The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications
Abstract
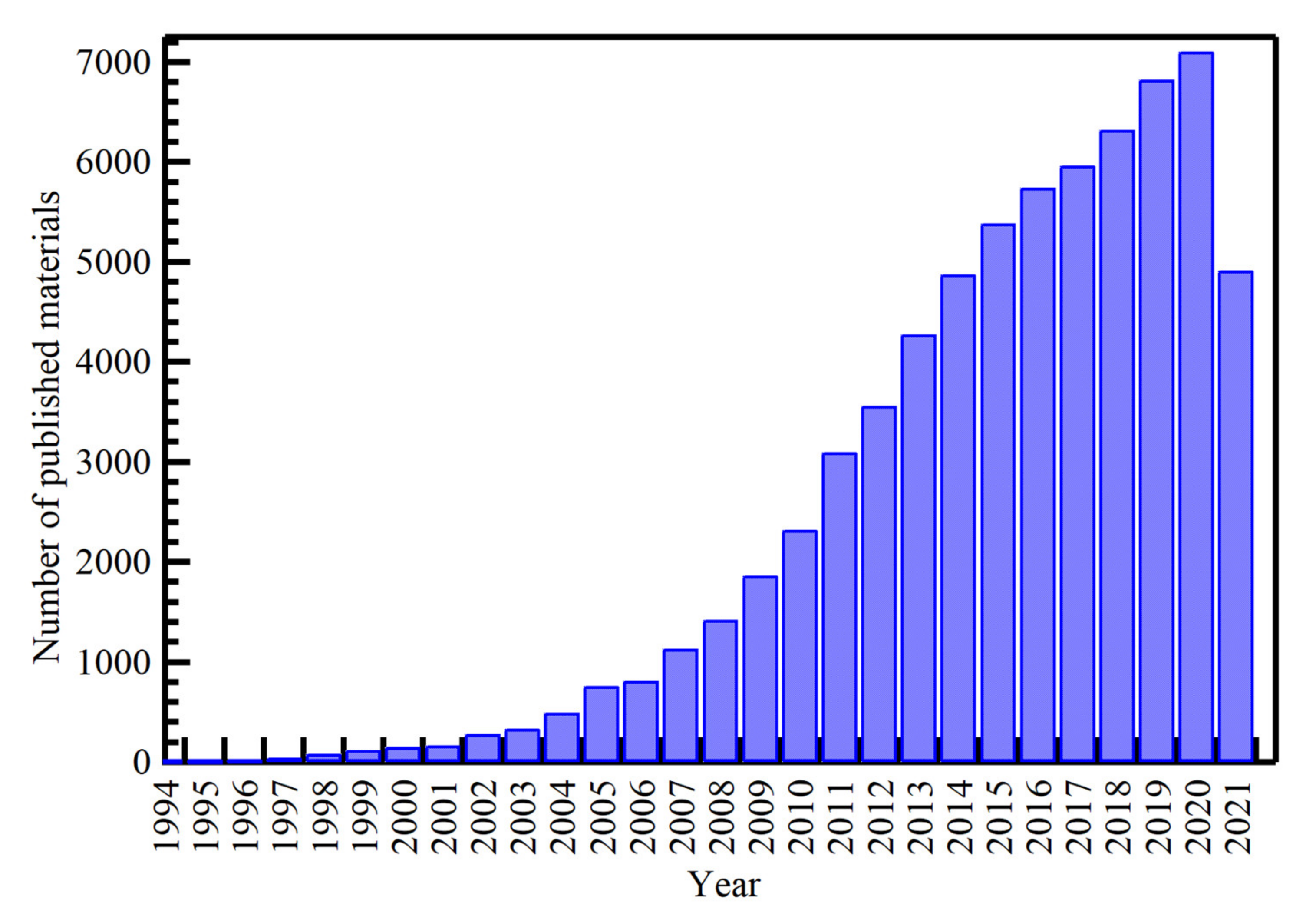
1. Introduction
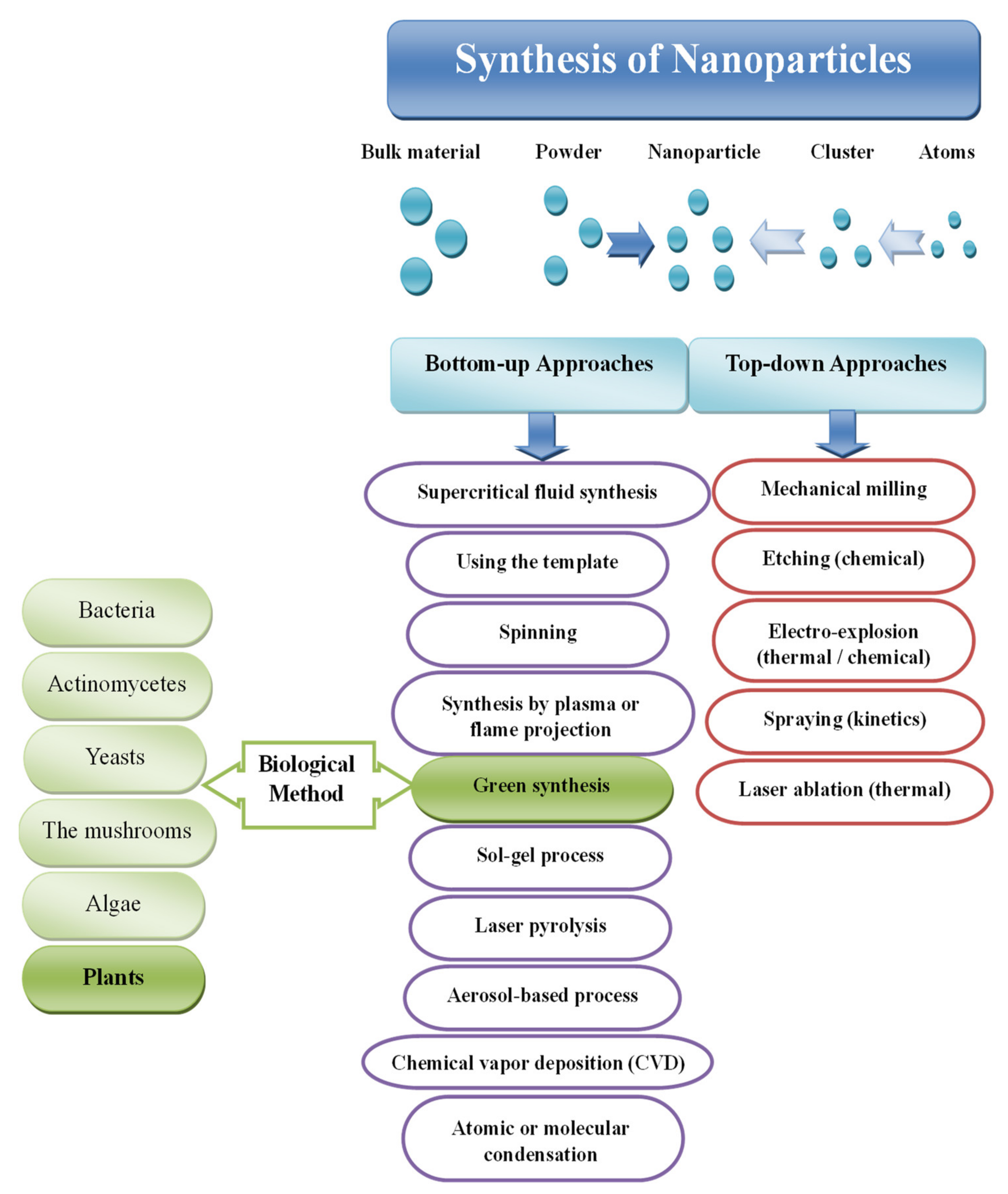
2. Metal Nanoparticles Synthesis
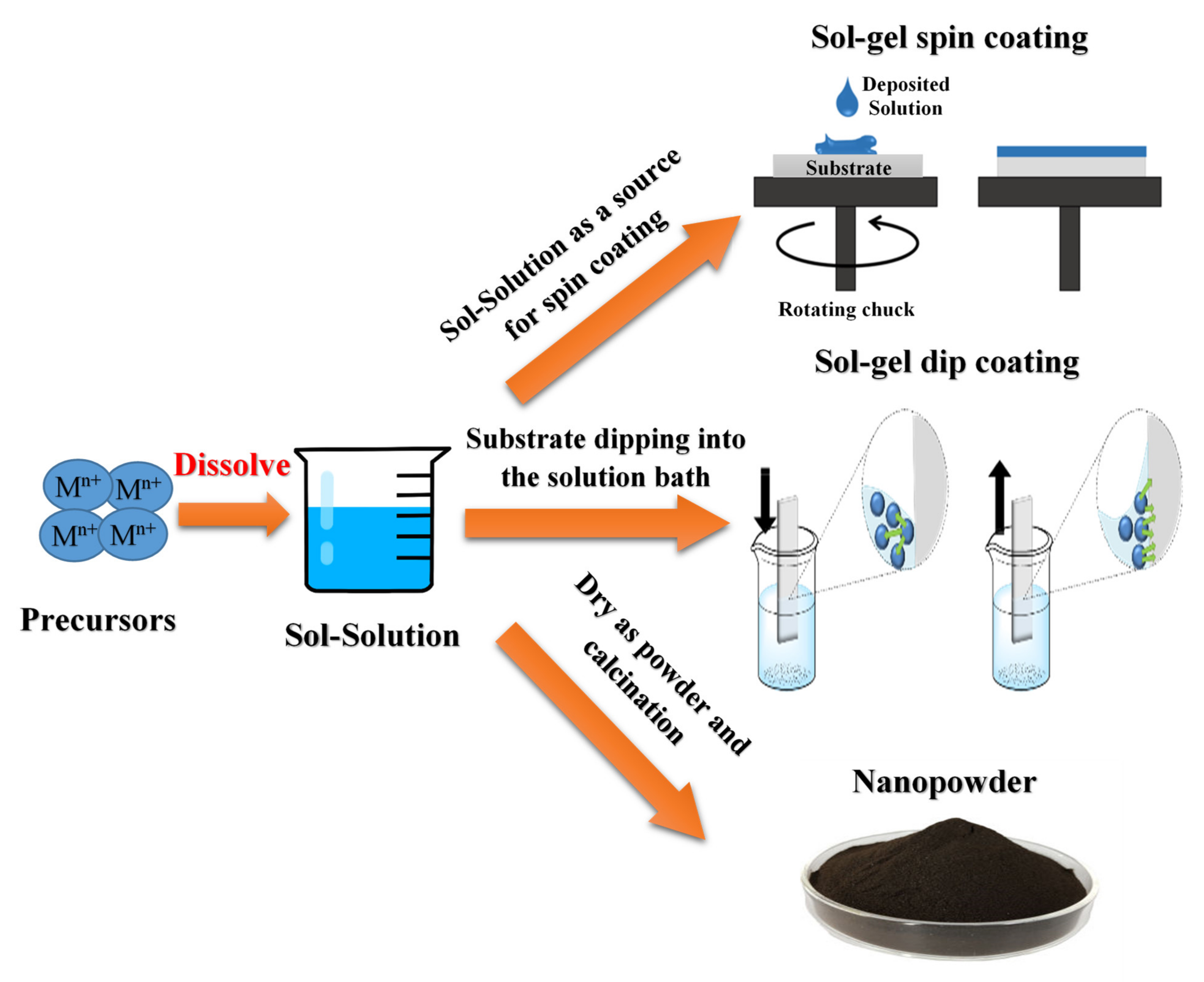
2.1. Sol-Gel Method
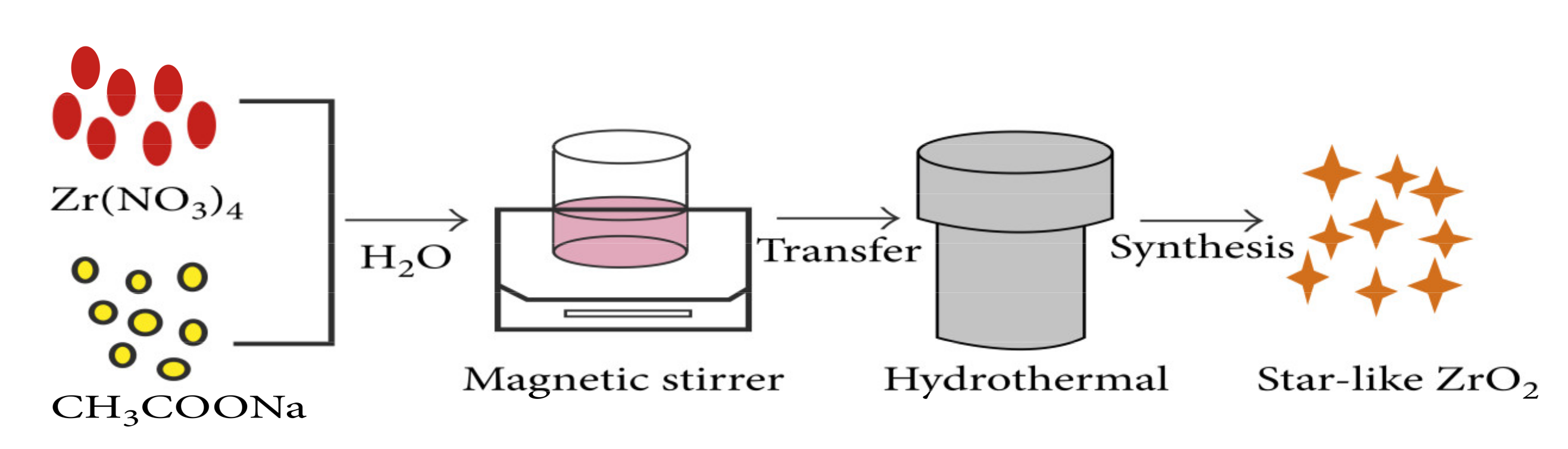
2.2. Hydrothermal Method
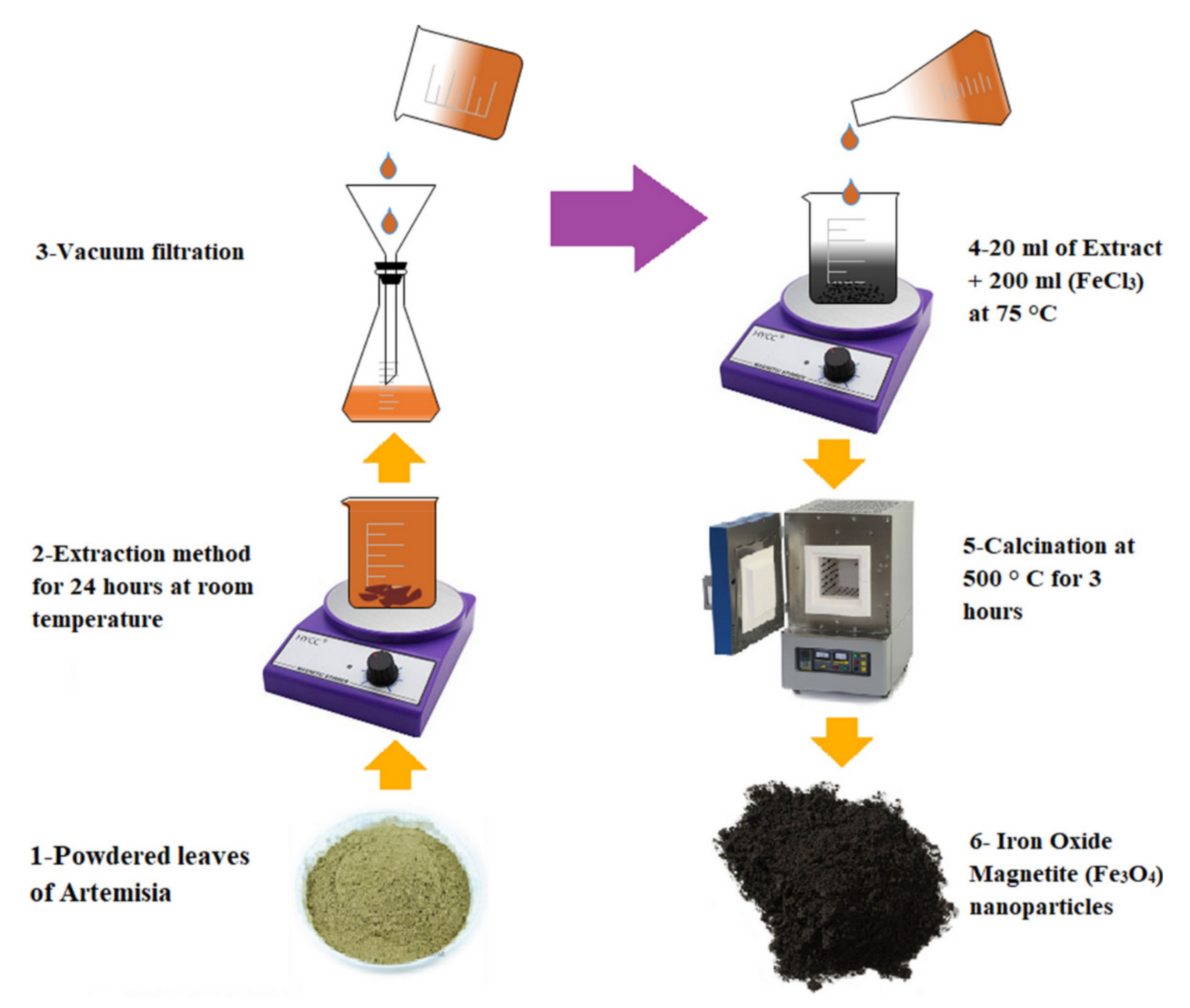
2.3. Green Synthesis Method
3. Silver Nanoparticles
3.1. History of Silver Nanoparticles
3.2. Synthesis of Silver Nanoparticles
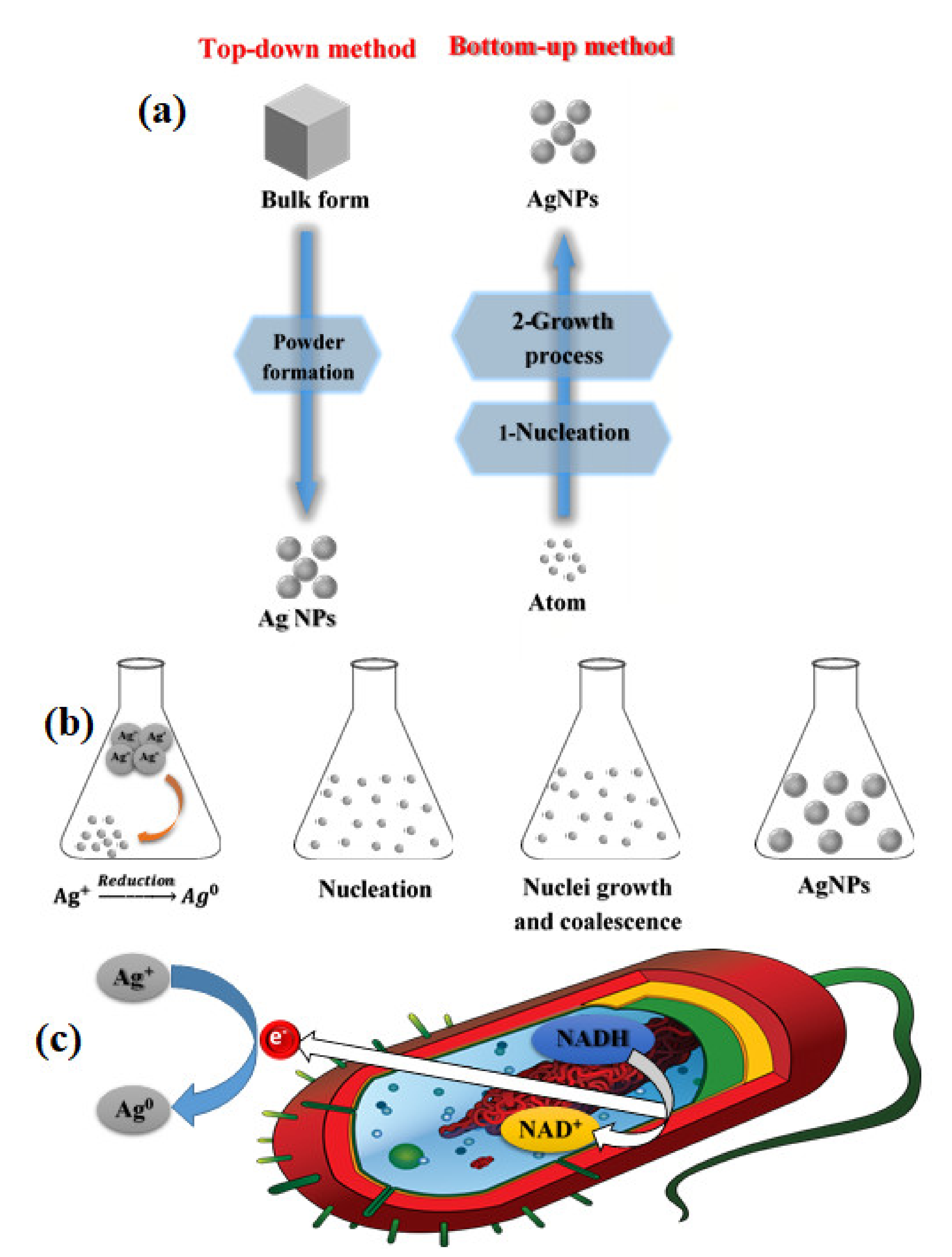
3.2.1. Top-Down and Bottom-Up Approaches
3.2.2. Physical Methods
3.2.3. Chemical Methods
3.2.4. Green Methods
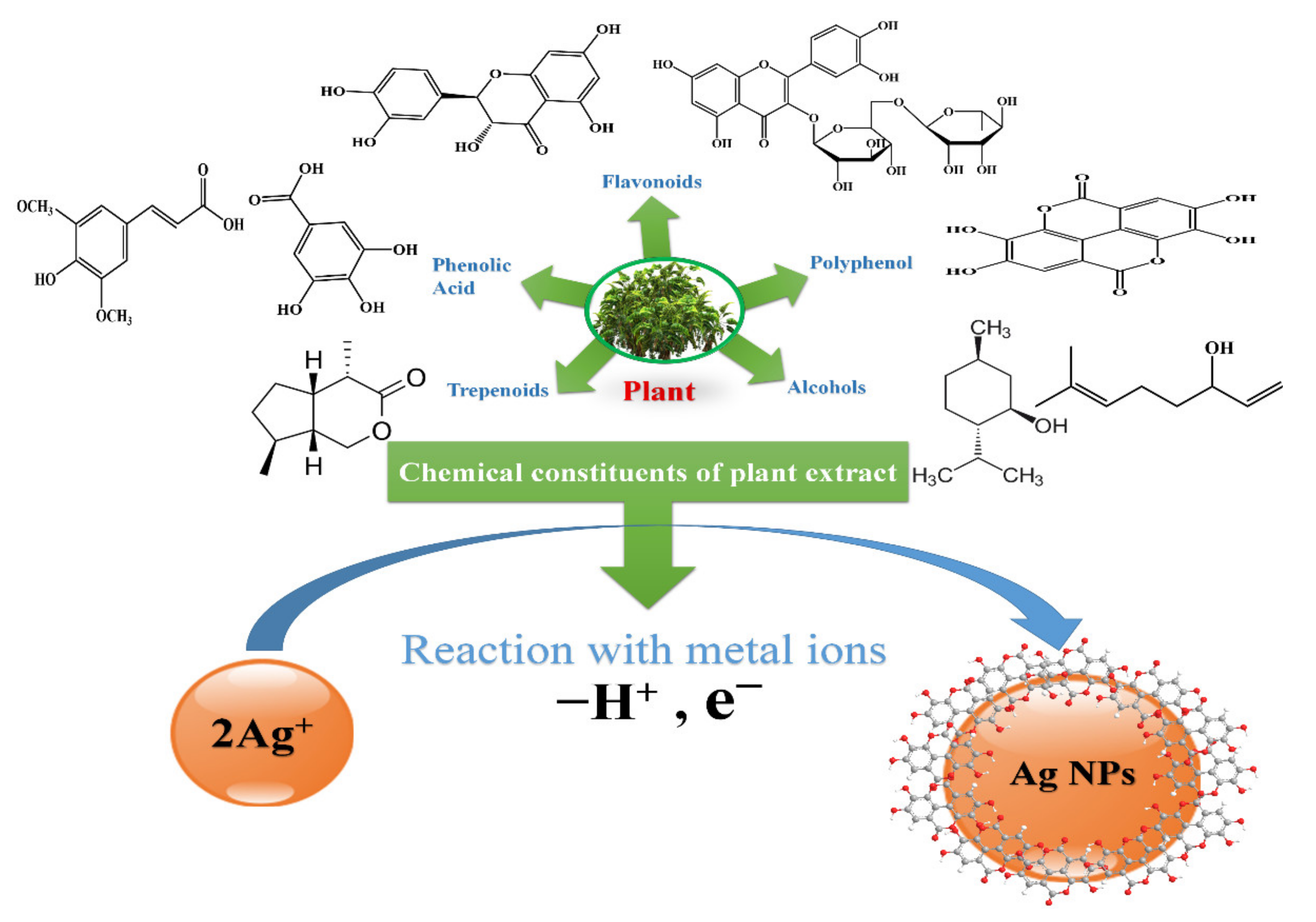
3.2.5. Green Synthesis by Plant Extract
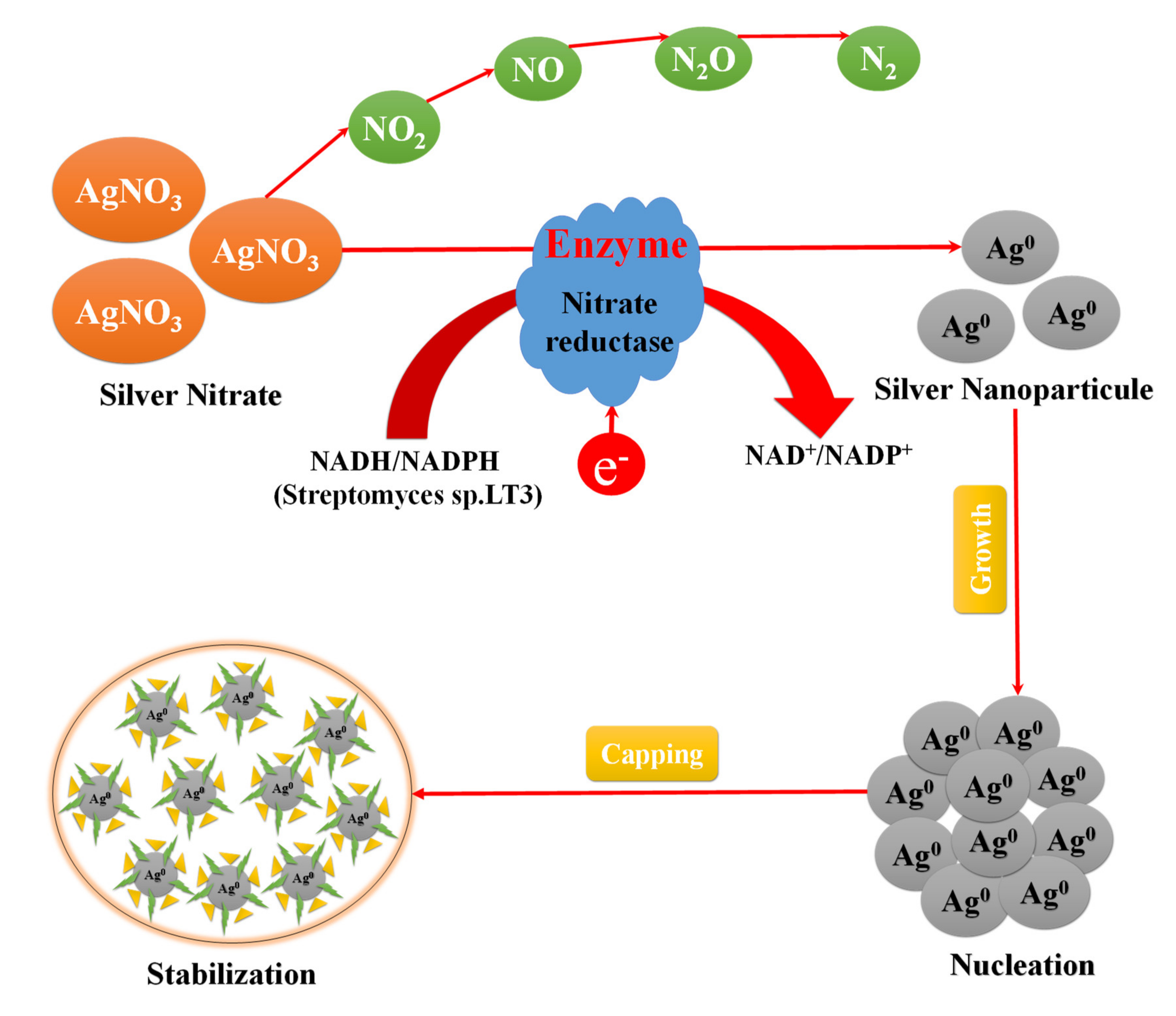
3.2.6. Green Synthesis Using Bacteria
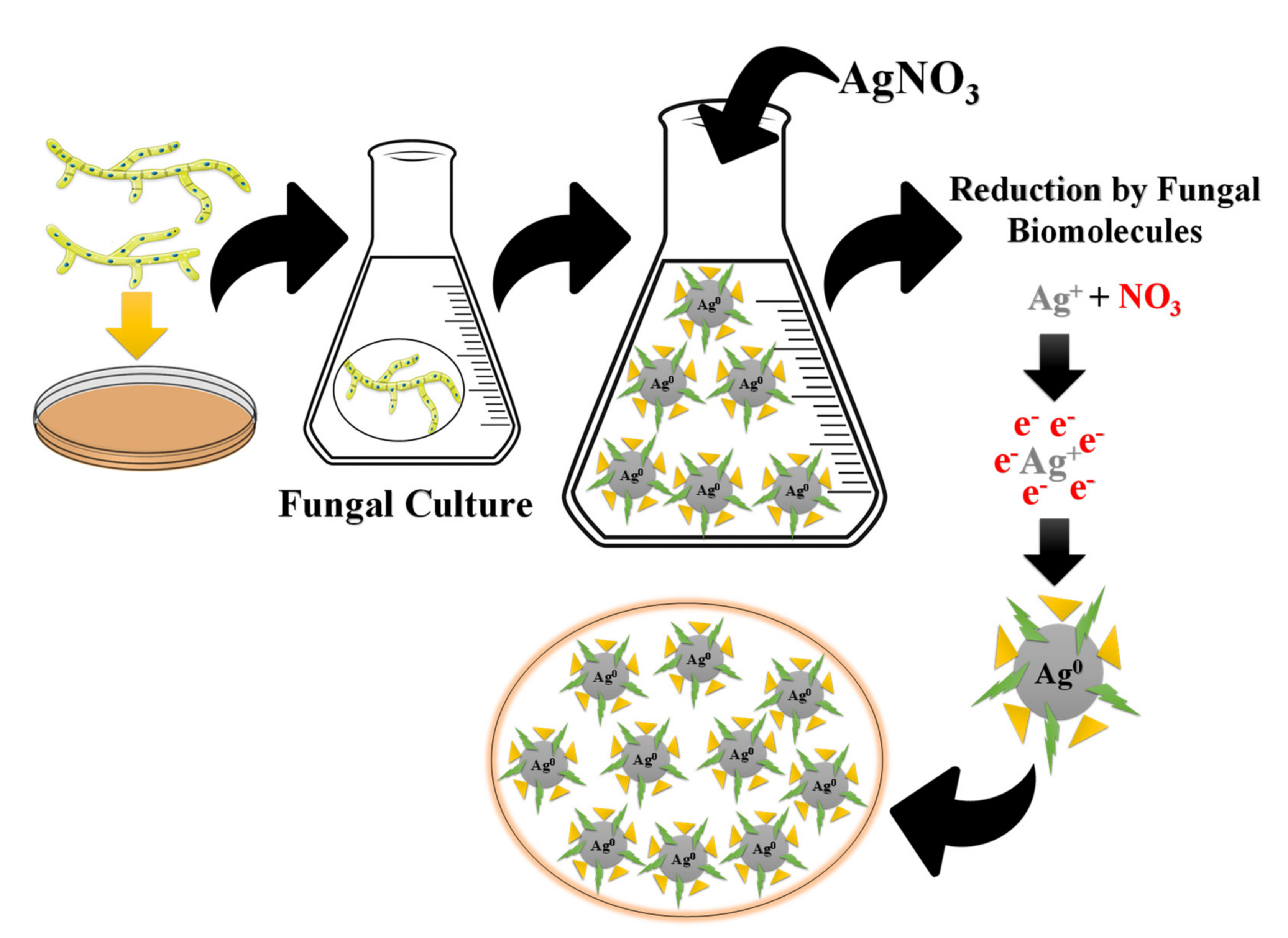
3.2.7. Green Synthesis Using Fungi
3.3. Characterizations of Silver Nanoparticles
3.3.1. Biological Characterizations
3.3.2. Electronic Characterizations
4. Applications of Silver Nanoparticles
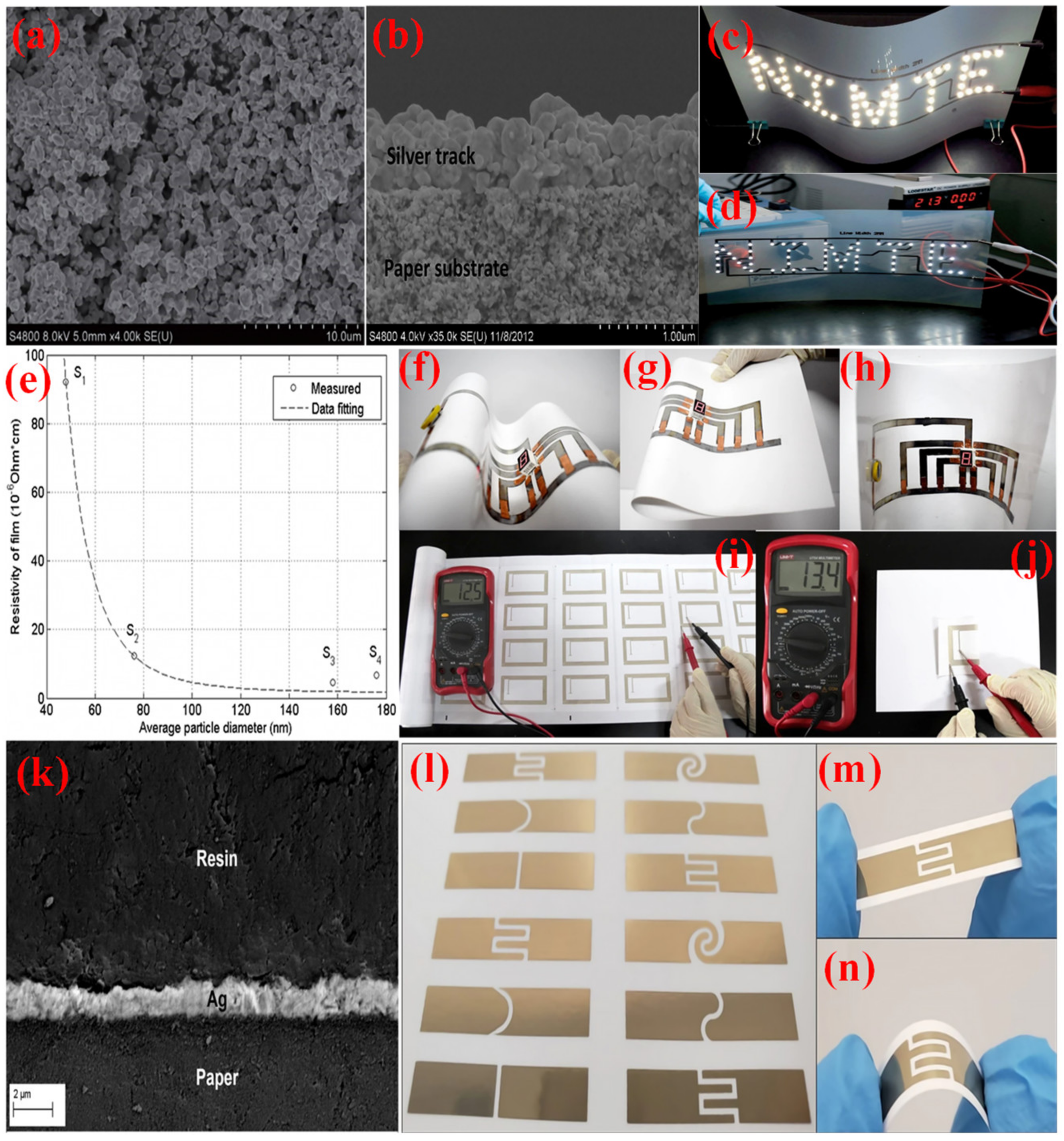
4.1. Electronic Applications
4.2. Sensing Applications
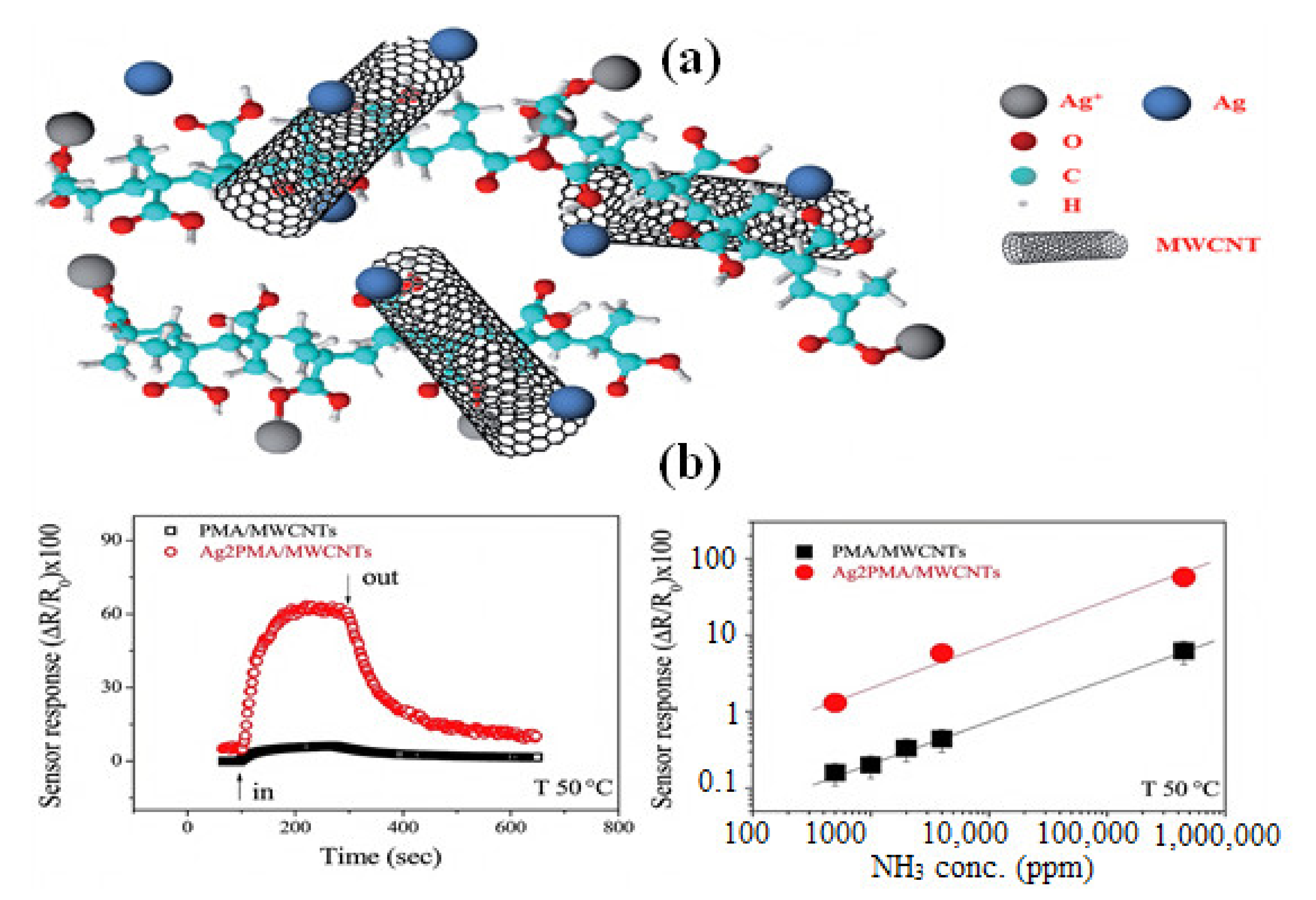
4.2.1. Gas Sensing Applications
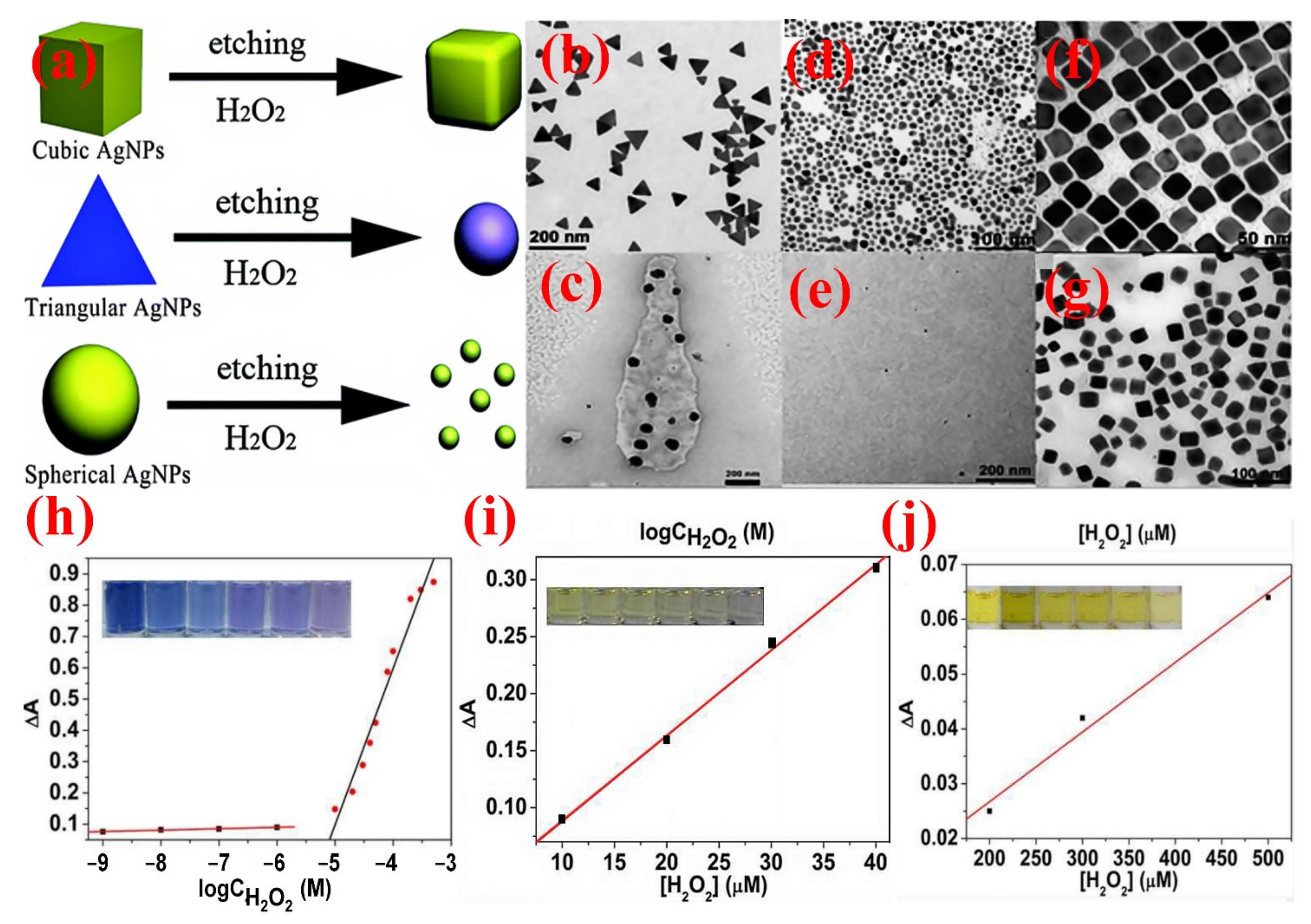
4.2.2. Hydrogen Peroxide Sensing Applications
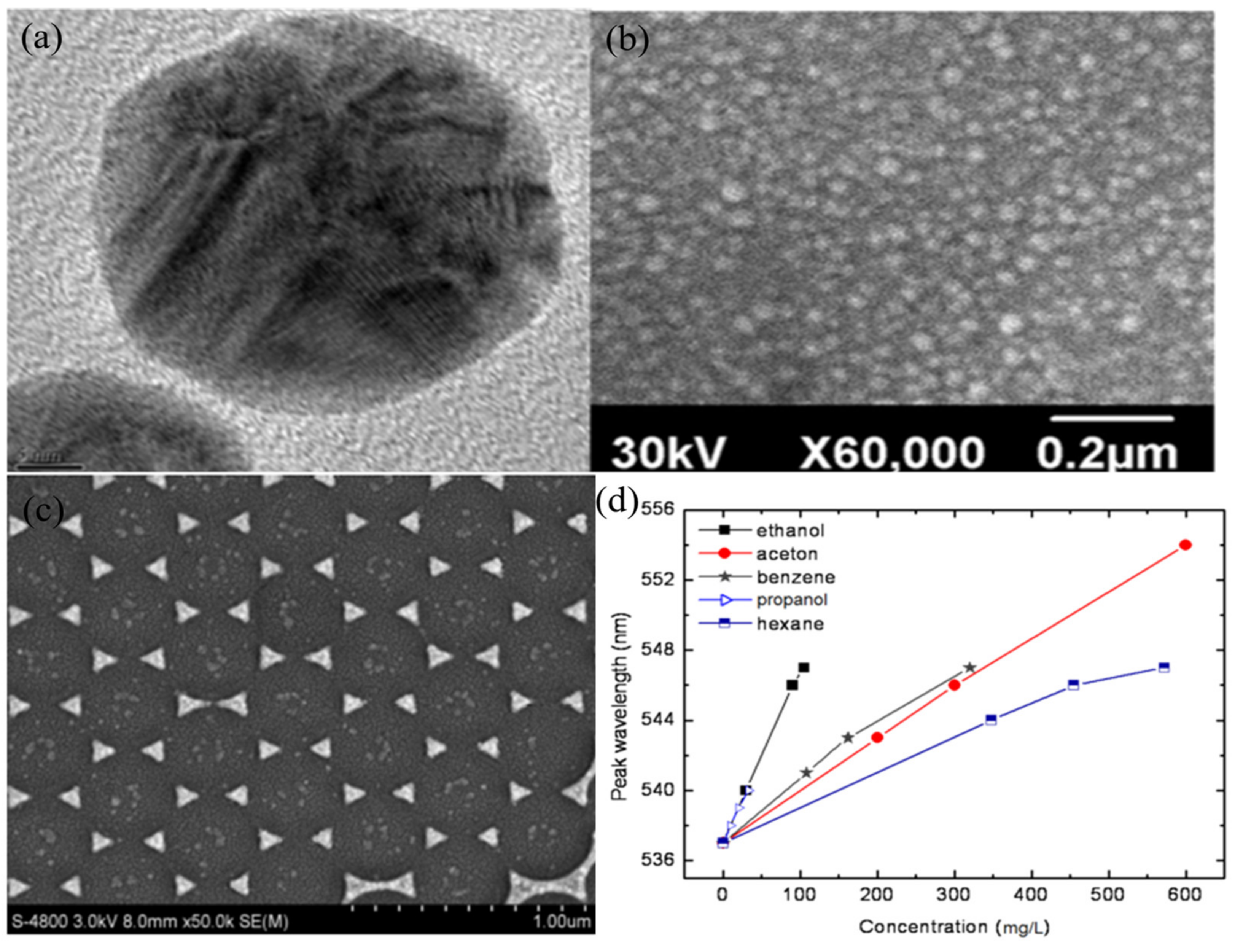
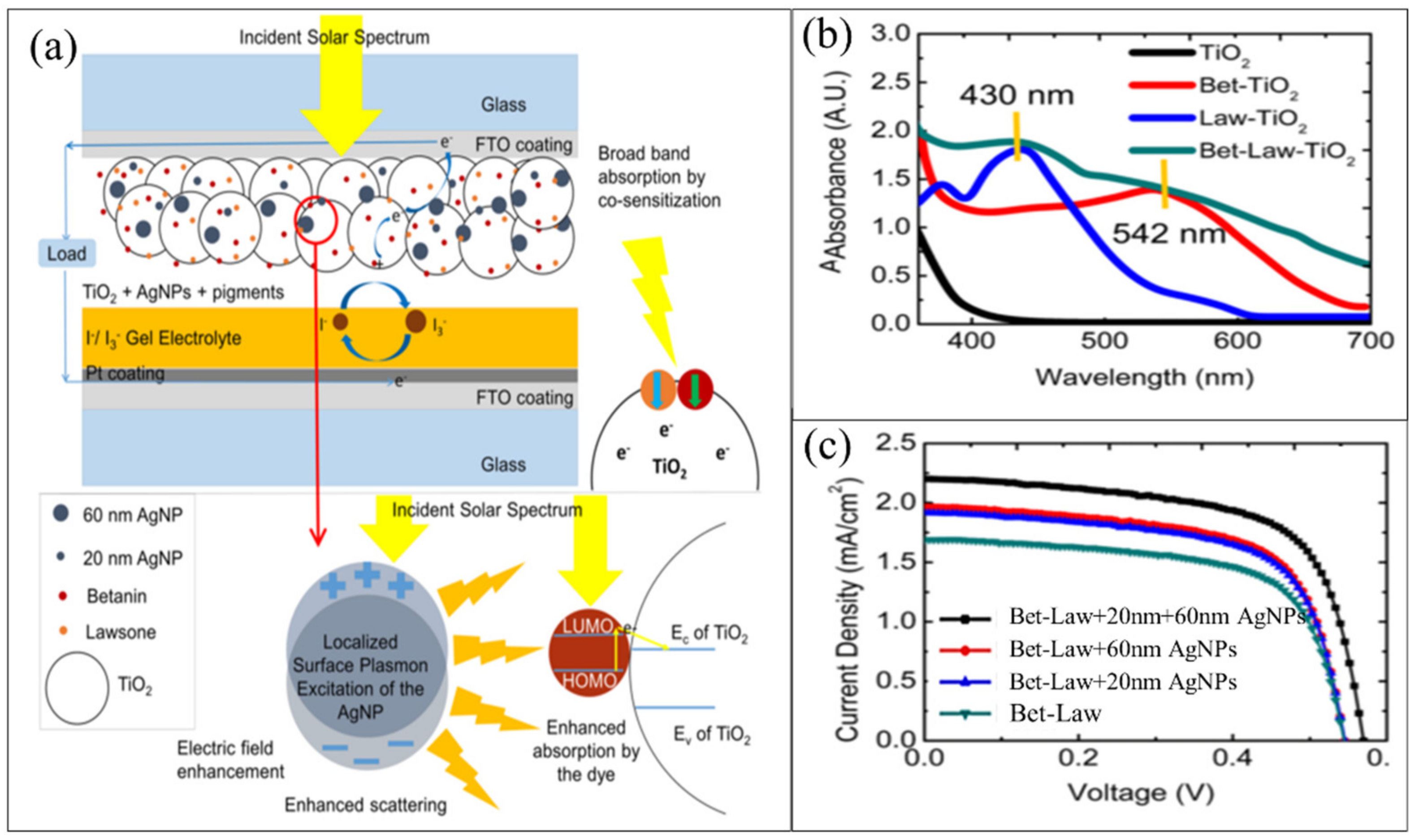
4.3. Solar Cell Applications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayesha, N.; Khanna, T.; Vohora, S. Silver preparations used in Indian systems of medicine: Neuropsychobehavioural effects. Indian J. Pharmacol. 1999, 31, 214. [Google Scholar]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent developments in the medicinal applications of silver-NHC complexes and imidazolium salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh Jalilian, A.C.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Nicole, C.; Mueller, B.N. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 2–10. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Walter, P.; Welcomme, E.; Hallégot, P.; Zaluzec, N.J.; Deeb, C.; Castaing, J.; Veyssière, P.; Bréniaux, R.; Lévêque, J.-L.; Tsoucaris, G. Early Use of PbS Nanotechnology for an Ancient Hair Dyeing Formula. Nano Lett. 2006, 6, 2215–2219. [Google Scholar] [CrossRef]
- Johnson-McDaniel, D.; Barrett, C.A.; Sharafi, A.; Salguero, T.T. Nanoscience of an Ancient Pigment. J. Am. Chem. Soc. 2013, 135, 1677–1679. [Google Scholar] [CrossRef]
- Nabavifard, S.; Jalili, S.; Rahmati, F.; Vasseghian, Y.; Ali, G.A.M.; Agarwal, S.; Gupta, V.K. Application of Dendrimer/Gold Nanoparticles in Cancer Therapy: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4231–4244. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Aboelazm, E.A.A.; Ali, G.A.M.; Chong, K.F. Cobalt oxide supercapacitor electrode recovered from spent lithium-ion battery. Chem. Adv. Mater. 2018, 3, 67–74. [Google Scholar]
- Fouad, O.A.; Makhlouf, S.A.; Ali, G.A.M.; El-Sayed, A.Y. Cobalt/silica nanocomposite via thermal calcination-reduction of gel precursors. Mater. Chem. Phys. 2011, 128, 70–76. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Makhlouf, A.S.H. Fundamentals of Waste Recycling for Nanomaterial Manufacturing. In Waste Recycling Technologies for Nanomaterials Manufacturing; Makhlouf, A.S.H., Ali, G.A.M., Eds.; Springer International Publishing: Cham, Switzerland; Basel, Switzerland, 2021; pp. 3–24. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Delphine Schaming, H.R. Nanotechnology: From the ancient time to nowadays. Found Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Fouad, O.A.; Ali, G.A.M.; El-Erian, M.A.I.; Makhlouf, S.A. Humidity sensing properties of cobalt oxide/silica nanocomposites prepared via sol-gel and related routes. Nano 2012, 7, 1250038. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Optical and Electrochemical Properties of Co3O4/SiO2 Nanocomposite. Adv. Mater. Res. 2016, 1133, 447–451. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Co3O4/SiO2 nanocomposites for supercapacitor application. J. Solid State Electrochem. 2014, 18, 2505–2512. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A. Structural, optical and electrical properties of sol-gel prepared mesoporous Co3O4/SiO2 nanocomposites. J. Alloys Compd. 2013, 579, 606–611. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Livage, J. Sol-gel processes. Curr. Opin. Solid State Mater. Sci. 1997, 2, 132–138. [Google Scholar] [CrossRef]
