Plasmonic Gold Nanomaterials as Photoacoustic Signal Resonant Enhancers for Cysteine Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
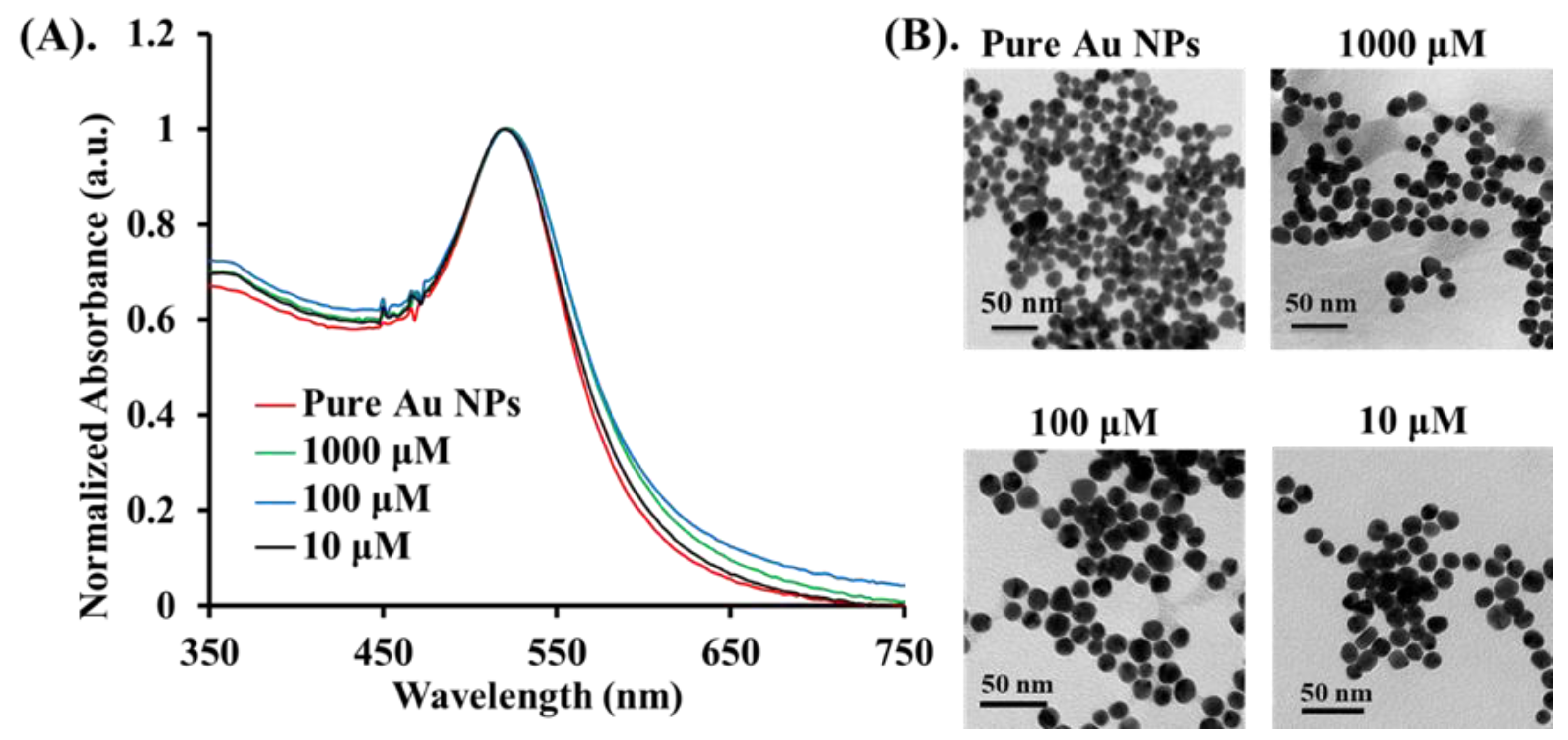
2.2. Synthesis of Gold Nanospheres (Au NPs)
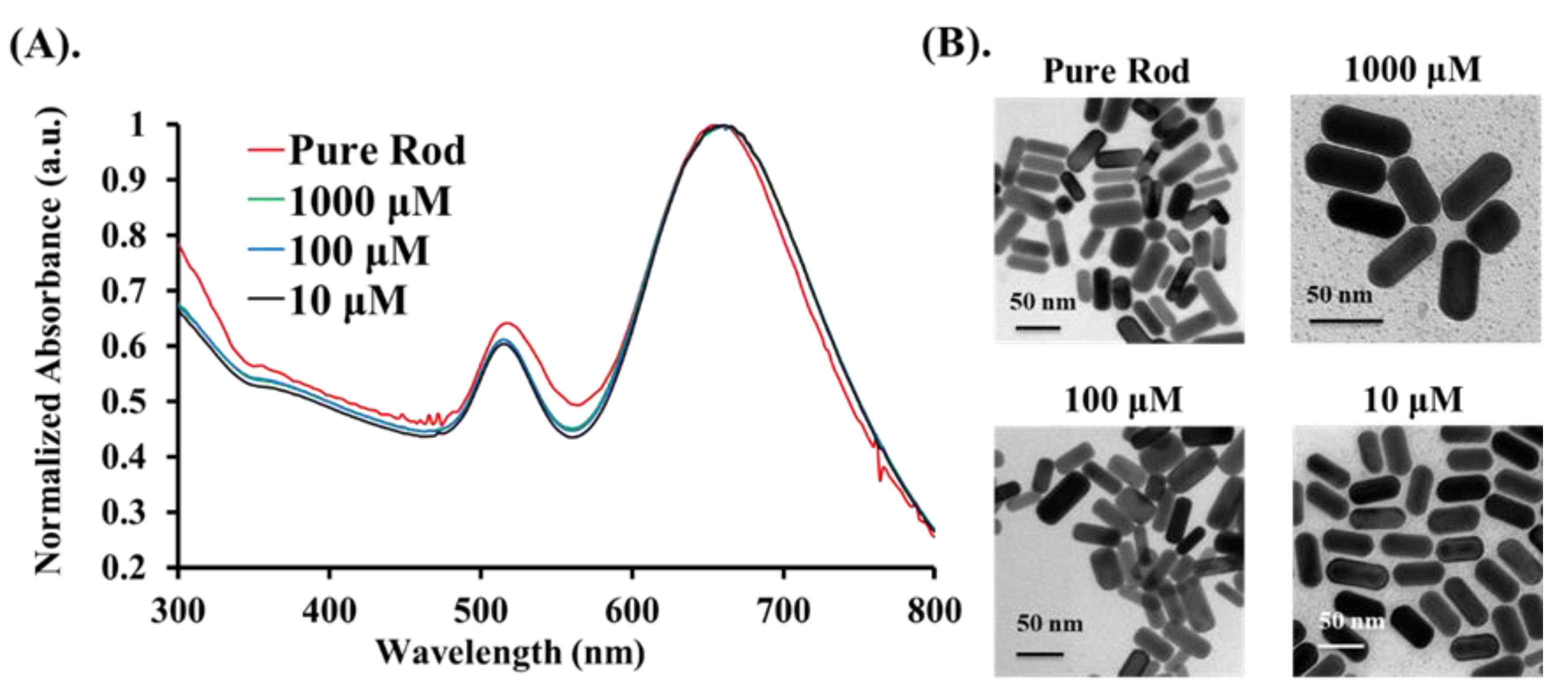
2.3. Synthesis of Gold Nanorods (Au NRs)
- (i)
- Synthesis of Gold Seeds (Au Seeds)
- (ii)
- Synthesis of Gold Growth Solution and Au NRs
2.4. L-Cysteine Capped Au Nanomaterials (Au@Cys NRs and Au@Cys NPs)
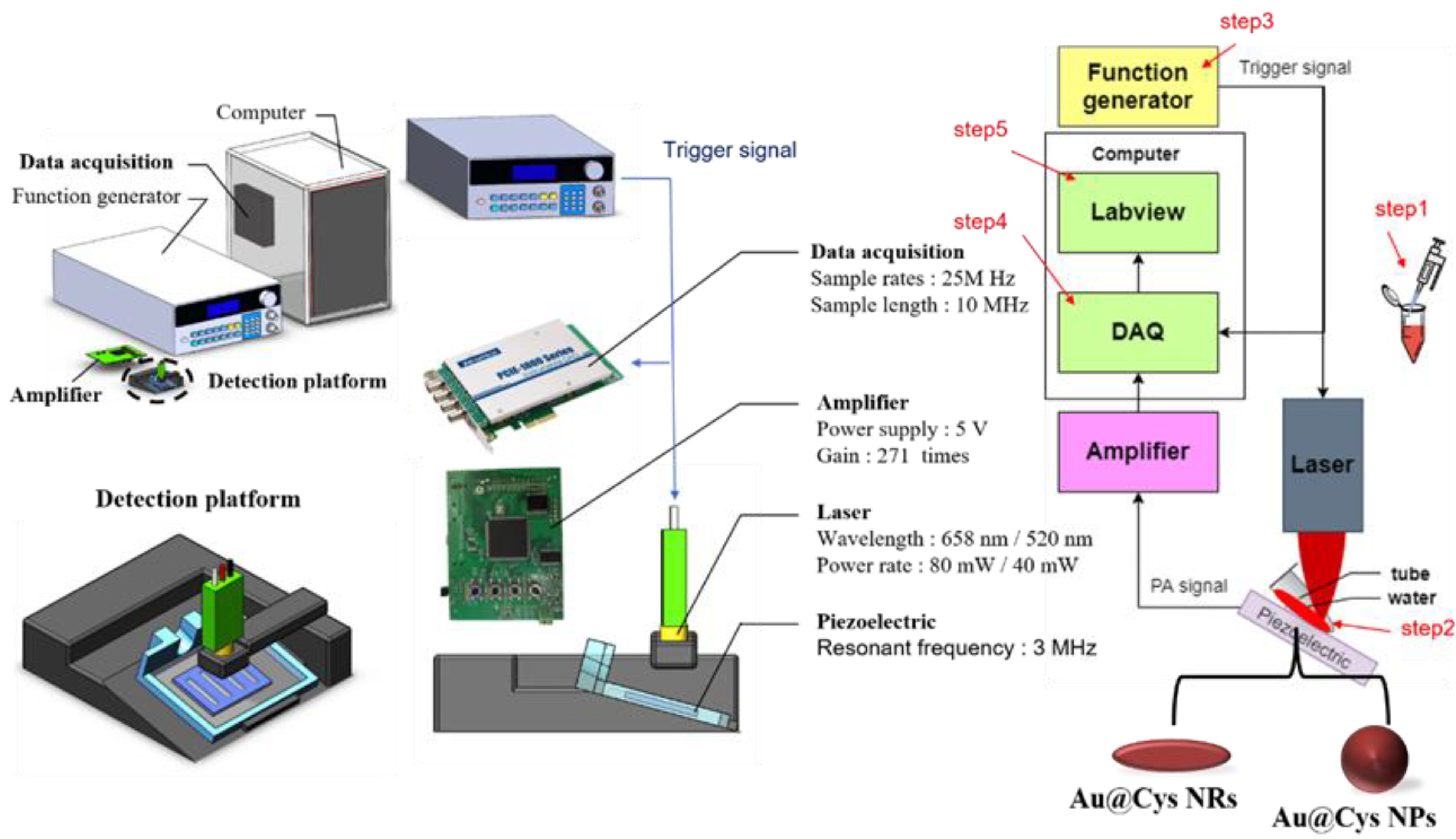
2.5. Photoacoustic System
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, Y.; Huo, F.; Ning, P.; Zhang, Y.; Chao, J.; Meng, X.; Yin, C. Dual-Site Fluorescent Probe for Visualizing the Metabolism of Cys in Living Cells. J. Am. Chem. Soc. 2017, 139, 3181–3185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Chen, J.; Li, G.; Yue, X.; Zhang, L.; Song, X.; Chen, W. Simultaneous detection of Cys/Hcy and H2S through distinct fluores-cence channels. Anal. Chim. Acta 2020, 1097, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, A.; Kundu, S.; Saha, S.; Sarkar, H.S.; Sahoo, P. Development of a new fluorescent probe for cysteine detection in processed food samples. Anal. Bioanal. Chem. 2019, 411, 6203–6212. [Google Scholar] [CrossRef]
