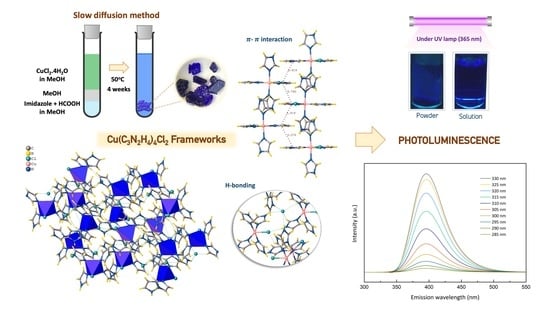
A Single Crystal Hybrid Ligand Framework of Copper(II) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedure of Copper Coordinate Complexes
2.3. Characterizations
2.3.1. Single-Crystal X-ray Crystallography (SC-XRD)
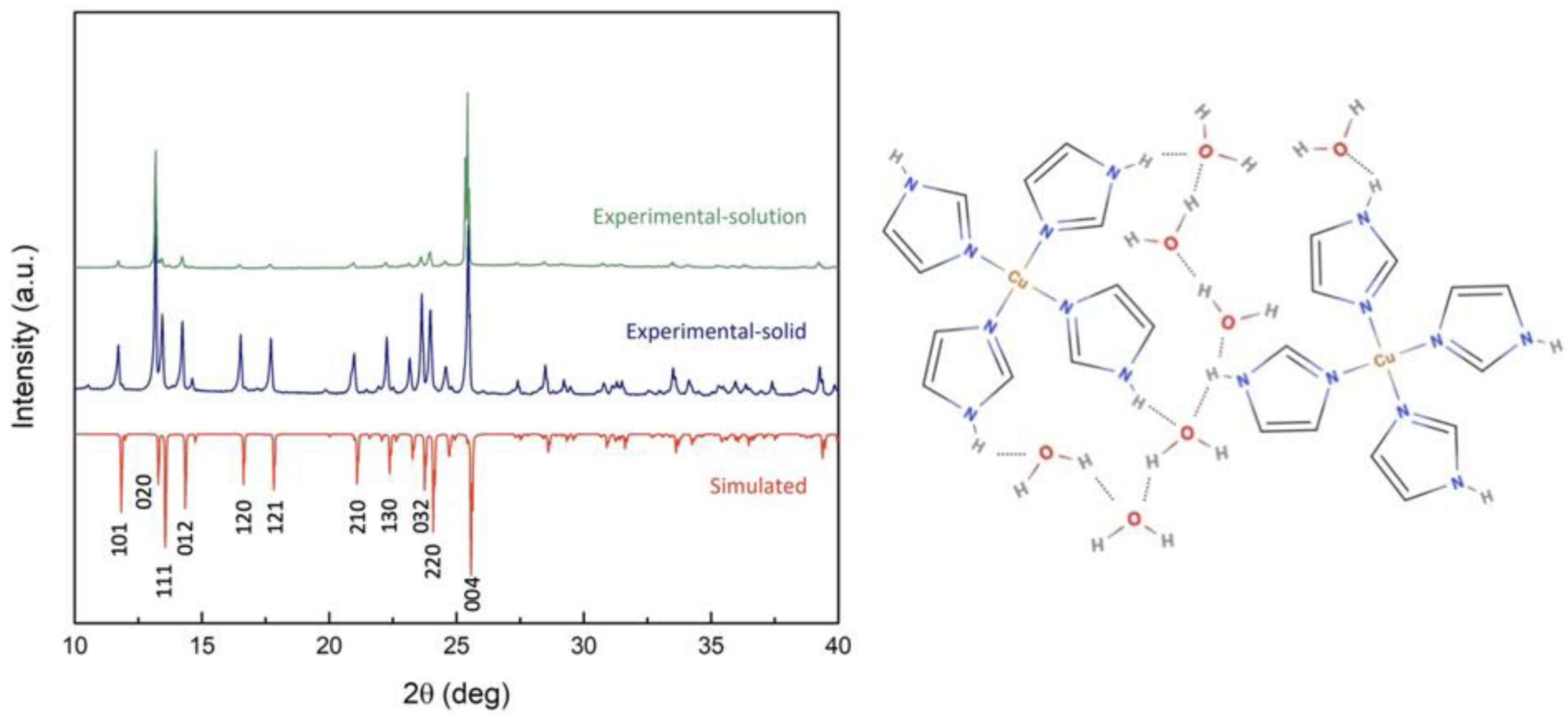
2.3.2. X-ray Diffraction (XRD)
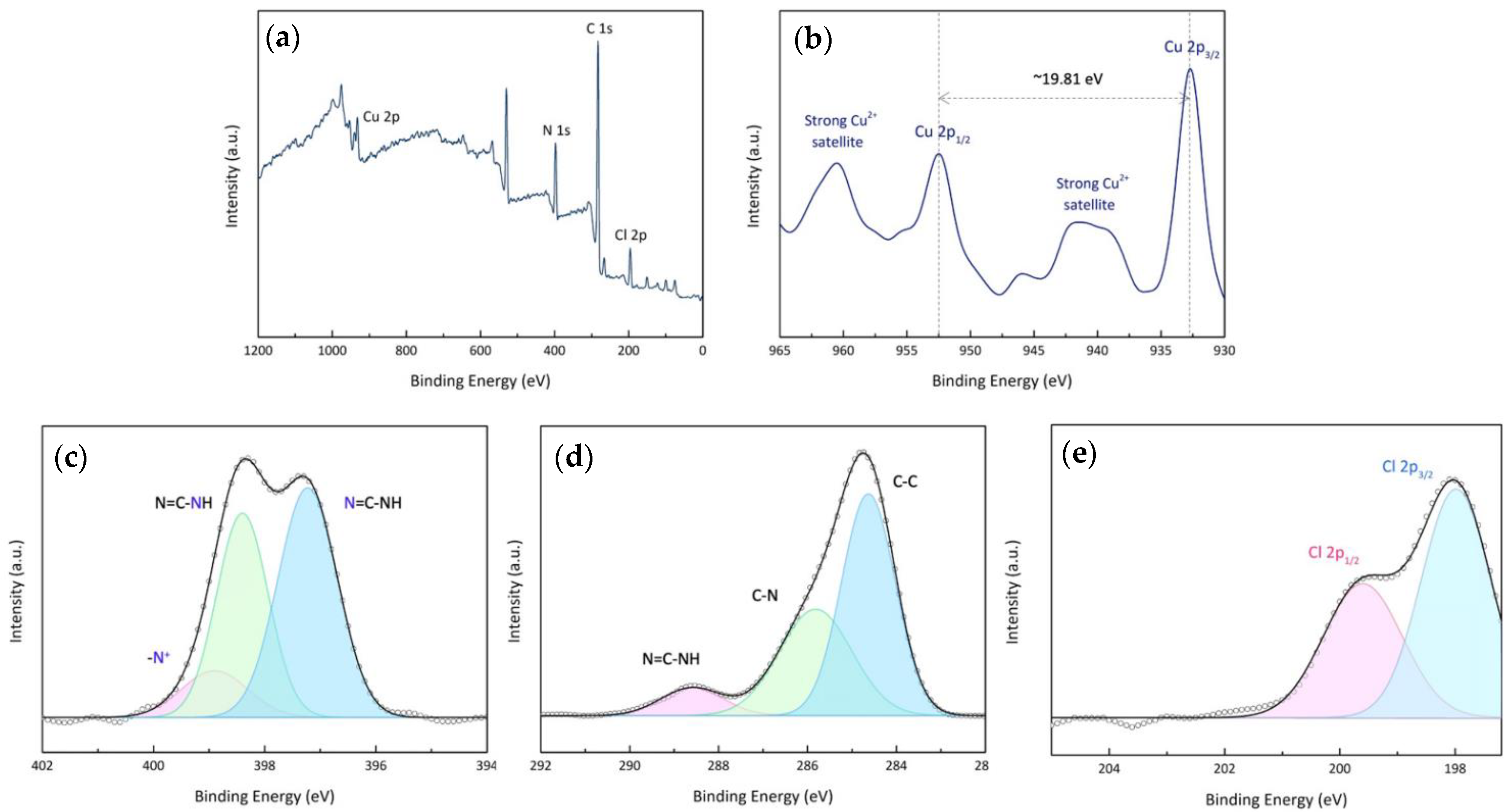
2.3.3. X-ray Photoelectron Spectroscopy
2.3.4. Element Analysis
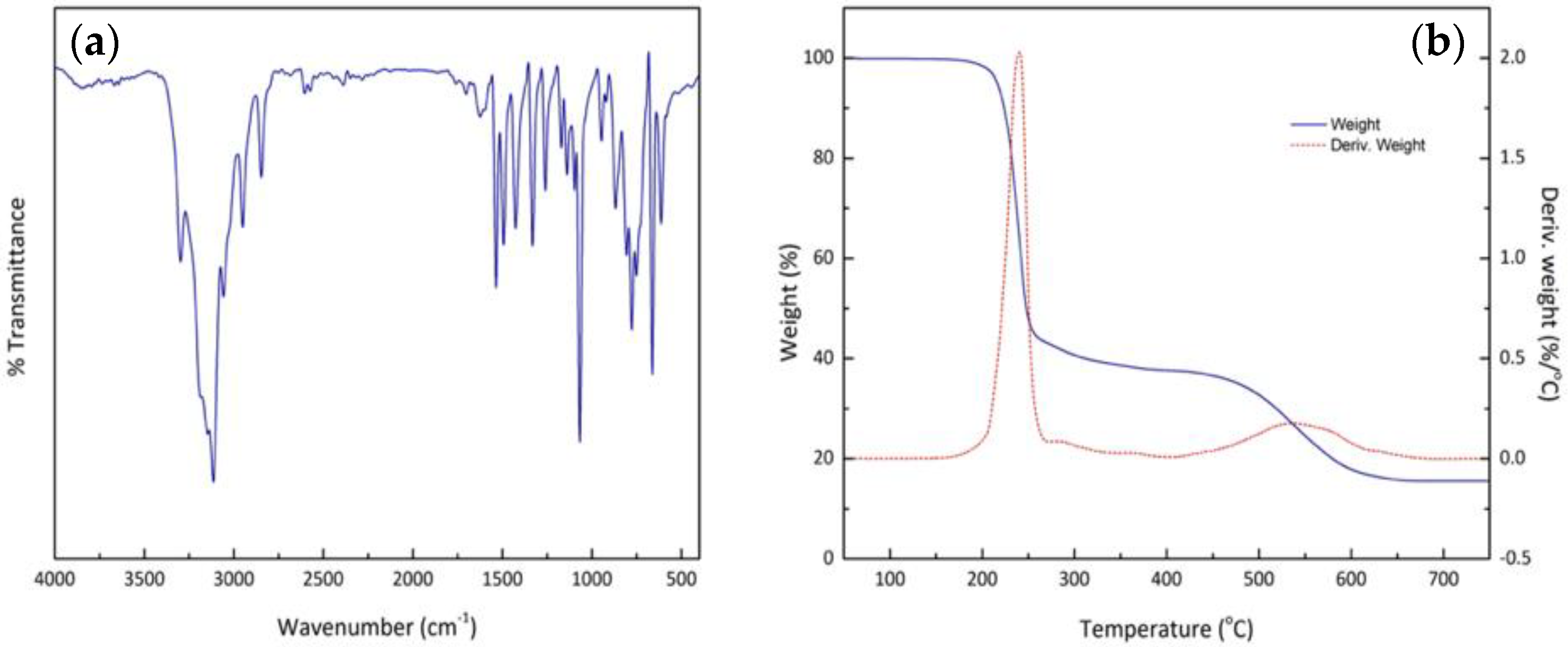
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.6. Thermal Analysis
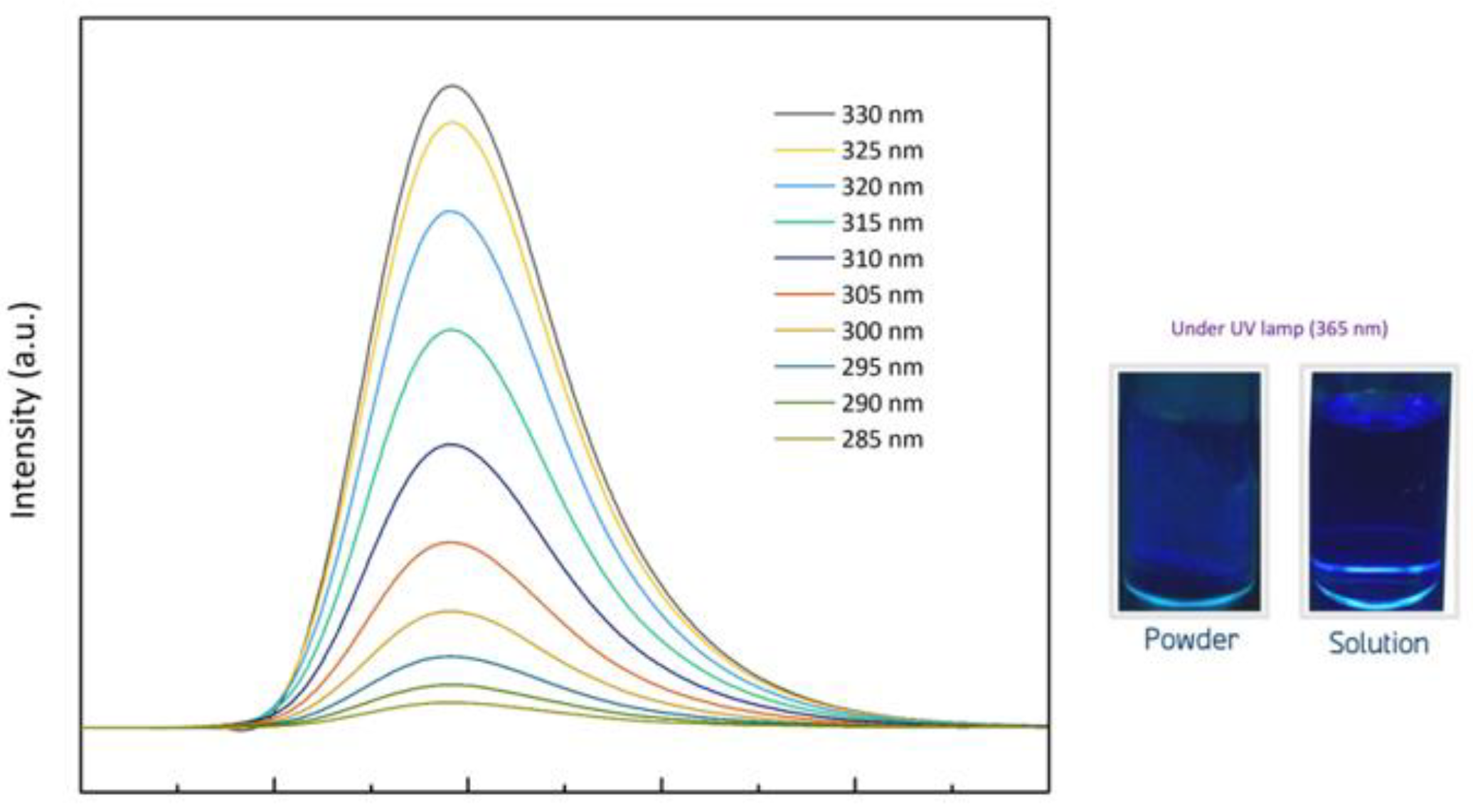
2.3.7. UV–Vis and Fluorescent Spectrophotometer
3. Results and Discussion
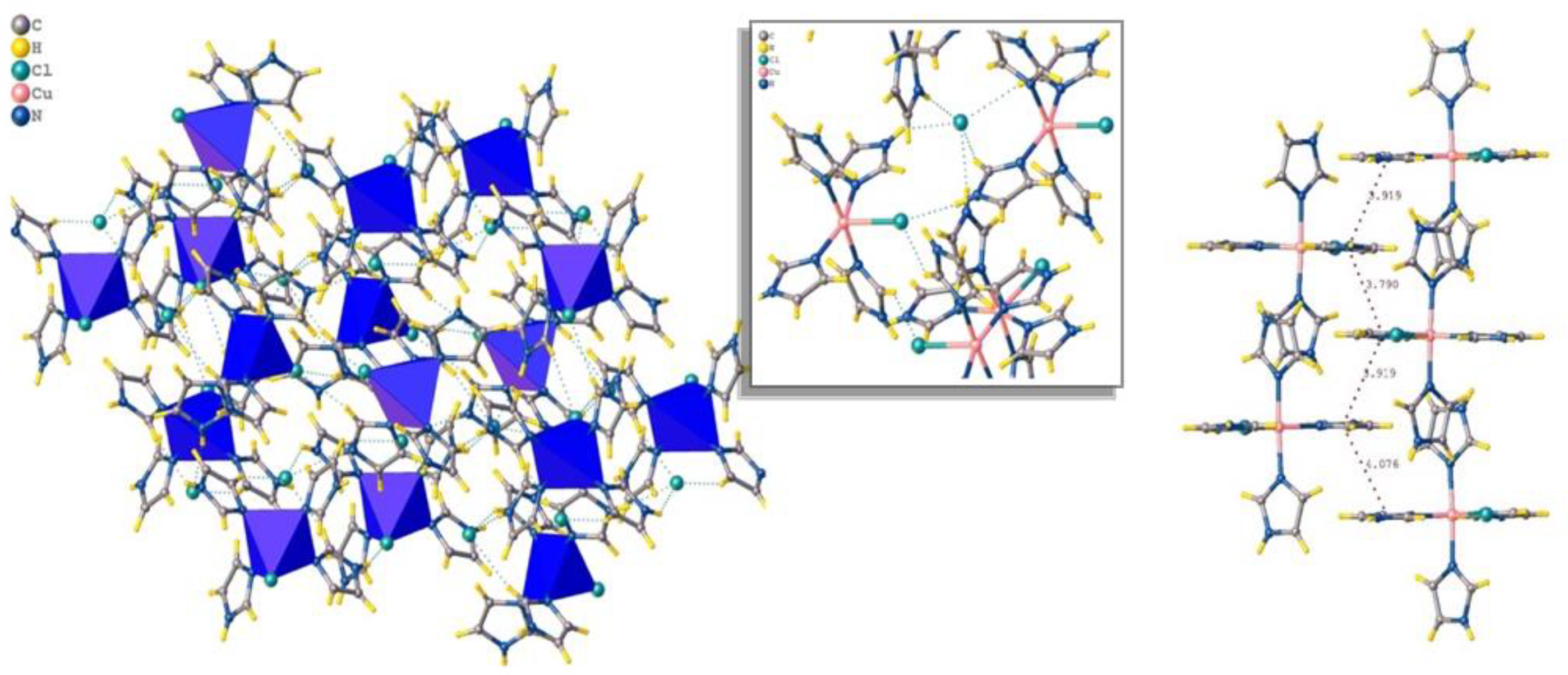
3.1. Molecular Structure and Crystallography Data of [Cu(II)(C3N2H4)4Cl]Cl, or [Cu(II)Im4Cl]Cl, Coordination Framework
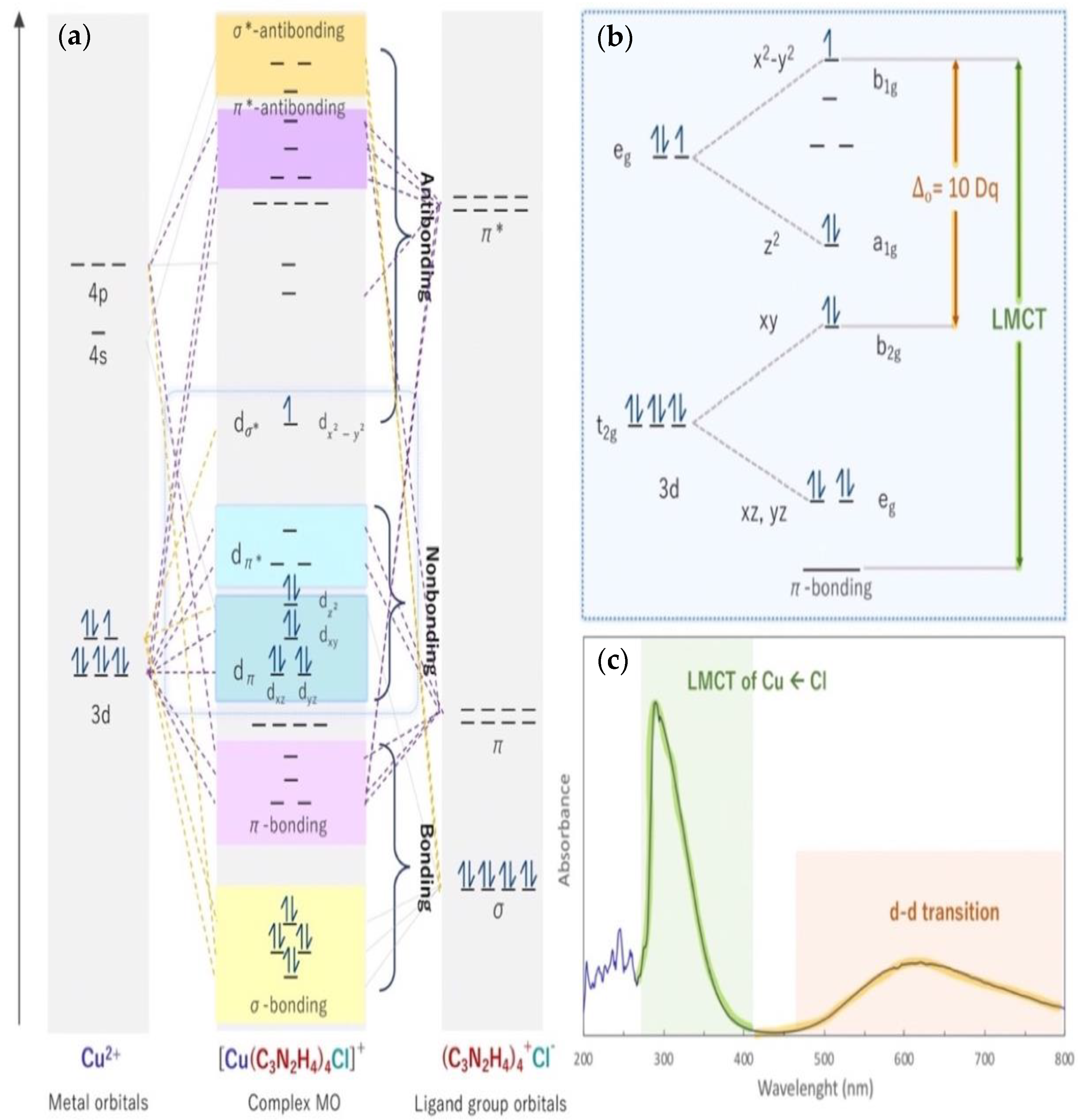
3.2. Electronic Structure of [Cu(II)(C3N2H4)4Cl]Cl Coordination Complexes
3.3. Valent State and Binding Energy of Copper Coordination Complexes
3.4. FTIR, Element Analysis and TGA Thermogram
3.5. Fluorescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascanu, V.; Miera, G.G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, R.T. Gas Adsorption and Storage in Metal−Organic Framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2019, 7, 300–339. [Google Scholar] [CrossRef]