- Abdel Ghafar, H.H.; Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A. Enhancement of adsorption efficiency of methylene blue on Co3O4/SiO2 nanocomposite. Desalination Water Treat. 2015, 53, 2980–2989. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Rhen, D.S.; Soldatov, A.V.; Ali, G.A.M.; Bakr, Z.H. Efficient and recyclable Cu incorporated TiO2 nanoparticle catalyst for organic dye photodegradation. Int. J. Thin Film. Sci. Technol. 2021, 10, 169–182. [Google Scholar]
- Guglielmi, M.; Carturan, G. Precursors for sol-gel preparations. J. Non-Cryst. Solids 1988, 100, 16–30. [Google Scholar] [CrossRef]
- Yao, Y.; Shu, H.; Tang, B.; Chen, S.; Lu, Z.; Xue, W. Synthesis, Characterization and Application of Some Axially Chiral Binaphthyl Phosphoric Acids in Asymmetric Mannich Reaction. Chin. J. Chem. 2015, 33, 601–609. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Sanmugam, A.; Vikraman, D. Facile methodology of sol-gel synthesis for metal oxide nanostructures. In Recent Applications in Sol-Gel Synthesis; IntechOpen: London, UK, 2017; pp. 1–17. [Google Scholar]
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.M.; Algarni, H.; Chong, K.F. Al3+ ion intercalation pseudocapacitance study of W18O49 nanostructure. J. Power Sources 2019, 438, 227028. [Google Scholar] [CrossRef]
- Tao, X.-Y.; Ma, J.; Hou, R.-L.; Song, X.-Z.; Guo, L.; Zhou, S.-X.; Guo, L.-T.; Liu, Z.-S.; Fan, H.-L.; Zhu, Y.-B. Template-Free Synthesis of Star-Like ZrO2 Nanostructures and Their Application in Photocatalysis. Adv. Mater. Sci. Eng. 2018, 2018, 8191095. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green biosynthesis and physicochemical characterization of Fe3O4 nanoparticles using Punica granatum L. fruit peel extract for optoelectronic applications. Text. Res. J. 2021, 00405175211006671. [Google Scholar] [CrossRef]
- Abderrhmane, B.; Salah Eddine, L. Plant-Mediated Synthesis of Iron Oxide Nanoparticles and Evaluation of the Antimicrobial Activity: A Review. Mini-Rev. Org. Chem. 2021, 18, 725–734. [Google Scholar]
- Bouafia, A.; Laouini, S.E.; Ouahrani, M.R. A review on green synthesis of CuO nanoparticles using plant extract and evaluation of antimicrobial activity. Asian J. Res. Chem. 2020, 13, 65–70. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Khelef, A.; Tedjani, M.L.; Guemari, F. Effect of Ferric Chloride Concentration on the Type of Magnetite (Fe3O4) Nanoparticles Biosynthesized by Aqueous Leaves Extract of Artemisia and Assessment of Their Antioxidant Activities. J. Clust. Sci. 2021, 32, 1033–1041. [Google Scholar] [CrossRef]
- Jung, W.K.; Kim, S.H.; Koo, H.C.; Shin, S.; Kim, J.M.; Park, Y.K.; Hwang, S.Y.; Yang, H.; Park, Y.H. Antifungal activity of the silver ion against contaminated fabric. Mycoses 2007, 50, 265–269. [Google Scholar] [CrossRef]
- Arora, S.; Jain, J.; Rajwade, M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, H.; Wang, W.; Gao, P.; Yao, J. Ethanol vapor sensing properties of triangular silver nanostructures based on localized surface plasmon resonance. Sensors 2011, 11, 8643–8653. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Farré, M.; Gajda-Schrantz, K.; Kantiani, L.; Barceló, D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem. 2009, 393, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Scown, T.M.; Santos, E.M.; Johnston, B.D.; Gaiser, B.; Baalousha, M.; Mitov, S.; Lead, J.R.; Stone, V.; Fernandes, T.F.; Jepson, M.; et al. Effects of Aqueous Exposure to Silver Nanoparticles of Different Sizes in Rainbow Trout. Toxicol. Sci. 2010, 115, 521–534. [Google Scholar] [CrossRef]
- Scopus. Available online: https://www.scopus.com/ (accessed on 20 August 2021).
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Van Hyning, D.L.; Zukoski, C.F. Formation Mechanisms and Aggregation Behavior of Borohydride Reduced Silver Particles. Langmuir 1998, 14, 7034–7046. [Google Scholar] [CrossRef]