- Chen, T.; Pei, X.; Yue, Y.; Huo, F.; Yin, C. An enhanced fluorescence sensor for specific detection Cys over Hcy/GSH and its bioimaging in living cells, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Chapter One—Mechanistic Chemical Perspective of Hydrogen Sulfide Signaling; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lehmann, A. Alterations in hippocampal extracellular amino acids and purine catabolites during limbic seizures induced by folate injections into the rabbit amygdala. Neuroscience 1987, 22, 573–578. [Google Scholar] [CrossRef]
- Thannhauser, T.; Sherwood, R.; Scheraga, H. Determination of the cysteine and cystine content of proteins by amino acid analysis: Application to the characterization of disulfide-coupled folding intermediates. Protein J. 1998, 17, 37–43. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Ye, Y.; Chen, W.; Song, X. A ratiometric fluorescent probe for simultaneous detection of Cys/Hcy and GSH. Org. Biomol. Chem. 2019, 17, 9631–9635. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, B.; Lu, C.; Lin, J.-M. Determination of cysteine, homocysteine, cystine, and homocystine in biological fluids by HPLC using fluorosurfactant-capped gold nanoparticles as postcolumn colorimetric reagents. J. Sep. Sci. 2013, 37, 30–36. [Google Scholar] [CrossRef]
