Silver Nanoparticle’s Toxicological Effects and Phytoremediation
Abstract
:1. Introduction
2. Applications of Silver Nanoparticles
3. Environmental and Toxicological Effects of AgNPs
3.1. Effects of AgNPs on Plants
3.2. The Effects of AgNPs on Soil Microorganisms
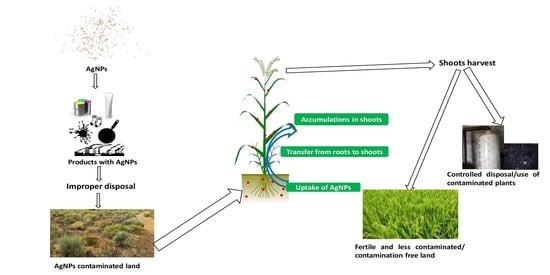
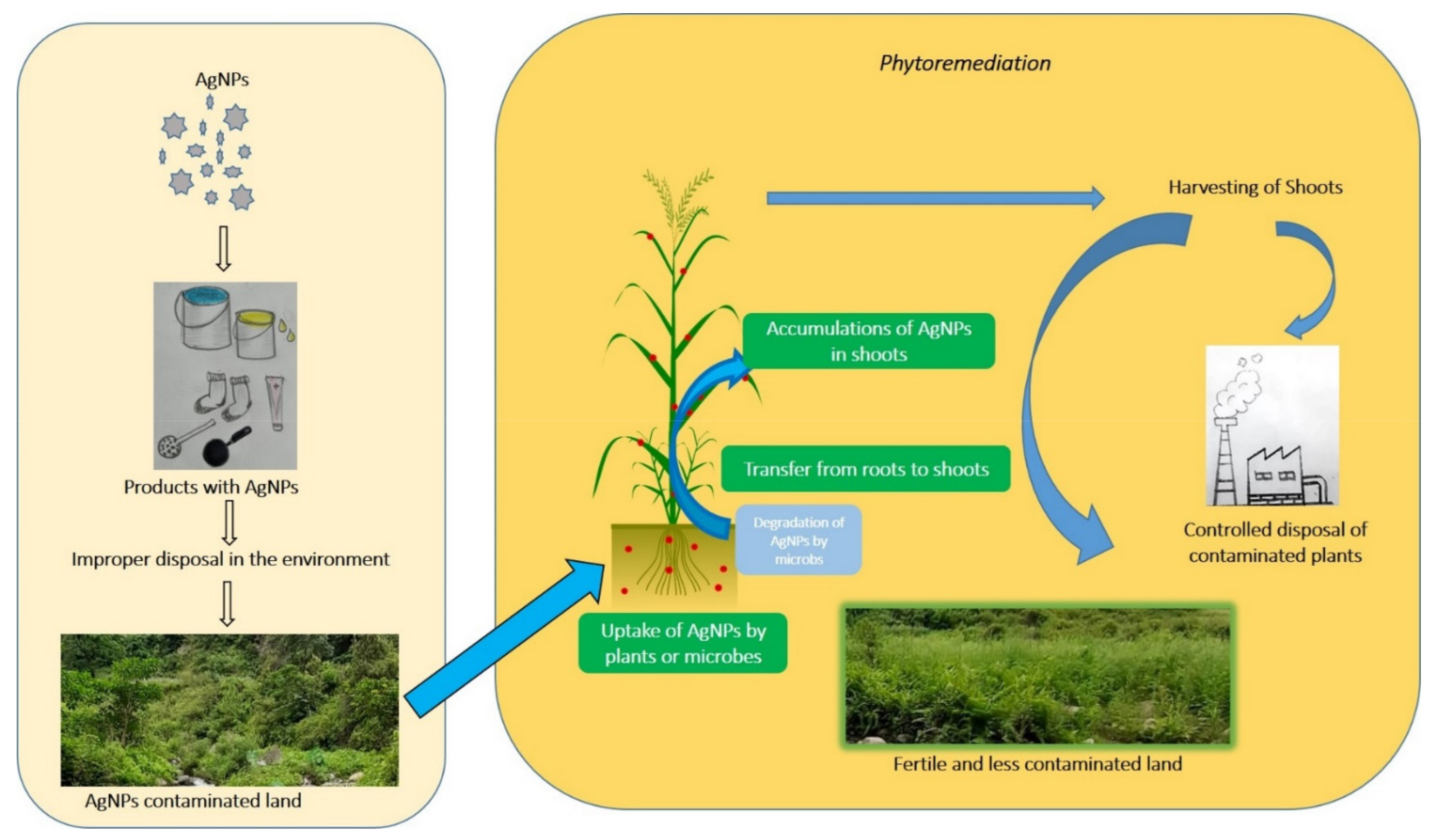
4. Nanoparticle’s Phytotechnology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [Green Version]
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.; Nilavukkarasi, M.; Praseetha, P. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crop. Prod. 2021, 170, 113762. [Google Scholar]
- Dhawan, A.; Shanker, R.; Das, M.; Gupta, K.C. Guidance for safe handling of nanomaterials. J. Biomed. Nanotechnol. 2011, 7, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Dillon, A.D.; Ghidiu, M.J.; Krick, A.L.; Griggs, J.; May, S.J.; Gogotsi, Y.; Barsoum, M.W.; Fafarman, A.T. Highly conductive optical quality solution-processed films of 2D titanium carbide. Adv. Funct. Mater. 2016, 26, 4162–4168. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Chandra, H.; Kumari, P.; Bontempi, E.; Yadav, S. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal. Agric. Biotechnol. 2020, 24, 101518. [Google Scholar] [CrossRef]
- Chinnamuthu, C.; Boopathi, P.M. Nanotechnology and agroecosystem. Madras Agric. J. 2009, 96, 17–31. [Google Scholar]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; De la Torre-Roche, R.; Castillo-Michel, H.; Pagano, L.; Hawthorne, J.; Musante, C.; Pignatello, J.; Uchimiya, M.; White, J.C. Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiol. Biochem. 2017, 110, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2020, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Samal, A.K.; Polavarapu, L.; Rodal-Cedeira, S.; Liz-Marzaán, L.M.; Peérez-Juste, J.; Pastoriza-Santos, I. Size Tunable Au@ Ag core–shell nanoparticles: Synthesis and surface-enhanced raman scattering properties. Langmuir 2013, 29, 15076–15082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonin, M.; Martins, J.M.; Uzu, G.; Spadini, L.; Navel, A.; Richaume, A. Low mobility of CuO and TiO2 nanoparticles in agricultural soils of contrasting texture and organic matter content. Sci. Total Environ. 2021, 783, 146952. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, K.; Sun, P.; Lin, D.; Shen, B.; Luo, Y. Co-transport of Pb2+ and TiO2 nanoparticles in repacked homogeneous soil columns under saturation condition: Effect of ionic strength and fulvic acid. Sci. Total Environ. 2016, 571, 471–478. [Google Scholar] [CrossRef]
- Courtois, P.; de Vaufleury, A.; Grosser, A.; Lors, C.; Vandenbulcke, F. Transfer of sulfidized silver from silver nanoparticles, in sewage sludge, to plants and primary consumers in agricultural soil environment. Sci. Total Environ. 2021, 777, 145900. [Google Scholar] [CrossRef] [PubMed]
- Torrent, L.; Marguí, E.; Queralt, I.; Hidalgo, M.; Iglesias, M. Interaction of silver nanoparticles with mediterranean agricultural soils: Lab-controlled adsorption and desorption studies. J. Environ. Sci. 2019, 83, 205–216. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, I.; Rijckaert, H.; De Gelder, L.; Van Driessche, I.; Vermeir, P. High variability in silver particle characteristics, silver concentrations, and production batches of commercially available products indicates the need for a more rigorous approach. Nanomaterials 2020, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Market Watch. Available online: https://www.marketwatch.com/press-release/silvernanoparticles-market-future-scope-demands-and-projectedindustry-growths-to-2024-2019-05-09 (accessed on 20 October 2019).
- Noori, A.; Ngo, A.; Gutierrez, P.; Theberge, S.; White, J.C. Silver nanoparticle detection and accumulation in tomato. J. Nanopart. Res. 2020, 22, 131. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver Nanoparticle-Based Nanocomposites for Combating Infectious Pathogens: Recent Advances and Future Prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef]
- Peharec Štefanić, P.; Košpić, K.; Lyons, D.M.; Jurković, L.; Balen, B.; Tkalec, M. Phytotoxicity of silver nanoparticles on tobacco plants: Evaluation of coating effects on photosynthetic performance and chloroplast ultrastructure. Nanomaterials 2021, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediat. 2020, 22, 40–51. [Google Scholar] [CrossRef]