- Zielinska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Suzanne, E.; Howson, A.R.; Scott, P.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J. Optically pure, water-stable metallo-helical flexicate’ assemblies with antibiotic activity. Nat. Chem. 2012, 4, 31–36. [Google Scholar]
- Hsu, S.L.-C.; Wu, R.-T. Preparation of Silver Nanoparticle with Different Particle Sizes for Low-Temperature Sintering. Int. Conf. Nanotechnol. Biosens. IPCBEE 2010, 2, 55–58. [Google Scholar]
- Rodríguez-Sánchez, L.; Blanco, M.C.; López-Quintela, M.A. Electrochemical Synthesis of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J Nanoparticle Res 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- Dikovska, A.O.; Alexandrov, M.T.; Atanasova, G.B.; Tsankov, N.T.; Stefanov, P.K. Silver nanoparticles produced by PLD in vacuum: Role of the laser wavelength used. Appl. Phys. A 2013, 113, 83–88. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Mogoantă, L.; Ficai, A.; Andronescu, E.; Grumezescu, A.M. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Tsuji, T.; Iryo, K.; Ohta, H.; Nishimura, Y. Preparation of Metal Colloids by a Laser Ablation Technique in Solution: Influence of Laser Wavelength on the Efficiencies of Colloid Formation. Jpn. J. Appl. Phys. 2000, 39, 981–983. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghettia, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Magnusson, K.D.; Malm, J.; Bovin, J.; Samuelson, L. Gold nanoparticles: Production, reshaping, and thermal charging. J. Nanoparticle Res. 1999, 1, 243–251. [Google Scholar]
- Jung, J.H.; Oh, H.; Noh, H.S.; Ji, J.H.; Kim, S.S. Metal nanoparticle generation using a small ceramic heater with a local heating area. Aerosol Sci. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Tsuji, T.; Kakita, T.; Tsuji, M. Preparation of nano-size particles of silver with femtosecond laser ablation in water. Appl. Surf. Sci. 2003, 206, 314–320. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]
- Elmira Solati, M.M.; Dorranian, D. Effects of laser pulse wavelength and laser fluence on the characteristics of silver nanoparticle generated by laser ablation. Appl Phys. A 2013, 112, 689–694. [Google Scholar] [CrossRef]
- Menazeaa, A.A.; Ahmed, M.K. Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: Preparation, characterization, and photoactivated antibacterial activity. Nano-Struct. Nano-Objects 2020, 22, 100464. [Google Scholar] [CrossRef]
- Ayman, M.; Mostafa, A.A.M. Polyvinyl Alcohol/Silver nanoparticles film prepared via pulsed laser ablation: An eco-friendly nano-catalyst for 4-nitrophenol degradation. J. Mol. Struct. 2020, 1212, 128125. [Google Scholar]
- Wagener, P.; Ibrahimkutty, S.; Menzel, A.; Plech, A.; Barcikowski, S. Dynamics of silver nanoparticle formation and agglomeration inside the cavitation bubble after pulsed laser ablation in liquid. Phys. Chem. Chem. Phys. 2013, 15, 3068–3074. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Ramezani, M. Silver and silver oxide nanoparticles: Synthesis and characterizationby thermal decomposition. Mater. Lett. 2014, 130, 259–262. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mir, N.; Mousavi-Kamazani, M.; Bagheri, S.; Salavati-Niasari, M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016, 6, 32539. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, P.; Srikanth, C.K.; Dixit, S. Synthesis of monodisperse silver nanoparticles and their self-assembly through simple thermal decomposition approach. Mater. Chem. Phys. 2010, 122, 402–407. [Google Scholar] [CrossRef]
- Ji, J.H.; Jung, J.H.; Yu, J.; Kim, S.S. Long-Term Stability Characteristics of Metal Nanoparticle Generator Using Small Ceramic Heater for Inhalation Toxicity Studies. Inhal. Toxicol. 2007, 19, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Merga, G.; Wilson, R.; Lynn, G.; Milosavljevic, B.H.; Meisel, D. Redox Catalysis on “Naked” Silver Nanoparticles. J. Phys. Chem. C 2007, 111, 12220–12226. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J.G. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Du, J.; Wang, S.; Zheng, J.; Yang, Q.; Chen, X. One-pot synthesis of polyacrylamide-gold nanocomposite. Mater. Chem. Phys. 2007, 106, 412–415. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What Factors Control the Size and Shape of Silver Nanoparticles in the Citrate Ion Reduction Method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E. Green synthesis of iron oxide nanoparticles by aqueous leaves extract of Mentha Pulegium, L.: Effect of ferric chloride concentration on the type of product. Mater. Lett. 2020, 265, 127364. [Google Scholar] [CrossRef]