- Hoogerheide, J.G.; Campbell, C.M. Determination of cysteine plus half-cystine in protein and peptide hydrolysates: Use of di-thiodiglycolic acid and phenylisothiocyanate derivatization. Anal. Biochem. 1992, 201, 146–151. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Guran, R.; Zitka, O.; Adam, V.; Krężel, A. Mass Spectrometry-Based Structural Analysis of Cysteine-Rich Met-al-Binding Sites in Proteins with MetaOdysseus R Software. J. Proteome Res. 2021, 20, 776–785. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Pisanu, E.; Sanna, M.; Sati, S.; Deiana, L.; Sengupta, S.; Carru, C. Determination of homocysteine thiolactone, reduced homocysteine, homocystine, homocysteine–cysteine mixed disulfide, cysteine and cystine in a reaction mixture by overimposed pressure/voltage capillary electrophoresis. Talanta 2010, 82, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, C. Spectrophotometric determination of cysteine and cystine in urine. Analyst 1990, 115, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.; Zhao, Y.; Wang, F.; Wu, J.; Liang, G. Using Bioluminescence Turn-On to Detect Cysteine in Vitro and in Vivo. Anal. Chem. 2018, 90, 4951–4954. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Recent advances in photoacoustic contrast agents for in vivo imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Karthikesh, M.S.; Yang, X. Photoacoustic image-guided interventions. Exp. Biol. Med. 2019, 245, 330–341. [Google Scholar] [CrossRef] [Green Version]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohammadi, M.; Yoon, S.J.; Yeager, D.; Emelianov, S. Photoacoustic