- Downes, C.; Marinescu, S.C. Electrocatalytic Metal–Organic Frameworks for Energy Applications. ChemSusChem 2017, 10, 4374–4392. [Google Scholar] [CrossRef] [Green Version]
- Nadar, S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, W.; Cong, J.; Cheng, M.; Zhang, B.; Chen, H.; Liu, P.; Li, R.; Safdari, M.; Kloo, L.; et al. Cu(II) Complexes as p-Type Dopants in Efficient Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 497–503. [Google Scholar] [CrossRef]
- Shu, Y.; Hao, J.-N.; Niu, D.; Li, Y. A smart luminescent metal–organic framework-based logic system for simultaneous analysis of copper ions and hydrogen sulfide. J. Mater. Chem. C 2020, 8, 8635–8642. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Persson, I.; Penner-Hahn, J.; Hodgson, K.O. An EXAFS spectroscopic study of solvates of copper(I) and copper(II) in acetonitrile, dimethyl sulfoxide, pyridine, and tetrahydrothiophene solutions and a large-angle x-ray scattering study of the copper(II) acetonitrile solvate in solution. Inorg. Chem. 1993, 32, 2497–2501. [Google Scholar] [CrossRef]
- Pasquarello, A.; Petri, I.; Salmon, P.S.; Parisel, O.; Car, R.; Tóth, E.; Powell, D.H.; Fischer, H.E.; Helm, L.; Merbach, A.E. First Solvation Shell of the Cu(II) Aqua Ion: Evidence for Fivefold Coordination. Science 2001, 291, 856–859. [Google Scholar] [CrossRef]
- Frank, P.; Benfatto, M.; Hedman, B.; Hodgson, K.O. The X-ray Absorption Spectroscopic Model of the Copper(II) Imidazole Complex Ion in Liquid Aqueous Solution: A Strongly Solvated Square Pyramid. Inorg. Chem. 2012, 51, 2086–2096. [Google Scholar] [CrossRef] [Green Version]
- Sadaoui-Kacel, S.; Zaater, S.; Bensouilah, N.; Djebbar, S. Novel repaglinide complexes with manganese(II), iron(III), copper(II) and zinc(II): Spectroscopic, DFT characterization and electrochemical behaviour. J. Struct. Chem. 2016, 57, 1519–1530. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states-I—Orbital degeneracy. Proc. R. Soc. Lond. 1937, 161, 220–235. [Google Scholar]
- Reinen, D.; Atanasov, M.; Köhler, P.; Babel, D. Jahn–Teller coupling and the influence of strain in Tg and Eg ground and excited states—A ligand field and DFT study on halide MIIIX6 model complexes [M = TiIII − CuIII; X = F−, Cl−]. Coord. Chem. Rev. 2010, 254, 2703–2754. [Google Scholar] [CrossRef]