- Noori, A.; White, J.C.; Newman, L.A. Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J. Nanopart. Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Courtois, P.; Rorat, A.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Levard, C.; Vandenbulcke, F. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ. Pollut. 2019, 253, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yousef, N.M.; Nafady, N.A. Application of biosynthesized silver nanoparticles for the control of land snail Eobania vermiculata and some plant pathogenic fungi. J. Nanomater. 2015, 2015, 218904. [Google Scholar] [CrossRef] [Green Version]
- Capek, I. Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv. Colloid Interface Sci. 2004, 110, 49–74. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [Green Version]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [Green Version]
- Burić, P.; Jakšić, Ž.; Štajner, L.; Sikirić, M.D.; Jurašin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, A.-J.; Luo, Z.; Chen, C.-S.; Chin, W.-C.; Santschi, P.H.; Quigg, A. Intracellular uptake: A possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE 2010, 5, e15196. [Google Scholar] [CrossRef] [Green Version]
- Siripattanakul-Ratpukdi, S.; Ploychankul, C.; Limpiyakorn, T.; Vangnai, A.S.; Rongsayamanont, C.; Khan, E. Mitigation of nitrification inhibition by silver nanoparticles using cell entrapment technique. J. Nanopart. Res. 2014, 16, 2218. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Klitzke, S.; Metreveli, G.; Peters, A.; Schaumann, G.E.; Lang, F. The fate of silver nanoparticles in soil solution—Sorption of solutes and aggregation. Sci. Total Environ. 2015, 535, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.-H.; Lee, B.-C.; Eom, I.-C.; Kim, P.; Yeo, M.-K. Uptake and bioaccumulation of titanium-and silver-nanoparticles in aquatic ecosystems. Mol. Cell. Toxicol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Howe, P.D.; Dobson, S. Silver and Silver Compounds: Environmental Aspects; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Galazzi, R.M.; Júnior, C.A.L.; de Lima, T.B.; Gozzo, F.C.; Arruda, M.A.Z. Evaluation of some effects on plant metabolism through proteins and enzymes in transgenic and non-transgenic soybeans after cultivation with silver nanoparticles. J. Proteom. 2019, 191, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Lazim, Z.M.; Salmiati; Samaluddin, A.R.; Salim, M.R.; Arman, N.Z. Toxicity of Silver Nanoparticles and Their Removal Applying Phytoremediation System to Water Environment: An Overview. J. Environ. Treat. Tech. 2020, 8, 978–984. [Google Scholar]
- Handy, R.D.; Shaw, B.J. Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007, 9, 125–144. [Google Scholar] [CrossRef]
- Owen, R.; Handy, R. Formulating the Problems for Environmental Risk Assessment of Nanomaterials; ACS Publications: Washington, DC, USA, 2007; pp. 5582–5588. [Google Scholar]
- Pérez-de-Luque, A.; Rubiales, D. Nanotechnology for parasitic plant control. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V. Advances in nanobiotechnology for agriculture. In Current Topics in Biotechnology & Microbiology; Dhingra, H.K., Nath Jha, P., Bajpai, P., Eds.; Lap Lambert Academic Publishing: Dudweller Landstr, Germany, 2011; pp. 156–167. [Google Scholar]
- Fernandes, J.P.; Mucha, A.P.; Francisco, T.; Gomes, C.R.; Almeida, C.M.R. Silver nanoparticles uptake by salt marsh plants–Implications for phytoremediation processes and effects in microbial community dynamics. Mar. Pollut. Bull. 2017, 119, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Shafer, M.M.; Overdier, J.T.; Armstong, D.E. Removal, partitioning, and fate of silver and other metals in wastewater treatment plants and effluent-receiving streams. Environ. Toxicol. Chem. Int. J. 1998, 17, 630–641. [Google Scholar] [CrossRef]
- Hanks, N.A.; Caruso, J.A.; Zhang, P. Assessing Pistia stratiotes for phytoremediation of silver nanoparticles and Ag (I) contaminated waters. J. Environ. Manag. 2015, 164, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Purcell, T.W.; Peters, J.J. Historical impacts of environmental regulation of silver. Environ. Toxicol. Chem. Int. J. 1999, 18, 3–8. [Google Scholar] [CrossRef]
- U.S. Environmnetal Proection Agency (EPA). National Primary Drinking Water Regulation Table, EPA 816-F-09-0004. 2009. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulation-table (accessed on 5 August 2021).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2011; p. 415. Available online: http://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf;jsessionid=7602427D0558C27BC51742431A74F67E?sequence=1 (accessed on 5 August 2021).
- Varner, K.; El-Badawy, A.; Feldhake, D.; Venkatapathy, R. State-of-the-Science Review: Everything Nanosilver and More; EPA/600/R-10/084:2010; US Environmental Protection Agency: Washington, DC, USA, 2010.
