Abstract
Amorphous CeO2-TiO2 nanoparticles synthesized by the H2O2-modified sol-gel method were investigated in terms of the Ce-O-Ce and Ti-O-Ti linkage, local structure, and redox properties. The decrease in the crystallinity of CeO2-TiO2 by H2O2 addition was confirmed. The metal–oxygen linkage analysis showed the difference in size of the metal–oxygen network between crystalline CeO2-TiO2 and amorphous CeO2-TiO2 due to the O22− formed by H2O2. The local structure of CeO2-TiO2 was analyzed with an extended X-ray absorption fine structure (EXAFS), and the oscillation changes in the k space revealed the disordering of CeO2-TiO2. The decrease in Ce-O bond length and the Ce-O peak broadening was attributed to O22− interfering with the formation of the extended metal–oxygen network. The temperature-programmed reduction of the H2 profile of amorphous CeO2-TiO2 exhibited the disappearance of the bulk oxygen reduction peak and a low-temperature shift of the surface oxygen reduction peak. The H2 consumption increased compared to crystalline CeO2-TiO2, which indicated the improvement of redox properties by amorphization.
1. Introduction
Metal oxides have been widely studied in from a number of research angles, including their uses in catalysts, in electrochemistry, and as gas sensors [1]. Cerium oxide, CeO2, is an important rare-earth oxide with a redox pair of Ce3+/Ce4+, which enables the storage/release of oxygen located at the tetrahedral site under an oxidation/reduction atmosphere [2]. However, CeO2 is mainly used with other metal oxides because of its low redox property, specific surface area, and the thermal stability of pure CeO2. TiO2 has been extensively investigated as a CeO2-TiO2 mixed oxide due to its advantages, such as a highly specific surface area, outstanding thermal and chemical stability, and nontoxicity. CeO2-TiO2 nanoparticles have attracted much interest for use in various areas where oxidation/reduction reactions of cerium ions are important, such as de-NOx catalysts, photocatalysts, and water gas shift catalysts [3,4,5]. CeO2-TiO2 nanoparticles have various properties depending on their crystallinity, and many studies have reported the improvement of the catalytic performance of CeO2 and TiO2 through amorphization [6,7].
Amorphous oxides have great potential, and their method of synthesis has been highlighted, as amorphous oxides have excellent physicochemical properties compared to crystalline oxides [8]. CeO2-TiO2 nanoparticles are synthesized by the sol-gel method, hydrothermal routes, and coprecipitation [9,10,11]. The sol-gel method is widely used and has the advantages of obtaining uniform particles and a highly specific area [12]. The structure of the oxide synthesized through this method is modified according to the degree of hydrolysis, which is affected by additives or the amount of H2O [13,14]. Modifying the hydrolysis and condensation reaction causes changes in structure—for example, in the crystallinity and microstructure [15,16,17]. Therefore, it is important to understand the mechanism of hydrolysis and condensation reactions in terms of local structure in the sol-gel reaction and to reveal the relationship between structural properties and physicochemical properties.
In this study, the metal–oxygen linkage, local structure, and redox properties of CeO2-TiO2 nanoparticles were synthesized by the H2O2-modified sol-gel method. The effect of H2O2 on the linkage in the sol-gel was verified by Fourier transform infrared spectroscopy. The local structure was analyzed using an extended X-ray absorption fine structure (EXAFS). The relationship between the local structure and the redox properties was examined by H2-temperature-programmed reduction.
