Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO
Abstract
:1. Introduction
2. Experimental
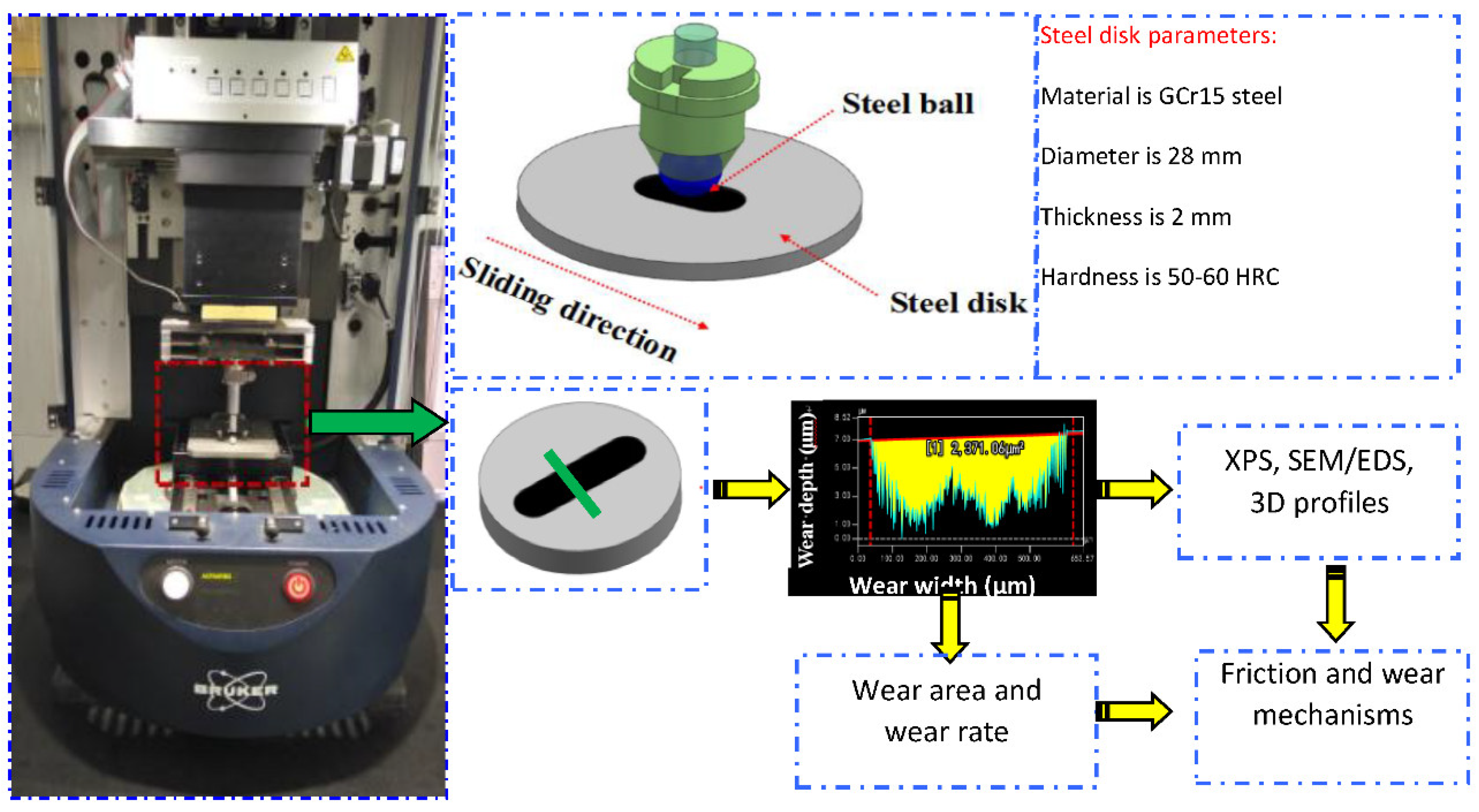
2.1. Raw Materials and Instruments
2.2. Analysis Method
3. Results and Discussion
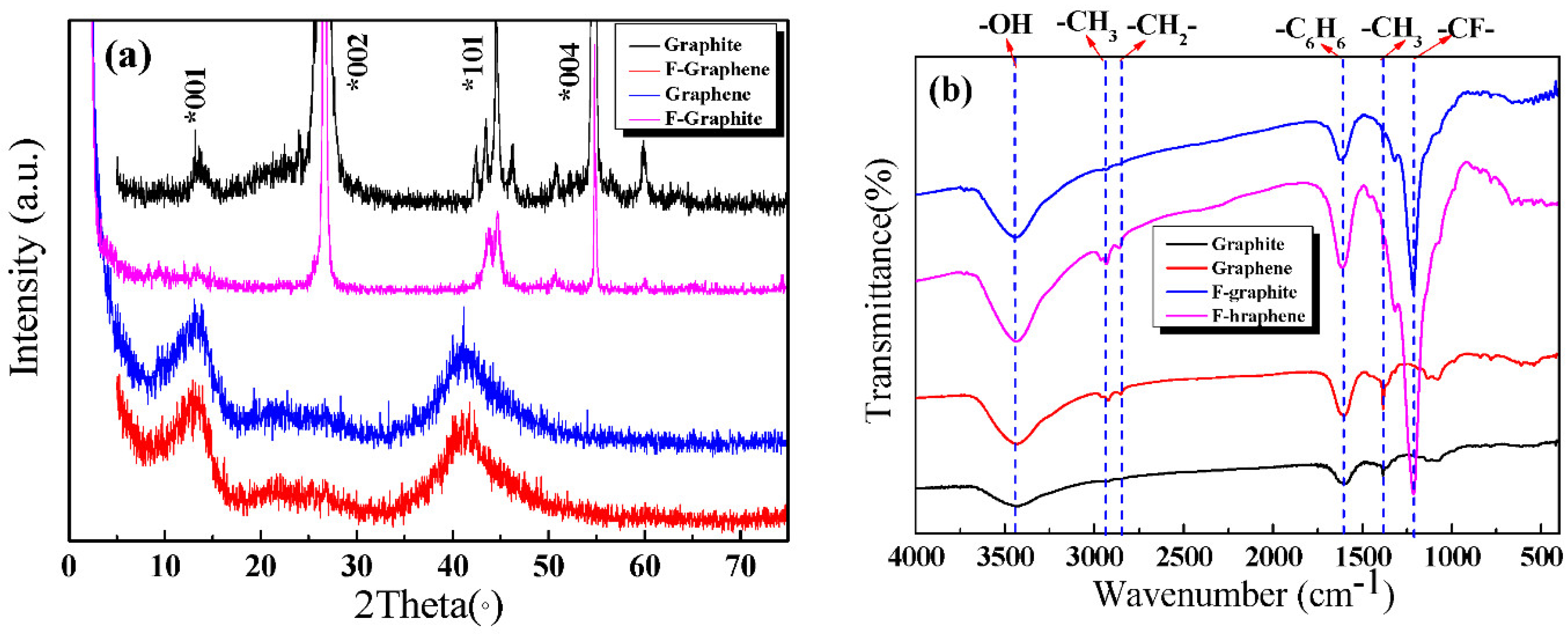
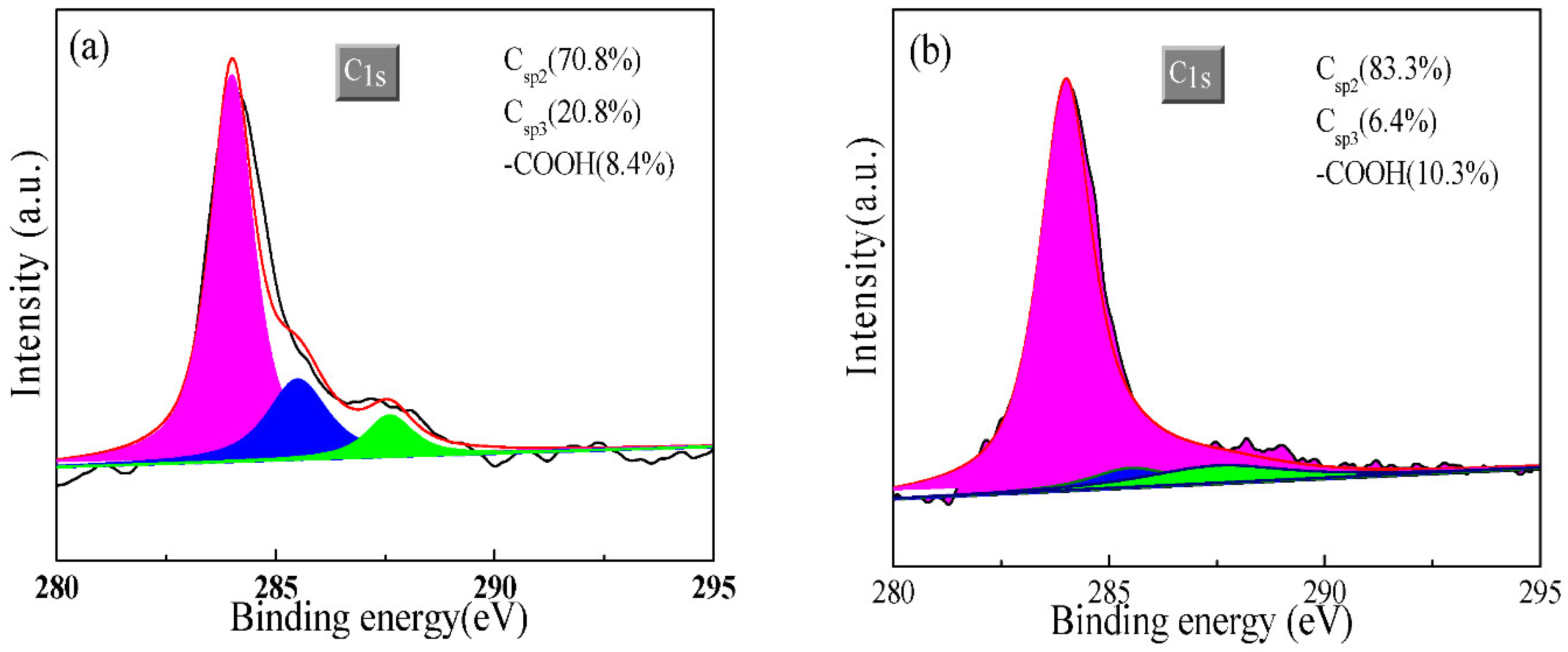
3.1. Characterization of Carbon Materials
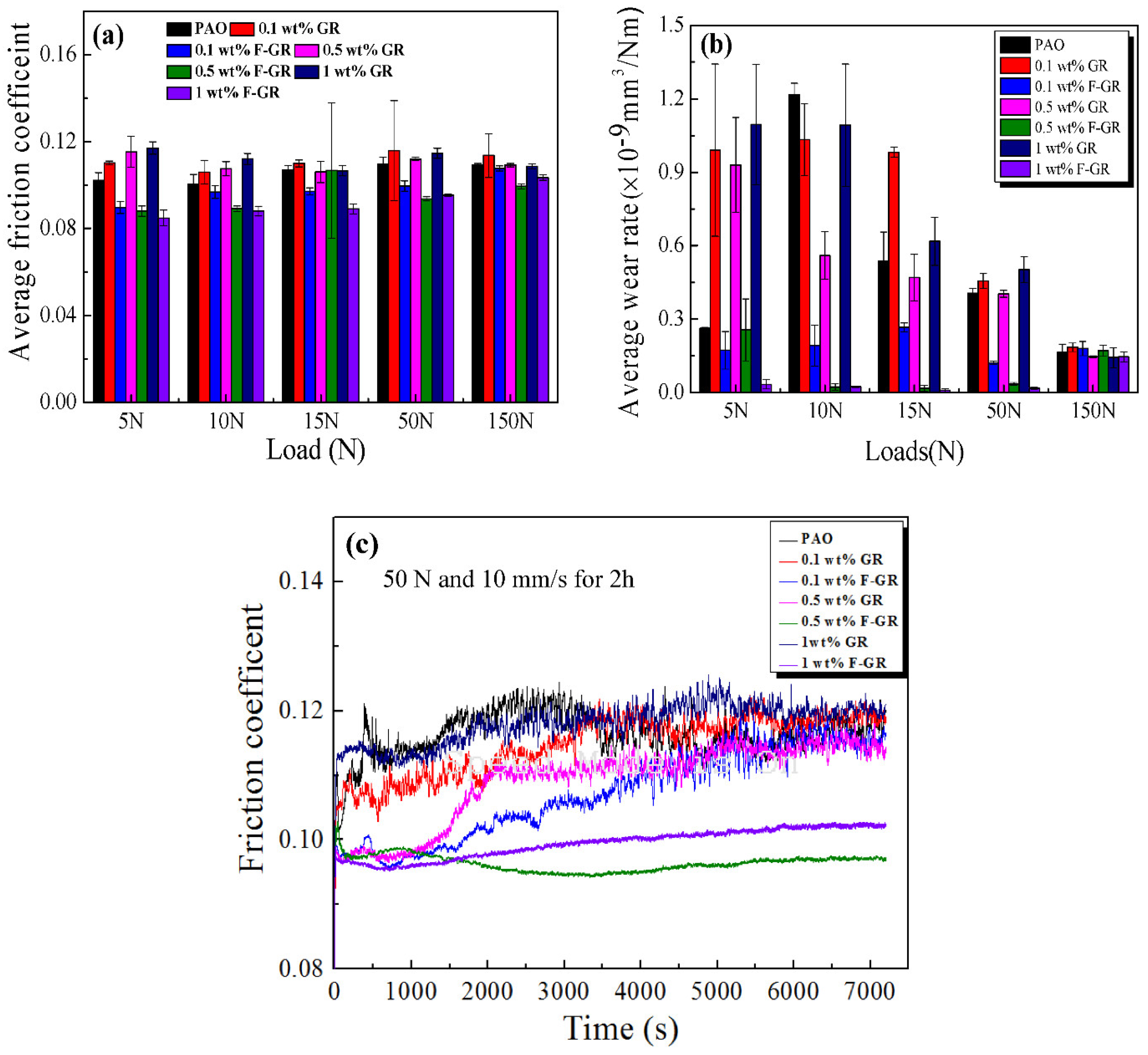
3.2. Anti-Wear and Anti-Friction Performances
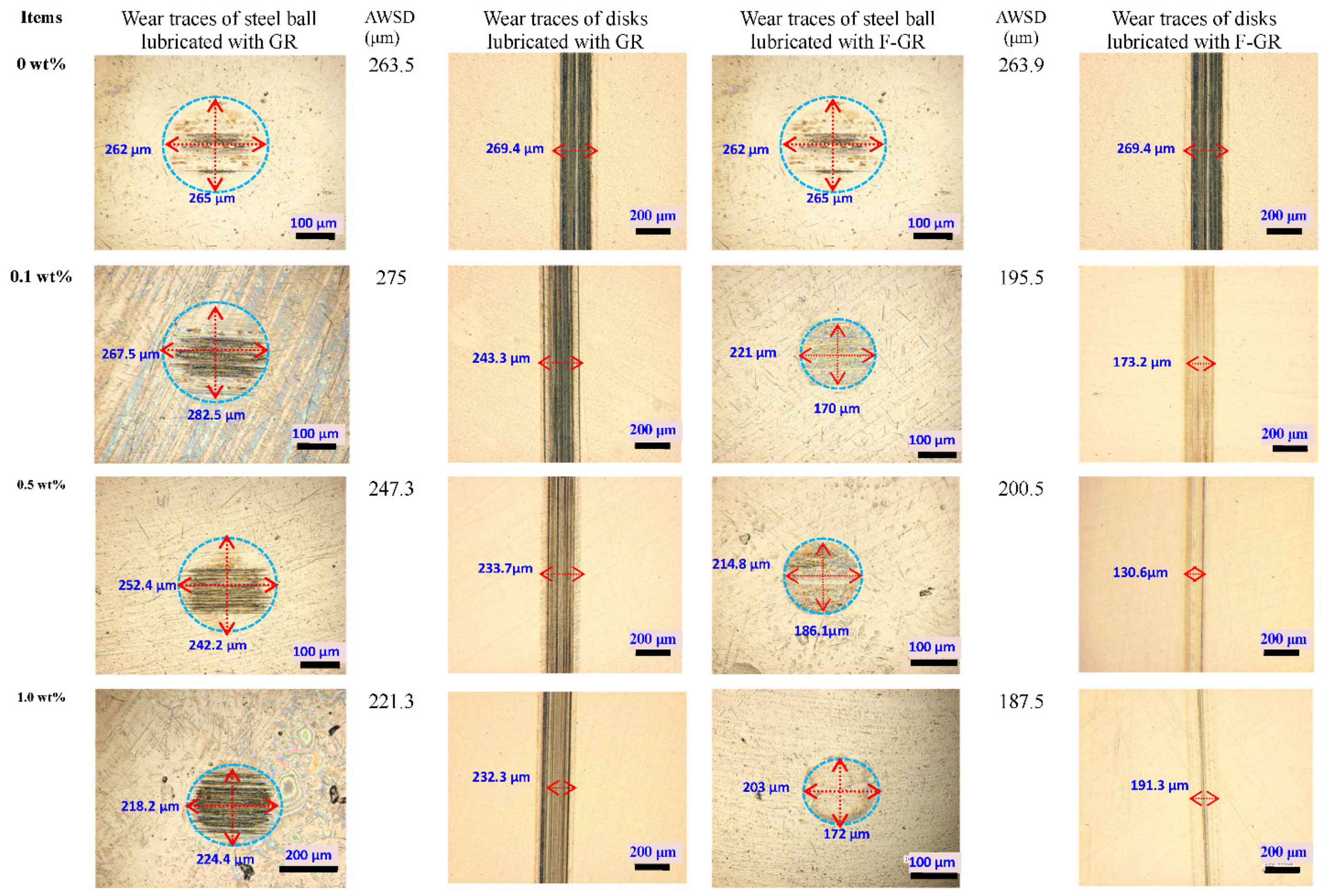
3.3. SEM/EDS Analysis
3.4. Friction and Wear Mechanism Analysis
4. Conclusions
- (1)
- GR has a better crystal structure than that of F-GR.
- (2)
- Two kinds of materials can improve the lubrication characteristics of PAO6 to some extent. The optimum addition level of F-GR was 1 wt% at 10 N, which resulted in the reduction of friction coefficient of 0.088 and wear rates of 0.033 × 10−9 mm3/Nm, which corresponded to decreases of 12.3% and 87%, respectively, relative to that of pure PAO6.
- (3)
- The wear resistance and friction reduction properties of F-GR for PAO6 were superior to GR. The accumulation of GR in PAO6 increased the average friction coefficient and wear rates at some conditions. Addition of graphite fluoride chips because of their small size, and a reasonable shear force quickly enter the wear interface achieving the