- Bin Li, T.; Hu, Y.L.; Li, J.K.; He, G.F. Chloridotetrakis (imidazole) copper (II) chloride. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m2536. [Google Scholar]
- Jian, F.F.; Tong, Y.P.; Xiao, L.H.; Wang, Q.X.; Jiao, K. Structure and thermal properties of transition metal imidazole chloride. Chin. J. Struct. Chem. 2004, 23, 979–984. [Google Scholar]
- Pato-Doldán, B.; Aguirre, L.C.G.; Bermúdez-García, J.M.; Sánchez-Andújar, M.; Fondado, A.F.; Mira, J.; Castro-García, S.; Señarís-Rodríguez, M.A. Coexistence of magnetic and electrical order in the new perovskite-like (C3N2H5)[Mn(HCOO)3] formate. RSC Adv. 2013, 3, 22404–22411. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Otieno, T.; Hatfield, M.J.; Asher, S.L.; McMullin, A.I.; Patrick, B.O.; Parkin, S. Structures Of Pentakis-(Imidazole)Copper(II) Hexafluoroarsenate Monohydrate And Chlorotetrakis-(Imidazole)Copper(II) Chloride. Synth. React. Inorg. Met. Chem. 2001, 31, 1587–1598. [Google Scholar] [CrossRef]
- Halcrow, M.A. Jahn–Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem. Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef] [Green Version]
- House, J.E. Chapter 17—Ligand fields and molecular orbitals. In Inorganic Chemistry, 3rd ed.; House, J.E., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 687–715. [Google Scholar]
