Preparation of Triple-Functionalized Montmorillonite Layers Promoting Thermal Stability of Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
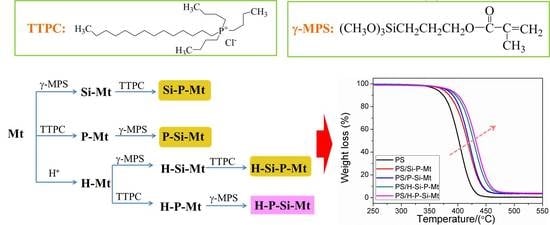
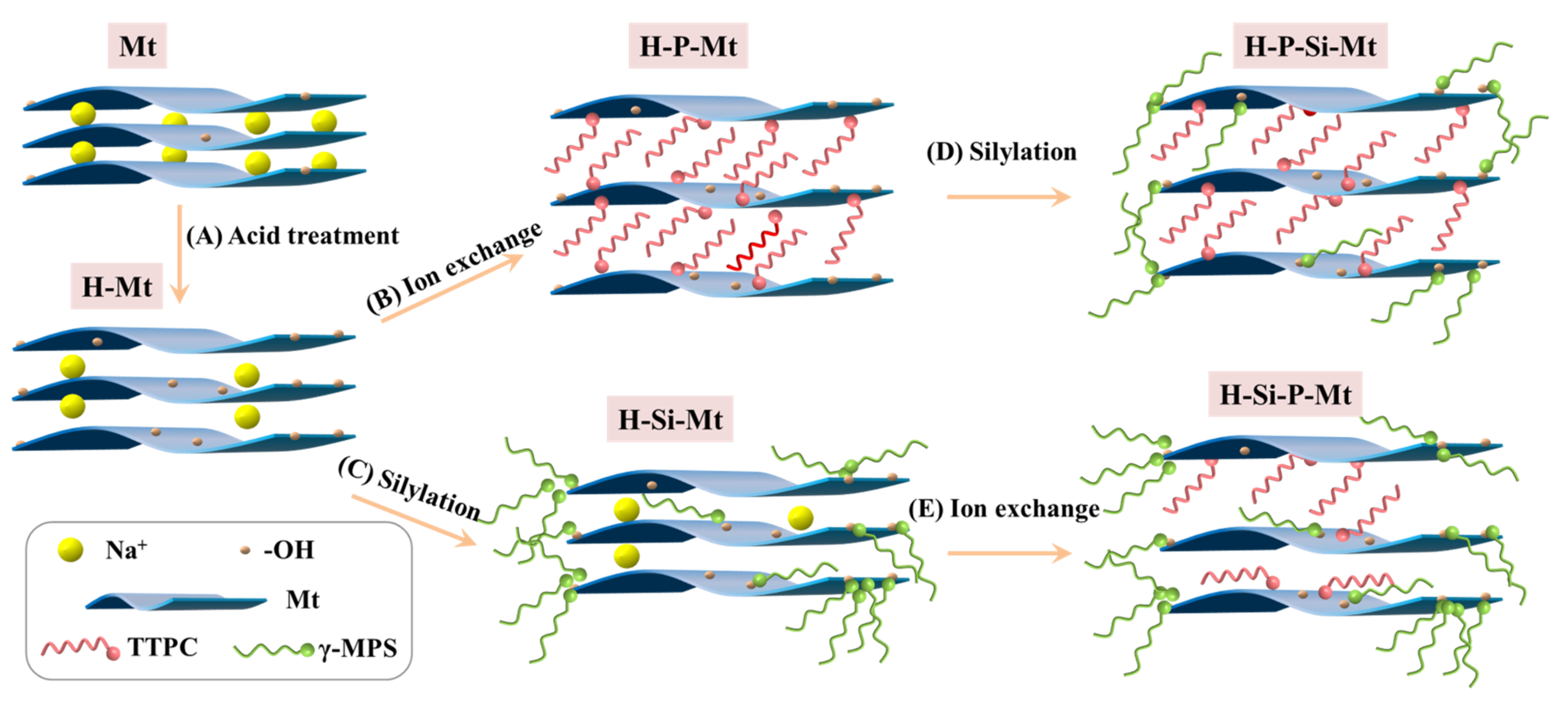
2.2. Acid Activation Reaction
2.3. Ion Exchange Reaction
2.4. Silylation Reaction
2.5. Synthesis of The Polystyrene (PS)/Organic Mt (OMt) Nanocomposites
2.6. Characterization
3. Results
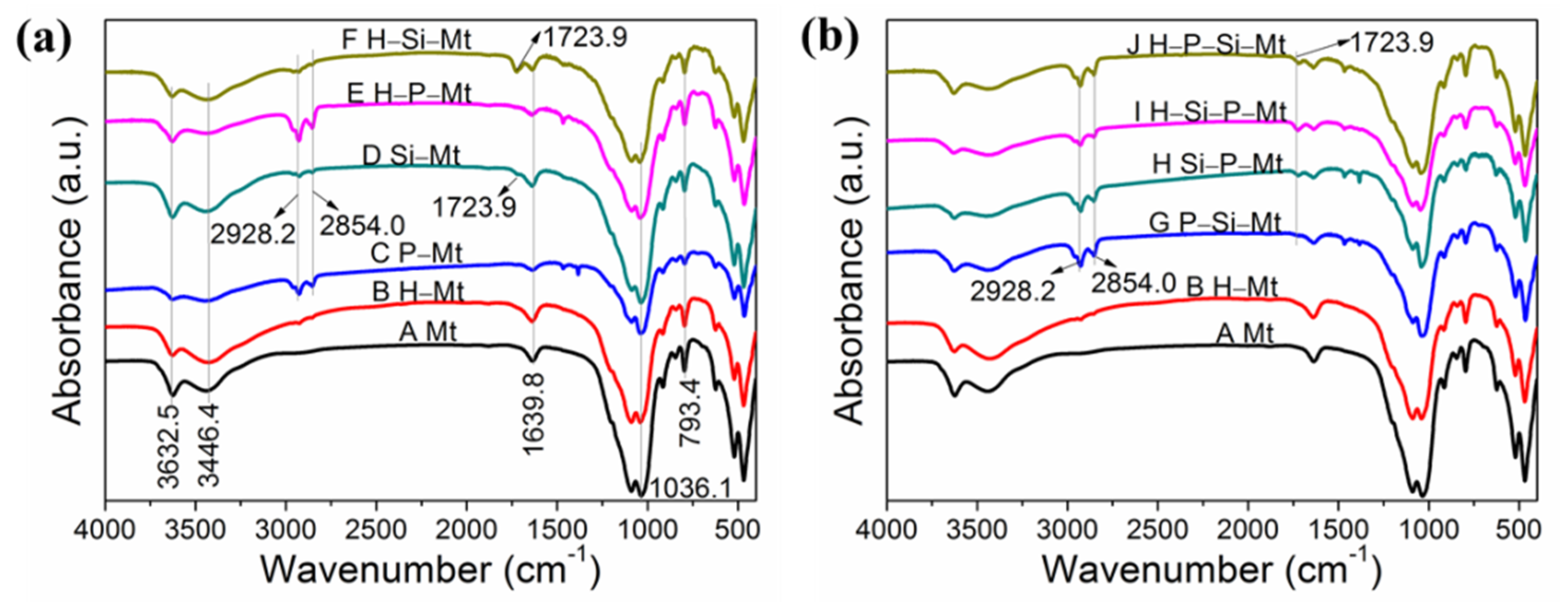
3.1. FTIR Analysis of Modified Mt
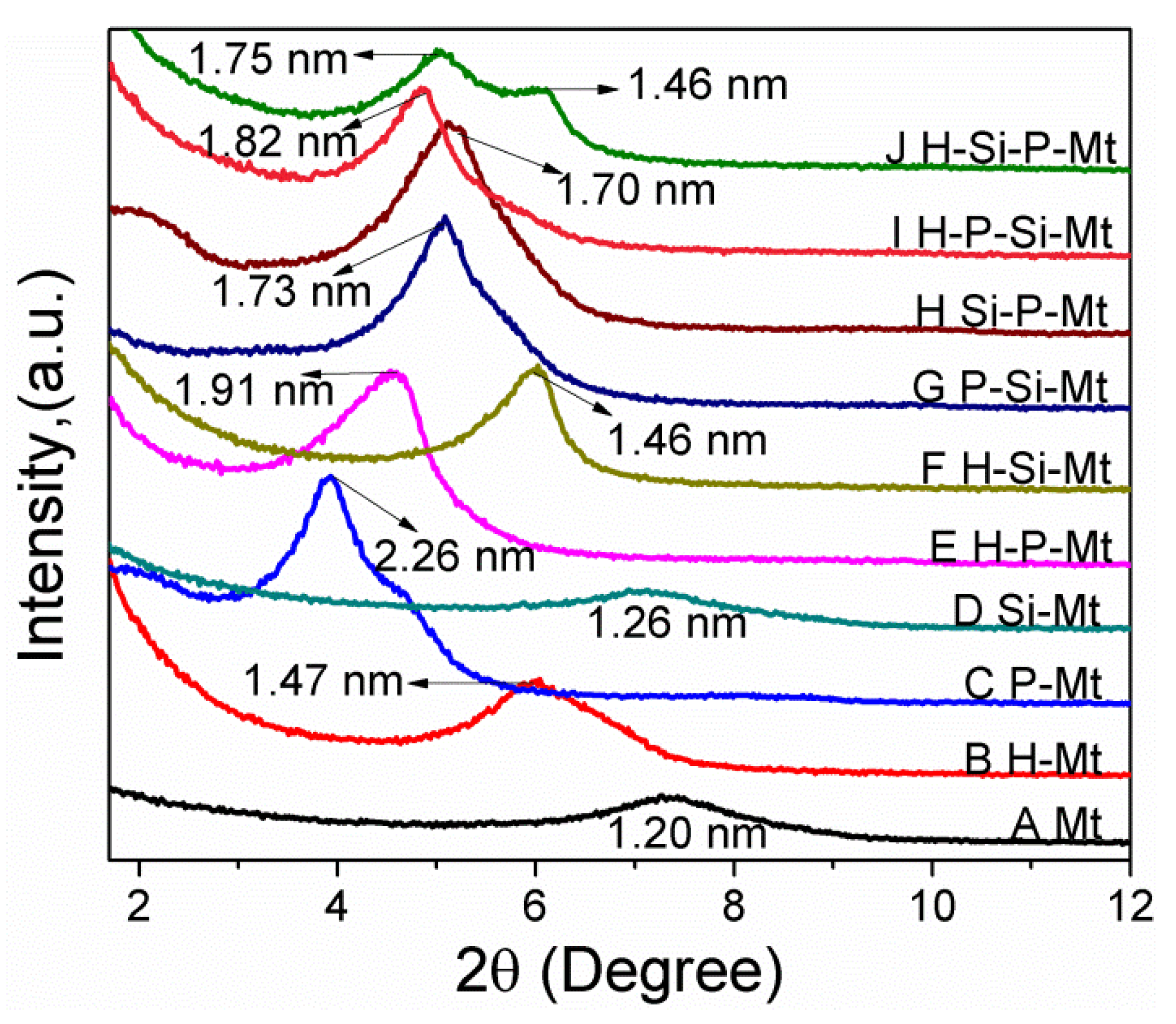
3.2. XRD Analysis of Modified Mt
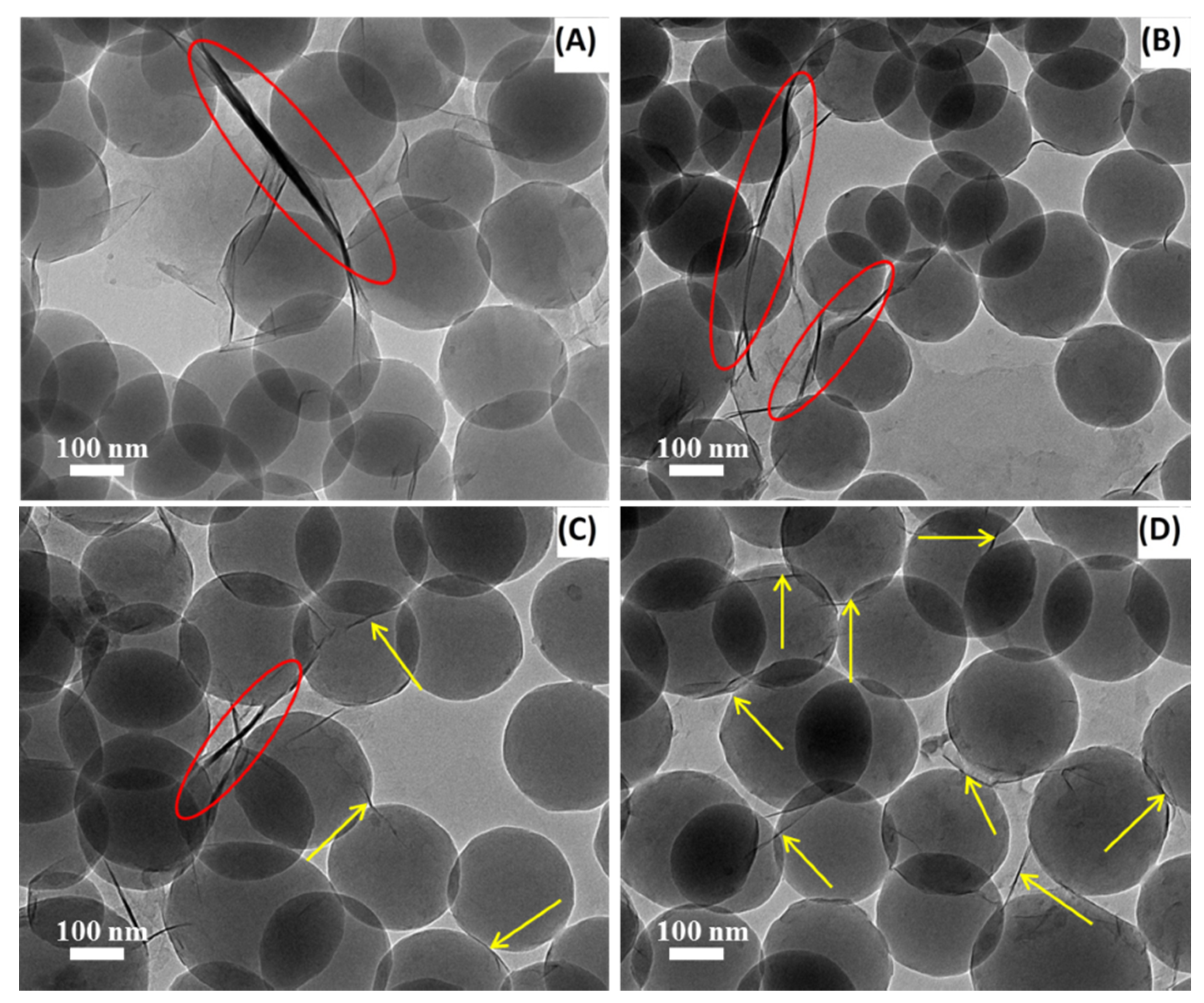
3.3. TEM Analysis of Modified Mt
3.4. BET and BJH Analysis of Modified Mt
3.5. XRF Analysis of Modified Mt
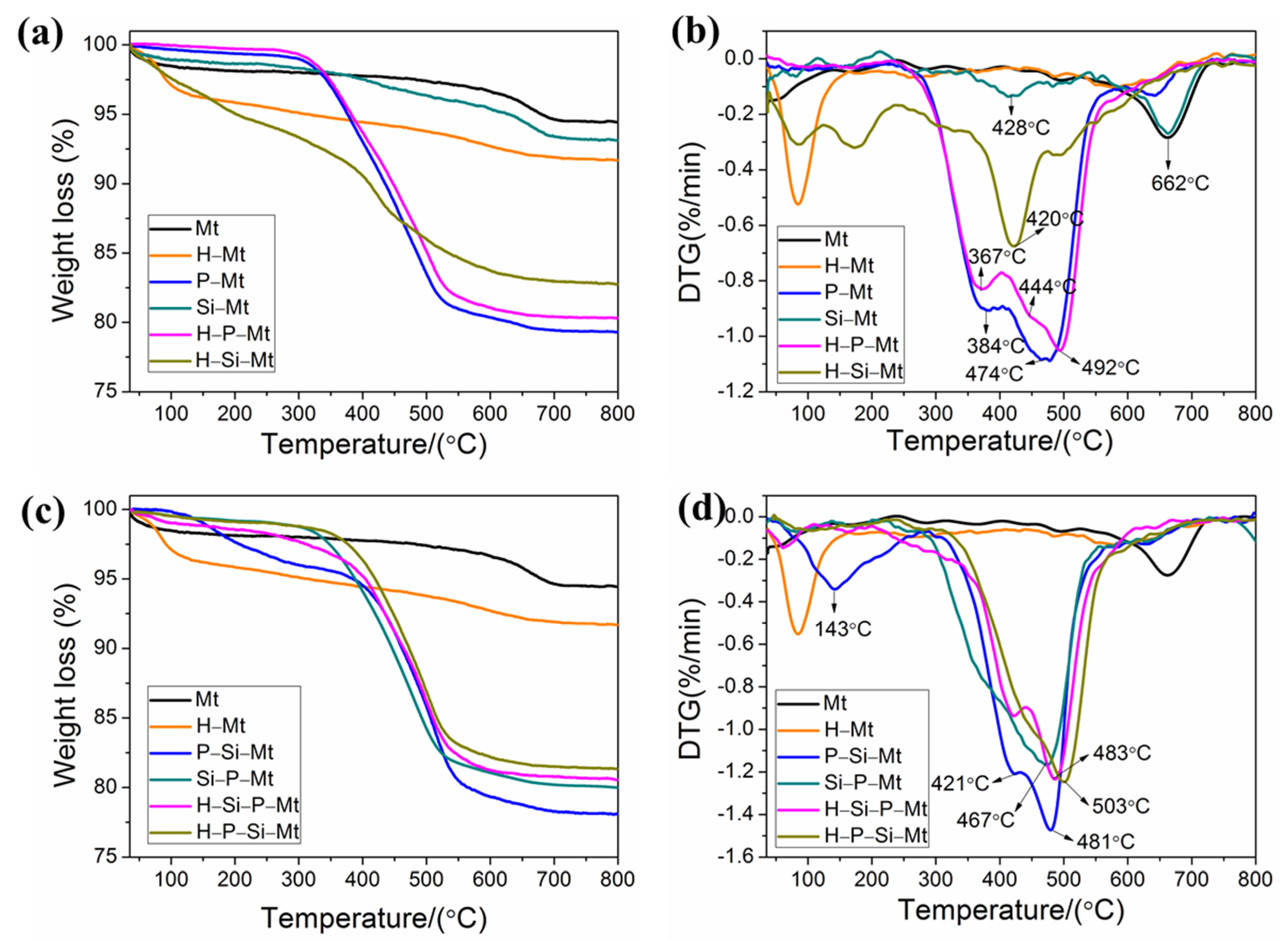
3.6. TGA-DTG Analysis of Modified Mt
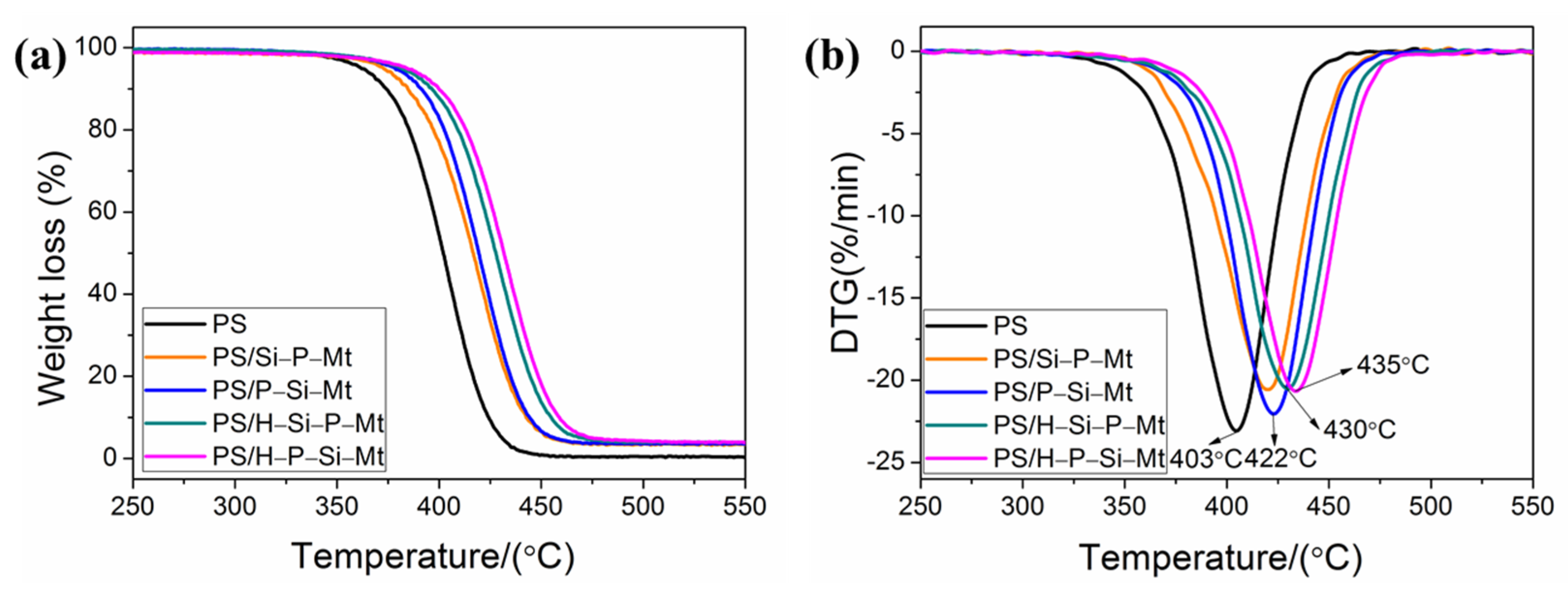
3.7. Thermal Properties of PS/OMt Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Tomsia, A.P.; Jiang, L.; Tang, B.Z.; Cheng, Q. Stiff and tough PDMS-MMT layered nanocomposites visualized by AIE luminogens. Nat. Commun. 2021, 12, 4539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, P.; Zhou, J.; Qi, S.; Yamauchi, Y.; Shi, R.; Fang, R.; Ishida, Y.; Wang, S.; Tomsia, A.P.; et al. Layered nanocomposites by shear-flow-induced alignment of nanosheets. Nature 2020, 508, 210–215. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving Thermal, Mechanical, and Barrier Properties of Feather Keratin/Polyvinyl Alcohol/Tris(hydroxymethyl)aminomethane Nanocomposite Films by Incorporating Sodium Montmorillonite and TiO2. Nanomatreials 2019, 9, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Hou, Y.; Yang, M. Scalable synthesis of multifunctional epidermis-like smart coatings. Adv. Funct. Mater. 2019, 29, 1903984. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, C.; Kabwe, F.B.; Wu, Q.; Li, C.; Zhang, J. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Xu, Y.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W. Novel multi amine-containing Gemini surfactant modified montmorillonite as adsorbents for removal of phenols. Appl. Clay Sci. 2018, 162, 204–213. [Google Scholar] [CrossRef]