effect of anti-wear and form a protective layer. The friction produced in the process of graphite fluoride debris can also be filled to contact surface grinding marks and bear part of the load to reduce wear and improve the effectiveness of the bearing capacity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Qu, J.; Barnhill, W.C.; Luo, H.; Meyer, H.M.; Leonard, D.N.; Landauer, A.K.; Kheireddin, B.; Gao, H.; Papke, B.L.; Dai, S. Synergistic Effects Between Phosphonium-Alkylphosphate Ionic Liquids and Zinc Dialkyldithiophosphate (ZDDP) as Lubricant Additives. Adv. Mater. 2015, 27, 4767–4774. [Google Scholar] [CrossRef]
- Sharma, V.; Johansson, J.; Timmons, R.B.; Prakash, B.; Aswath, P.B. Tribological Interaction of Plasma-Functionalized Polytetrafluoroethylene Nanoparticles with ZDDP and Ionic Liquids. Tribol. Lett. 2018, 66, 107. [Google Scholar] [CrossRef]
- Niste, V.B.; Tanaka, H.; Sugimura, J. The Importance of Temperature in Generating ZDDP Tribofilms Efficient at Preventing Hydrogen Permeation in Rolling Contacts. Tribol. Trans. 2018, 61, 930–937. [Google Scholar] [CrossRef]
- Shi, S.-C.; Wu, J.-Y.; Peng, Y.-Q. Transfer layer formation in MoS2/hydroxypropyl methylcellulose composite. Wear 2018, 408–409, 208–213. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, W.; Zhang, C. Microstructure Evolution and Tribological Performance of Cu-WS2 self-lubricating Composites. Wear 2018, 412–413, 109–119. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, S.; Hu, M.; Gao, X.; Jiang, D.; Sun, J. Tribological Properties of WS2/MoS2-Ag Composite Films Lubricated with Ionic Liquids under Vacuum Conditions. Tribol. Int. 2017, 115, 389–396. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Mohamed, K.A.A.; Hou, X.; Mohamed, A.A.; Gulzar, M.; Elsheikh, A.H. Novel Approach of the Graphene Nanolubricant for Energy Saving via Antifriction/wear in Automobile Engines. Tribol. Int. 2018, 124, 209–229. [Google Scholar]
- Wang, X.; Zhang, Y.; Yin, Z.; Su, Y.; Zhang, Y.; Cao, J. Experimental Research on Tribological Properties of Liquid Phase Exfoliated Grapheme as an Additive in SAE 10W-30 lubricating oil. Tribol. Int. 2019, 135, 29–37. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; et al. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303–205307. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, Y.; Lang, H. A Novel Approach to Decrease Friction of Grapheme. Carbon 2017, 118, 233–240. [Google Scholar] [CrossRef]
- Ramón-Raygoza, E.; Rivera-Solorio, C.; Giménez-Torres, E.; Cortés, D.M.; Cardenas-Alemán, E.; Sampedro, R.C. Development of nanolubricant based on impregnated multilayer graphene for automotive applications: Analysis of tribological properties. Powder Technol. 2016, 302, 363–371. [Google Scholar] [CrossRef]