Imaging for Cancer Detection and Staging. Curr. Mol. Imag. 2013, 2, 89–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, L. Photoacoustic Imaging and Spectroscopy; FL CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Abelha, T.; Dreiss, C.; Green, M.; Dailey, L. Conjugated polymers as nanoparticle probes for fluorescence and photoacoustic imag-ing. J. Mater. Chem. B 2020, 8, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Langer, G.; Bouchal, K.-D.; Grün, H.; Burgholzer, P.; Berer, T. Two-photon absorption-induced photoacoustic imaging of Rhoda-mine B dyed polyethylene spheres using a femtosecond laser. Opt. Express. 2013, 21, 22410–22422. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Yang, Y.; He, Z.; Wu, C.; Zhang, W.; Zhang, W.; Liu, J.; Li, P.; Tang, B. In situ photoacoustic imaging of cysteine to reveal the mechanism of limited GSH synthesis in pulmonary fibrosis. Chem. Commun. 2019, 55, 9685–9688. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-coated gold nanorods as photoacoustic signal nano-amplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2020, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Skrabalak, S.E.; Li, Z.-Y.; Xia, Y.; Wang, L. Photoacoustic Tomography of a Rat Cerebral Cortex in vivo with Au Nanocages as an Optical Contrast Agent. Nano Lett. 2007, 7, 3798–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Huang, S.W.; O’Donnell, M.; Day, K.C.; Day, M.L.