- Silva, D.T.C.; Arruda, I.E.S.; França, L.M.; França, D.B.; Fonseca, M.G.; Soares, M.F.L.R.; Iborra, C.V.; Soares-Sobrinho, J.L. Tamoxifen/montmorillonite system-Effect of the experimental conditions. Appl. Clay Sci. 2019, 180, 105142. [Google Scholar] [CrossRef]
- Huskić, M.; Žigon, M.; Ivanković, M. Comparison of the properties of clay polymer nanocomposites prepared by montmorillonite modified by silane and by quaternary ammonium salts. Appl. Clay Sci. 2013, 85, 109–115. [Google Scholar] [CrossRef]
- Gou, J.; Zhang, L.; Li, C. A new method combining modification of montmorillonite and crystal regulation to enhance the mechanical properties of polypropylene. Polym. Test. 2020, 82, 106236. [Google Scholar] [CrossRef]
- Ray, S.S. Recent trends and future outlooks in the field of clay-containing polymer nanocomposites. Macromol. Chem. Phys. 2014, 215, 1162–1179. [Google Scholar] [CrossRef]
- Hária, J.; Horvátha, F.; Rennera, K.; Móczóa, J.; Pukánszky, B. Comparison of the reinforcing effect of various micro- and nanofillers in PA6. Polym. Test. 2018, 72, 178–186. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Z.; Tang, J.; Zhang, M.; Liao, L. Effects of variables on the dispersion of cationic-anionic organomontmorillonites and characteristics of Pickering emulsion. RSC Adv. 2016, 6, 9678–9685. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Sun, K.; Sun, Y.; Pan, C.; Zhao, Y. Organoclays prepared from montmorillonites with tetramethylolphosphonium chloride in different pH conditions. Powder Technol. 2013, 247, 178–187. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.; Luo, Z.; Yang, S. Enhanced removal of bisphenol A from aqueous solution by organo-montmorillonites modified with novel gemini pyridinium surfactants containing long alkyl chain. Chem. Eng. J. 2016, 285, 27–38. [Google Scholar] [CrossRef]
- Calderon, J.U.; Lennox, B.; Kamal, M.R. Thermally stable phosphonium-montmoril- lonite organoclays. Appl. Clay Sci. 2008, 40, 90–98. [Google Scholar] [CrossRef]