- Zhao, J.; Mao, J.; Li, Y.; He, Y.; Luo, J. Friction-induced nano-structural evolution of graphene as a lubrication additive. Appl. Surf. Sci. 2018, 434, 21–27. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-situ exfoliated graphene for high-performance water-based lubricants. Carbon 2016, 96, 1181–1190. [Google Scholar] [CrossRef]
- Wu, P.; Wang, H.; Li, B.; Wang, X.; Liu, X. Characterization of Fluorinated Graphene and Its Reinforced Aromatic Polyamide Film. Polym. Mater. Sci. Eng. 2016, 9, 59–64. [Google Scholar]
- Wang, Z.; Wang, J.; Li, Z.; Gong, P.; Liu, X.; Zhang, L.; Ren, J.; Wang, H.; Yang, S. Synthesis of fluorinated graphene with tunable degree of fluorination. Carbon 2012, 50, 5403–5410. [Google Scholar] [CrossRef]
- Sun, J.; Du, S. Application of graphene derivatives and their nanocomposites in tribology and lubrication: A review. RSC Adv. 2019, 9, 40642–40661. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Lao, M.; Pan, D. Facile Synthesis and Spectroscopic Characterization of Fluorinated Graphene with Tunable C/F Ratio via Zn Reduction. Appl. Surf. Sci. 2017, 400, 339–346. [Google Scholar] [CrossRef]