- Olshin, P.; Myasnikova, O.S.; Kashina, M.; Gorbunov, A.O.; Bogachev, N.; Kompanets, V.O.; Chekalin, S.V.; Pulkin, S.A.; Kochemirovsky, V.A.; Skripkin, M.Y.; et al. The electronic spectra and the structures of the individual copper(II) chloride and bromide complexes in acetonitrile according to steady-state absorption spectroscopy and DFT/TD-DFT calculations. Chem. Phys. 2018, 503, 14–19. [Google Scholar] [CrossRef]
- Cheruzel, L.E.; Cecil, M.R.; Edison, S.E.; Mashuta, M.; Baldwin, M.; Buchanan, R.M. Structural and Spectroscopic Characterization of Copper(II) Complexes of a New Bisamide Functionalized Imidazole Tripod and Evidence for the Formation of a Mononuclear End-On Cu−OOH Species. Inorg. Chem. 2006, 45, 3191–3202. [Google Scholar] [CrossRef]
- Mereshchenko, A.S.; Olshin, P.; Karimov, A.M.; Skripkin, M.; Burkov, K.A.; Tveryanovich, Y.; Tarnovsky, A.N. Photochemistry of copper(II) chlorocomplexes in acetonitrile: Trapping the ligand-to-metal charge transfer excited state relaxations pathways. Chem. Phys. Lett. 2014, 615, 105–110. [Google Scholar] [CrossRef]