- Mishra, A.K.; Allauddin, S.; Narayan, R.; Aminabhavi, T.M.; Raju, K.V.S.N. Characterization of surface-modified montmorillonite nanocomposites. Ceram. Int. 2012, 38, 929–934. [Google Scholar] [CrossRef]
- Mittal, V. Modification of montmorillonites with thermally stable phosphonium cations and comparison with alkylammonium montmorillonites. Appl. Clay Sci. 2012, 56, 103–109. [Google Scholar] [CrossRef]
- Hojiyev, R.; Ulcay, Y.; Çelik, M.S.; Carty, W.M. Effect of CEC coverage of hexadecyltributylphosphonium modified montmorillonite on polymer compatibility. Appl. Clay Sci. 2017, 141, 204–211. [Google Scholar] [CrossRef]
- Kooli, F. Exfoliation properties of acid-activated montmorillonites and their resulting organoclays. Langmuir 2009, 25, 724–730. [Google Scholar] [CrossRef]
- Zatta, L.; Ramos, L.P.; Wypych, F. Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric acid acid with methanol. Appl. Clay Sci. 2013, 80–81, 236–244. [Google Scholar] [CrossRef]
- Alvi, M.U.; Zulfiqar, S.; Yavuz, C.T.; Kweon, H.S.; Sarwar, M.I. Influence of aminosilane coupling agent on aromatic polyamide/intercalated clay nanocomposites. Ind. Eng. Chem. Res. 2013, 52, 6908–6915. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay: The effect of dispersing media on intercalation and chemical grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Mamat, M.; Bee, S.T.; Sin, L.T.; Hui, D.; Rahmat, A.R. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part. B Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Yu, C.; Ke, Y.; Hu, X.; Zhao, Y.; Deng, Q.; Lu, S. Effect of bifunctional montmorillonite on the thermal and tribological properties of polystyrene/montmorillonite nanocomposites. Polymers 2019, 11, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, D.A.; Ollier, R.P.; Alvarez, V.A.; Schroeder, W.F.; Cyras, V.P. Modification of bentonite by combination of reactions of acid-activation, silylation and ionic exchange. Appl. Clay Sci. 2014, 99, 254–260. [Google Scholar] [CrossRef]