- Gudimalla, A.; Jose, J.; Varghese, R.J.; Thomas, S. Green Synthesis of Silver Nanoparticles Using Nymphae odorata Extract Incorporated Films and Antimicrobial Activity. J. Polym. Environ. 2021, 29, 1412–1423. [Google Scholar] [CrossRef]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, Z.L.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- Kiani, M.; Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Fatahi, Y.; Dinarvand, R.; Webster, T.J. Improved Green Biosynthesis of Chitosan Decorated Ag- and Co3O4-Nanoparticles: A Relationship Between Surface Morphology, Photocatalytic and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102331. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Zheng, Y.; Wang, Z.; Ma, Z.; Yang, Q.; Yao, B.; Zhao, Y.; Zhang, H. A green approach for synthesizing silver nanoparticles, and their antibacterial and cytotoxic activities. New J. Chem. 2018, 42, 2882–2888. [Google Scholar] [CrossRef]
- Laid, T.M.; Abdelhamid, K.; Eddine, L.S.; Bouafia, A. Optimizing the biosynthesis parameters of iron oxide nanoparticles using central composite design. J. Mol. Struct. 2021, 1229, 129497. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Salah Eddine, L.; Bouafia, A.; Alonso-González, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Laouini, S.E.; Bouafia, A.; Soldatov, A.V.; Algarni, H.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green Synthesized of Ag/Ag2O Nanoparticles Using Aqueous Leaves Extracts of Phoenix dactylifera L. and Their Azo Dye Photodegradation. Membranes 2021, 11, 468. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Yadav, S.; Khurana, J.M. Cinnamomum tamala leaf extract-mediated green synthesis of Ag nanoparticles and their use in pyranopyrazles synthesis. Chin. J. Catal. 2015, 36, 1042–1046. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Tarannum, N.; Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926–34948. [Google Scholar] [CrossRef]
- Chand, K.; Cao, D.; Eldin Fouad, D.; Hussain Shah, A.; Qadeer Dayo, A.; Zhu, K.; Nazim Lakhan, M.; Mehdi, G.; Dong, S. Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab. J. Chem. 2020, 13, 8248–8261. [Google Scholar] [CrossRef]
- Maghimaaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111806. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef]
- Jenifer, A.A.; Anjugam, M.; Malaikozhundan, B.; Iswarya, A. Green Synthesis and Characterization of Silver Nanoparticles (AgNPs) Using Leaf Extract of Solanum nigrum and Assessment of Toxicity in Vertebrate and Invertebrate Aquatic Animals. J. Clust. Sci. 2020, 31, 989–1002. [Google Scholar] [CrossRef]
- Ituen, E.; Ekemini, E.; Yuanhua, L.; Singh, A. Green synthesis of Citrus reticulata peels extract silver nanoparticles and characterization of structural, biocide and anticorrosion properties. J. Mol. Struct. 2020, 1207, 127819. [Google Scholar] [CrossRef]
- Kazlagić, A.; Abud, O.A.; Ćibo, M.; Hamidović, S.; Borovac, B.; Omanović-Mikličanin, E. Green synthesis of silver nanoparticles using apple extract and its antimicrobial properties. Health Technol. 2020, 10, 147–150. [Google Scholar] [CrossRef]
- Lakhana, M.N.; Chena, R.; Shara, A.H.; Chanda, K.; Shahb, A.H.; Ahmeda, M.; Alic, I.; Ahmedd, R.; Liua, J.; Takahashia, K.; et al. Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and antidiatom activity. J. Microbiol. Methods 2020, 173, 105934. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, M.A.; Parray, M.u.d.; Mehdi, S.H.; Alzahrani, K.A.; Alshehri, A.A.; Malik, M.A.; Patel, R. Green synthesis of silver nanoparticles from Delonix regia leaf extracts: In-vitro cytotoxicity and interaction studies with bovine serum albumin. Mater. Chem. Phys. 2020, 242, 122493. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Gopinath, V.; Priyadharsshini, N.M.; Ali, D.M.; Velusamy, P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf. B Biointerfaces 2013, 102, 232–237. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, W.; Yasumira, A.A.N. Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. E-J. Chem. 2019, 6, 61–70. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Haefeli, C.; Franklin, C.; Hardy, K. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J. Bacteriol. 1984, 158, 389–392. [Google Scholar] [CrossRef]