- Bhargava, G.; Ramanarayanan, T.; Bernasek, S.L. Imidazole−Fe Interaction in an Aqueous Chloride Medium: Effect of Cathodic Reduction of the Native Oxide. Langmuir 2009, 26, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kostko, O.; Xu, B.; Ahmed, M. Local electronic structure of histidine in aqueous solution. Phys. Chem. Chem. Phys. 2021, 23, 8847–8853. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Sarkar, D.; Madras, G. Hierarchical Design of CuS Architectures for Visible Light Photocatalysis of 4-Chlorophenol. ACS Omega 2017, 2, 4009–4021. [Google Scholar] [CrossRef] [Green Version]
- Drolet, D.P.; Manuta, D.M.; Lees, A.J.; Katnani, A.; Coyle, G.J. FT-IR and XPS study of copper(II) complexes of imidazole and benzimidazole. Inorganic. Chim. Acta 1988, 146, 173–180. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Różycka-Sokołowska, E.; Michalska, D. Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis(imidazole)copper(II) chloride. J. Mol. Struct. 2012, 1028, 49–56. [Google Scholar] [CrossRef]
- Majoube, M.; Vergoten, G. Assignment of normal modes for imidazole on the basis of 3-21G and 4-21G Ab Initio Force Fields. J. Mol. Struct. 1992, 266, 345–352. [Google Scholar] [CrossRef]