- Yi, D.; Yang, H.; Zhao, M.; Huang, L.; Camino, G.; Frache, A.; Yang, R. A novel, low surface charge density, anionically modified montmorillonite for polymer nanocomposites. RSC Adv. 2017, 7, 5980–5988. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.Q.; Ke, Y.C.; Pei, Y.; Zhang, G.L. Effects of highly exfoliated montmorillonite layers on the properties of clay reinforced terpolymer nanocomposite plugging microspheres. J. Appl. Polym. Sci. 2017, 134, 44894. [Google Scholar] [CrossRef]
- Chen, G.; Kim, H.; Shim, J.; Yoon, J. Role of epoxy groups on clay surface in the improvement of morphology of poly(L-lactide)/clay composites. Macromolecules 2005, 38, 3738–3744. [Google Scholar] [CrossRef]
- Angaji, M.T.; Rafiee, R.; Hemmati, M.; Abdollahi, M.; Razavi Aghjeh, M.K. Parametric studies on the grafting of poly (methyl methacrylate) onto organophilic montmorillonite using silylated clay platelets. J. Macromol. Sci. Part. B 2014, 53, 957–974. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, M.; Ye, Y.; Yang, S. Modification of reduced-charge montmorillonites by a series of Gemini surfactants: Characterization and application in methyl orange removal. Appl. Surf. Sci. 2015, 324, 807–816. [Google Scholar] [CrossRef]
- Yao, Q.Z.; Zhou, Y.M. Surface functional imprinting of bensulfuron-methyl at surface of silica nanoparticles linked by silane coupling agent. J. Inorg. Organomet. Polym. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Ren, H.; Tian, S.; Zhu, M.; Zhao, Y.; Li, K.; Ma, Q.; Ding, S.; Gao, J.; Miao, Z. Modification of montmorillonite by Gemini surfactants with different chain lengths and its adsorption behavior for methyl orange. Appl. Clay Sci. 2018, 151, 29–36. [Google Scholar] [CrossRef]
- Romanzini, D.; Piroli, V.; Frache, A.; Zattera, A.J.; Amico, S.C. Sodium montmorillonite modified with methacryloxy and vinylsilanes: Influence of silylation on the morphology of clay/unsaturated polyester nanocomposites. Appl. Clay Sci. 2015, 114, 550–557. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Benbayer, C.; Saidi-Besbes, S.; Givenchy, E.T.; Amigoni, S.; Guittard, F.; Derdour, A. Synergistic effect of organoclay fillers based on fluorinated surfmers for preparation of polystyrene nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42347. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Zhang, Z.; Wang, T.; Pan, F. Preparation and Characterization of Poly (methyl methacrylate)/Reactive Montmorillonit Nanocomposites. Polym. Compos. 2016, 37, 2396–2403. [Google Scholar] [CrossRef]