- Bernas, L.; Winkelmann, K.; Palmer, A. Phytoremediation of silver species by waterweed (Egeria densa). Chemist 2017, 90, 7–13. [Google Scholar]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart. Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef]
- Lapresta-Fernández, A.; Fernández, A.; Blasco, J. Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. TrAC Trends Anal. Chem. 2012, 32, 40–59. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. Int. J. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Brooks, M.; Gerfen, J.R.; Wang, Q.; Fotis, C.; Sparer, A.; Ma, X.; Berg, R.H.; Geisler, M. Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials 2014, 4, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Çekiç, F.Ö.; Ekinci, S.; İnal, M.S.; Özakça, D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk. J. Biol. 2017, 41, 700–707. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Reprint of: Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2017, 110, 33–49. [Google Scholar] [CrossRef]
- Gupta, I.R.; Anderson, A.J.; Rai, M. Toxicity of fungal-generated silver nanoparticles to soil-inhabiting Pseudomonas putida KT2440, a rhizospheric bacterium responsible for plant protection and bioremediation. J. Hazard. Mater. 2015, 286, 48–54. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Zia-ur-Rehman, M.; Rizwan, M.; Rinklebe, J. Biochar-induced immobilization and transformation of silver-nanoparticles affect growth, intracellular-radicles generation and nutrients assimilation by reducing oxidative stress in maize. J. Hazard. Mater. 2020, 390, 121976. [Google Scholar] [CrossRef]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Schober, Y.; Römpp, A.; Ghassempour, A.; Spengler, B. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol. Environ. Saf. 2014, 108, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Peharec Štefanić, P.; Jarnević, M.; Cvjetko, P.; Biba, R.; Šikić, S.; Tkalec, M.; Cindrić, M.; Letofsky-Papst, I.; Balen, B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019, 26, 22529–22550. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Zou, X.; Li, P.; Huang, Q.; Zhang, H. The different response mechanisms of Wolffia globosa: Light-induced silver nanoparticle toxicity. Aquat. Toxicol. 2016, 176, 97–105. [Google Scholar] [CrossRef]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Li, S.; Yin, L.; Huang, J.; Chen, C. Toxicity of silver nanoparticles to Arabidopsis: Inhibition of root gravitropism by interfering with auxin pathway. Environ. Toxicol. Chem. 2017, 36, 2773–2780. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Li, Y.; Qu, Q.; Ye, Y.; Peijnenburg, W.; Zhang, Z.; Xu, N.; Lu, T.; Sun, L.; Qian, H. Offspring toxicity of silver nanoparticles to Arabidopsis thaliana flowering and floral development. J. Hazard. Mater. 2020, 386, 121975. [Google Scholar] [CrossRef]
- Minogiannis, P.; Valenti, M.; Kati, V.; Kalantzi, O.-I.; Biskos, G. Toxicity of pure silver nanoparticles produced by spark ablation on the aquatic plant Lemna minor. J. Aerosol Sci. 2019, 128, 17–21. [Google Scholar] [CrossRef]
- Mylona, Z.; Panteris, E.; Moustakas, M.; Kevrekidis, T.; Malea, P. Physiological, structural and ultrastructural impacts of silver nanoparticles on the seagrass Cymodocea nodosa. Chemosphere 2020, 248, 126066. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yilmaz, A.; Karaman, A.; Aysin, F.; Aksakal, O. Monitoring chemically and green-synthesized silver nanoparticles in maize seedlings via surface-enhanced Raman spectroscopy (SERS) and their phytotoxicity evaluation. Talanta 2021, 225, 121952. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R. Phytotoxicity of silver nanoparticles on Vicia faba: Evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Kushwaha, H.R.; Kumar, K.; Verma, P.K. Comparative structural modeling of a monothiol GRX from chickpea: Insight in iron–sulfur cluster assembly. Int. J. Biol. Macromol. 2012, 51, 266–273. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, A.; Yadav, S. Thioredoxins as Molecular Players in Plants, Pests, and Pathogens. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 107–125. Available online: https://doi.org/10.1007/978-981-15-2467-7_6 (accessed on 20 July 2021).