- Hou, K.; Gong, P.; Wang, J.; Yang, Z.; Ma, L.; Yang, S. Construction of Highly Ordered Fluorinated Graphene Composite Coatings with Various Fluorine Contents for Enhanced Lubrication Performance. Tribol. Lett. 2015, 60, 1–12. [Google Scholar] [CrossRef]
- Uchsteiner, A.; Lerf, A.; Pieper, J. Water Dynamics in Graphite Oxide Investigated with Neutron Scattering. J. Phys. Chem. B 2006, 110, 22328–22338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, Y.; Che, J. Preparation and Application of Fluorinated Fossil ink. New Chem. Mater. 2017, 3, 10–12. [Google Scholar]
- Hou, K.; Gong, P.; Wang, J.; Yang, Z.; Wang, Z.; Yang, S. Structural and tribological characterization of fluorinated graphene with various fluorine contents prepared by liquid-phase exfoliation. RSC Adv. 2014, 4, 56543–56551. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.-Z.; Kim, S.-P.; Shenoy, V.B.; Sheehan, P.E.; Robinson, J.; Carpick, R.W. Fluorination of Graphene Enhances Friction Due to Increased Corrugation. Nano Lett. 2014, 14, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Yang, L.; Ouyang, L.; Liu, J.; Wang, H.; Zhu, M. A new method for few-layer graphene preparation via plasma-assisted ball milling. J. Alloys Compd. 2017, 728, 578–584. [Google Scholar] [CrossRef]