- Murray, H. Traditional and new applications for kaolin smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.; Luan, X.; Ji, H.; Xia, Z. Characterization of anion–cationic surfactants modified montmorillonite and its application for the removal of methyl orange. Chem. Eng. J. 2011, 171, 1150–1158. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Ric, G.I.; Miñana, C.C.; Bermejo, J.S. Characterization of montmorillonites modified with organic divalent phosphonium cations. Appl. Clay Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Su, L.; Tao, Q.; He, H.; Zhu, J.; Yuan, P.; Zhu, R. Silylation of montmorillonite surfaces: Dependence on solvent nature. J. Colloid Interf. Sci. 2013, 391, 16–20. [Google Scholar] [CrossRef]
- Xie, W.; Xie, R.; Pan, W.P.; Hunter, D.; Koene, B.; Tan, L.S.; Vaia, R. Thermal stability of quaternary phosphonium modified montmorillonites. Chem. Mater. 2002, 14, 4837–4845. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, W.; Ma, Y.; Ma, L.; Zhou, Q.; Yuan, P.; Liu, D.; He, H. The influence of alkyl chain length on surfactant distribution within organo-montmorillonites and their thermal stability. J. Therm. Anal. Calorim. 2012, 109, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.A.; Dahmouche, K.; Soares, B.G. Nanostructure and dynamic mechanical properties of silane-functionalized montmorillonite/epoxy nanocomposites. Appl. Clay Sci. 2011, 54, 151–158. [Google Scholar] [CrossRef]
- Shen, W.; He, H.; Zhu, J.; Yuan, P.; Frost, R.L. Grafting of montmorillonite with different functional silanes via two different reaction systems. J. Colloid Interface Sci. 2007, 313, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Tao, Q.; He, H.; Zhu, J.; Yuan, P. Locking effect: A novel insight in the silylation of montmorillonite surfaces. Mater. Chem. Phys. 2012, 136, 292–295. [Google Scholar] [CrossRef]