- Husseiny, M.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Hietzschold, S.; Walter, A.; Davis, C.; Taylor, A.A.; Sepunaru, L. Does Nitrate Reductase Play a Role in Silver Nanoparticle Synthesis? Evidence for NADPH as the Sole Reducing Agent. ACS Sustain. Chem. Eng. 2019, 7, 8070–8076. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.d. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Lucas, L.J.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Kierans, M.; Staines, A.M.; Bennett, H.; Gadd, G.M. Silver tolerance and accumulation in yeasts. Biol. Met. 1991, 4, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Guo, Z.; Wang, Z.; Yang, L.; Fang, Y.; Xin, Z.; Li, X.; Chen, Y.; Cao, M.; Zhang, Q.; et al. Nano-Silver Ink of High Conductivity and Low Sintering Temperature for Paper Electronics. Nanoscale Res. Lett. 2019, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assuncao, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef]
- Umadevi, M.; Christy, J. Optical, structural and morphological properties of silver nanoparticles and its influence on the photocatalytic activity of TiO2. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 111, 80–85. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Mlozniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, U. A review on applications of nanoparticles for the preconcentration of environmental pollutants. J. Mater. Chem. 2009, 19, 8279. [Google Scholar] [CrossRef]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M.; Elshafei, A.M. Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J. Genet. Eng. Biotechnol. 2019, 17, 1–13. [Google Scholar] [CrossRef]
- Kumar, M.; Devi, P.; Kumar, A. Structural analysis of PVP capped silver nanoparticles synthesized at room temperature for optical, electrical and gas sensing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 5014–5020. [Google Scholar] [CrossRef]
- Liu, T.; Rong, Y.; Xiong, Y.; Mei, A.; Hu, Y.; Sheng, Y.; Jiang, P.; Hou, X.; Duan, M.; Guan, Y.; et al. Spacer improvement for efficient and fully printable mesoscopic perovskite solar cells. RSC Adv. 2017, 7, 10118–10123. [Google Scholar] [CrossRef]
- Kjellander, B.K.C.; Smaal, W.T.T.; Myny, K.; Genoe, J.; Dehaene, W.; Heremans, P.; Gelinck, G.H. Optimized circuit design for flexible 8-bit RFID transponders with active layer of ink-jet printed small molecule semiconductors. Org. Electron. 2013, 14, 768–774. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.B.; Jeon, S.; Chung, D.Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, H.M.; Myllylä, R.A.; Jabbour, G.E. Inkjet printing of light emitting quantum dots. Appl. Phys. Lett. 2009, 94, 073108. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, Y.; Bu, N.; Wang, X.; Xiong, Y. Inkjet printing for flexible electronics: Materials, processes and equipments. Chin. Sci. Bull. 2010, 55, 3383–3407. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Liu, C. Optimization of inkjet printed PEDOT:PSS thin films through annealing processes. Org. Electron. 2012, 13, 1532–1540. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Y.; Liang, J.; Wan, X.; Chen, Y. Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res. 2011, 4, 675–684. [Google Scholar] [CrossRef]
- Mo, L.; Liu, D.; Li, W.; Li, L.; Wang, L.; Zhou, X. Effects of dodecylamine and dodecanethiol on the conductive properties of nano-Ag films. Appl. Surf. Sci. 2011, 257, 5746–5753. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Duoss, E.B.; Motala, M.J.; Guo, X.; Park, S.-I.; Xiong, Y.; Yoon, J.; Nuzzo, R.G.; Rogers, J.A.; Lewis, J.A. Omnidirectional Printing of Flexible, Stretchable, and Spanning Silver Microelectrodes. Science 2009, 323, 1590–1593. [Google Scholar] [CrossRef]