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef] [Green Version]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Feng, L.; Xuan, Z.; Ma, J.; Chen, J.; Cui, D.; Su, C.; Guo, J.; Zhang, Y. Preparation of gold nanorods with different aspect ratio and the optical response to solution refractive index. J. Exp. Nanosci. 2015, 10, 258–267. [Google Scholar] [CrossRef]
- Pearson, K.X. Contributions to the mathematical theory of evolution.—II. Skew variation in homogeneous material. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1895, 186, 343–414. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.W.; Ou, T.K.; Chang, C.-C. Histogram Analysis of Photoacoustic Effect Changes on Different Liquid Samples. In Proceedings of the Third International Conference on Medical and Health Informatics 2019—ICMHI 2019, Association for Computing Machinery (ACM), Suzhou, China, 14–18 October 2019; pp. 17–20. [Google Scholar]
- Tompkins, W.J.; Afonso, V.X. (Eds.) Biomedical Digital Signal Processing: C-Language Examples and Laboratory Experiments for the IBM PC; Prentice-Hall, Inc.: Enlgewood Cliffs, NJ, USA, 1993. [Google Scholar]
- Ma, X.; Guo, Q.; Xie, Y.; Ma, H. Green chemistry for the preparation of l-cysteine functionalized silver nanoflowers. Chem. Phys. Lett. 2016, 652, 148–151. [Google Scholar] [CrossRef]