- Pereira, P.; Gaspar, H.; Fernandes, L.; Bernardo, G. Impact of fullerenes on the thermal stability of melt processed polystyrene and poly(methyl methacrylate) composites. Polym. Test. 2015, 47, 130–136. [Google Scholar] [CrossRef]
- Pereira, P.; Fernandes, L.; Gaspar, H.; Bernardo, G. Melt processed polyethylene/fullerene nanocomposites with highly improved thermo-oxidative stability. Polym. Test. 2015, 45, 124–131. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The thermal degradation of polystyrene nanocomposite. Polymer 2005, 46, 2933–2942. [Google Scholar] [CrossRef]
- Bourbigot, S.; Gilman, J.W.; Wilkie, C.A. Kinetic analysis of the thermal degradation of polystyreneemontmorillonite nanocomposite. Polym. Degrad. Stab. 2004, 84, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.V.; Pugazhenthi, G. Properties and thermal degradation kinetics of polystyrene/organoclay nanocomposites synthesized by solvent blending method: Effect of processing conditions and organoclay loading. J. Appl. Polym. Sci. 2011, 120, 1322–1336. [Google Scholar] [CrossRef]
- Wang, H.W.; Chang, K.; Yeh, J.; Liou, S. Synthesis and dielectric properties of polystyrene–clay nanocomposite materials. J. Appl. Polym. Sci. 2004, 91, 1368–1373. [Google Scholar] [CrossRef]
- Qi, R.; Jin, X.; Nie, J.; Yu, W.; Zhou, C. Synthesis and properties of polystyrene–clay nanocomposites via in situ intercalative polymerization. J. Appl. Polym. Sci. 2005, 97, 201–207. [Google Scholar] [CrossRef]
- Qiu, L.; Qu, B. Preparation and characterization of surfactant-free polystyrene/layered double hydroxide exfoliated nanocomposite via soap-free emulsion polymerization. J. Colloid Interf. Sci. 2006, 301, 347–351. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, W.; Qu, B. Structural characterisation and thermal properties of exfoliated polystyrene/ZnAl layered double hydroxide nanocomposites prepared via solution intercalation. Polym. Degrad. Stab. 2005, 87, 433–440. [Google Scholar] [CrossRef]
- Kuljanin-Jakovljević, J.; Marinović-Cincović, M.; Stojanović, Z.; Krkljes, A.; Abazović, N.D.; Comor, M.I. Thermal degradation kinetics of polystyrene/cadmium sulfide composites. Polym. Degrad. Stab. 2009, 94, 891–897. [Google Scholar] [CrossRef]