2. Experimental Procedure
2.1. Sample Preparation
The samples were synthesized by the sol-gel method with a molar ratio of Ce:Ti = 3:7; cerium nitrate and titanium isopropoxide (TTIP, Ti[OCH(CH3)2]4, Sigma-Aldrich, St. Louis, MO, USA, 97%) were used as precursors of CeO2 and TiO2, respectively. TTIP was added to ethanol while stirring. Here, we added H2O2 at a volume ratio of TTIP:35% H2O2 = 1:5 after the addition of TTIP. Distilled water was poured into the mixed solution to start the hydrolysis of TTIP. Cerium nitrate hexahydrate (Ce(NO3)3 6H2O, Sigma-Aldrich, St. Louis, USA, MO, 99%) was added to the mixed solution, then ammonia hydroxide solution (NH4OH 25% in H2O, Junsei, Tokyo, Japan) was slowly added until the pH of the mixed solution reached approximately 10. The filtered precipitate was dried at 80 °C and calcined at 550 °C to obtain a CeO2-TiO2 powder. The prepared oxides were named CT and CT_H2O2 according to the H2O2 addition.
2.2. Characterization
Powder X-ray diffraction (XRD) was measured by an X-ray diffractometer (X’pert pro MPD, Almelo, The Netherlands) with Cu Kα (40 kV, 40 mA) radiation. High-resolution transmission electron microscopy (HR-TEM) images and selected-area electron diffraction (SAED) patterns were obtained using field emission (JEOL JEM-2100, Tokyo, Japan) at the KBSi Busan Center. Fourier transform infrared spectroscopy (FTIR) spectra were recorded using a Vertex 80 v FTIR spectrometer (Bruker, Billericay, MA, USA) over the range of 400~4000 cm−1 with KBr pellets.
X-ray absorption spectroscopy experiments were conducted at the Ce L3-edge using the extended X-ray absorption fine structure (EXAFS) facility of the 10C-wide XAFS beam line at the Pohang Accelerator Laboratory (Pohang, Korea). The storage ring was operated at 2.5 GeV with an injection current of 251 mA using a Si (111) double-crystal monochromator. The X-ray absorption spectroscopy (XAFS) data at the Ce L3 edge were collected at room temperature in transmission mode. The background intensity was removed and normalized using Fourier transform under k-weight 3 to measure the interatomic distance. The XAFS data were analyzed using the Athena and Artemis software packages.
The Brunauer−Emmett−Teller (BET) surface area was measured by N2 adsorption/desorption with a Micromeritics 2020 M instrument (Micromeritics, Norcross, GA, USA). H2 temperature-programmed reduction (H2-TPR) was performed using a Quantachrome with a Chem BET chemisorption analyzer (Micromeritics, Norcross, GA, USA) with 100 mg of the sample in a quartz tube reactor with a thermal conductivity detector (TCD). The sample was preheated from room temperature to 200 °C for 1 h and then cooled down to room temperature. TPR was performed at 10 °C/min up to 800 °C using 10 vol% H2 in He gas. CuO was used as a reference for calibration to quantify the total amount of H2 consumed.
3. Results and Discussion
The crystallinity and phase of the synthesized CeO2-TiO2 were investigated by XRD analysis, as shown in Figure 1, and each pattern corresponded to CT and CT_H2O2. Anatase TiO2 and CeO2 peaks were confirmed in the CT pattern, revealing that CT consisted of CeO2 and TiO2 and that there were no secondary peaks. On the other hand, the broadening of those peaks was observed in the pattern of CT_H2O2 compared to CT, indicating that CT_H2O2 had a low crystallinity of CeO2 and TiO2 or consisted of very small nanoparticles. This finding is similar to that of previous studies, indicating that CT_H2O2 had very low crystallinity through a broad peak and a low intensity [18,19]. Further analysis was performed to examine the structure of the CeO2-TiO2 changed by H2O2.
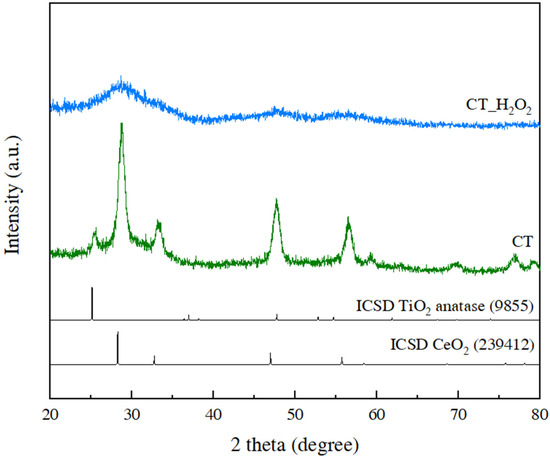
Figure 1.