- Abdel-Aziz, H.M.; Rizwan, M. Chemically synthesized silver nanoparticles induced physio-chemical and chloroplast ultrastructural changes in broad bean seedlings. Chemosphere 2019, 235, 1066–1072. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Kruszka, D.; Sawikowska, A.; Selvakesavan, R.K.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver nanoparticles affect phenolic and phytoalexin composition of Arabidopsis thaliana. Sci. Total Environ. 2020, 716, 135361. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Ko, Y.-S.; Lee, S.J.; Le, T.S.; Woo, K.; Ko, G. Disinfection of various bacterial pathogens using novel silver nanoparticle-decorated magnetic hybrid colloids. Sci. Total Environ. 2017, 609, 289–296. [Google Scholar] [CrossRef]
- Uddin, I.; Ahmad, K.; Khan, A.A.; Kazmi, M.A. Synthesis of silver nanoparticles using Matricaria recutita (Babunah) plant extract and its study as mercury ions sensor. Sens. Bio-Sens. Res. 2017, 16, 62–67. [Google Scholar] [CrossRef]
- Samarajeewa, A.; Velicogna, J.; Princz, J.; Subasinghe, R.; Scroggins, R.; Beaudette, L. Effect of silver nano-particles on soil microbial growth, activity and community diversity in a sandy loam soil. Environ. Pollut. 2017, 220, 504–513. [Google Scholar] [CrossRef]
- Grün, A.-L.; Straskraba, S.; Schulz, S.; Schloter, M.; Emmerling, C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. J. Environ. Sci. 2018, 69, 12–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Li, M.; Cheng, J.; Liu, W.; Feng, Y. Influence of Silver nanoparticles on nutrient removal and microbial communities in SBR process after long-term exposure. Sci. Total Environ. 2016, 569, 234–243. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Kwak, J.I.; An, Y.-J. Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere 2012, 88, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Abdulsada, Z.; Kibbee, R.; Örmeci, B.; DeRosa, M.; Princz, J. Impact of anaerobically digested silver and copper oxide nanoparticles in biosolids on soil characteristics and bacterial community. Chemosphere 2021, 263, 128173. [Google Scholar] [CrossRef]
- Zhai, Y.; Hunting, E.R.; Wouters, M.; Peijnenburg, W.J.; Vijver, M.G. Silver nanoparticles, ions, and shape governing soil microbial functional diversity: Nano shapes micro. Front. Microbiol. 2016, 7, 1123. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, S.; Seena, S. Can low concentrations of metal oxide and Ag loaded metal oxide nanoparticles pose a risk to stream plant litter microbial decomposers? Sci. Total Environ. 2019, 653, 930–937. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judy, J.D.; Kirby, J.K.; Creamer, C.; McLaughlin, M.J.; Fiebiger, C.; Wright, C.; Cavagnaro, T.R.; Bertsch, P.M. Effects of silver sulfide nanomaterials on mycorrhizal colonization of tomato plants and soil microbial communities in biosolid-amended soil. Environ. Pollut. 2015, 206, 256–263. [Google Scholar] [CrossRef]
- Sosnowska, M.E.; Jankiewicz, U.; Kutwin, M.; Chwalibog, A.; Gałązka, A. Influence of salts and metal nanoparticles on the activity and thermal stability of a recombinant chitinase from Stenotrophomonas maltophilia N4. Enzym. Microb. Technol. 2018, 116, 6–15. [Google Scholar] [CrossRef]
- Batista, D.; Pascoal, C.; Cássio, F. How do physicochemical properties influence the toxicity of silver nanoparticles on freshwater decomposers of plant litter in streams? Ecotoxicol. Environ. Saf. 2017, 140, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Beddow, J.; Stolpe, B.; Cole, P.; Lead, J.R.; Sapp, M.; Lyons, B.P.; Colbeck, I.; Whitby, C. Effects of engineered silver nanoparticles on the growth and activity of ecologically important microbes. Environ. Microbiol. Rep. 2014, 6, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, C.; Yang, Y.; Moreira Soares, H.; Alvarez, P.J. Silver nanoparticles temporarily retard NO2− production without significantly affecting N2O release by Nitrosomonas europaea. Environ. Toxicol. Chem. 2015, 34, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.; Giska, J.; Radniecki, T.; Semprini, L. Effects of short-and long-term exposure of silver nanoparticles and silver ions to Nitrosomonas europaea biofilms and planktonic cells. Chemosphere 2018, 206, 606–614. [Google Scholar] [CrossRef]
- Grün, A.-L.; Emmerling, C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on major soil bacterial phyla of a loamy soil. Environ. Sci. Eur. 2018, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Palmer, G.R.; Shah, V.; Walker, V.K. The effect of silver nanoparticles on seasonal change in arctic tundra bacterial and fungal assemblages. PLoS ONE 2014, 9, e99953. [Google Scholar] [CrossRef] [PubMed]