- Yan, Y.W.; Huang, J.Q.; Guan, Y.H.; Shang, K.; Jian, R.; Wang, Y. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35–42. [Google Scholar] [CrossRef]
- Pal, M.K.; Singh, B.; Gautam, J. Thermal stability and UV-shielding properties of polymethyl methacrylate and polystyrene modified with calcium carbonate nanoparticles. J. Therm. Anal. Calorim. 2012, 107, 85–96. [Google Scholar] [CrossRef]
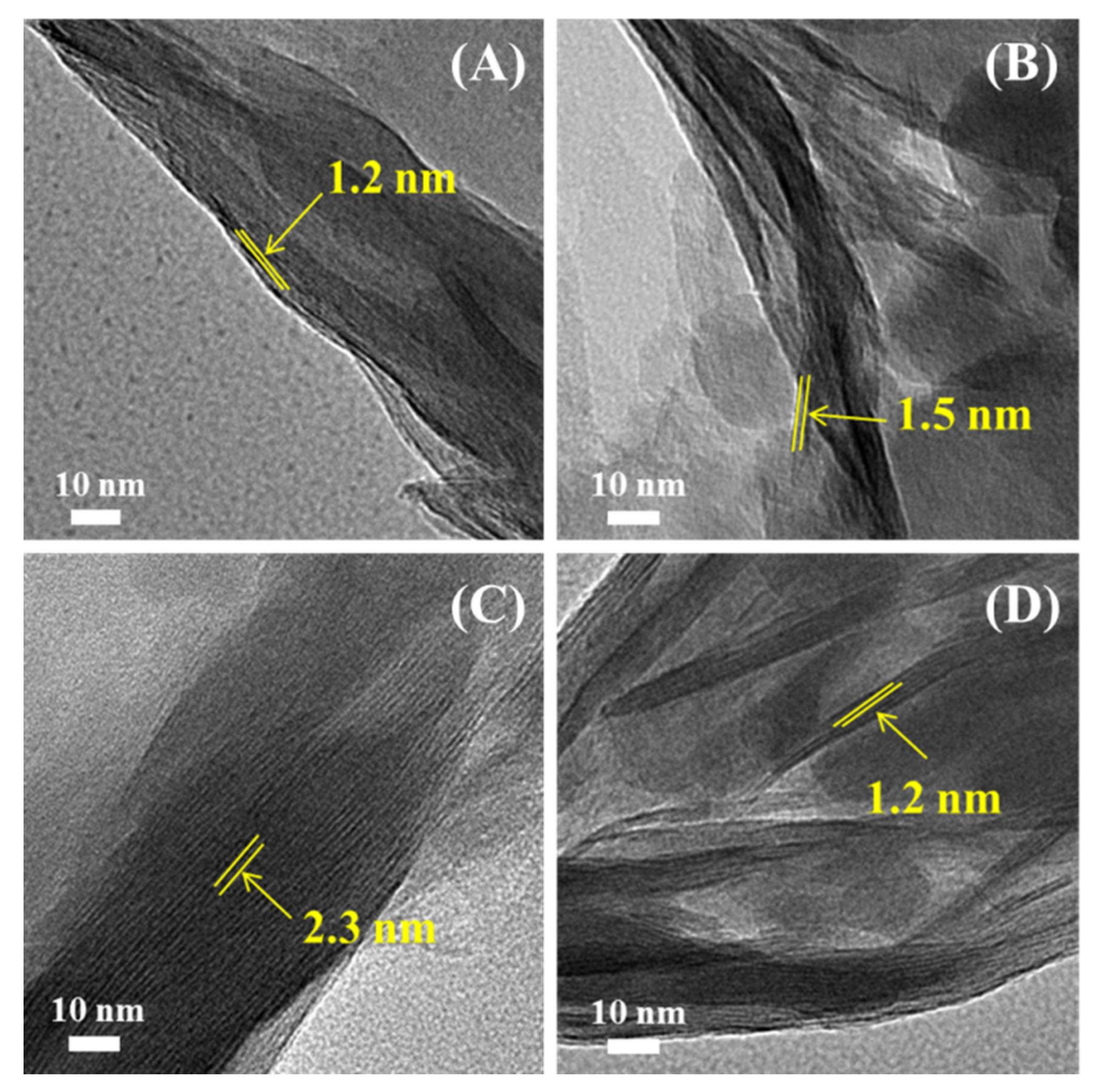
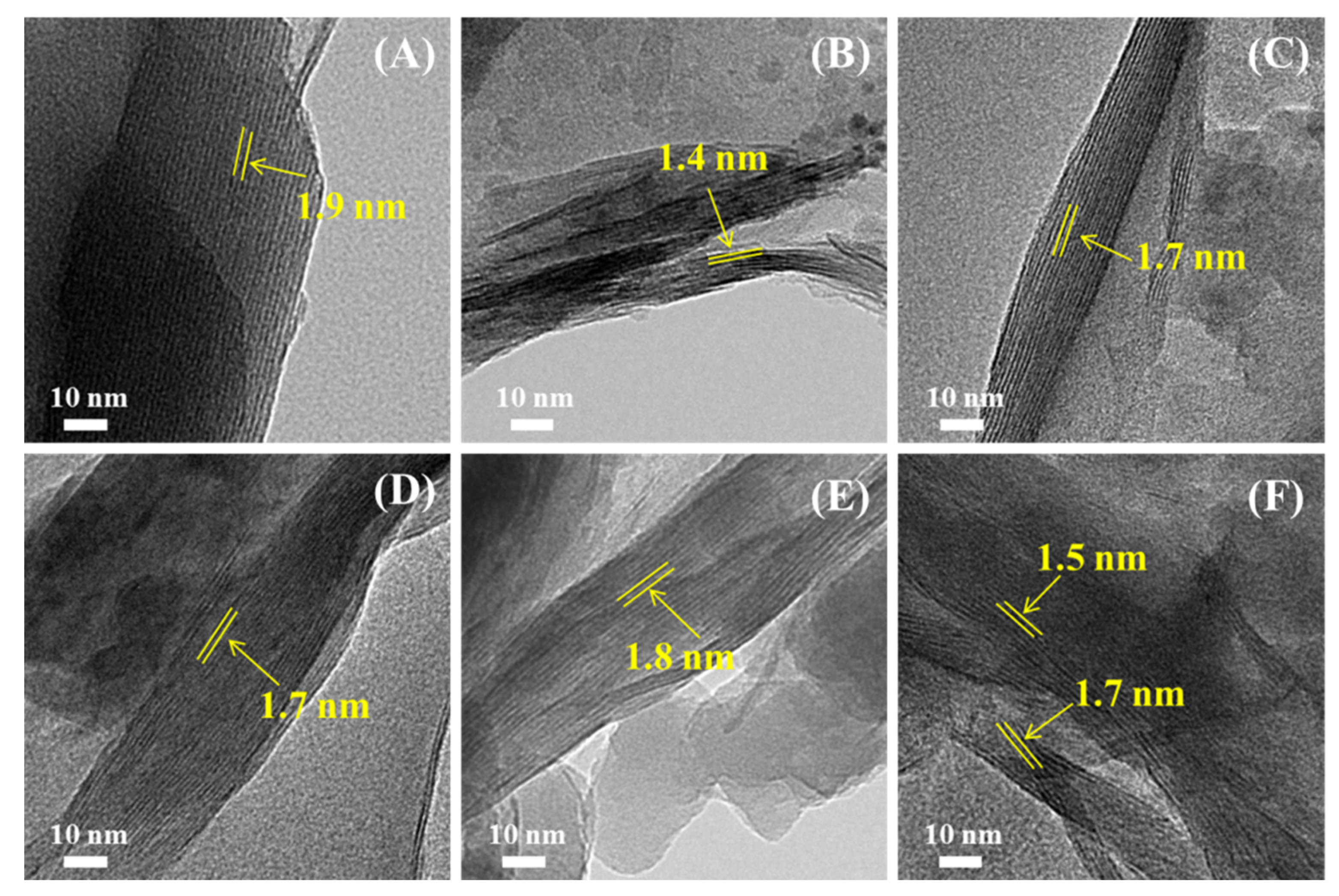
| Sample | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) |
|---|---|---|
| Mt | 40.36 | 0.173 |
| H-Mt | 105.93 | 0.259 |
| P-Mt | 30.12 | 0.163 |
| Si-Mt | 68.87 | 0.191 |
| H-P-Mt | 80.09 | 0.244 |
| H-Si-Mt | 108.29 | 0.260 |
| P-Si-Mt | 33.90 | 0.168 |
| Si-P-Mt | 31.62 | 0.154 |
| H-Si-P-Mt | 56.58 | 0.212 |
| H-P-Si-Mt | 95.25 | 0.238 |
| Sample | Composition (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | Na2O | CaO | Fe2O3 | K2O | P2O5 | TiO2 | Others a | |
| Mt | 72.9 | 14.7 | 4.1 | 3.2 | 1.5 | 1.3 | 0.3 | 0 | 0.1 | 1.9 |
| H-Mt | 81.0 | 13.2 | 2.7 | 0.4 | 0.2 | 1.3 | 0.3 | 0 | 0.1 | 0.8 |
| P-Mt | 71.6 | 14.5 | 4.1 | 1.5 | 1.5 | 1.5 | 0.3 | 2.9 | 0.1 | 2.0 |
| Si-Mt | 75.3 | 13.6 | 3.8 | 2.5 | 1.3 | 1.0 | 0.3 | 0 | 0.1 | 2.1 |
| H-P-Mt | 77.7 | 13.2 | 2.8 | 0.1 | 0.2 | 1.2 | 0.3 | 3.7 | 0.1 | 0.7 |
| H-Si-Mt | 83.1 | 12.0 | 2.5 | 0.3 | 0.2 | 1.3 | 0.3 | 0 | 0.1 | 0.2 |
| P-Si-Mt | 72.8 | 14.3 | 4.0 | 0.9 | 1.6 | 1.6 | 0.3 | 2.7 | 0.1 | 1.7 |
| Si-P-Mt | 71.5 | 14.5 | 4.0 | 1.1 | 1.5 | 1.5 | 0.3 | 3.4 | 0.1 | 2.1 |
| H-Si-P-Mt | 81.4 | 12.3 | 2.4 | 0.1 | 0.1 | 1.1 | 0.3 | 1.8 | 0.1 | 0.4 |
| H-P-Si-Mt | 78.7 | 13.3 | 2.7 | 0.1 | 0.2 | 1.3 | 0.3 | 2.5 | 0.1 | 0.8 |
| Sample | Temperature Range | ||
|---|---|---|---|
| <200 °C | 200–600 °C | 30–800 °C | |
| Mt | 1.9 | 1.4 | 5.6 |
| H-Mt | 4.2 | 2.8 | 8.3 |
| P-Mt | 0.6 | 19.0 | 20.7 |
| Si-Mt | 1.4 | 3.2 | 6.8 |
| H-P-Mt | 0.2 | 18.8 | 19.6 |
| H-Si-Mt | 5.0 | 11.4 | 17.2 |
| P-Si-Mt | 2.4 | 18.5 | 21.9 |
| Si-P-Mt | 0.9 | 18.1 | 19.9 |
| H-Si-P-Mt | 1.5 | 17.2 | 19.4 |