XRD patterns of CT and CT_H2O2.
The microstructure and crystallinity of CT and CT_H2O2 were verified through TEM images and SAED patterns, as displayed in Figure 2. Particles of approximately 10 nm with lattice fringes were observed in the TEM image of CT, indicating that they had a crystalline structure (Figure 2a). These crystalline structures were composed of a CeO2 (111) plane with an interplanar spacing of 0.31 nm and a TiO2 (101) plane with a 0.35 nm interplanar spacing. The SAED pattern of CT showed ring patterns, meaning that CT existed in crystalline form (Figure 2b). In contrast, an amorphous structure was found in the TEM image of CT_H2O2 (Figure 2c), and a diffuse ring pattern was confirmed (Figure 2d). Based on these results, H2O2 addition was the cause of the amorphization of CeO2-TiO2.
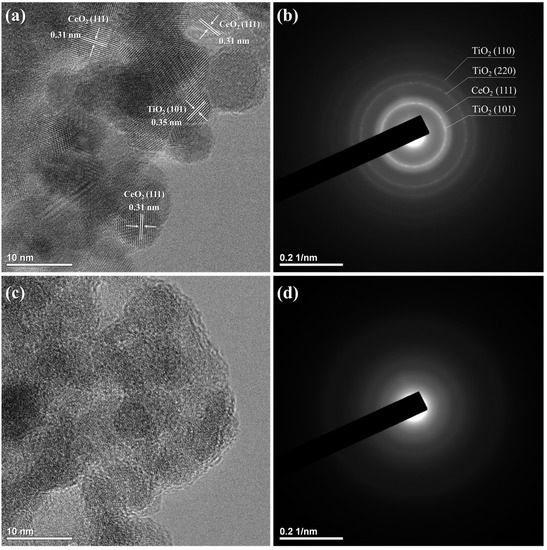
Figure 2.
TEM images and SAED patterns of (a,b) CT and (c,d) CT_H2O2.
FTIR analysis was carried out to investigate the effect of H2O2 on CeO2-TiO2 linkage formation in sol-gel chemistry. The calcination process is generally necessary in the sol-gel synthesis of oxides, and impurities—including OH groups and organic matter—are removed when calcination is performed for precipitation [20]. The FTIR spectra of CT and CT_H2O2 before and after calcination are shown in Figure 3, and all peaks were classified through the literature [21,22,23,24,25]. The characteristic absorption peak of TTIP was in the range of 1085–1050 cm−1. Since characteristic peaks were not detected for all samples, all TTIPs participated in the sol-gel reaction. A reduced CH3 peak at 1338 cm−1 revealed that the organic matter was removed by calcination. Peaks assigned to the -OH group appeared at 3542 cm−1 and 1630 cm−1, and the peak intensities of the samples before calcination were greater than those after calcination. It should be noted that CT_H2O2 had stronger OH peaks than CT and had an O–O peak representing a peroxy group at 900 cm−1. In addition, the Ce-O22− peak at 842 cm−1 demonstrated that H2O2 formed O22− [26]. The difference between the OH peaks of CT and CT_H2O2 is explained by O22− (peroxy ions).