- Schlich, K.; Beule, L.; Hund-Rinke, K. Single versus repeated applications of CuO and Ag nanomaterials and their effect on soil microflora. Environ. Pollut. 2016, 215, 322–330. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Li, J.; Cui, L.; Xu, B.; Zhang, H.; Yu, C.-P. Interaction of silver nanoparticles with pure nitrifying bacteria. Chemosphere 2013, 90, 1404–1411. [Google Scholar] [CrossRef]
- Huang, J.; Chong, C.; Runqing, L.; Wenzhu, G. Effects of silver nanoparticles on soil ammonia-oxidizing microorganisms under temperatures of 25 and 5 °C. Pedosphere 2018, 28, 607–616. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ni, J.; Sun, Y.; Xue, L.; Feng, Y.; Yu, Y.; Lin, X.; Yang, L. Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles. Chemosphere 2016, 147, 195–202. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, X.; He, S.; Dong, G.; Chen, M.; Wang, J.; Lin, X. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Khalaf, D.M. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agric. Ecosyst. Environ. 2016, 218, 163–177. [Google Scholar] [CrossRef]
- McGee, C.; Storey, S.; Clipson, N.; Doyle, E. Soil microbial community responses to contamination with silver, aluminium oxide and silicon dioxide nanoparticles. Ecotoxicology 2017, 26, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Collins, D.; Walker, V.K.; Shah, S. The impact of engineered cobalt, iron, nickel and silver nanoparticles on soil bacterial diversity under field conditions. Environ. Res. Lett. 2014, 9, 024001. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Shah, V.; Walker, V.K. Influence of a nanoparticle mixture on an arctic soil community. Environ. Toxicol. Chem. 2012, 31, 131–135. [Google Scholar] [CrossRef]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella Jr, M.F.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J. Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario. PLoS ONE 2013, 8, e57189. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, C.; Wilkinson, K.J.; Desrosiers, M.; Sauvé, S. Effects of silver nanoparticles on soil enzyme activities with and without added organic matter. Environ. Toxicol. Chem. 2014, 33, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55. [Google Scholar] [CrossRef]
- Kumari, P.; Rastogi, A.; Shukla, A.; Srivastava, S.; Yadav, S. Prospects of genetic engineering utilizing potential genes for regulating arsenic accumulation in plants. Chemosphere 2018, 211, 397–406. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Liu, W.; Li, X.-L.; Shen, S.-G.; Liang, S.-X.; Liu, C.; Shan, L. Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 2016, 95, 25–29. [Google Scholar] [CrossRef]
- Fulekar, M. Bioremediation Technology: Recent Advances; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Evangelou, M.W.; Papazoglou, E.G.; Robinson, B.H.; Schulin, R. Phytomanagement: Phytoremediation and the Production of Biomass for Economic Revenue on Contaminated Land. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 115–132. Available online: https://doi.org/10.1007/978-3-319-10395-2_9 (accessed on 22 April 2020).
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytoremediat. 2016, 18, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, S.; Murugan, N.; Ragavendran, C.; Thangabalu, R.; Natarajan, D. Phytoremediation potential of some agricultural plants on heavy metal contaminated mine waste soils, salem district, tamilnadu. Int. J. Phytoremediat. 2016, 18, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Bennicelli, R.; Stępniewska, Z.; Banach, A.; Szajnocha, K.; Ostrowski, J. The ability of Azolla caroliniana to remove heavy metals (Hg(II), Cr(III), Cr(VI)) from municipal waste water. Chemosphere 2004, 55, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Elmachliy, S.; Chefetz, B.; Tel-Or, E.; Vidal, L.; Canals, A.; Gedanken, A. Removal of silver and lead ions from water wastes using Azolla filiculoides, an aquatic plant, which adsorbs and reduces the ions into the corresponding metallic nanoparticles under microwave radiation in 5 min. Water Air Soil Pollut. 2011, 218, 365–370. [Google Scholar] [CrossRef]
- Macek, T.; Macková, M.; Káš, J. Exploitation of plants for the removal of organics in environmental remediation. Biotechnol. Adv. 2000, 18, 23–34. [Google Scholar] [CrossRef]
- Glick, B.R. Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003, 21, 383–393. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, S.; Bondar, O. Bioremediation and Phytoremediation: Best Approach for Rehabilitation of Soils for Future Use. In Remediation Measures for Radioactively Contaminated Areas; Gupta, D., Voronina, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 201–221. Available online: https://doi.org/10.1007/978-3-319-73398-2_9 (accessed on 20 April 2020).