| H-P-Si-Mt | 0.8 | 16.9 | 18.6 |
| Sample | T−5% (°C) | T−50% (°C) | Tmax (°C) |
|---|---|---|---|
| PS | 359 | 401 | 403 |
| PS/Si-P-Mt | 374 | 416 | 419 |
| PS/P-Si-Mt | 379 | 420 | 422 |
| PS/H-Si-P-Mt | 382 | 427 | 430 |
| PS/H-P-Si-Mt | 386 | 432 | 435 |
| Filler | Method | Content (wt.%) | ΔTonset (°C) | ΔT−50% (°C) | ΔTmax (°C) | Ref. |
|---|---|---|---|---|---|---|
| Organoclay | Bulk polymerization | 5 | 50 | [50] | ||
| Organoclay | Bulk polymerization | 3 | 50 | [51] | ||
| Organoclay | Solvent blending | 5 | 16 | 24 | [52] | |
| Organoclay | Solution polymerization | 5 | 51.1 | [53] | ||
| Organoclay | In situ intercalative polymerization | 10 | 109.5 | [54] | ||
| Fullerene | Melt extrusion | 1 | 7.0 | 4.5 | 2.2 | [47] |
| Layered double hydroxide | Soap-free emulsion polymerization | 1 | 53.3 | [55] | ||
| ZnAl-layered double hydroxide | Solution intercalation | 10 | 39 | [56] | ||
| Cadmium sulfide | Melt extrusion | 10 | 95 | [57] | ||
| Aluminum hypophosphite | Melt extrusion | 30 | –23 | 6 | [58] | |
| Calcium carbonate | Solution casting | 4 | 15 | [59] | ||
| Organoclay | Soap-free emulsion polymerization | 3 | 27 | 31 | 32 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Hu, X.; Lu, S.; Ke, Y.; Luo, J. Preparation of Triple-Functionalized Montmorillonite Layers Promoting Thermal Stability of Polystyrene. Nanomaterials 2021, 11, 2170. https://doi.org/10.3390/nano11092170
Yu C, Hu X, Lu S, Ke Y, Luo J. Preparation of Triple-Functionalized Montmorillonite Layers Promoting Thermal Stability of Polystyrene. Nanomaterials. 2021; 11(9):2170. https://doi.org/10.3390/nano11092170
Chicago/Turabian StyleYu, Chengcheng, Xu Hu, Shichao Lu, Yangchuan Ke, and Jianbin Luo. 2021. "Preparation of Triple-Functionalized Montmorillonite Layers Promoting Thermal Stability of Polystyrene" Nanomaterials 11, no. 9: 2170. https://doi.org/10.3390/nano11092170
APA StyleYu, C., Hu, X., Lu, S., Ke, Y., & Luo, J. (2021). Preparation of Triple-Functionalized Montmorillonite Layers Promoting Thermal Stability of Polystyrene. Nanomaterials, 11(9), 2170. https://doi.org/10.3390/nano11092170