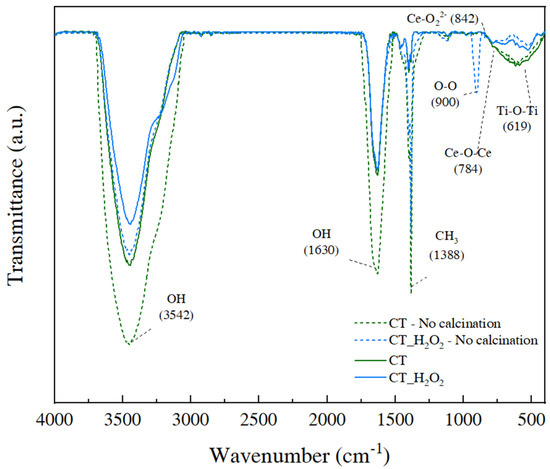
Figure 3.
FTIR spectra of CT and CT_H2O2 before calcination.
The Ce-O-Ce and Ti-O-Ti peaks in the fingerprint region of 800 to 400 cm−1 indicate the metal–oxygen linkage and bonding network. The one large peak in this region is due to the Ce-O-Ti linkage, confirming that the cerium species react via a cross-link and covalently bond on the Ti-O-Ti [25,27]. A wide peak in this region in the CT spectrum indicated a large network [28]. The intensity of this peak decreased after adding H2O2, and the intensity of this peak indicates the size of the Ce-O-Ce or Ti-O-Ti network. This decrease occurred because O22− interfered with the hydrolysis and condensation reactions and thus affected the formation of the Ti-O-Ti network.
EXAFS is a powerful technique for analyzing the local structure of amorphous materials [26]. The EXAFS k3χ data and Fourier transform (F.T.) of Ce L3 edge are shown in Figure 4. It was confirmed that CT had a long-range order but that CT_H2O2 had a short-range order resulting from the broken order. As corresponding results, the oscillation differences between CT and CT_H2O2 were clearly seen in the red dotted circle of the k space at the Ce L3-edge (Figure 4a), and severe noise due to the disordered structure of the amorphous phase was observed. EXAFS F.T. data at the Ce L3 edge (Figure 4b) provide information on the bond length and the disordering around the Ce atoms. The first and third peaks are assigned to the 1st shell and 2nd shell, respectively, indicating the distances between the Ce-O and Ce-Ce atoms. The second peak is the Ce-Ti bond assigned to an interaction between Ce-Ti forming amorphous Ce [29]. In CT, the bonding length of Ce-O was 1.84 Å and that of Ce-Ce was 3.53 Å. For CT_H2O2, the bond length of Ce-O decreased to 1.68 Å, and no Ce-Ce peak was observed. Additionally, the broadening of the 1st shell peak was confirmed with the addition of H2O2, which is related to amorphization due to disordering, revealing the disordering of the oxygen around Ce [30]. The peak intensity ratio of the 1st shell and the 2nd shell (I2nd/I1st) indicates the degree of amorphousness, and this ratio decreased from 0.56 to 0.15, indicating amorphization by H2O2.
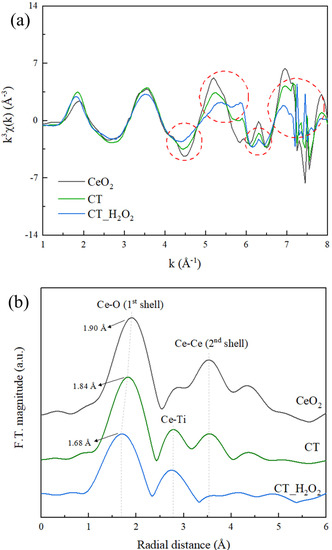
Figure 4.
(a) Extended X-ray absorption fine structure (EXAFS) k3χ data and (b) Fourier transform of the EXAFS k3χ data at the Ce L3-edge EXAFS of CT and CT_H2O2.
This disordering occurs because the strong nucleophilicity of O22− causes strong bonding with Ti ions, which interferes with the binding of OH (Figure 5). In more detail, this could be described as the hydrolysis reaction (Equations (1) and (2)) and condensation reaction (Equations (3) and (4)) in sol-gel chemistry [15,17].