- Sharma, P.; Pandey, S. Status of phytoremediation in world scenario. Int. J. Environ. Bioremediat. Biodegrad. 2014, 2, 178–191. [Google Scholar]
- Huang, Z.; Zeng, Z.; Chen, A.; Zeng, G.; Xiao, R.; Xu, P.; He, K.; Song, Z.; Hu, L.; Peng, M. Differential behaviors of silver nanoparticles and silver ions towards cysteine: Bioremediation and toxicity to Phanerochaete chrysosporium. Chemosphere 2018, 203, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Romeh, A.A.A. Green silver nanoparticles for enhancing the phytoremediation of soil and water contaminated by fipronil and degradation products. Water Air Soil Pollut. 2018, 229, 1–13. [Google Scholar] [CrossRef]
- Saraswathi, V.S.; Kamarudheen, N.; BhaskaraRao, K.; Santhakumar, K. Phytoremediation of dyes using Lagerstroemia speciosa mediated silver nanoparticles and its biofilm activity against clinical strains Pseudomonas aeruginosa. J. Photochem. Photobiol. B Biol. 2017, 168, 107–116. [Google Scholar] [CrossRef]
- Abbas, S.; Nasreen, S.; Haroon, A.; Ashraf, M.A. Synhesis of silver and copper nanoparticles from plants and application as adsorbents for naphthalene decontamination. Saudi J. Biol. Sci. 2020, 27, 1016–1023. [Google Scholar] [CrossRef]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Shrivastava, P. The role of corporations in achieving ecological sustainability. Acad. Manag. Rev. 1995, 20, 936–960. [Google Scholar] [CrossRef]
- Wigger, K.A.; Shepherd, D.A. We’re All in the Same Boat: A Collective Model of Preserving and Accessing Nature-Based Opportunities. Entrep. Theory Pract. 2020, 44, 587–617. [Google Scholar] [CrossRef]
- Laguir, I.; Stekelorum, R.; El Baz, J. Going green? Investigating the relationships between proactive environmental strategy, GSCM practices and performances of third-party logistics providers (TPLs). Prod. Plan. Control 2020, 1–14. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Frérot, H.; Faucon, M.P.; Willems, G.; Godé, C.; Courseaux, A.; Darracq, A.; Verbruggen, N.; Saumitou-Laprade, P. Genetic architecture of zinc hyperaccumulation in Arabidopsis halleri: The essential role of QTL × environment interactions. New Phytol. 2010, 187, 355–367. [Google Scholar] [CrossRef]
- Caille, N.; Zhao, F.; McGrath, S. Comparison of root absorption, translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytol. 2005, 165, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Kuyukina, M.; Ivshina, I. Advanced Rhodococcus biocatalysts for environmental biotechnologies. Catalysts 2019, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Rai, P.K.; Kim, K.-H.; Lee, S.S.; Lee, J.-H. Molecular mechanisms in phytoremediation of environmental contaminants and prospects of engineered transgenic plants/microbes. Sci. Total Environ. 2020, 705, 135858. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Cota-Ruiz, K.; Delgado-Rios, M.; Martínez-Martínez, A.; Núñez-Gastelum, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Current findings on terrestrial plants—Engineered nanomaterial interactions: Are plants capable of phytoremediating nanomaterials from soil? Curr. Opin. Environ. Sci. Health 2018, 6, 9–15. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Xu, W.; Liu, G.; Song, M.; Tan, Z.; Mao, Y.; Yin, Y.; Cai, Y.; Liu, J.; Jiang, G. Transformation and uptake of silver nanoparticles and silver ions in rice plant (Oryza sativa L.): The effect of iron plaque and dissolved iron. Environ. Sci. Nano 2020, 7, 599–609. [Google Scholar] [CrossRef]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
| Species | Size (Diameter in nm) | Concentration mg L−1 | Impacts | References |
|---|---|---|---|---|
| Solanum tuberosum L. | 20 | 2, 10, 20 | Increase in superoxide anion (O2¯) and reactive oxygen species (ROS); Significant induction in the activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT) | [74] |
| Oryza sativa L. | 20 | 0.2, 0.5, 1 | Significant decrease in fresh weights, root elongation, shoot and root, carotenoids contents, total chlorophyll; Increased level of hydrogen peroxide (H2O2) and lipid peroxidation (MDA) in shoots and roots, increased foliar proline accumulation, and decreased sugar contents | [69] |
| Wolffia globosa | 10 | 1, 2, 5, 8, 10 | Elevated level of malondialdehyde (MDA), ROS content, and SOD activity. Decrease in chlorophyll a, carotenoids, and soluble protein | [75] |
| Lycopersicon esculentum | 10–15 | 100, 1000 | Reduced in fruits productivity, significant decreases in root growth, chlorophyll contents, and increased activity of higher SOD content | [76] |