- Hu, E.; Dearn, K.D.; Yang, B.; Song, R.; Xu, Y.; Hu, X. Tribofilm formation and characterization of lubricating oils with biofuel soot and inorganic fluorides. Tribol. Int. 2016, 107, 163–172. [Google Scholar] [CrossRef]
- Kogut, L.; Etsion, I. Elastic-Plastic Contact Analysis of a Sphere and a Rigid Flat. J. Appl. Mech. 2002, 69, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Huang, P. Tribology Course, 1st ed.; High Education Publishing Ltd.: Beijing, China, 2008; ISBN 978-7-04-023351-3. [Google Scholar]
- Wang, B.; Hu, E.; Tu, Z.; David, K.D.; Hu, K.; Hu, X.; Yang, W.; Guo, J.; Cai, W.; Qian, W.; et al. Characterization and tribological properties of rice husk carbon nanoparticles Co-doped with sulfur and nitrogen. Appl. Surf. Sci. 2018, 462, 944–954. [Google Scholar] [CrossRef]
- Hu, E.; Hu, X.; Liu, T.; Liu, Y.; Song, R.; Chen, Y. Investigation of morphology, structure and composition of biomass-oil soot particles. Appl. Surf. Sci. 2013, 270, 596–603. [Google Scholar] [CrossRef]
- Ma, J.; Larsen, R.M. Comparative Study on Dispersion and Interfacial Properties of Single Walled Carbon Nanotube/polymer composites Using Hansen Solubility Parameters. ACS Appl. Mater. Interfaces 2013, 4, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 4, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Zhang, Y.; Kim, P.; Pinczuk, A. Electric Field