- Fukasawa, T.; Shinto, H.; Aoki, H.; Ito, S.; Ohshima, M. Size-dependent effect of gold nanospheres on the acoustic pressure pulses from laser-irradiated suspensions. Adv. Powder Technol. 2014, 25, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, G.I.; Hondow, N.; Gardiner, P.H. Factors influencing the surface functionalization of citrate stabilized gold nanoparticles with cysteamine, 3-mercaptopropionic acid or l-selenocystine for sensor applications. Chemosensors 2020, 8, 80. [Google Scholar] [CrossRef]
- Perera, G.S.; Athukorale, S.A.; Perez, F.; Pittman, C.U., Jr.; Zhang, D. Facile displacement of citrate residues from gold nanoparticle surfaces. J. Colloid Interface Sci. 2018, 511, 335–343. [Google Scholar] [CrossRef]
- Liu, C.-P.; Chen, K.-C.; Su, C.-F.; Yu, P.-Y.; Lee, P.-W. Revealing the active site of gold nanoparticles for the peroxidase-like activity: The determination of surface accessibility. Catalysts 2019, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef]
- Masim, F.C.P.; Liu, H.-L.; Porta, M.; Yonezawa, T.; Balčytis, A.; Juodkazis, S.; Hsu, W.-H.; Hatanaka, K. Enhanced photoacoustics from gold nano-colloidal suspensions under femtosecond laser excitation. Opt. Express 2016, 24, 14781–14792. [Google Scholar] [CrossRef]
- Strang, G. Edwin “Jed” Herman, Calculus Volume 1; Chapters 6 and 7; OpenStax: Houston, TX, USA, 2016. [Google Scholar]