| Lycopersicon esculentum | 20 | 10, 20, 30 | Decrease in biomass, increased oxidative stress indicators content (H2O2 and MDA), induced antioxidative stress responses such as flavonoids, anthocyanins, CAT, peroxidase (POD), reduced chlorophyll content, upregulation of the expression of membrane transporters and xylem cells enlargement | [24] |
| Lycopersicon esculentum | 7–14 | 10 | Lower fruit production, induction of oxidative stress, decrease in photosynthetic rate, elevated activity of antioxidant enzymes | [70] |
| Arabidopsis thaliana | 10 | 0.2, 0.5, 3 | Root growth inhibition, decreased in chlorophyll content and disruption of the thylakoid membrane structure; alteration of transcription of antioxidant and aquaporin channels | [68] |
| Arabidopsis thaliana | 20 | 10–150 | Inhibition of Arabidopsis root gravitropism, lower auxin accumulation in root tips, also, downregulation of the expression of auxin receptor-related genes | [77] |
| Arabidopsis thaliana Populus deltoids × nigra, DN-34 | 5, 10, 25 | 0.01–100 | Toxicity of AgNPs increased with decreasing nanoparticles size; however, the stimulatory effect on fresh weight, evapotranspiration, and root elongation at sublethal concentrations | [78] |
| Arabidopsis thaliana | 10 | 12.5 | delay in flowering, decrease in petal development and vegetative growth, and pollen viability, downregulation of genes involved in floral development | [79] |
| Lemna minor | 10–80 | 0.005–0.04 | number of fronds decreased, growth reduction, chlorosis in leaves | [80] |
| Cymodocea nodosa | 35 | 0.0002–0.2 | length of leaves decreased, induction of oxidative stress indicator (H2O2), and antioxidative enzymes activity, less actin and tubulin filaments | [81] |
| Zea mays (seedlings) | 49 | 7.5 | Inhibition of root and leaf growth, induction of O2¯, H2O2, and MDA, increased activity of antioxidative enzymes (SOD, GR, APX), | [82] |
| Vicia faba | 25, 50, 75 | 100 | Size dependent growth decrease, leaf necrosis and damage, and stomatal conductivity decrease | [83] |
| Species | Size | Concentration | Major Functions | References |
|---|---|---|---|---|
| Escherichia coli and Nitrifying bacteria | 16 nm | 0.1–1 mg L−1 | AgNPs inhibit respiration and nitrification (lack of change in dissolved oxygen); the effect varies depending on the size and bioavailability of the NPs. | [109] |
| Ammonia-oxidizing bacteria (AOB) | 118 ± 11 nm | 0.5–50 mg L−1 | Silver treatments affect ammonia-oxidizing bacteria (AOB), reducing Nitrification potential rates. | [110] |
| Bacillus subtilis | 27 nm | 0.5–50 mg L−1 | The growth of B. subtilis is affected by AgNPs depending on the size of the AgNPs which probably plays a role in toxicity differences. | [110] |
| Nitrosomonas europaea | AgNPs (35 nm)/AgNO3 | 0.075–0.75 mg L−1 | The oxidation and production of NO2 by regulating the gene expression (nitric oxide reductase, ammonia monooxygenase, and nitrite reductase). | [111] |
| Nitrosomonas europaea | AgNPs (25.5 nm)/Ag+ | 0.1 mL/h of solution, 0.05–2 ppm Ag+, 1.5–20 ppm AgNPs | Low concentrations of AgNPs cause enzymatic inhibition (of ammonia monooxygenase enzyme) and high concentrations cause cell death. | [112] |
| Soil microbial community | BAM-N001 (20 nm)/AgNO3 | 0.01 mg/kg | The toxicity of AgNPs increases over time (possibly due to Ag+ release). AgNPs cause a decrease in biomass and the activity of soil microorganisms. | [97] |
| Soil bacterial phyla | BAM-N001 (20 nm)/AgNO3 | 0.01 mg/kg | Long-term exposure to AgNPs cause a decrease in several phyla of bacteria, affecting important functions of soil like nitrification or organic carbon transformation. | [113] |
| Bacterial and fungal assemblages | AgNPs (20 nm)/ AgMPs (3000 nm) | 0.066% and 6.6% | Small particles are more toxic (cause a decrease in respiration, signature bacterial fatty acids, changes in richness and evenness in bacterial and fungal DNA sequence assemblages). | [114] |
| Heterotrophic bacterial and nitrifying communities | NM-300K (15 nm) | 1.67 and 5 mg/kg supplied in one or three applications | Single application has a stronger effect on potential nitrification than split doses (i.e., same dose applied in 3 doses), whereas of respiration an opposite pattern is observed. | [115] |
| Azotobacter vinelandii | 10 and 50 nm | 2 and 10 mg L−1 for nano-Ag 10; 10 and 100 mg L−1 for nano-Ag 50 | 10 and 50 nm AgNPs induced apoptosis by 20.23% and 3.14%, reduced cell number, structural damage, inhibition of biological nitrogen fixation (BNF), ROS generation | [116] |