Effect Tuning of Electron-Phonon Coupling in Graphene. Phys. Rev. Lett. 2007, 16, 166802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, E.; Hu, X.; Liu, T.; Fang, L.; Dearn, K.D.; Xu, H. The role of soot particles in the tribological behavior of engine lubricating oils. Wear 2013, 304, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Sandeep, C.S.; Senetakis, K. Effect of Young’s Modulus and Surface Roughness on the Inter-Particle Friction of Granular Materials. Materials 2018, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Sandeep, C.; Senetakis, K. An experimental investigation of the microslip displacement of geological materials. Comput. Geotech. 2019, 107, 55–67. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Mao, J.; He, Y.; Luo, J. Synthesis of thermally reduced graphite oxide in sulfuric acid and its application as an efficient lubrication additive. Tribol. Int. 2017, 116, 303–309. [Google Scholar] [CrossRef]
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Liang, H.; Bu, Y.; Zhang, J.; Cao, Z.; Liang, A. Graphene Oxide Film as Solid Lubricant. ACS Appl. Mater. Interfaces 2013, 5, 6369–6375. [Google Scholar] [CrossRef]
- Hu, E.; Hu, X.; Liu, T.; Song, R.; Dearn, K.D.; Xu, H. Role of TiF3 catalyst in the tribological properties of biofuel soot-contaminated liquid paraffin. Tribol. Int. 2014, 77, 122–131. [Google Scholar] [CrossRef]