- Jung, I.; Jo, Y.H.; Kim, I.; Lee, H.M. A Simple Process for Synthesis of Ag Nanoparticles and Sintering of Conductive Ink for Use in Printed Electronics. J. Electron. Mater. 2011, 41, 115–121. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Printing Conductive Nanomaterials for Flexible and Stretchable Electronics: A Review of Materials, Processes, and Applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef] [PubMed]
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, 125–149. [Google Scholar] [CrossRef]
- Kala, P.V.; Rao, B.T.; Srinivasarao, K. Structural, optical and gas sensing properties of TiO2-MoO3 thin films. Int. J. Thin Film. Sci. Technol. 2019, 8, 163–174. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Huang, K.T.; Liu, C.Y. Determination of organophosphorous pesticides by a novel biosensor based on localized surface plasmon resonance. Biosens. Bioelectron. 2006, 22, 513–518. [Google Scholar] [CrossRef]
- Bewley, R. FARO: A new type of neutron spectrometer with flux and resolution optimized. Rev. Sci. Instrum. 2019, 90, 075106. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, C.; Han, B.; Wang, E. Enzyme Colorimetric Assay Using Unmodified Silver Nanoparticles. Anal. Chem. 2008, 80, 7051–7055. [Google Scholar] [CrossRef]
- Ghanbari, R.; Safaiee, R.; Sheikhi, M.H.; Golshan, M.M.; Horastani, Z.K. Graphene Decorated with Silver Nanoparticles as a Low-Temperature Methane Gas Sensor. ACS Appl. Mater. Interfaces 2019, 11, 21795–21806. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci Pollut. Res. Int. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Imamura, A.; Yumoto, T. Dynamics of fruit-body production and mycorrhiza formation of ectomycorrhizal ammonia fungi in warm temperate forests in Japan. Mycoscience 2008, 49, 42–55. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Highly sensitive methanol chemical sensor based on undoped silver oxide nanoparticles prepared by a solution method. Microchim. Acta 2012, 178, 99–106. [Google Scholar] [CrossRef]
- Choudhury, A. Polyaniline/silver nanocomposites: Dielectric properties and ethanol vapour sensitivity. Sens. Actuators B Chem. 2009, 138, 318–325. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, F.; Spadaro, D.; Trocino, S.; Neri, G. Development of an ammonia sensor based on silver nanoparticles in a poly-methacrylic acid matrix. J. Mater. Chem. C 2014, 2, 5778. [Google Scholar] [CrossRef]
- Rithesh Raj, D.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Ammonia sensing properties of tapered plastic optical fiber coated with silver nanoparticles/PVP/PVA hybrid. Opt. Commun. 2015, 340, 86–92. [Google Scholar] [CrossRef]
- Banihashemian, S.M.; Hajghassem, H.; Nikfarjam, A.; Azizi Jarmoshti, J.; Abdul Rahman, S.; Boon Tong, G. Room temperature ethanol sensing by green synthesized silver nanoparticle decorated SWCNT. Mater. Res. Express 2019, 6, 065602. [Google Scholar] [CrossRef]
- Martin, B.; Sedelmeier, J.; Bouisseau, A.; Fernandez-Rodriguez, P.; Haber, J.; Kleinbeck, F.; Kamptmann, S.; Susanne, F.; Hoehn, P.; Lanz, M.; et al. Toolbox study for application of hydrogen peroxide as a versatile, safe and industrially-relevant green oxidant in continuous flow mode. Green Chem. 2017, 19, 1439–1448. [Google Scholar] [CrossRef]
- Teong, S.P.; Li, X.; Zhang, Y. Hydrogen peroxide as an oxidant in biomass-to-chemical processes of industrial interest. Green Chem. 2019, 21, 5753–5780. [Google Scholar] [CrossRef]
- Kamrani, M.S.; Seifpanahi-Shabani, K.; Seyed-Hakimi, A.; Ali, G.A.M.; Agarwa, S.; Gupta, V.K. Degradation of cyanide from gold processing effluent by H2O2, NaClO and Ca(ClO)2 combined with sequential catalytic process. Bulg. Chem. Commun. 2019, 51, 384–393. [Google Scholar] [CrossRef]
- Ryoo, D.; Xu, X.; Li, Y.; Tang, J.A.; Zhang, J.; van Zijl, P.C.M.; Liu, G. Detection and Quantification of Hydrogen Peroxide in Aqueous Solutions Using Chemical Exchange Saturation Transfer. Anal. Chem. 2017, 89, 7758–7764. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, X. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuators B Chem. 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Karimi, A.; Husain, S.W.; Hosseini, M.; Azar, P.A.; Ganjali, M.R. Rapid and sensitive detection of hydrogen peroxide in milk by Enzyme-free electrochemiluminescence sensor based on a polypyrrole-cerium oxide nanocomposite. Sens. Actuators B Chem. 2018, 271, 90–96. [Google Scholar] [CrossRef]