| Nitrosomonas europaea ATCC-19718 | 7 ± 3 (PVA doped) 40 ± 14 (Na2ATP doped) | 1, 5 and 10 mg L−1 | Capping and size dependent decrease in NH3 oxidation, cell wall damage, and disintegrated nuclei | [117] |
| Ammonia oxidizing microorganisms | 15 nm | 1, 10, and 100 µg g−1 dry soil | 10 and 100 μg g−1 AgNPs significantly inhibited soil urease activity and nitrification | [118] |
| Soil microbial activity | 20.4 and 10 nm | 0.1, 1, and 10 mg kg−1 soil | Decreased soil microbial metabolic activity, nitrification ability, and the abundances of ammonia-oxidizing bacteria at 0.1–10 mg kg−1 AgNPs | [119] |
| Mycorrhizal clover (Trifolium repens) | 20.6 ± 3.1 (AgNPs) | 0.01–1 mg kg−1 | Drastic decrease in biomass of mycorrhizal clover, root nutrient acquisition of AMF, and glomalin content | [120] |
| Glomus aggregatum-Faba bean | 5–50 | 800 μg kg−1 sandy soil-loam mixture | Lowered mycorrhizal colonization, glomalin content, and mycorrhizal responsiveness | [121] |
| AMF (unspecified)-Tomato | 2 and 15 nm | 12–36 mg kg−1 soil | Dose dependent AgNPs decrease in AMF colonization | [25] |
| Soil microbial activity | 20 nm ± 10 | 50 mg kg−1 | Decrease in urease and dehydrogenase activity, bacterial and archaeal amoA gene abundance in soil | [122] |
| Soil microbial activity | 2–50 nm (average 35 nm) | 550 mg/pot | Pyrosequence analysis showed no significant effect on soil microbial richness; however, individual analysis affected bacterial groups | [123] |
| Soil microbial activity | 15–20 nm | 220 mg kg−1 | Decrease in C and N biomass and modification of microbial community structure | [124] |
| Soil microbial activity | 21 ± 17 nm | 0.14 mg kg−1 | AgNPs caused a modification in the bacterial community | [125] |
| Soil microbial activity | 10 and 50 nm | 1600, or 3200 µg Ag kg−1 dry soil | Decreases in enzymatic activities due to AgNPs | [126] |
| Soil microbial activity | 20.08 nm ± 2.24 | 1–1000 mg kg1 | AgNPs induced effects on enzymes | [99] |
| Plant | Type of NPs | Description | Effect | Reference |
|---|---|---|---|---|
| Phragmites australis | AgNPs | It accumulated silver only in roots and in leaves and stems there was no metal accumulation | Phytoremediation in estuarine areas, Phytostabilization | [49] |
| Pistia stratiotes | AgNPs | Extracted silver from source in short period of time, Easy handling and can be used in polyculture | Phytoremediation of water source | [51] |
| Egeria densa | AgNPs | Plants absorbed AgNPs at concentrations as low as 5 ppm, bioaccumulation proportional to concentration of NPs | Phytoremediation of water source | [56] |
| Phanerochaete chrysosporium | AgNPs | Uptake of NPs in presence of cysteine amino acids | Phytoremediation of aquatic source | [144] |
| Zea mays | AgNPs | NPs increase the bioremediation potential of three PGPRs isolated from municipal wastewater | Phytoremediation of municipal wastewater | [134] |
| Ipomoea carnea, Plantago major, Camellia sinensis | AgNPs | Green synthesized NPs from few medicinal plants displayed bioremediation potential | Fipronil (an insecticide) contaminated water | [145] |
| Lagerstroemia speciosa | AgNPs | methyl orange and methylene blue showing 310- and 290-min degradation time, respectively | methyl orange and methylene blue dyes | [146] |
| Aloe barbedensis, Azadirachta indica and Coriandrum sativum | AgNPs and CuNPs | Green synthesized NPs from three plants decontaminated naphthalene in water | Wastewater remediation | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihtisham, M.; Noori, A.; Yadav, S.; Sarraf, M.; Kumari, P.; Brestic, M.; Imran, M.; Jiang, F.; Yan, X.; Rastogi, A. Silver Nanoparticle’s Toxicological Effects and Phytoremediation. Nanomaterials 2021, 11, 2164. https://doi.org/10.3390/nano11092164
Ihtisham M, Noori A, Yadav S, Sarraf M, Kumari P, Brestic M, Imran M, Jiang F, Yan X, Rastogi A. Silver Nanoparticle’s Toxicological Effects and Phytoremediation. Nanomaterials. 2021; 11(9):2164. https://doi.org/10.3390/nano11092164
Chicago/Turabian StyleIhtisham, Muhammad, Azam Noori, Saurabh Yadav, Mohammad Sarraf, Pragati Kumari, Marian Brestic, Muhammad Imran, Fuxing Jiang, Xiaojun Yan, and Anshu Rastogi. 2021. "Silver Nanoparticle’s Toxicological Effects and Phytoremediation" Nanomaterials 11, no. 9: 2164. https://doi.org/10.3390/nano11092164
APA StyleIhtisham, M., Noori, A., Yadav, S., Sarraf, M., Kumari, P., Brestic, M., Imran, M., Jiang, F., Yan, X., & Rastogi, A. (2021). Silver Nanoparticle’s Toxicological Effects and Phytoremediation. Nanomaterials, 11(9), 2164. https://doi.org/10.3390/nano11092164