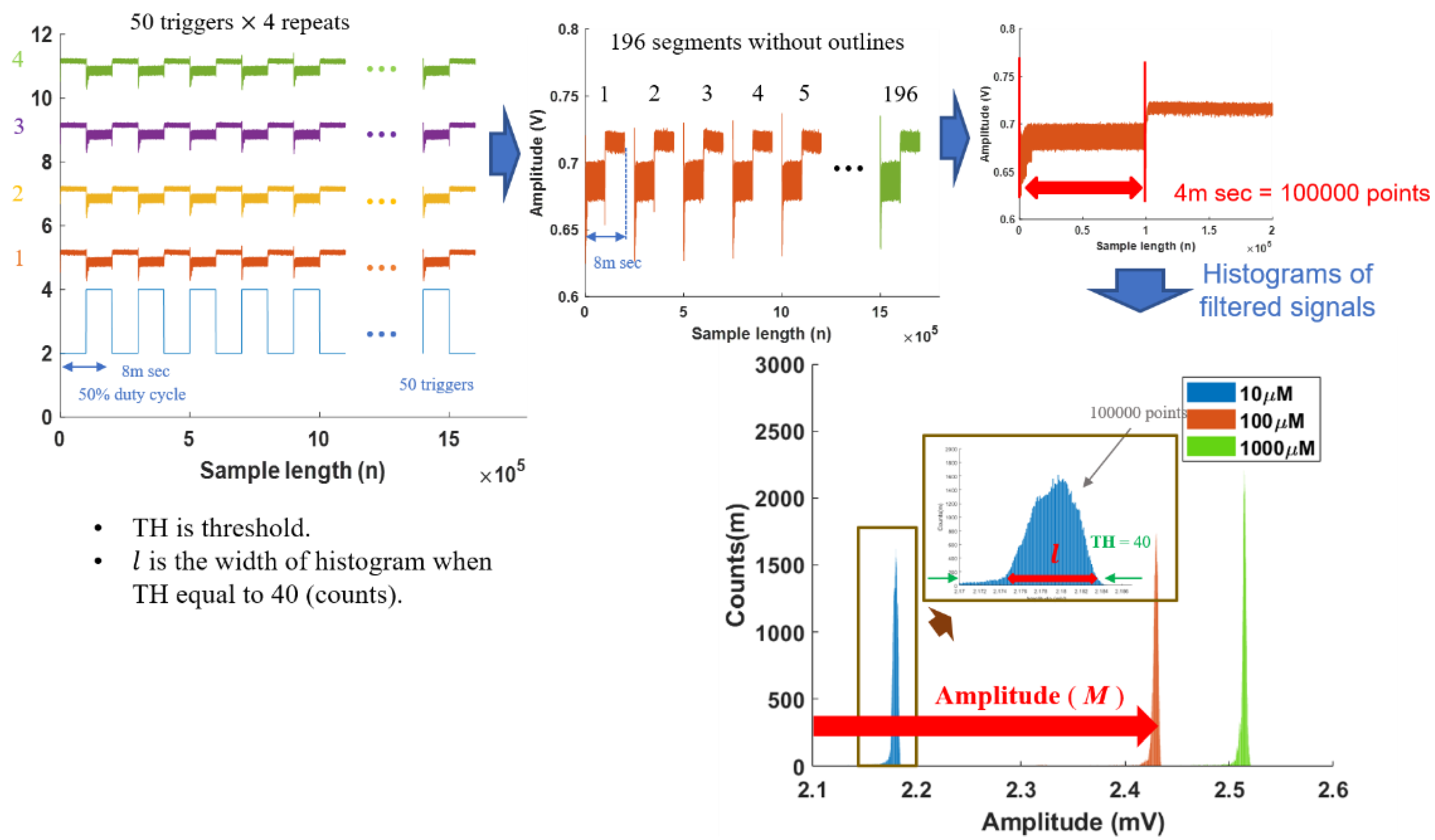
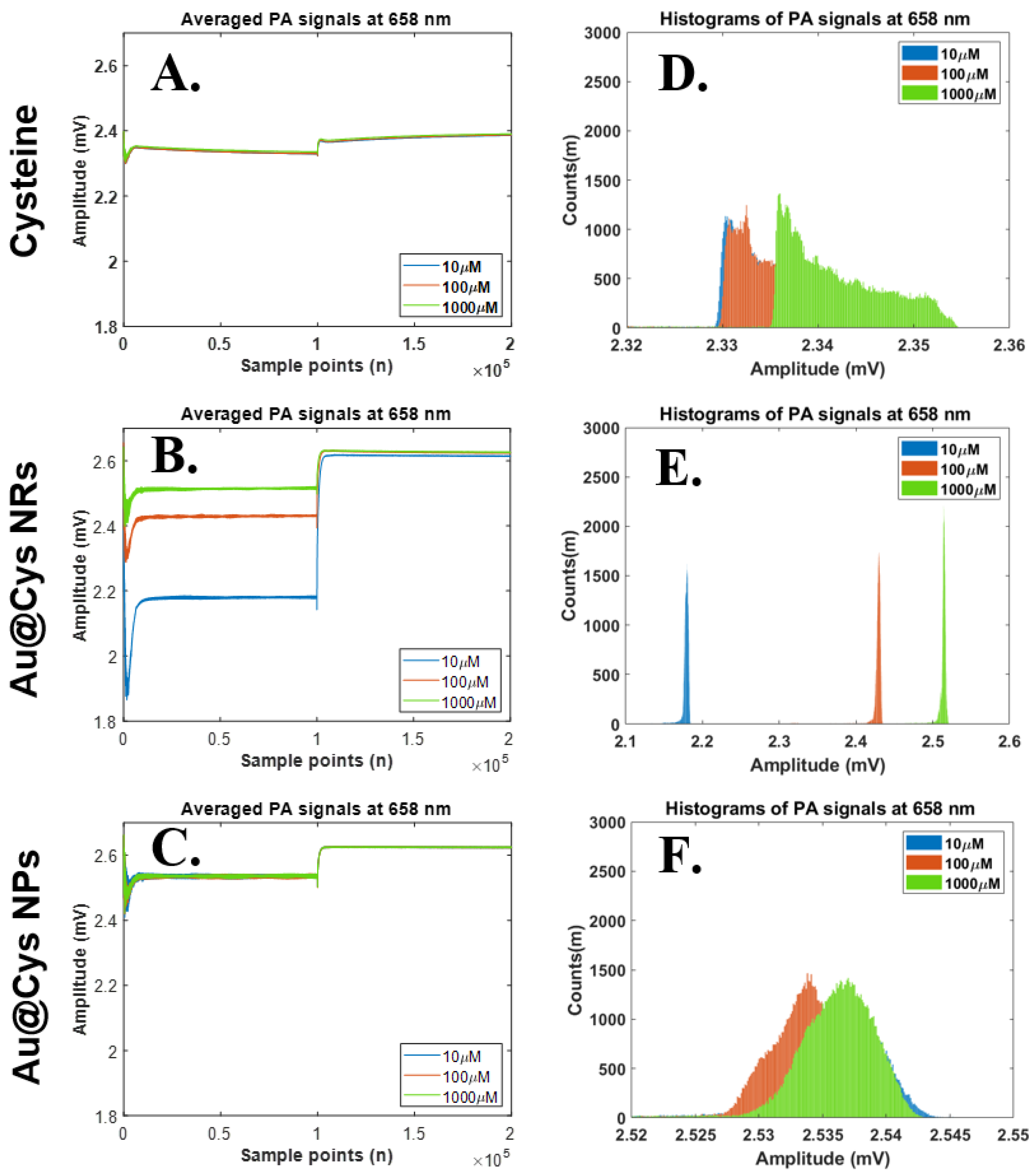


| Amplitude (M) N = 196 | |||||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (mV) (1) | p Value | ||||||
| CONC (μM) | Cys | Cys@Au NRs | Cys@Au NPs | CONC vs. CONC | Cys | Cys@Au NRs | Cys@Au NPs |
| 658 nm laser | 658 nm laser | ||||||
| 10 | 2.3367 ± 0.0005 | 2.1691 ± 0.0070 | 2.5338 ± 0.0015 | 10 vs. 100 | ns | **** | **** |
| 100 | 2.3367 ± 0.0066 | 2.4251 ± 0.0621 | 2.5324 ± 0.0020 | 10 vs. 1000 | **** | **** | **** |
| 1000 | 2.3416 ± 0.0107 | 2.5117 ± 0.0014 | 2.5346 ± 0.0032 | 100 vs. 1000 | **** | **** | **** |
| 520 nm laser | 520 nm laser | ||||||
| 10 | 2.3757 ± 0.0038 | 1.7860 ± 0.1144 | 2.3350 ± 0.0019 | 10 vs. 100 | **** | **** | **** |
| 100 | 2.3807 ± 0.0108 | 1.5584 ± 0.07012 | 2.4030 ± 0.0351 | 10 vs. 1000 | * | *** | **** |
| 1000 | 2.3767 ± 0.0070 | 1.8821 ± 0.3577 | 2.4244 ± 0.0145 | 100 vs. 1000 | **** | **** | ns |
| Histogram Width (l) N = 196 | |||||||
| Mean ± SD (mV) (1) | p Value | ||||||
| CONC (μM) | Cys | Cys@Au NRs | Cys@Au NPs | CONC vs. CONC | Cys | Cys@Au NRs | Cys@Au NPs |
| 658 nm laser | 658 nm laser | ||||||
| 10 | 9.3034 ± 0.4402 | 31.9986 ± 5.0176 | 46.7316 ± 3.3908 | 10 vs. 100 | ** | **** | ns |
| 100 | 9.4693 ± 0.6127 | 33.6338 ± 4.8570 | 46.7199 ± 3.3764 | 10 vs. 1000 | **** | **** | **** |