- Tritt-Goc, J.; Jankowska, I.; Pogorzelec-Glaser, K.; Pankiewicz, R.; Ławniczak, P. Imidazole-doped nanocrystalline cellulose solid proton conductor: Synthesis, thermal properties, and conductivity. Cellulose 2017, 25, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Gratzel, M.; Mhaisalkar, S.; Soci, C.; et al. Lead-Free MA2CuClxBr4–x Hybrid Perovskites. Inorg. Chem. 2016, 55, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Wu, W.; Xie, J.; Xie, D. Two copper complexes with imidazole ligands: Syntheses, crystal structures and fluorescence. Russ. J. Inorg. Chem. 2010, 55, 384–389. [Google Scholar] [CrossRef]

| Crystallography Data | |
|---|---|
| Empirical formula | C12H16N8Cl2Cu |
| Formula weight | 406.77 |
| Temperature/K | 273.15 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 8.8707(4) |
| b/Å | 13.3224(8) |
| c/Å | 13.9250(6) |
| α/° | 90 |
| β/° | 90.094(2) |
| γ/° | 90 |
| Volume/Å3 | 1645.64(14) |
| Z | 4 |
| ρcalcg/cm3 | 1.6417 |
| μ/mm−1 | 1.663 |
| F(000) | 830.7 |
| Crystal size/mm3 | 0.42 × 0.4 × 0.32 |
| Radiation | Mo Kα (λ = 0.71073) |
| 2Θ range for data collection/° | 5.44 to 61.14 |
| Index ranges | −10 ≤ h ≤ 12, −19 ≤ k ≤ 6, −19 ≤ l ≤ 15 |
| Reflections collected | 7881 |
| Independent reflections | 4439 [Rint = 0.0395, Rsigma = 0.0566] |
| Data/restraints/parameters | 4439/0/217 |