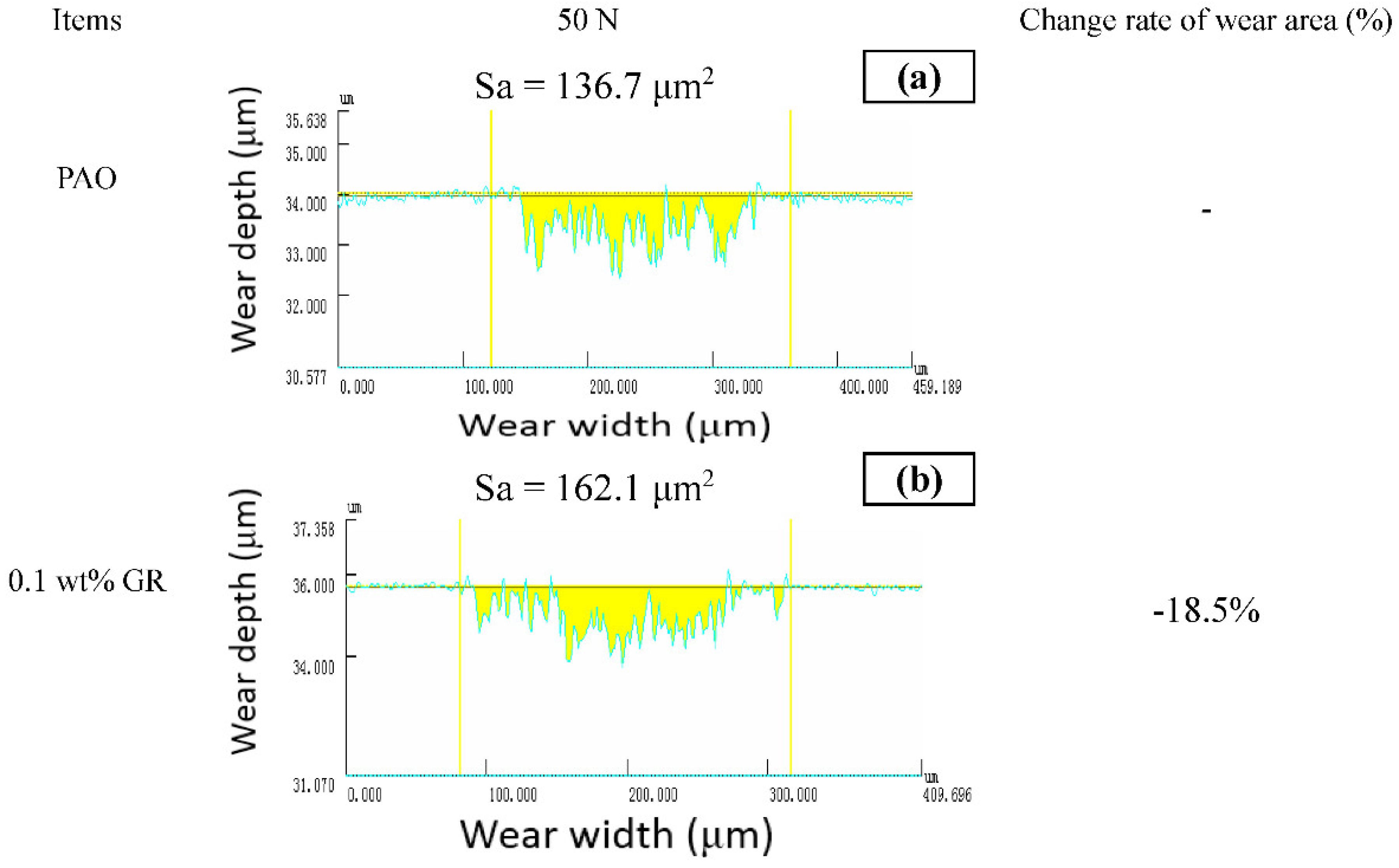

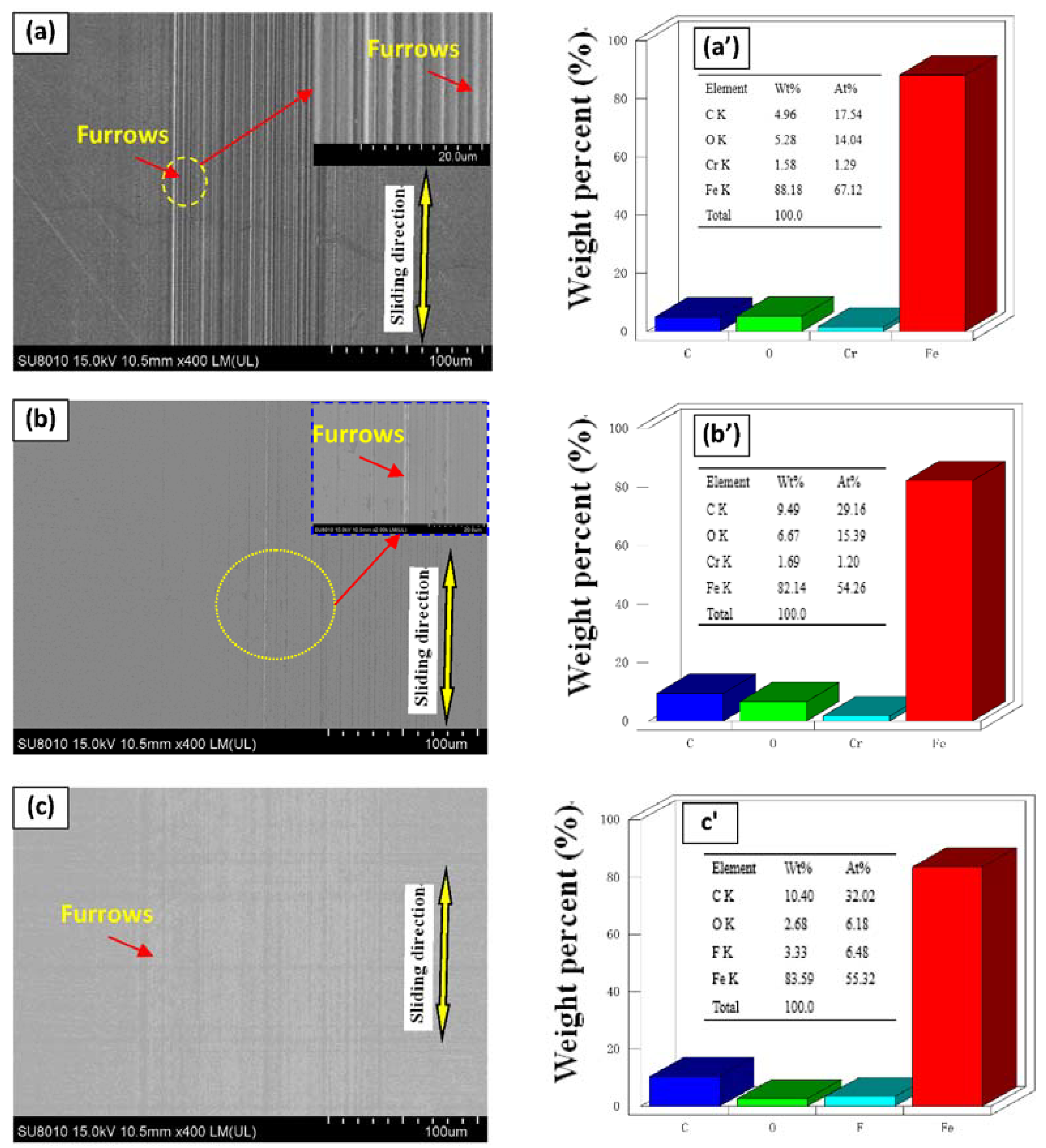
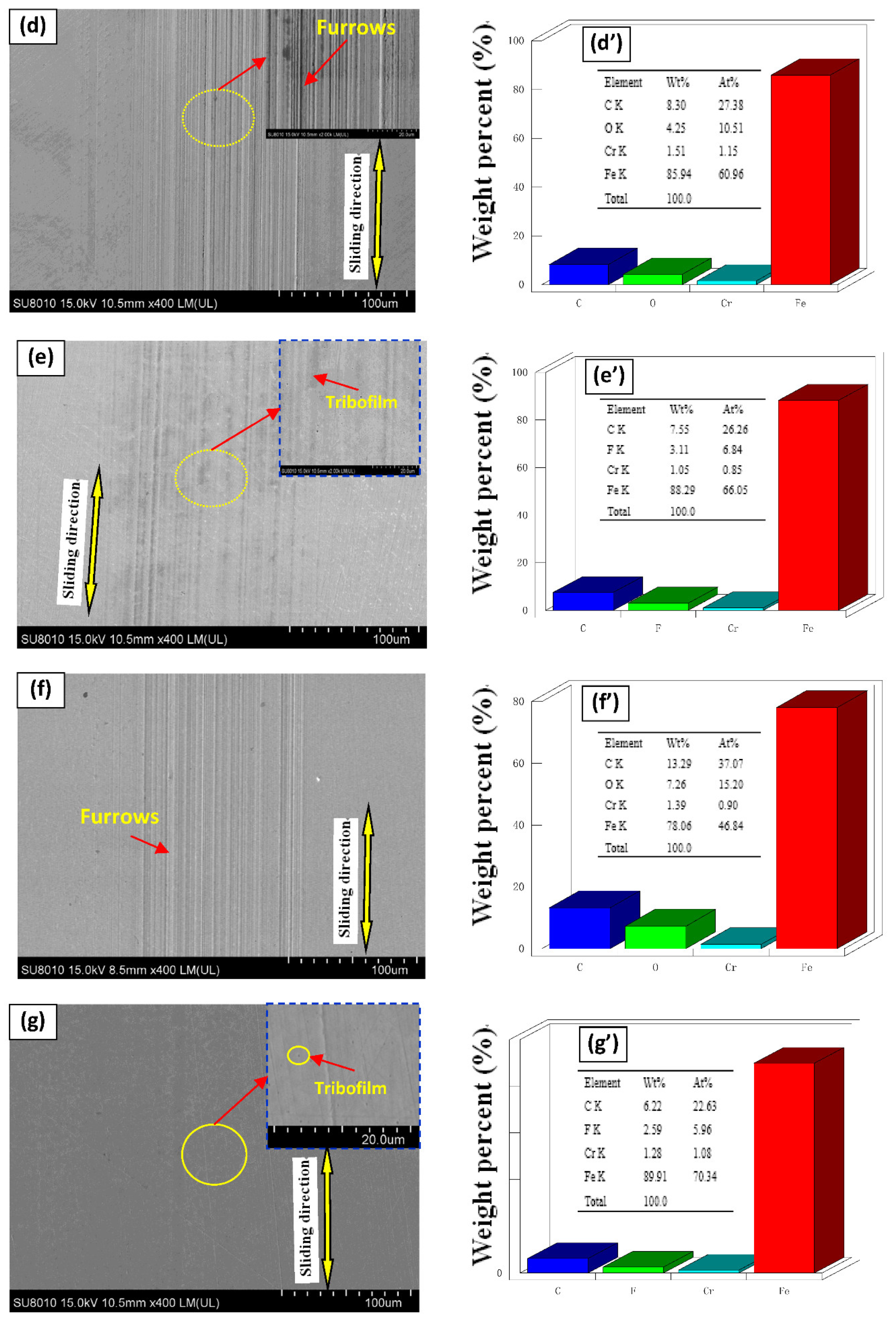



| Items | Element Atom Contents (%) | ||||
|---|---|---|---|---|---|
| C | O | Fe | Cr | F | |
| PAO6 | 23 | 35.22 | 35.11 | 3.05 | - |
| PAO6 + 0.1 wt% GR | 25.67 | 38.16 | 30.13 | 2.18 | - |
| PAO6 + 0.1 wt% F-GR | 27.87 | 34.32 | 27.79 | 2.6 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Hu, E.; Zhong, H.; Wang, J.; Subedi, A.; Hu, K.; Hu, X. Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO. Nanomaterials 2021, 11, 2126. https://doi.org/10.3390/nano11082126
Chen Y, Hu E, Zhong H, Wang J, Subedi A, Hu K, Hu X. Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO. Nanomaterials. 2021; 11(8):2126. https://doi.org/10.3390/nano11082126
Chicago/Turabian StyleChen, Yanjie, Enzhu Hu, Hua Zhong, Jianping Wang, Ayush Subedi, Kunhong Hu, and Xianguo Hu. 2021. "Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO" Nanomaterials 11, no. 8: 2126. https://doi.org/10.3390/nano11082126
APA StyleChen, Y., Hu, E., Zhong, H., Wang, J., Subedi, A., Hu, K., & Hu, X. (2021). Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO. Nanomaterials, 11(8), 2126. https://doi.org/10.3390/nano11082126