| 1000 | 12.0049 ± 0.9178 | 35.9976 ± 4.9866 | 53.4999 ± 4.1349 | 100 vs. 1000 | **** | **** | **** |
| 520 nm laser | 520 nm laser | ||||||
| 10 | 7.6219 ± 0.7148 | 14.3424 ± 6.1438 | 15.3343 ± 6.0414 | 10 vs. 100 | ns | **** | ns |
| 100 | 7.6477 ± 0.7394 | 15.6709 ± 6.0875 | 15.3304 ± 6.0847 | 10 vs. 1000 | **** | **** | **** |
| 1000 | 8.3673 ± 0.5976 | 18.0120 ± 6.5396 | 17.3117 ± 6.0219 | 100 vs. 1000 | **** | **** | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.-W.; Ou, T.-K.; Lin, B.-Y.; Chien, Y.-H. Plasmonic Gold Nanomaterials as Photoacoustic Signal Resonant Enhancers for Cysteine Detection. Nanomaterials 2021, 11, 1887. https://doi.org/10.3390/nano11081887
Shen T-W, Ou T-K, Lin B-Y, Chien Y-H. Plasmonic Gold Nanomaterials as Photoacoustic Signal Resonant Enhancers for Cysteine Detection. Nanomaterials. 2021; 11(8):1887. https://doi.org/10.3390/nano11081887
Chicago/Turabian StyleShen, Tsu-Wang, Ting-Ku Ou, Bo-Yan Lin, and Yi-Hsin Chien. 2021. "Plasmonic Gold Nanomaterials as Photoacoustic Signal Resonant Enhancers for Cysteine Detection" Nanomaterials 11, no. 8: 1887. https://doi.org/10.3390/nano11081887
APA StyleShen, T.-W., Ou, T.-K., Lin, B.-Y., & Chien, Y.-H. (2021). Plasmonic Gold Nanomaterials as Photoacoustic Signal Resonant Enhancers for Cysteine Detection. Nanomaterials, 11(8), 1887. https://doi.org/10.3390/nano11081887