| Goodness-of-fit on F2 | 1.072 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0359, wR2 = 0.0868 |
| Final R indexes [all data] | R1 = 0.0520, wR2 = 0.0959 |
| Largest diff. peak/hole/e Å−3 | 0.58/−0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoensuk, S.; Tan, J.; Sain, M.; Manuspiya, H. A Single Crystal Hybrid Ligand Framework of Copper(II) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution. Nanomaterials 2021, 11, 2281. https://doi.org/10.3390/nano11092281
Charoensuk S, Tan J, Sain M, Manuspiya H. A Single Crystal Hybrid Ligand Framework of Copper(II) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution. Nanomaterials. 2021; 11(9):2281. https://doi.org/10.3390/nano11092281
Chicago/Turabian StyleCharoensuk, Suwitra, Jing Tan, Mohini Sain, and Hathaikarn Manuspiya. 2021. "A Single Crystal Hybrid Ligand Framework of Copper(II) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution" Nanomaterials 11, no. 9: 2281. https://doi.org/10.3390/nano11092281
APA StyleCharoensuk, S., Tan, J., Sain, M., & Manuspiya, H. (2021). A Single Crystal Hybrid Ligand Framework of Copper(II) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution. Nanomaterials, 11(9), 2281. https://doi.org/10.3390/nano11092281