Hydrolysis: Ti(OiPr)4 + 2H2O → Ti(OH)4 + 4iPrOH,
Ti(OiPr)4 + H2O2 + 2H2O → Ti(OO)2−2 + 4iPrOH + 6H+,
Condensation: -Ti-OH + HO-Ti → -Ti-O-Ti- + H2O,
-Ti-OH + iPrO-Ti- → -Ti-O-Ti- + iPrOH.
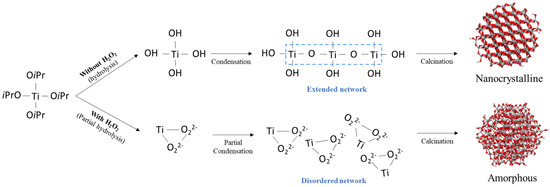
Figure 5.
Schematic showing the effect of H2O2 on the local structure.
TTIP (Ti(OiPr)4) reacts with H2O and a hydrolysis reaction occurs to form -Ti-OH (Equation (1)). The condensation reaction also simultaneously occurs to release one H2O or iPrOH molecule, forming an extended network of Ti-O-Ti (Equations (3) and (4)). In the H2O2 addition, O22− is strongly bound to Ti ions, eventually inhibiting the binding to OH (Equation (2)). As a result, the hydrolysis and condensation reactions of TTIP would have partially occurred. It is concluded that the extended network of Ti-O-Ti was not formed due to partial hydrolysis by O22−, leading to amorphization.
The redox properties of CeO2-TiO2 according to amorphization were analyzed by H2-TPR, and the results are illustrated in Figure 6. The H2 consumption and specific surface area were 1110 mmol/g and 103.0 m2/g for CT, and 1354 mmol/g and 113.1 m2/g for CT_H2O2, respectively as shown in Table 1. This is due to the smaller particle size of CT_H2O2 or the formation of dangling bonds due to the disordering of Ce-O [31,32,33]. These two facts suggest that the active surface area of CT_H2O2 is larger. CT had two peaks observed at 490 °C and 767 °C, which corresponded to the reduction from Ce4+ to Ce3+ by the surface oxygen of CeO2 and the lattice oxygen of CeO2, respectively [34]. CT_H2O2 showed a peak due to the surface oxygen at only 468 °C and 558 °C, and there was no reduction peak assigned to the lattice CeO2, which implies that CT_H2O2 is amorphous [35,36]. The surface CeO2 peak shifted to low temperature by amorphization, and the H2 consumption increased, confirming the improvement of the redox property. The atoms of the crystalline oxide are tightly bonded by strong intermolecular forces, while the atoms of the amorphous oxide are loosely bonded. Consequently, the improvement of the redox property was because the Ce-O-Ce bond was weak and easily reduced by the disordered structure of CT_H2O2.
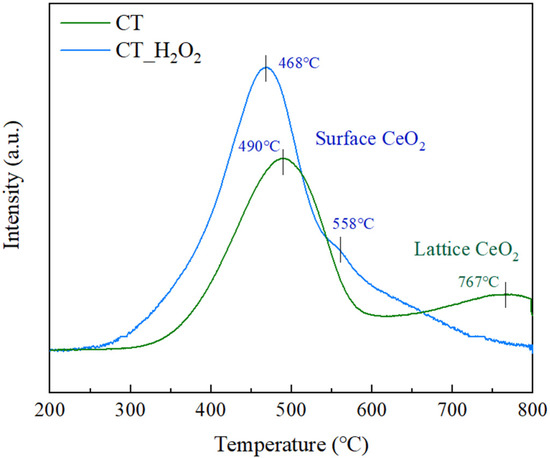
Figure 6.
H2-TPR profiles of CT and CT_H2O2.

Table 1.
H2-TPR results and BET surface area of CT and CT_H2O2.