- Lee, J.H.; Huynh-Nguyen, B.-C.; Ko, E.; Kim, J.H.; Seong, G.H. Fabrication of flexible, transparent silver nanowire electrodes for amperometric detection of hydrogen peroxide. Sens. Actuators B Chem. 2016, 224, 789–797. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hieda, K.; Ikeda, K.; Tamiya, E. Hydrogen peroxide detection with a silver nanoparticle grating chip fabricated by plasmonic plating. Anal. Methods 2019, 11, 2991–2995. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L. Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal. Methods 2016, 8, 6691–6695. [Google Scholar] [CrossRef]
- Srikhao, N.; Kasemsiri, P.; Lorwanishpaisarn, N.; Okhawilai, M. Green synthesis of silver nanoparticles using sugarcane leaves extract for colorimetric detection of ammonia and hydrogen peroxide. Res. Chem. Intermed. 2021, 47, 1269–1283. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, C.; Shi, H.; Li, C.; Wang, L.; Huang, W.; Dong, X. A hydrogen peroxide electrochemical sensor based on silver nanoparticles decorated three-dimensional graphene. Appl. Phys. Lett. 2014, 104, 243704. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Kundu, M.; Sasidharan, M. Electrochemical detection of hydrogen peroxide based on silver nanoparticles via amplified electron transfer process. J. Mater. Sci. 2018, 53, 8328–8338. [Google Scholar] [CrossRef]
- Sangno, R.; Maity, S.; Mehta, R.K. Plasmonic Effect Due to Silver Nanoparticles on Silicon Solar Cell. Procedia Comput. Sci. 2016, 92, 549–553. [Google Scholar] [CrossRef][Green Version]
- Bonsak, J.; Mayandi, J.; Thøgersen, A.; Stensrud Marstein, E.; Mahalingam, U. Chemical synthesis of silver nanoparticles for solar cell applications. Phys. Status Solidi 2011, 8, 924–927. [Google Scholar] [CrossRef]
- Dzhafarov, T.D.; Pashaev, A.M.; Tagiev, B.G.; Aslanov, S.S.; Ragimov, S.H.; Aliev, A.A. Influence of silver nanoparticles on the photovoltaic parameters of silicon solar cells. Adv. Nano Res. 2015, 3, 133–141. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Xiang, W.; Gu, A.; Hu, X.; Saana, I.A.; Zhao, X. Carbon black/silicon nitride nanocomposites as high-efficiency counter electrodes for dye-sensitized solar cells. New J. Chem. 2018, 42, 11715–11723. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Xiang, W.; Li, Z.; Amiinu, I.S.; Zhao, X. Yolk-shell m-SiO2@ Nitrogen doped carbon derived zeolitic imidazolate framework high efficient counter electrode for dye-sensitized solar cells. Electrochim. Acta 2018, 292, 276–284. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Xiang, W.; Amiinu, I.S.; Li, Z.; Yu, R.; Zhao, X. ZnO-nitrogen doped carbon derived from a zeolitic imidazolate framework as an efficient counter electrode in dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1976–1987. [Google Scholar] [CrossRef]
- Ahmed, A.S.A.; Xiang, W.; Saana Amiinu, I.; Zhao, X. Zeolitic-imidazolate-framework (ZIF-8)/PEDOT:PSS composite counter electrode for low cost and efficient dye-sensitized solar cells. New J. Chem. 2018. [Google Scholar] [CrossRef]
- Ahmad, S.; Yum, J.H.; Butt, H.J.; Nazeeruddin, M.K.; Gratzel, M. Efficient platinum-free counter electrodes for dye-sensitized solar cell applications. ChemPhysChem 2010, 11, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Q.; He, B.; Chen, H. Graphene-incorporated quasi-solid-state dye-sensitized solar cells. RSC Adv. 2015, 5, 43402–43407. [Google Scholar] [CrossRef]
- Photiphitak, C.; Rakkwamsuk, P.; Muthitamongkol, P.; Sae-Kung, C.; Thanachayanont, C. Effect of Silver Nanoparticle Size on Efficiency Enhancement of Dye-Sensitized Solar Cells. Int. J. Photoenergy 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Kislov, D.A. Effect of Plasmonic Silver Nanoparticles on the Photovoltaic Properties of Graetzel Solar Cells. Phys. Procedia 2015, 73, 114–120. [Google Scholar] [CrossRef]
