptor ligands for the treatment of metabolic syndrome and atherosclerosis: Analysis of experimental and clinical data. Vestn. Ross. Akad. Med. Nauk. 2017, 72, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Hu, P.; Lin, J.; Xia, W.; Zhang, R. Activating Cannabinoid Receptor 2 Protects Against Diabetic Cardiomyopathy Through Autophagy Induction. Front. Pharmacol. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- van Eenige, R.; Ying, Z.; Tambyrajah, L.; Pronk, A.C.M.; Blomberg, N.; Giera, M.; Wang, Y.; Coskun, T.; van der Stelt, M.; Rensen, P.C.N.; et al. Cannabinoid type 1 receptor inverse agonism attenuates dyslipidemia and atherosclerosis in APOE∗3-Leiden.CETP mice. J. Lipid Res. 2021, 62, 100070. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P. Cannabinoid CB1 receptor antagonists for atherosclerosis and cardiometabolic disorders: New hopes, old concerns? Arterioscler. Thromb. Vasc. Biol. 2009, 29, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Steffens, S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin. Immunopathol. 2009, 31, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Alfulaij, N.; Meiners, F.; Michalek, J.; Small-Howard, A.L.; Turner, H.C.; Stokes, A.J. Cannabinoids, the Heart of the Matter. J. Am. Heart Assoc. 2018, 7, e009099. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pastor, E.; Andrade, F.; Sánchez-Pastor, J.M.; Elizalde, A.; Huerta, M.; Virgen-Ortiz, A.; Trujillo, X.; Rodríguez-Hernández, A. Cannabinoid receptor type 1 activation by arachidonylcyclopropylamide in rat aortic rings causes vasorelaxation involving calcium-activated potassium channel subunit alpha-1 and calcium channel, voltage-dependent, L type, alpha 1C subunit. Eur. J. Pharmacol. 2014, 729, 100–106. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, B.K.; Choi, J.Y.; Ryu, T.; Roh, W.S.; Song, S.Y. Role of calcium channels responsible for phenylephrine-induced contraction in rat aorta 3 days after acute myocardial infarction. Korean J. Anesthesiol. 2014, 66, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Aronstam, R.S.; Chen, D.R.; Huang, Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. In Vitro 2010, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Yin, Q.; Cui, G.; Shao, L. Endothelial Barrier Dysfunction Induced by Zinc Oxide Nanoparticles InVivo and InVitro and Their Mechanism of Crossing the Endothelial Barrier. J. Biomed. Nanotechnol. 2019, 15, 443–461. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Sarott, R.C.; Viray, A.E.G.; Pfaff, P.; Sadybekov, A.; Rajic, G.; Katritch, V.; Carreira, E.M.; Frank, J.A. Optical Control of Cannabinoid Receptor 2-Mediated Ca2+ Release Enabled by Synthesis of Photoswitchable Probes. J. Am. Chem. Soc. 2021, 143, 736–743. [Google Scholar] [CrossRef]