4. Conclusions
We studied the effect of H2O2 on the Ce-O-Ti linkage in the sol-gel reaction in terms of local structure for the first time. The decrease in the crystallinity of CeO2-TiO2 with the addition of H2O2 was verified through XRD peak broadening, and it was confirmed that the SAED pattern of CeO2-TiO2 became a diffuse pattern. CeO2 and crystalline TiO2 were observed for CT, whereas an amorphous structure was observed for CT_H2O2. In the FTIR spectrum of CT_H2O2, the O22− peak attributed to H2O2 was observed, and the broadening of the metal–oxygen peak showed that the size of the metal–oxygen network decreased due to O22−. The local structure of the synthesized samples was analyzed using EXAFS. Severe noise and oscillation differences in the k space were observed by the disordered structure in CT_H2O2; furthermore, the decrease in the Ce-O bond length and the broadening of the Ce-O peak in R space were identified. It was found that O22− interfered with the formation of the metal–oxygen extended network. In the H2-TPR analysis to investigate the redox property according to the amorphization, a low-temperature shift in the surface CeO2 reduction peak and an increase in H2 consumption indicated an improvement in the redox property due to amorphization. The improved redox property of amorphous CeO2-TiO2 resulted from the metal–oxygen bond being weakened by disordered oxygen.
Author Contributions
Conceptualization, H.L.; Formal analysis, M.K.; Investigation, M.K., G.P. and H.L.; Methodology, M.K. and G.P.; Supervision, H.L.; Visualization, M.K.; Writing–original draft, M.K.; Writing–review & editing, G.P. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Ministry of Trade, Industry, and Energy (MOTIE) as “Development of NOx Removal Catalyst with Wide Temperature Window (No.20005721)”. This research was supported by the BK21 FOUR Program (NO.4120200513801) funded by the Ministry of Education(MOE, Korea) and National Research Foundation of Korea(NRF).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Trovarelli, A.; Boaro, M.; Rocchini, E.; de Leitenburg, C.; Dolcetti, G. Some recent developments in the characterization of ceria-based catalysts. J. Alloys Compd. 2001, 323, 584–591. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef]
- Fang, N.; Ding, Y.; Liu, C.; Chen, Z. Effect of crystalline/amorphous structure on light absorption and carrier separationof CeO2-TiO2 heterojunctions. Appl. Surf. Sci. 2018, 452, 49–57. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2–TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Curry-Hyde, H.E.; Musch, H.; Baiker, A. Selective catalytic reduction of nitric oxide over amorphous and crystalline chromia: I. Comparative study of activities. Appl. Catal. 1990, 65, 211–223. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Xu, W.; Li, J. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Li, L.; Shao, Q.; Huang, X. Amorphous oxide nanostructures for advanced electrocatalysis. Chem. Eur. J. 2020, 26, 3943–3960. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol–gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Zhao, Z.; Zhou, W.; Wang, D.; Kang, X.; Liu, H.; Wang, J.; Chen, S.; Cai, H.; et al. Enhanced photocatalytic performances of CeO2/TiO2 nanobelt heterostructures. Small 2013, 9, 3864–3872. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Frenzer, G.; Maier, W.F. Amorphous porous mixed oxides: Sol-gel ways to a highly versatile class of materials and catalysts. Annu. Rev. Mater. Res. 2006, 36, 281–331. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Søgaard, E.G. Sol–gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.I.; Ahn, B.Y.; Pramanik, N.C.; Kim, H.; Hong, S.I. Preparation of nanosized rutile TiO2 from an aqueous peroxotitanate solution. J. Am. Ceram. Soc. 2006, 89, 1147–1149. [Google Scholar] [CrossRef]