- Saadmim, F.; Forhad, T.; Sikder, A.; Ghann, W.; Ali, M.M.; Sitther, V.; Ahammad, A.J.S.; Subhan, M.A.; Uddin, J. Enhancing the Performance of Dye Sensitized Solar Cells Using Silver Nanoparticles Modified Photoanode. Molecules 2020, 25, 4021. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Pesala, B. Plasmonic enhancement of betanin-lawsone co-sensitized solar cells via tailored bimodal size distribution of silver nanoparticles. Sci. Rep. 2020, 10, 8240. [Google Scholar] [CrossRef] [PubMed]
| Physical Method | Applications | Shape | Size (nm) | Reference |
|---|---|---|---|---|
| Laser ablation | Antibacterial efficiency | Semi-spherical | 14 | [62] |
| Spherical | 13–32 | [63] | ||
| Antibacterial activity | 5–30 | [64] | ||
| Catalytic degradation activity | Spherical-like | 17 | [65] | |
| Spherical | 8–10 | [66] | ||
| Thermal decomposition | 40–50 | [67] | ||
| 5–15 | [68] | |||
| Cubic/hexagonal | 3.0–4.5 | [69] | ||
| Small Ceramic Heater | Inhalation toxicity studies | Spherical | 14 | [70] |
| Source | Bioreducing Agent of Silver Nitrate | The Mechanism for the Synthesis |
|---|---|---|
| Plants | Alkaloids, Terpenes, Steroids and Saponins, Flavonoids and Tannins, Alcohol, Phenolic Acids. | Electrostatic interaction between the functional groups of a respective constituent of plant extract and Ag+ |
| Bacteria | Bacillus Cereus, Bacillus licheniformis, Staphylococcus aureus, Enterobacteriaceae, Pantoeaananatis, Proteus mirabilis | In the extra-cellular synthesis of Ag NPs by Bacillus subtilis, the synthesis of Ag NPs was observed in the reaction mixture after 6 h contact time at room temperature. |
| Fungi | Proteins, peptides enzymes, napthoquinones, NADH, NADPH, peptides, nitrogenous biomacromolecules, | Intracellular and extracellular synthesis of Ag NPs |
| Biopolymers | Cellulose, Chitosan, polypeptides, alginate, lignin, protein | Electrostatic interaction between Ag+ ion and polar groups attached to the polymer |
| Plant Sources | Part | Applications | Operating Conditions | Size/Shape | Reference |
|---|---|---|---|---|---|
| Onion, tomato, Acacia catechu | Pieces | Photocatalytic application | 16.987 g of AgNO3 in 1 L of distilled water, 10 mL extract, stirred for 10 min. and kept for 24 h at room temperature | 32.1, 22.6, 14.5 nm, Spherical | [91] |
| Curcuma longa L. | Leaf | Antibacterial activity | 10 mL of Curcuma longa L. leaf extrac, 90 mL of 1 mM AgNO3. | 15–40 nm, spherical | [92] |
| Phaseolus vulgaris L. | Seeds | Photocatalytic activity, antimicrobial activity | Extract (1, 2, 3, and 4 mL), AgNO3 (0.01 M, 50 mL), (300 rpm) for 30 min | 10–20 nm, spherical | [93] |
| Solanum nigrum L. | Leaves | Ecotoxicity Studies | 1 mL leaf extract, AgNO3 (10−3 M, 50 mL), stirred continuously at room temperature | 10–50 nm, spherical | [94] |
| f Citrus reticulata | Peels | Biocide and anticorrosion properties | 1 mM AgNO3, ratio 1:1 with tangerine peels extract | 39.6–56.1 nm, round | [95] |
| Apple | Fruits | Antibacterial activity | AgNO3 (1 mM, 90 mL), 10 mL of extract, at 70 °C. | 45–110 nm, spherical shape | [96] |
| Clove | Buds | Antibacterial and antidiatom activity | AgNO3 (1 mM, 400 mL), 80 mL of extract, darkness for 24 h | 9.42 nm | [97] |
| Delonix regia | Leaf | In vitro cytotoxicity and interaction studies with bovine serum albumin | Leaf extract and an aqueous solution of 1 mM AgNO3 (20:80, V/V). | 72.77 nm, non-uniform | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. https://doi.org/10.3390/nano11092318
Bouafia A, Laouini SE, Ahmed ASA, Soldatov AV, Algarni H, Feng Chong K, Ali GAM. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials. 2021; 11(9):2318. https://doi.org/10.3390/nano11092318
Chicago/Turabian StyleBouafia, Abderrhmane, Salah Eddine Laouini, Abdelaal S. A. Ahmed, Alexander V. Soldatov, Hamed Algarni, Kwok Feng Chong, and Gomaa A. M. Ali. 2021. "The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications" Nanomaterials 11, no. 9: 2318. https://doi.org/10.3390/nano11092318
APA StyleBouafia, A., Laouini, S. E., Ahmed, A. S. A., Soldatov, A. V., Algarni, H., Feng Chong, K., & Ali, G. A. M. (2021). The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials, 11(9), 2318. https://doi.org/10.3390/nano11092318