- Aweimer, A.; Jettkant, B.; Monsé, C.; Hagemeyer, O.; van Kampen, V.; Kendzia, B.; Gering, V.; Marek, E.M.; Bünger, J.; Mügge, A.; et al. Heart rate variability and cardiac repolarization after exposure to zinc oxide nanoparticles in healthy adults. J. Occup. Med. Toxicol. 2020, 28, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, F.; Zhang, L.; Zhang, C.; Peng, H.; Lan, F.; Peng, S.; Liu, C.; Guo, J. Zinc Oxide Nanoparticles Induce Mitochondrial Biogenesis Impairment and Cardiac Dysfunction in Human iPSC-Derived Cardiomyocytes. Int. J. Nanomed. 2020, 15, 2669–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, S.; Sprague, J.E.; Oh, J.; Riek, A.E.; Chin, K.; Garcia, M.; Bernal-Mizrachi, C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS ONE 2013, 8, e54625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Cassis, L.A.; Daugherty, A. Atherosclerosis and arterial blood pressure in mice. Curr. Drug Targets 2007, 8, 1181–1189. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group | Total Cholesterol mg/dL | HDL-C mg/dL | LDL-C mg/dL | Triglycerides mg/dL | Atherogenic Index |
|---|---|---|---|---|---|
| Control | 52 ± 2 | 23 ± 2 | 38 ± 3 | 32 ± 2 | 0.14 ± 0.04 |
| ZnONPs- 1 month | 46 ± 3 | 21 ± 1 | 19 ± 2 a | 48 ± 3 a | 0.34 ± 0.04 a |
| ZnONPs- 2 moths | 57 ± 3 | 31 ± 4 | 36 ± 2 | 43 ± 3 a | 0.15 ± 0.07 |
| ZnONPs- 3 months | 67 ± 6 a | 40 ± 2 a | 29 ± 3 a | 31 ± 1 | 0.00 ± 0.11 |
| Systolic Blood Pressure (mmHg) | |||
|---|---|---|---|
| Control | Rats treated with ZnONPs | ||
| One-month | Two-months | Three-months | |
| 116 ± 3 | 125 ± 9 | 129 ± 4 | 137 ± 4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos-Gutiérrez, A.; Rodríguez-Hernández, A.; Álvarez-Valadez, M.d.R.; Limón-Miranda, S.; Andrade, F.; Figueroa-Gutiérrez, A.; Díaz-Reval, I.; Apolinar-Iribe, A.; Castro-Sánchez, L.; Alamilla, J.; et al. ZnO Nanoparticles Induce Dyslipidemia and Atherosclerotic Lesions Leading to Changes in Vascular Contractility and Cannabinoid Receptors Expression as Well as Increased Blood Pressure. Nanomaterials 2021, 11, 2319. https://doi.org/10.3390/nano11092319
Ceballos-Gutiérrez A, Rodríguez-Hernández A, Álvarez-Valadez MdR, Limón-Miranda S, Andrade F, Figueroa-Gutiérrez A, Díaz-Reval I, Apolinar-Iribe A, Castro-Sánchez L, Alamilla J, et al. ZnO Nanoparticles Induce Dyslipidemia and Atherosclerotic Lesions Leading to Changes in Vascular Contractility and Cannabinoid Receptors Expression as Well as Increased Blood Pressure. Nanomaterials. 2021; 11(9):2319. https://doi.org/10.3390/nano11092319
Chicago/Turabian StyleCeballos-Gutiérrez, Adriana, Alejandrina Rodríguez-Hernández, María del Rosario Álvarez-Valadez, Saraí Limón-Miranda, Felipa Andrade, Alejandro Figueroa-Gutiérrez, Irene Díaz-Reval, Alejandro Apolinar-Iribe, Luis Castro-Sánchez, Javier Alamilla, and et al. 2021. "ZnO Nanoparticles Induce Dyslipidemia and Atherosclerotic Lesions Leading to Changes in Vascular Contractility and Cannabinoid Receptors Expression as Well as Increased Blood Pressure" Nanomaterials 11, no. 9: 2319. https://doi.org/10.3390/nano11092319
APA StyleCeballos-Gutiérrez, A., Rodríguez-Hernández, A., Álvarez-Valadez, M. d. R., Limón-Miranda, S., Andrade, F., Figueroa-Gutiérrez, A., Díaz-Reval, I., Apolinar-Iribe, A., Castro-Sánchez, L., Alamilla, J., Sánchez-Pastor, E., & Virgen-Ortiz, A. (2021). ZnO Nanoparticles Induce Dyslipidemia and Atherosclerotic Lesions Leading to Changes in Vascular Contractility and Cannabinoid Receptors Expression as Well as Increased Blood Pressure. Nanomaterials, 11(9), 2319. https://doi.org/10.3390/nano11092319