- Shen, C.; Shaw, L.L. FTIR analysis of the hydrolysis rate in the sol–gel formation of gadolinia-doped ceria with acetylacetonate precursors. J. Sol-Gel Sci. Technol. 2010, 53, 571–577. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Gao, J.; Xie, F. An amorphous TiO2 sol sensitized with H2O2 with the enhancement of photocatalytic activity. J. Alloys Compd. 2010, 497, 420–427. [Google Scholar] [CrossRef]
- Gao, Y.; Masuda, Y.; Seo, W.S.; Ohta, H.; Koumoto, K. TiO2 nanoparticles prepared using an aqueous peroxotitanate solution. Ceram. Int. 2004, 30, 1365–1368. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Nanostructured TiO2–CeO2 mixed oxides by an aqueous sol–gel process: Effect of Ce: Ti molar ratio on physical and sensing properties. Sens. Actuators B Chem. 2010, 150, 631–640. [Google Scholar] [CrossRef]
- Sharma, N.; Deepa, M.; Varshney, P.; Agnihotry, S.A. FTIR and absorption edge studies on tungsten oxide based precursor materials synthesized by sol–gel technique. J. Non-Cryst. Solids 2002, 306, 129–137. [Google Scholar] [CrossRef]
- Damatov, D.; Mayer, J.M. (Hydro)peroxide ligands on colloidal cerium oxide nanoparticles. Chem. Commun. 2016, 52, 10281–10284. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-gel-assisted microwave-derived synthesis of anatase Ag/TiO2/GO nanohybrids toward efficient visible light phenol degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Wandre, T.M.; Gaikwad, P.N.; Tapase, A.S.; Garadkar, K.; Vanalakar, S.A.; Lokhande, P.D.; Sasikala, R.; Hankare, P.P. Sol–gel synthesized TiO2–CeO2 nanocomposite: An efficient photocatalyst for degradation of methyl orange under sunlight. J. Mater. Sci. Mater. Electron. 2016, 27, 825–833. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Nicolae, C.A.; Frone, A.N.; RĂDIŢOIU, A.; Raduly, F.M.; ŞOMOGHI, R.; Gabor, R.A.; Căprărescu, S. Synthesis and morpho-structural properties of TiO2-based materials. J. Optoelectron. Adv. Mater. 2019, 21, 281–286. [Google Scholar]
- Ksapabutr, B.; Gulari, E.; Wongkasemjit, S. Sol–gel derived porous ceria powders using cerium glycolate complex as precursor. Mater. Chem. Phys. 2006, 99, 318–324. [Google Scholar] [CrossRef]
- Teo, B.K. EXAFS: Basic Principles and Data Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 9. [Google Scholar]
- Jo, S.H.; Lee, H. Local structure and short-range ordering of MnO2–Ce(1−x) ZrxO2/TiO2. Mater. Charact. 2015, 110, 102–108. [Google Scholar] [CrossRef]
- Jo, S.H.; Lee, I.; Park, H.; Lee, H. Experimental evidence and mechanism of the oxygen storage capacity in MnO2-Ce(1−x)ZrxO2/TiO2 catalyst for low-temperature SCR. Ceram. Int. 2017, 43, 5182–5188. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, M.; Cha, J.; Kim, H.; Lee, H. Local atomic structure and enhanced catalytic activity of W doped CeWTiOx catalysts. J. Solid State Chem. 2020, 292, 121689. [Google Scholar] [CrossRef]
- Neri, G.; Pistone, A.; Milone, C.; Galvagno, S. Wet air oxidation of p-coumaric acid over promoted ceria catalysts. Appl. Catal. B Environ. 2002, 38, 321–329. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.L.; Fei, Z.Y.; Chen, X.; Tang, J.H.; Cui, M.F.; Qiao, X. Structure and properties of amorphous CeO2@TiO2 catalyst and its performance in the selective catalytic reduction of NO with NH3. J. Fuel Chem. Technol. 2016, 44, 954–960. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, Y.; Wang, M.; Tao, Z.; Liu, Q.; Chen, X.; Cui, M.; Zhang, Z.; Tang, J.; Qiao, X. Precisely fabricating Ce-O-Ti structure to enhance performance of Ce-Ti based catalysts for selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 353, 930–939. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).